Abstract
Teaching experiments involving edible, biodegradable calcium alginate beads serve as an attractive model system to introduce upper secondary age students to core chemistry topics through innovations in sustainable consumer products. A teaching experiment is described that engages students with the synthesis of calcium alginate hydrogel beads from sodium alginate and calcium lactate, two food-safe and renewable materials. The beads’ outer membranes are a result of ionic interactions between carboxylate groups from alginate strands and the divalent calcium cations between them, thus forming cross-linked polymers. Protonation of the carboxylate groups on the alginate strands decreases crosslinking density affecting bead formation. First, various concentrations of citric acid are used to lower the pH of the sodium alginate solution and the effect on the calcium alginate bead formation is observed. A correlation between pH and bead shape and firmness is derived. This information is then used to explore juices with varying natural acidities. The experiment is amenable to implementation in the classroom or as an at-home activity. Learning outcomes include acid-base reactions, chemical bonding, polymer structures, and green chemistry concepts. Students consider the environmental challenges of traditional plastics used in packaging and how innovative new commercial products are attempting to provide solutions.
Introduction
Plastics are ubiquitous materials we use in our daily lives. These materials remain an essential component in the packaging industry helping prolong the shelf life of food and other consumer products. The durable quality of plastics unfortunately contributes to the damage they cause to the environment (Schneiderman & Hillmyer, 2017). Traditional plastics derived from fossil fuels take centuries to biodegrade, accumulating in landfills and polluting the natural environment (Geyer, Jambeck, & Law, 2017). Solutions to combat this global issue involve the development of sustainable and environmentally friendly alternatives to petroleum-based plastics. These materials are designed to be biodegradable or compostable that break down into benign subunits such as carbon dioxide and water. Biodegradable materials are degraded in natural environments such as soil or aquatic systems. Compostable plastics are broken down under controlled industrial conditions (Wissinger et al., 2020). Polylactic acid (PLA), derived from corn, is an example of a compostable bioplastic.
An example of a biodegradable product is the edible, hydrogel pods sourced from seaweed derivatives produced by The Skipping Rocks Laboratory Notpla Limited (2022). The gel-like water spheres begin to biodegrade in approximately four to six weeks and are envisioned as an alternative to single-use plastic water bottles as illustrated by their use in the 2019 London Marathon (OohoWater Admin, 2019; Patel, 2019). The Skipping Rocks Laboratory has applied this technology to other innovative products such as condiment sachets and food containers all aiming to reduce the plastic footprint through degradable packaging. Showcasing examples of these current innovations in sustainability and green chemistry through teaching experiments is an effective tool in engaging students. These guided-inquiry experiments connect students to global issues addressed by the UN Sustainable Development Goals (United Nations Department of Economic and Social Affairs, 2022) and provide a framework to develop problem-solving skills. Goals 3 (Good Health and Well-being), 12 (Responsible Consumption and Production), and 14 (Life Below Water) are directly tied to plastics. Herein we report a teaching experiment that introduces students at the upper secondary age group to the chemistry behind the formation of edible water beads derived from alginate, a linear anionic polysaccharide that can be extracted from algae.
Background
Alginate is an attractive model system to explore in a teaching lab due its tunable properties that are desired in various industries ranging from food, medicine, and construction. As a hydrogel, alginate can absorb a large amount of water molecules. Alginate can be woven into a fiber for wound dressings (Aderibigbe & Buyana, 2018). Formation of alginate gel beads through spherification have been incorporated into imitation food and encapsulation of biomolecules (Jeong, Kim, Lee, Cho, & Kim, 2020). Thin film and coatings derived from alginate are used in food packaging to improve shelf life (Parreidt, Müller, & Schmid, 2018). Alginate capsules containing sunflower oil were utilized for self-healing properties to rejuvenate asphalt (Al-Mansoori, Norambuena-Contreras, Micaelo, & Garcia, 2018).
The life cycle of calcium alginate beads meets many of the criteria described in the Sustainable Polymer Framework from the National Science Foundation (NSF) Center for Sustainable Polymers (Wissinger et al., 2020) and summarized in Figure 1. The reagents used in this experiment are or can be potentially obtained from natural feedstocks. The process to synthesize calcium alginate can be completed at room temperature. The reagents are non-toxic and inexpensive and food-grade quality of the materials can be purchased online enabling the chemistry to be safely conducted at home in the kitchen. For end-of-use the alginate beads are biodegradable. The aforementioned features make studying alginate very accessible for lower and upper secondary age groups. While there are several demos found online showcasing the basics of synthesizing calcium alginate, the teaching experiments described here are translated into curricula that meet learning standards set for upper secondary age students.
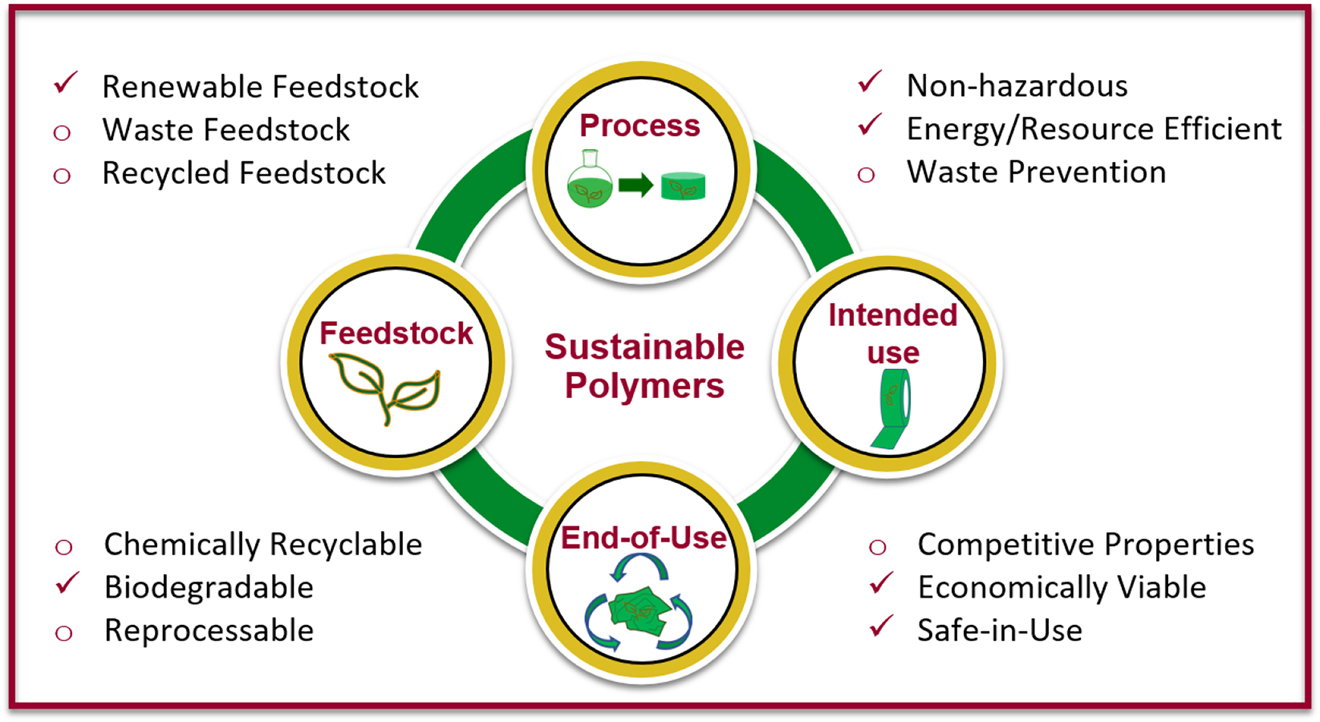
The criteria addressed by the alginate experiment curriculum that align with the sustainable polymer framework (adapted from Wissinger et al. 2020).
The structure of alginate is comprised of two uronic acid blocks, a β-D-mannuronate (M) and α-L-guluronate (G) residue linked through a 1,4-glycosidic bond. The alcohol functionality at the C6 position is fully oxidized to a carboxylic acid. The polymer has three different arrangements that provide a unique structure: A flat ribbonlike block of repeating MM residues, an egg-box block of repeating GG residues, and a helical shaped region of alternating MG residues (Lee & Mooney, 2012; Zhang, Cheng, & Ao, 2021). The conformational shape of the GG block allows for the carboxylate anions to strongly coordinate to multivalent cations connecting two separate polymer chains. Alginate beads can be synthesized by reacting its sodium salt form with calcium lactate via a double displacement reaction (Scheme 1). The crosslinking coordination of the divalent cation to the carboxylate groups is the key focus of this teaching lab.

Synthesis of calcium alginate with calcium coordinated to the α-L-guluronate (G) residues.
Classroom experiments involving alginate have been successfully incorporated for lower, upper and freshman/sophomore age groups. These past experiments demonstrate the versatile properties of alginate polymers as they are prepared in a variety of different forms:
Worms (Waldman et al., 1998)
Spaghetti (Erdal, Hakkarainen, & Blomqvist, 2019)
Chitosan alginate discs (Ward & Wyllie, 2019)
Slime (Boyd, 2021)
One notable parameter that was not studied in these teaching experiments was the investigation of pH on bead formation. This phenomenon presents as a great opportunity to use in a classroom teaching experiment. Alginate polymers are known for being pH responsive and the direct impact of pH effects on bead shape and the calcium interactions has been well studied (Chuang et al., 2017; Liang et al., 2004; Zheng et al., 2017). Students are exploring this effect using citric acid, which is derived from natural resources and friendly to use.
This new addition of calcium alginate bead investigation is designed for upper secondary students to explore the effect of pH on calcium alginate bead formation using varying concentrations of citric acid (Scheme 2). Similar to calcium lactate and sodium alginate, food grade citric acid is readily available for purchase and can also be safely used in the experiment. When citric acid reacts with sodium alginate, the carboxylate groups on the polymer can be protonated depending on the pH. The pKa values of the carboxyl groups on the mannuronate and guluronate blocks are 3.38 for and 3.65 respectively (Francis et al., 2013). Recall that the G-blocks of alginate are the site of coordinations with divalent cations. When pH is above the pKa of the carboxylic acid there will be a higher concentration of its deprotonated form. When pH is below the pKa, the carboxylic acid will be primarily in its protonated state. Lowering the pH of the alginate below the pKa of the guluronate residue has a major impact on bead shape. At a lower pH, calcium alginate beads form a more oblate, less spherical shape (Chuang et al., 2017). The protonation of the carboxylate anions on the G residues disrupts the coordination with the calcium cations reducing the available sites for crosslinking. This weakens the crosslinking network lowering the resistance to deformation under the bead’s own weight (Figure 2). A second effect that contributes to the oblateness of the bead shape is caused by the reduction of interfacial tension at a lower pH (Yoo & Mitragotri, 2010).

Acid-base reaction between sodium alginate and citric acid.

Left: calcium alginate formed at pH 3.45. Right: calcium alginate formed at pH 7.2.
The learning objectives of this teaching experiment for upper secondary age students involve discussing the need for finding alternatives to traditional plastics and introduction to green chemistry and sustainability. This teaching experiment invokes and brings awareness to the plastics problem at a level suitable for its audience. The topics of polymer structure, chemical bonding, solutions and solubility, and acid-base chemistry are incorporated into the experiment. In the first part of this multi-day experiment, students are required to consider how pH relates to protonation and how this can affect the structure of an ionizable polymer. Students will mix solutions of varying concentrations and directly observe that more solute in a solution results in a higher concentration. Furthermore, the relationship between concentration and pH is observed when an acidic solution diluted with water results in a higher pH. In the second part, students undertake an exploratory investigation taking the knowledge obtained from the previous studies and predict which juice beverage can form beads. Since this experiment uses food safe materials, students explore juices which can be tied to pH as they naturally contain organic acids. Students are asked to determine whether juices could substitute for water and create new environmentally-friendly ways to deliver thirst quenching beverages without the use of plastic bottles.
Experimental overview
Materials and design (see Supplementary Material for details regarding procedures and setup)
This chemistry experiment explores the effect of pH on calcium alginate beads and their practical application to hold liquids such as juices or condiments. This two-part experiment can take place in two 50 min class sessions. Part I examines the effect of pH on calcium alginate beads filled with water. This knowledge is then applied to predict and test the viability of beads filled with juice.
To synthesize calcium alginate beads, a solution of sodium alginate and the contents of the bead is dropped or placed into calcium lactate solution and allowed to react for 15 min or more. Two methods are given to prepare the beads. The first utilizes a standard plastic pipette that is cut to create a larger opening for delivery of the sodium alginate solutions. The second uses a teaspoon that is gently placed liquid side up just below the surface of the calcium lactate for 5–10 s to allow initiation of the outer shell membrane formation. The teaspoon is then gently inverted to empty the contents into the calcium lactate solution. Food coloring is used to improve visibility of beads when recovered from the lactate baths.
Part I – pH control with citric acid
Students work in groups of five and begin by preparing five sodium alginate + citric acid solutions in water. The pH of each solution is measured and recorded. Each solution, ranging in pH between ∼6 and ∼3 are added to a beaker containing 0.06% w/v calcium lactate via a cut pipette or teaspoon. The beads sit in solution for at least 15 min while the calcium alginate outer membrane forms. After 15 min, observations of the beads in solution are made. After removal from solution, the texture, shape, and firmness of the beads are analyzed at each pH and reported in a table.
Part II – Investigation of juice-beverage beads
Part II of the experiment encourages students to begin thinking about the practical applications of sodium alginate beads and to share and compile data. From Part I, a relationship between pH and bead formation is drawn. Students consider the tartness of various juices such as cranberry, apple, and lemon juice and correlate with the order of pH and bead formation and shape. Pairs are assigned one type of juice and work with two sodium alginate solutions, a non-diluted juice solution and a diluted juice solution. Again, the pH of each solution is determined and the same protocol as that used for water is followed to prepare calcium alginate juice filled beads. Students examine the beads and share data with other groups to collect data on each type of juice.
Potential extension – contact angle analysis
Instructors can choose to include an optional extension that involves measuring the contact angle of the calcium alginate beads. ImageJ software, which can be downloaded online for free through the National Institutes of Health (https://imagej.nih.gov/ij/download.html), allows quantification of contact angle through the Contact Angle Plug-in (https://imagej.nih.gov/ij/plugins/contact-angle.html). Photographs of the calcium alginate beads can be used to determine contact angles using a reported literature procedure (Buahom, 2018) and as described in the Supplementary material.
Hazards
Sodium alginate, calcium lactate, and citric acid can be purchased in food grade. Juice beverages can be obtained at local markets. Even though juice beverages and food grade reagents will be used in the experiment, students should wear proper lab attire, goggles, and gloves accordingly to instill safety practices. Citric acid is a minor skin irritant. Exposure to skin requires washing with water. Beads prepared in the lab using equipment and glassware should not be consumed. Consumption of beads is only allowed when kitchen equipment is used to conduct the experiment. Possible food allergies must be taken in consideration for ingesting beads that contain juices.
Discussion/results
Part I – pH control with citric acid
Figure 3 illustrates the beads formed from the dropper and spoon methods giving different sized beads. The spoon method gives beads of approximately 2.5–3.5 cm in diameter whereas the dropper affords beads with diameters of approximately 0.5–0.6 cm. Each method has varying degrees of technique involved. The dropper method provides better control in delivery resulting in more precise and consistent shapes. The spoon method is less consistent with size and shape, however the larger appearance of the beads makes it easier to identify significant changes in bead shape when changing the pH. Turkey basters and boba straws were also shown to effectively transfer the alginate solutions into the calcium lactate baths.

Left image: beads formed with spoon. Right image: beads formed with pipette.
Representative student data expected for Part I are shown in Tables 1 and 2 (see Supplementary material for more information). Beads with no citric acid and the highest pH are the firmest and roundest. As pH is lowered, the beads start losing their structural integrity, becoming less spheroid in shape and are easily broken when gently squeezed. The oblateness of the alginate beads is more apparent as the pH nears and eventually becomes lower than the pKa of the carboxyl groups (3.38) of the G block. Using food coloring dye is essential to help observe the results of the experiment.
Example of data and observations using the pipette dropping method.
| Sodium alginate + citric acid % w/v | pH | Photo | Observations |
|---|---|---|---|
| 0.00 | 6.00 |

|
Round, firm, smooth, retains shape |
| 0.40 | 3.77 |

|
Round and smooth, squishy, does not fully retain shape |
| 0.80 | 3.45 |

|
Spread out, oblate, squishy |
| 1.20 | 3.25 |

|
Very oblate, does not retain shape |
| 1.60 | 3.13 |

|
Flat, squishy, not a bead |
Example of data and observations using the teaspoon method.
| Sodium alginate + citric acid % w/v | pH | Photo | Observations |
|---|---|---|---|
| 0.00 | 6.00 |

|
Round, firm, smooth, retains shape |
| 0.40 | 3.77 |

|
Round and smooth, squishy, does not fully retain shape |
| 0.80 | 3.45 |

|
Spread out, oblate, squishy |
| 1.20 | 3.25 |

|
Very oblate, does not retain shape |
| 1.60 | 3.13 |

|
Flat, squishy, not a bead |
Part II – Investigation of juice-beverage beads
Table 3 illustrates the data and observations expected for juices. When analyzing the non-diluted alginate-juice solutions, students will notice that alginate-apple juice readily forms a bead with a pH above four ensuring that the carboxyl groups on the G-block remain deprotonated. Cranberry juice and lemon juice are relatively more acidic compared to apple juice. Particularly for lemon juice, no bead is formed at all. Dilution of lemon and cranberry juices raises the pH high enough to allow for successful formation of alginate-juice beads.
Example of data and observations for alginate-juice experiment using teaspoon method.
| Sodium alginate + juice solution | pH | Observations (in solution) | Photo | Observations (out of solution) |
|---|---|---|---|---|
| Apple juice: non-diluted | 4.17 | Formed beads |

|
Round, but very squishy bead |
| Apple juice: diluted | 4.94 | Formed beads |

|
Round, firm bead |
| Cranberry juice: non-diluted | 3.55 | Dispersed, very clumpy, stayed at the top of solution |

|
Clumpy, flat, not a bead |
| Cranberry juice: diluted | 4.41 | Drops stayed together, formed beads |

|
Round, squishy bead |
| Lemon juice: non-diluted | 3.15 | Drops dispersed immediately |

|
No beads formed |
| Lemon juice: diluted | 3.83 | Drops spread out, but did not disperse completely |

|
Oblate, very squishy bead |
Extension – contact angle analysis
Contact angle analysis can provide further information on the effects of pH on bead shape. Usage of contact angle is commonly applied for water droplets where their relative wettability, how the material spreads over a solid surface, is determined. Droplets that possess low wettability have contact angles in between 180° and 90°, have hydrophobic surfaces where there is less affinity to maintain contact with the surface. Materials with high wettability, have a strong affinity for the solid and spreads out and contact angles less than 90°. Table 4 and Figure 4 represent an example of contact angles study on the beverage-alginate beads. The dilutions of alginate-cranberry juice show a decreasing trend in contact angle as pH lowered. This aligns with the oblateness of the beads as they become less spheroid in shape.
Analysis of contact angles of beverage-alginate beads.
| Entry | Beverage | Beverage:H2O | pH of juice + alginate | Contact angle° |
|---|---|---|---|---|
| 1 | Apple | – | 4.12 | 127.1 |
| 2 | Cranberry | 1:3 | 3.33 | 85.3 |
| 3 | Cranberry | 1:4 | 3.53 | 109.9 |
| 4 | Cranberry | 1:9 | 3.76 | 140.9 |
| 5 | Lemon | 1:9 | 3.44 | 78.2 |
| 6 | Water | – | 6.92 | 146.8 |

Left image: photo taken from ImageJ software with contact angle plug-in. Yellow lines were added to aid in visualization of location of contact angle. Angle θ, calculated from ImageJ is used to determine contact angle. Right image: photos of beads used to determine contact angle.
Implementation
Due to the ongoing Covid-19 Pandemic, classroom implementation of the full experiment was delayed due to the challenges with handling social distancing guidelines and the change from in-person to online virtual classes. The general experiment involving the synthesis of calcium alginate was successfully conducted virtually in fall 2020 with a group of upper secondary age students. A virtual lecture was provided discussing the plastics problem, basic polymer structure, reaction chemistry, and overview of the experimental procedure. Premade kits containing all the essential reagents were provided to students beforehand. In place of glassware, students used containers they had available in the kitchen of their homes. We plan to implement this teaching experiment in the upcoming academic year assuming classes are in-person or provide inexpensive pH probes for at-home learning.
Conclusions
We report a teaching experiment that brings awareness to sustainable solutions to single-use plastic bottles and packaging. Applications of green chemistry focus on the derivation of products from renewable feedstocks (Principle #7) and design for degradation (Principle #10) while also exploring new sustainable commercial products in lab. The investigation into the pH effect on calcium alginate beads provides an engaging and versatile experiment for upper secondary age groups with appropriate level science learning outcomes that cover acid/base chemistry and bonding. Students are exposed to solutions to real-world problems associated with petroleum-based plastics through the curricula with a vision of how a career in a Science, Technology, Math, and Engineering (STEM) field can contribute to a sustainable future.
Funding source: NSF Center for Sustainable Polymers 10.13039/100000001
Award Identifier / Grant number: CHE-1901635
Funding source: University of Minnesota MRSEC 10.13039/100000001
Award Identifier / Grant number: DMR-2011401
Funding source: Research Experiences for Undergraduates 10.13039/100000001
Award Identifier / Grant number: DMR-1852044
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the NSF Center for Sustainable Polymers, CHE-1901635. This work was supported (partially) by the Research Experiences for Teachers (RET) Program of the National Science Foundation under Award Number DMR-1852044 and through the University of Minnesota MRSEC under Award Number DMR-2011401.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
Supplementary material
Information regarding experimental procedures and lab worksheets are available here: https://doi.org/10.1515/cti-2021-0027.
References
Aderibigbe, B. A., & Buyana, B. (2018). Alginate in wound dressings. Pharmaceutics, 10(2), 42. https://doi.org/10.3390/pharmaceutics10020042.Search in Google Scholar PubMed PubMed Central
Al-Mansoori, T., Norambuena-Contreras, J., Micaelo, R., & Garcia, A. (2018). Self-healing of asphalt mastic by the action of polymeric capsules containing rejuvenators. Construction and Building Materials, 161, 330–339. https://doi.org/10.1016/j.conbuildmat.2017.11.125.Search in Google Scholar
Boyd, D. A. (2021). Safer and greener polymer demonstrations for STEM outreach. ACS Polymers Au, 1(2), 67–75. https://doi.org/10.1021/acspolymersau.1c00019.Search in Google Scholar
Buahom, P. (2018). Measuring the contact angle using ImageJ with contact angle plug-in. ResearchGate. Retrieved from https://www.researchgate.net/publication/328733959_Measuring_the_Contact_Angle_using_ImageJ_with_Contact_Angle_plug-in.Search in Google Scholar
Chuang, J.-J., Huang, Y.-Y., Lo, S. H., Hsu, T.-F., Huang, W.-Y., Huang, S.-L., & Lin, Y.-S. (2017). Effects of pH on the shape of alginate particles and its release behavior. International Journal of Polymer Science, 2017, 3902704. https://doi.org/10.1155/2017/3902704.Search in Google Scholar
Corcoran, E., & Wissinger, J. E., (2020). Earth-friendly plastics. Celebrating Chemistry, Chemists Celebrate Earth Week, 8. Retrieved from https://www.acs.org/content/dam/acsorg/education/outreach/celebratingchemistry/%0A2020-ccew-celebrating-chemistry-english.pdf.Search in Google Scholar
Corcoran, E. R., Lydon, C., Enright, M. C., Buenaflor, J. P., Anderson, K., & Wissinger, J. E. (2022). Thirst for a solution: Alginate biopolymer experiments for the middle and high school classroom. Journal of Chemical Education, 99(2), 1021–1025. https://doi.org/10.1021/acs.jchemed.1c00905.Search in Google Scholar
Erdal, N. B., Hakkarainen, M., & Blomqvist, A. G. (2019). Polymers, giant molecules with properties: An entertaining activity introducing polymers to young students. Journal of Chemical Education, 96(8), 1691–1695. https://doi.org/10.1021/acs.jchemed.8b00918.Search in Google Scholar
Francis, N. L., Hunger, P. M., Donius, A. E., Riblett, B. W., Zavaliangos, A., Wegst, U. G. K., & Wheatley, M. A. (2013). An ice-templated, linearly aligned chitosan-alginate scaffold for neural tissue engineering. Journal of Biomedical Materials Research – Part A, 101(12), 3493–3503. https://doi.org/10.1002/jbm.a.34668.Search in Google Scholar PubMed
Geyer, R., Jambeck, J. R., & Law, K. L. (2017). Production, use, and fate of all plastics ever made. Science Advances, 3(7), 25–29. https://doi.org/10.1126/sciadv.1700782.Search in Google Scholar PubMed PubMed Central
Jeong, C., Kim, S., Lee, C., Cho, S., & Kim, S.-B. (2020). Changes in the physical properties of calcium alginate gel beads under a wide range of gelation temperature conditions. Foods, 9(2), 180. https://doi.org/10.3390/foods9020180.Search in Google Scholar PubMed PubMed Central
Lee, K. Y., & Mooney, D. J. (2012). Alginate: Properties and biomedical applications. Progress in Polymer Science, 37(1), 106–126. https://doi.org/10.1016/j.progpolymsci.2011.06.003.Search in Google Scholar PubMed PubMed Central
Liang, H.-F., Hong, M.-H., Ho, R.-M., Chung, C.-K., Lin, Y.-H., Chen, C. H., & Sung, H. W. (2004). Novel method using a temperature-sensitive polymer (methylcellulose) to thermally gel aqueous alginate as a pH-sensitive hydrogel. Biomacromolecules, 5(5), 1917–1925. https://doi.org/10.1021/bm049813w.Search in Google Scholar PubMed
Notpla Limited. (2022). We make packaging disappear. Retrieved from https://www.notpla.com/.Search in Google Scholar
OohoWater Admin. (2019). Ooho Water, the edible bottle. Retrieved from http://www.oohowater.com/.Search in Google Scholar
Parreidt, T. S., Müller, K., & Schmid, M. (2018). Alginate-based edible films and coatings for food packaging applications. Foods, 7(10), 1–38. https://doi.org/10.3390/foods7100170.Search in Google Scholar PubMed PubMed Central
Patel, P. (2019). Edible packaging. ACS Central Science, 5(12), 1907–1910. https://doi.org/10.1021/acscentsci.9b01251.Search in Google Scholar PubMed PubMed Central
Schneiderman, D. K., & Hillmyer, M. A. (2017). 50th anniversary perspective: There is a great future in sustainable polymers. Macromolecules, 50(10), 3733–3749. https://doi.org/10.1021/acs.macromol.7b00293.Search in Google Scholar
United Nations Department of Economic and Social Affairs. (2022). Sustainable development, the 17 goals. Retrieved from https://sdgs.un.org/goals.10.18356/9789210018098Search in Google Scholar
Waldman, A. S., Schechinger, L., Govindarajoo, G., Nowick, J. S., Pignolet, L. H., & Labuza, T. (1998). The alginate demonstration: Polymers, food science, and ion exchange. Journal of Chemical Education, 75(11), 1430–1431. https://doi.org/10.1021/ed075p1430.Search in Google Scholar
Ward, A. M., & Wyllie, G. R. A. (2019). Bioplastics in the general chemistry laboratory: Building a semester-long research experience. Journal of Chemical Education, 96(4), 668–676. https://doi.org/10.1021/acs.jchemed.8b00666.Search in Google Scholar
Wissinger, J. E., Ellison, C. J., Dichtel, W. R., Trotta, J. T., Chang, A. B., Yang, A., & Bunyard, C. W. (2020). Sustainable polymer framework. Minnesota: NSF Center for Sustainable Polymers, University of Minnesota Digital Conservancy. Retrieved from http://hdl.handle.net/11299/211643.Search in Google Scholar
Yoo, J.-W., & Mitragotri, S. (2010). Polymer particles that switch shape in response to a stimulus. Proceedings of the National Academy of Sciences of the United States of America, 107(25), 11205–11210. https://doi.org/10.1073/pnas.1000346107.Search in Google Scholar PubMed PubMed Central
Zhang, H., Cheng, J., & Ao, Q. (2021). Preparation of alginate-based biomaterials and their applications in biomedicine. Marine Drugs, 19(5), 1–24. https://doi.org/10.3390/md19050264.Search in Google Scholar PubMed PubMed Central
Zheng, H., Gao, M., Ren, Y., Lou, R., Xie, H., Yu, W., … Ma, X. (2017). An improved pH-responsive carrier based on EDTA-Ca-alginate for oral delivery of Lactobacillus rhamnosus ATCC 53103. Carbohydrate Polymers, 155, 329–335. https://doi.org/10.1016/j.carbpol.2016.08.096.Search in Google Scholar PubMed
© 2022 Jeffrey P. Buenaflor et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Frontmatter
- Guest Editorial
- Introduction to the special issue on Green Chemistry
- Special Issue Papers
- Simple experiments with immobilized enzymes as a contribution to green and sustainable chemistry education in the high school laboratory
- Learning Green Chemistry and its principles from Nature’s process and development of green procedures mimicking nature
- SpottingScience – a digital learning environment to introduce Green Chemistry to secondary students and the public
- Student explorations of calcium alginate bead formation by varying pH and concentration of acidic beverage juices
- Good Practice Report
- Simple green organic chemistry experiments with the kitchen microwave for high school chemistry classrooms
- Invited Paper
- Basic concept and application of conducting polymers for environmental protection
- Good Practice Report
- Distance learning: an interdisciplinary experiment on Rayleigh scattering
- Special Issue Paper
- Development of teaching material for green and sustainable chemistry in Japan
Articles in the same Issue
- Frontmatter
- Guest Editorial
- Introduction to the special issue on Green Chemistry
- Special Issue Papers
- Simple experiments with immobilized enzymes as a contribution to green and sustainable chemistry education in the high school laboratory
- Learning Green Chemistry and its principles from Nature’s process and development of green procedures mimicking nature
- SpottingScience – a digital learning environment to introduce Green Chemistry to secondary students and the public
- Student explorations of calcium alginate bead formation by varying pH and concentration of acidic beverage juices
- Good Practice Report
- Simple green organic chemistry experiments with the kitchen microwave for high school chemistry classrooms
- Invited Paper
- Basic concept and application of conducting polymers for environmental protection
- Good Practice Report
- Distance learning: an interdisciplinary experiment on Rayleigh scattering
- Special Issue Paper
- Development of teaching material for green and sustainable chemistry in Japan

