Abstract
As a result of social distancing measures in response to the Covid-Sars 2 pandemic, our school sent chemistry kits to the students’ homes for remote experimentation. This allowed the performance of ∼25 experiments per person in each of the Fall 2020 and Spring 2021 semesters in an elective chemistry course. Students were requested to design some experiments of their own and then have the entire group reproduce them. One such experiment consisted of the anodic indirect electrogeneration of colloidal sulfur by solution acidification to produce thiosulfate disproportionation. This was evidenced by the well-known Rayleigh scattering phenomenon. Here, the trajectory and polarization state of light are modified by its interaction with a medium containing particles of smaller diameter than the wavelengths of incident light. If white light interacts with this medium, the smaller wavelengths (e.g., blue, violet) are radially scattered while the longer wavelengths (e.g., orange, red) pass through the suspension. Such scattering is responsible for beautiful sunsets and blue skies and is produced here by an indirect electrochemical process that generates colloidal sulfur. Students evidence the scattering of light shone from simple cell phone flashlights. The entire procedure is performed in a 2-h class session. Key student outcomes are presented.
Introduction
Distance learning became crucial during the SARS-Covid-19 pandemic. We explored a non-traditional distance learning mode that involved the sending of microscale chemistry kits (Ibanez, 2011) to the students’ homes for remote experimentation. This allowed the performance of ∼25 experiments per person in each of the Fall-2020 and Spring-2021 semesters in an elective undergraduate chemistry course. As part of this course, students were requested to design experiments on their own and have the entire group reproduce them in their homes. Published outcoming examples include the fabrication of a fuel cell from solar electrolysis (Cesin-AbouAtme et al., 2021), bismuth plating by galvanic displacement (Aguilar-Charfen et al., 2021), tin electroplating (Herrera-Loya et al., 2022), homemade colloidal silver (Covarrubias-Montero and Ibanez, 2022), and homemade CO2 capture and conversion (Acuña-Girault et al., 2022). The present experiment, Rayleigh scattering, accomplishes the intersection of physics, environmental science, and electrochemistry.
Rayleigh scattering is the dispersion of light caused by the presence of isotropic particles acting as electric dipoles in a fluid through which it passes (Bender, 1952). The trajectory and polarization state of the incident electromagnetic radiation are modified by its interaction with such particles whose diameters are smaller than the wavelength of incident light. Induced dipole oscillations in Rayleigh scattering occur when particle diameters are up to about one-tenth of the wavelength of the scattered light (Ahn & Whitten, 2005). The dispersion intensity, I depends on several factors and is inversely proportional to the fourth power of the wavelength, λ: I α (1/λ 4) (Bender, 1952; Nave, 2016; Plumb, 1971). Because colloidal particle diameters are typically in the range 1–200 nm (Del Mazo-Vivar, 2016), when white light impinges on a colloid the shorter wavelengths (violet and blue) are dispersed more efficiently (i.e., radially scattered), whereas the longer wavelengths (orange and red) pass through the colloidal suspension with little or no dispersion. Such scattering is responsible for the beautiful sunsets and blue skies (Nave, 2016; Plumb, 1971).
Rayleigh scattering educational experiments are relatively scarce. One such experiment is aimed at observing the rotation of polarized light in an optically active medium, and the scattered light that tracks the directional radiation pattern of an oscillating dipole in an optically inactive medium (Avalos-Pecina et al., 1999). Another experiment allows direct determination of wavelength dependence of the Rayleigh scattering cross section (Chakraborti, 2007). A related experiment demonstrates that during fluorescence, the emitted wavelength is independent of the excitation wavelength, whereas the wavelength of Rayleigh scattered light increases with increasing excitation wavelength (Clarke & Oprysa, 2004).
In the present experiment, students reproduced this phenomenon at home with the contents of a kit sent to their homes by our school, together with easily available household items. They observed Rayleigh scattering by elemental sulfur indirectly electrogenerated by anodically-produced protons which acidify a thiosulfate solution. This in turn undergoes disproportionation into S(0) + S(IV). When shining light from a cell phone flashlight, the longer wavelengths pass through the solution, and the shorter wavelengths are radially scattered. The experiment should be useful for general, inorganic, environmental, green, and physical chemistry introductory lab courses, and can be performed either at school or at home. The simplicity of the required equipment and procedures should allow its implementation in high schools as well.
Experimental
Water electrolysis will be utilized to generate the necessary acidic medium for the thiosulfate disproportionation. Thiosulfate is obtained from an aquarium store in the form of a dechlorinator (typically an alkaline thiosulfate solution). In this case, we used Clorkill® (a 0.12 M NaHCO3, 0.63 M Na2S2O3 solution). Due to the presence of bicarbonate, the basic medium generated by this salt should be initially neutralized to shorten the time required for the protons from water electrolysis to neutralize its basicity. The acidic medium for the required thiosulfate acidification will be provided by the anodic half-cell in the water electrolysis process.
Preparation and use of the electrochemical cell
Two 10-mL beakers (A and B) will contain the anolyte and catholyte, respectively. Add ∼5 mL of water to beaker A, followed by 10 drops of Clorkill® and 3–4 drops of phenolphthalein indicator (1% solution). A pink coloration indicates that the solution is basic, mainly because of its NaHCO3 content. To speed up the experiment, neutralize this basic solution by adding white kitchen vinegar dropwise up to the pink-to-transparent endpoint. Once this point is reached, 1–2 extra drops of vinegar may be added. Then, pour 10–15 mL of water in a 50 mL beaker and add a spatula-tip of table salt (preferably of the non-iodized type to prevent side reactions). Stir to dissolve. Transfer ∼5–6 mL of this solution to beaker B. Put in contact the contents of beakers A and B through a salt bridge made of an ∼8 to 10 cm piece of textile yarn (previously soaked for a couple of minutes inside the 50 mL beaker in the NaCl solution). See Figure 1A.
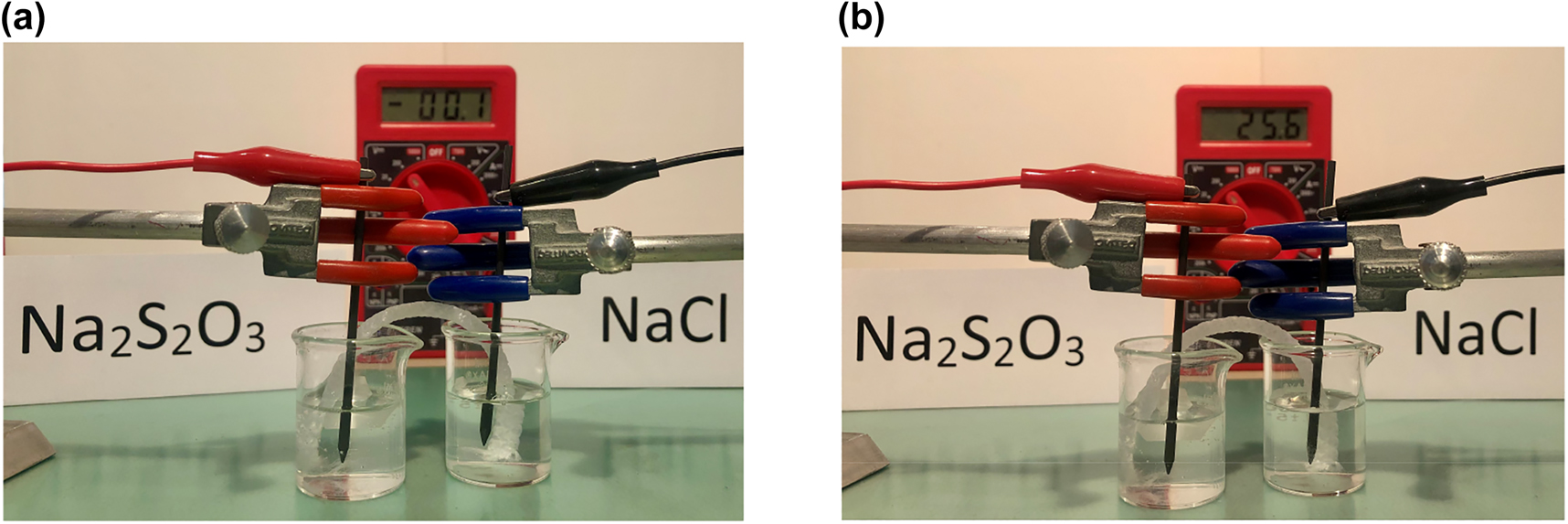
Electrolysis set up.
(a) Before connecting the power source. (b) After connecting the power source. (Note some hydrogen bubbling in the cathodic side).
Connect a 6–8 cm long graphite lead (e.g., Steadtler Mars HB, 2 mm in diameter) to an alligator clip and immerse the lead into the solution of beaker A (that contains the Clorkill®; this will be the anolyte). Do the same with another graphite lead for beaker B (the solution here will be the catholyte). Connect the alligator clips to an AC-to-DC adjustable voltage power adapter (e.g., Steren model ELI-035). Connect the positive end of the power source to the graphite lead in beaker A, and the negative to that in beaker B. Then, connect the adapter (at a nominal voltage setting of 12 V, which gives a true voltage of ∼25 V) to a regular home electricity outlet (i.e., 120 V). See Figure 1B. (A 9-V dry cell unfortunately does not yield the desired results within a reasonable timeframe).
Demonstration of Rayleigh scattering
After 10–20 min of electrolysis time, a colloidal suspension starts forming in the anolyte. Unplug the electrical connections and allow the freshly formed suspension to age for another 10–30 min. Stir (e.g., with a toothpick). Then, place a white surface behind the beakers. Shine white light (e.g., from a cell phone’s flashlight) in front of beaker A to project it onto the white surface. Observe the dispersion effect and write down your observations.
Results and discussion
Protons from vinegar neutralize the excess basicity caused in Clorkill® by the bicarbonate ions:
Then, the main electrolysis reactions are:
Oxidation:
Reduction:
Global reaction:
Reaction (2) furnishes the protons needed by the thiosulfate ions to disporportionate into S(0) and S(IV) species. The main reactions generally accepted are (Chakraborti, 2007; UMass-Amherst, 2011):
H2SO3 represents the equilibrium between SO2 and H2O. The Tyndall effect originated by the colloid produced by elemental sulfur can be observed with the aid of a common red laser. See Figure 2.
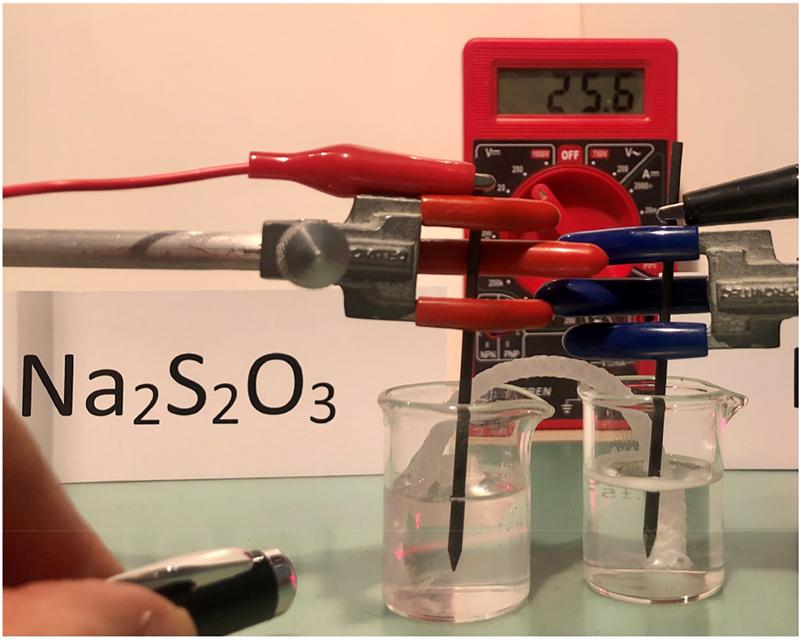
Demonstration of the Tyndall effect. (Also note more hydrogen bubbles at the surface of the catholyte).
As elemental sulfur is produced, this and its polysulfide moieties in equilibrium grow and produce a slightly milky colloidal suspension that is ready to initiate Rayleigh’s scattering. When light shines on it, a bluish tone is observed from the wavelengths radially scattered while a yellowish tone is observed passing through. As particles grow larger, the unscattered light becomes more orange-reddish. See Figure 3.

Rayleigh scattering in a colloidal suspension.
This phenomenon has been dramatically proven elsewhere with a more sophisticated set up by using 407-nm violet and 670-nm red diode lasers to monitor the growth of colloidal sulfur particles and examine reaction kinetics. Preferential scattering of violet light was observed at the beginning of the reaction (i.e., small particle sizes), whereas an intense red-light scattering occurred as particle size increased (Ahn & Whitten, 2005).
At the end of the course we analyzed key learning outcomes through a voluntary anonymous survey that was responded by 92% of the students (i.e., 11 out of 12). The main outcomes are now presented in Table 1 (Ibanez & Contreras-Ruiz, 2021).
Student outcomes.
| Responses and percentages | Strongly Agree | Agree | Undecided | Disagree | Strongly Disagree |
|---|---|---|---|---|---|
|
Specific knowledge acquired during the course
|
|||||
| With the practice at home I improved my mastery of the topics on environmental, organic, inorganic electrochemistry, energy storage, electrochemical theory, and corrosion. | 45 | 55 | 0 | 0 | 0 |
| I learned to design sound scientific procedures to generate new experiments or solve basic experimental problems. | 82 | 18 | 0 | 0 | 0 |
| I understood and applied green chemistry principles such as decreasing the amounts of substances used and minimizing the generation of waste. | 55 | 45 | 0 | 0 | 0 |
| Using scientific data published in reliable sources helped me better understand or report the results I observed. | 45 | 36 | 18 | 0 | 0 |
| I learned the correct use of basic glassware and laboratory equipment following the proper techniques. | 91 | 9 | 0 | 0 | 0 |
| I discussed with my classmates and with the professor the scientific or mathematical theories and models developed through data analysis to explain some of the observed phenomena. | 18 | 45 | 36 | 0 | 0 |
|
|
|||||
| Competencies developed during the course | |||||
|
|
|||||
| I further developed scientific reasoning, particularly in electrochemistry and corrosion. | 55 | 45 | 0 | 0 | 0 |
| I generated oral and written arguments consistent with scientific practice. | 55 | 45 | 0 | 0 | 0 |
| I made progress in my ability to prepare laboratory reports and audiovisual resources aligned with good scientific practices. | 73 | 27 | 0 | 0 | 0 |
| I managed my time and space efficiently in my home laboratory, and I improved in developing teamwork skills. | 45 | 36 | 18 | 0 | 0 |
| I understood and applied the principles of ethical behavior in academia and in scientific practice. | 82 | 9 | 9 | 0 | 0 |
| I developed learning and autonomous study skills applied to electrochemistry and corrosion. | 55 | 45 | 0 | 0 | 0 |
|
|
|||||
| Safety skills practiced during the course | |||||
|
|
|||||
| I knew and used the safety standards and the proper handling of chemicals in a home laboratory. | 100 | 0 | 0 | 0 | 0 |
| I participated in chemical practice in a conscious manner regarding safety and the environment. | 82 | 18 | 0 | 0 | 0 |
Conclusions
Distance learning can be enhanced by performing live experiments at home. Electrolysis can provide basic or acidic media as required, depending on the compartment selected to this end. When an acidic medium is produced in a thiosulfate solution, a chemical disproportionation occurs, and the resulting sulfur colloidal suspension produces an easily observable Rayleigh scattering of white incident light.
Safety and hazards
During the production of S(s), a small amount of SO2(g) is also produced. This gas is moderately toxic and therefore the experiment should be performed in a well-ventilated area. When testing the Tyndall effect with a red laser make sure not to observe its reflections on the beaker´s wall, as these can hurt the eye (Ibanez, 2003). Use general eye and skin protection. The residues generated are considered non-toxic and should be discarded according to local regulations.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Acuña-Girault, A., Gómez del Campo-Rábago, X., Contreras-Ruiz, M. A., & Ibanez, J. G. (2022). CO2 capture and conversion: A homemade experimental approach. Journal of Technology and Science Education. In press.10.3926/jotse.1610Search in Google Scholar
Aguilar-Charfen, J. L., Castro-Sayago, I., Turnbull-Agraz, J., & Ibanez, J. G. (2021). Homemade bismuth plating by galvanic displacement from bismuth subsalicylate tablets: A chemistry experiment for distance learning. Chemistry Teacher International, 3, 423–429. https://www.degruyter.com/document/doi/10.1515/cti-2021-0002/html.10.1515/cti-2021-0002Search in Google Scholar
Ahn, H., & Whitten, J. E. (2005). Monitoring particle growth: Light scattering using red and violet diode lasers. Journal of Chemical Education, 82, 909–911. https://pubs.acs.org/doi/abs/10.1021/ed082p909.10.1021/ed082p909Search in Google Scholar
Avalos-Pecina, M., Smith, C. A., Johnson, K., & Snetsinger, P. (1999). A classroom demonstration of Rayleigh light scattering in optically active and inactive systems. Journal of Chemical Education, 76, 1230–1233. https://doi.org/10.1021/ed076p1230.Search in Google Scholar
Bender, M. (1952). The use of light scattering for determining particle size and molecular weight and shape. Journal of Chemical Education, 29, 15–23. https://doi.org/10.1021/ed029p15.Search in Google Scholar
Cesin-AbouAtme, T., Lopez-Almeida, C. G., Molina-Labastida, G., & Ibanez, J. G. (2021). Light-emitting diodes as voltage generators: Demonstrating the fuel cell principle with low cost, magnetically enhanced homemade solar electrolysis. Journal of Chemical Education, 98, 3045–3049.10.1021/acs.jchemed.1c00093Search in Google Scholar
Chakraborti, S. (2007). Verification of the Rayleigh scattering cross section. American Journal of Physics, 75, 824–826. https://doi.org/10.1119/1.2752825.Search in Google Scholar
Clarke, R. J., & Oprysa, A. (2004). Fluorescence and light scattering. Journal of Chemical Education, 81, 705–707. https://doi.org/10.1021/ed081p705.Search in Google Scholar
Covarrubias-Montero, A. S., & Ibanez, J. G. (2022). Distance learning: Homemade colloidal silver. Natural Science and Advanced Technology Education (Bulgaria), 31(1), 102–108. https://doi.org/10.53656/nat2022-1.05.Search in Google Scholar
Del Mazo-Vivar, A. (2016). Rayleigh scattering. Revista Eureka sobre enseñanza y divulgación de las ciencias (in Spanish), 13, 505–510. https://doi.org/10.25267/Rev_Eureka_ensen_divulg_cienc.2016.v13.i2.19.Search in Google Scholar
Herrera-Loya, M. R., Cervantes-Herrera, L. M., Gutierrez-Vallejo, S., & Ibanez, J. G. (2022). Leaded or unleaded? Homemade microscale tin electroplating. Chemistry Teacher International, 4, 97–102. https://doi.org/10.1515/cti-2021-0024.Search in Google Scholar
Ibanez, J. G. (2003). Take care when using that laser pointer. Journal of Chemical Education, 80, 30.10.1021/ed080p30.1Search in Google Scholar
Ibanez, J. G. (2011). Spreading the good news of chemistry: Macroscale appreciation for a microscale approach. Journal of Chemical Education, 88, 127–129. https://doi.org/10.1021/ed1010745.Search in Google Scholar
Ibanez, J. G., & Contreras-Ruiz, M. A. (2021). Innovation in teaching methodologies for distance classes in practical Chemistry. Best Practice Jesuit Higher Education, 2.1, 97–100.Search in Google Scholar
Nave, R. (2016). Hyperphysics: Blue Sky. Retrieved from http://hyperphysics.phy-astr.gsu.edu/hbase/atmos/blusky.html [Accessed 22 Mar 2022].Search in Google Scholar
Plumb, R. C. (1971). Blue skies and the Tyndall effect. Journal of Chemical Education, 48, 389.10.1021/ed048p389.1Search in Google Scholar
UMass-Amherst (2011). Lecture Demonstrations. Retrieved from https://lecturedemos.chem.umass.edu/solutions13_4.html [Accessed 22 Mar 2022].Search in Google Scholar
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Frontmatter
- Guest Editorial
- Introduction to the special issue on Green Chemistry
- Special Issue Papers
- Simple experiments with immobilized enzymes as a contribution to green and sustainable chemistry education in the high school laboratory
- Learning Green Chemistry and its principles from Nature’s process and development of green procedures mimicking nature
- SpottingScience – a digital learning environment to introduce Green Chemistry to secondary students and the public
- Student explorations of calcium alginate bead formation by varying pH and concentration of acidic beverage juices
- Good Practice Report
- Simple green organic chemistry experiments with the kitchen microwave for high school chemistry classrooms
- Invited Paper
- Basic concept and application of conducting polymers for environmental protection
- Good Practice Report
- Distance learning: an interdisciplinary experiment on Rayleigh scattering
- Special Issue Paper
- Development of teaching material for green and sustainable chemistry in Japan
Articles in the same Issue
- Frontmatter
- Guest Editorial
- Introduction to the special issue on Green Chemistry
- Special Issue Papers
- Simple experiments with immobilized enzymes as a contribution to green and sustainable chemistry education in the high school laboratory
- Learning Green Chemistry and its principles from Nature’s process and development of green procedures mimicking nature
- SpottingScience – a digital learning environment to introduce Green Chemistry to secondary students and the public
- Student explorations of calcium alginate bead formation by varying pH and concentration of acidic beverage juices
- Good Practice Report
- Simple green organic chemistry experiments with the kitchen microwave for high school chemistry classrooms
- Invited Paper
- Basic concept and application of conducting polymers for environmental protection
- Good Practice Report
- Distance learning: an interdisciplinary experiment on Rayleigh scattering
- Special Issue Paper
- Development of teaching material for green and sustainable chemistry in Japan


