Molecular School – a pre-university chemistry school
-
Bernardo A. Nogueira
, Alexandre D. Silva
, Maria Inês P. Mendes
, Ana Dora R. Pontinha
, Carlos Serpa
, Mário J. F. Calvete
, Alexandra Rocha-Gonçalves
, Pedro J. B. S. Caridade
und Sérgio P. J. Rodrigues
Abstract
The planning, implementation and results of the first edition of the Molecular School are presented, as the first pre-university school project held in Portugal. This is not, however, a strictly Portuguese project, since it can be replicated in other countries at the secondary school level, with minor adjustments. Herein, the pilot edition of Molecular School is detailed and discussed, where 36 secondary school students have participated. The plan for the second edition, to be held in the first semester of 2021, with the confirmed participation of around 100 students, is further presented. Briefly, the project is divided in two modules: theoretical and laboratory work. These were prepared in a complementary way and performed to achieve the same purpose: deliver a wider vision of what chemistry really is. Hence, the classes were designed having in mind the applications that chemistry has in our everyday life, in the different academic research fields and in industry. A better preparation and training at the laboratory level was also a goal of this project. The enthusiasm, happiness and the motivation shown by the students, and their eagerness to participate in the future editions of the Molecular School, were clear signs of this project success.
Introduction
Background
Chemistry is a science with high impact in the economic growth and in improving the quality of life (Emsley, 1996, 1998). Throughout the years, chemistry had numerous breakthroughs in different areas such as energy and environment, food and agriculture, health sciences and medicine. Despite of all this, in Portugal, a chemical product is commonly recognized as a pollutant almost by definition and, in a certain way, chemistry is erroneously assigned as an undesirable, troublesome and dangerous science. Indeed, it is still very common to see advertisements and opinion-makers referring generically to “chemicals” as synthetic, toxic, harmful and unsafe compounds, preventing us from having better quality of life. This type of standard lexis in the non-scientific community decreases the acknowledgment of chemistry as a central science and as a major knowledge area in this era. This opinion also reduces the recognition of the role of chemists in the development of the society. Unfortunately, this chemophobia impression is not only felt in Portugal, but worldwide (Bensaude-Vincent & Simon, 2008; Hartings & Fahy, 2011; Saleh, Bearth, & Siegrist, 2019; Siegrist & Bearth, 2019).
Aiming to clear the “young minds” in all what is related to chemistry, Molecular Junior Enterprise (Molecular JE) was created in 2017, by a group of chemistry students from the Department of Chemistry of the University of Coimbra (mostly PhD Students). Molecular JE is a junior enterprise working in the field of chemistry in Portugal, whose main goal is the disclosure of chemistry as a natural and central science. A junior enterprise is a non-profit organization composed and directed exclusively by university students (from undergraduate to PhD), with a specific purpose: Molecular JE aims to improve the knowledge and enjoyment of chemistry as a single concept through initiatives for different targeted audiences. One of the projects of this junior enterprise is the organization of the Molecular School (Escola Molecular, in Portuguese), the first pre-university chemistry school held in Portugal. By pre-university school, we mean a non-formal education space, developed and organised by university actors and held in the university environment.
Although there have been reported some cases of University-Secondary School cooperation programs (Mernoff et al., 2017; Ragsdale, 1982; 2019), and also some summer chemistry camps for secondary school students, held at University campuses (Exstrom & Mosher, 2000; Robbins & Schoenfisch, 2005; Schwarz, Frenzel, Richter, Täuscher, & Kubsch, 2016), to the best of our knowledge, this is the first time that a pre-university school, as the herein defined and described is organized and reported.
According to the UNESCO Draft Proposal and Plan for a United Nations Literacy Decade, the education of science should aim to cultivate scientific literacy in the students so that they can value the importance of scientific and technological developments in a social, economic and environmental perspective (STSE perspective) (2001). However, according to N. Costa et al. (Costa, Marques, & Kempa, 2000), the knowledge of science teachers on research findings is generally quite limited. We also need to bear in mind that chemistry is one of the most difficult subjects and, therefore, students must be motivated and have a good knowledge prior to beginning post compulsory studies (Coe, Searle, Barmby, Jones, & Higgins, 2008). In the traditional education of sciences there is a predominance of expositive lectures that aim to deliver information from the teacher to the students (Johnstone, 1993). Nevertheless, as M. Figueiredo et al. noted, this method is not appropriate for the study of most topics in chemistry because “there are many skills involved in being a chemist, including observation, discussion and data-collection which cannot be developed in theoretical lectures” (Figueiredo, Esteves, Neves, & Vicente, 2016). For this reason, it is of fundamental importance to allow students to experience the full range of chemical events, still in a controlled environment. In fact, chemistry is frequently taught as a combination of symbols, formulas and equations about objects and phenomena which are invisible to the naked eye and are therefore somehow abstract concepts that makes it even more difficult to be understood (Taber & García-Franco, 2010). Indeed, the conceptual understanding of chemistry requires the observation of phenomena at three different levels: macroscopic (observing, manipulating, testing and describing the properties of materials), sub-microscopic (abstract understanding and explaining observations in terms of non-visible substances as atoms, ions and molecules) and symbolic (converting the understanding of observations into chemical equations, expression analogies and modelling kits) (Johnstone, 1982). Recently, educational works have highlighted that bringing together the three levels of chemical phenomena observation is crucial for effective learning in chemistry (Baptista, Martins, Conceição, & Reis, 2019; Talanquer, 2011). Nonetheless, the transition from the macroscopic into both sub microscopic and symbolic levels is found to be hard for the general students’ population and thus, this process should be carefully accompanied and directed (Prain, Tytler, & Peterson, 2009).
These are the main issues we have tried to answer in this non-formal education project, designed for students of the 10th, 11th and 12th grade of the Portuguese secondary school (equivalently, respectively, to the fifth, lower and upper sixth in the UK and sophomore, junior and senior grades of high-school in the US). In fact, in Molecular School, we are specifically focused on teaching the different chemistry concepts directly connected to chemical research and on the fundamental importance of practical work. By practical work we mean the Millar’s notion that describes it as any type of science teaching or learning activity where students work either individually or in small groups to perform manipulation and observation of real objects and materials (Millar, 2010). Unfortunately, this is the major weakness in Portuguese physics and chemistry curricula at the secondary school level. These two subjects are joined and taught in a single subject at the 10th and 11th grades, where chemistry is taught only during one-half of the school year. Considering the amount of time spent in each subject, presently there is only time for six sessions of 135 min per school year to perform lab work in the chemistry field (Figueiredo et al., 2016). Until the 2003/04 school year, the component of laboratory work in chemistry teaching was greater in Portugal, with a specific subject of Laboratory Techniques presented in each secondary school year curriculum, allowing students to better acquire laboratory practical skills (Figueiredo et al., 2016). Since then, there is no longer this possibility for students to be familiarized with laboratory procedures outside the main chemistry course at secondary schools, and therefore it was our intention to give them this opportunity in the university environment. In this project, small groups of students (operating as individual classes) were allowed to have several protocols to perform, with the supervision of PhD students. As Figueiredo et al. mentioned, the execution of practical work may indeed be determinant to motivate students to continue studies in the chemistry scientific areas and to choose future careers related to this topic (Figueiredo et al., 2016). In fact, to accomplish such goal, the practical work must be mainly laboratory work. In such case, the students must feel free to conduct the different stages of lab work development with the highest degree of independence possible. This will include the execution and the interpretation of results, since this is the best way to gain scientific knowledge (Figueiredo et al., 2016) and understand the intrinsic sub microscopic and symbolic levels.
The laboratory work teaching methodologies can be divided in three groups. The first one consists of experimental demonstrations performed by teachers (the most used in Portuguese secondary school education, due to a certain shortage of time and material resources). This methodology is the most limiting for the students, since they do not have the possibility to develop any of the skills that are needed to perform laboratory work (Figueiredo et al., 2016; Logar & Ferk-Savec, 2011). The second methodology involves the execution of laboratory recipes performed step-by-step, where students concentrate their thoughts on concluding one stage at a time. For this reason, sometimes students cannot develop a deep understanding of the experiments (Hofstein & Lunetta, 2004). Nonetheless, it is probably the best way for students to be safely familiarized with the laboratory environment and with the specific tasks that are essential in lab work. It is also the predominant methodology we have used for the group of beginner students, i.e., the students from the 10th grade of the secondary school. In the third methodology, the students themselves may plan and execute the experimental work, based on a specific scientific (chemical) purpose, under the supervision of a specialist. This is the methodology which better increases the learning achievements of the students and the one that is most likely to develop a strong personal interest towards chemistry (Tarhan & Sesen, 2010). Consequently, it is the main methodology that we will employ for 11th and 12th grade students in the subsequent editions of the Molecular School.
To summarize, the Molecular School was designed to fulfil two major objectives in order to increase the motivation of the students in learning chemistry: (1) to teach chemical concepts related to everyday life situations and directly linked with avant-garde research projects and (2) to improve the practical work performed by the secondary school students, with a hands-on approach in an university environment.
Methods
Participants
A total of 36 secondary school students participated in this pre-university school. Specifically, the school enrolled 28 students of the 10th grade (15–16 years old), 4 of the 11th grade (16–17 years old) and 4 of the 12th grade (17–18 years old) from the Portuguese cities of Coimbra, Proença-a-Nova, Lousã, Miranda do Corvo, Oliveira do Hospital, Pombal and Maia.
The participants were selected from individual applications sent to Molecular JE email address. These applications were publicized mainly through digital media (social networks and emailing secondary schools). Therefore, the students choose to become part of the project by suggestion from their secondary school teachers, or relatives, or by running into the publicity of the initiative in social networks. It should be mentioned that the participation of the students was entirely free of charges. Laboratory safety dress code was strictly followed and lab coats, safety goggles and gloves were provided to the students. A Certificate of Participation was given to every participant, at the end of Molecular School.
Project leadership
The project planning was carried out jointly by Molecular JE and the Department of Chemistry of the Faculty of Sciences and Technology of the University of Coimbra. The design and the preparation of the initiative was executed by the students that constitute Molecular JE, with the endorsement and the advice of the Department of Chemistry Directive Board. The theoretical lessons were lectured by Professors and PhD researchers of the Department of Chemistry, and by PhD Researchers working in the industry, who were chosen and invited by the Molecular JE administration. In the laboratory part of the project, the coordination was held by PhD students from the Department of Chemistry that are part of Molecular JE, helped by volunteer master and undergraduate students, from the same institution, who were specifically trained to help in each session by the members of the organizing commission.
Project goals
The main goals of Molecular School were, on one hand, to deliver a wider vision of what chemistry really is and to focus on the applications that chemistry has in everyday life in our society. Some specific examples that were used in the different theoretical lessons are detailed in the Project description. On the other hand, it was also sought to give the students the possibility to learn with researchers at the university or industry and work in a hands-on experience in a laboratory environment.
Results and discussion
Project description
The school was held in six modules, always using non-working days (five Saturdays and one national holiday), between the 2nd of March and the 27th of April of 2019. Every session was divided in one theoretical lesson (1.5 h), followed by a coffee-break, and an experimental activity in the laboratory (2 h). Each session had one specific topic that comprised several different subtopics and contents (Table 1). The choice of these topics was made considering the Portuguese chemistry curricula of the 10th grade of the secondary school (Fiolhais et al., 2014), and lessons were prepared accordingly, however considering a different approach. Our distinct approach focused mainly on the influence that the subjects taught have in our everyday life and their relevance to the state-of-the art research and development, as it will be described henceforth. At the same time, several practical experiments related to the topic were introduced during the classes (Table 2).
Main topics, subtopics and contents of the different lessons held in the first edition of the Molecular School.
| Main topic | Subtopic | Contents | |
|---|---|---|---|
| Lesson 1 | Chemical elements | Materials | Physical states; Protons, neutrons and electrons |
| Solutions | Solutions in different physical states; Solvent and solute; Unit conversion | ||
| Chemical elements | Atomic number and chemical symbols; Mass number; Isotopes; Relative atomic mass; Chemical formulas | ||
| Periodic table | Groups, periods and blocks; Atomic radii and ionization energy | ||
|
|
|||
| Lesson 2 | Radiation; Energy; Spectra | Electromagnetic radiation; Visible electromagnetic spectrum; Continuous and discontinuous spectra; Photons; Emission and absorption spectra; Photoelectric effect; Photoelectron spectroscopy | |
|
|
|||
| Lesson 3 | Electron energy on atoms | Hydrogen atom spectrum; Bohr’s atomic model; Energy levels; Orbitals and electronic clouds; Fundamental and excited states; Electronic configurations; Quantic model of the atom; Energy quantization; Electronic transitions; Ionization energy; Aufbau principle; Pauli’s exclusion principle; Hund’s rules | |
|
|
|||
| Lesson 4 | Chemical bond | Lewis representation; Covalent bonding; Bonding energy; Bonding length; Bonding angles; Molecular geometries; Molecular polarity: Organic and biological molecules structure | |
|
|
|||
| Lesson 5 | Gases and dispersions | Temperature, pressure and density; Avogadro’s law; Moles; Avogadro’s constant; Molar volume; Molar mass; Solutions: colloids and suspensions; Concentrations (mass concentration, mass percentage, volume percentage, ppm); Aqueous solutions dilutions | |
|
|
|||
| Lesson 6 | Chemical transformations | Chemistry and the atmosphere | The layers of the atmosphere; The evolution of the atmosphere; The current atmosphere: components and hazards |
| Chemical transformations | Bonding energy; Chemical reactions; Endo- and exo-processes; Enthalpy variation; Radicals; Atomization and ionization; Chemical species stability | ||
| Ozone | The ozone layer; The ozone as a solar filter; Ozone formation and decomposition; CFCs | ||
List of the different experiments performed in each lesson and the respective contents.
| Experiment name | Contents | |
|---|---|---|
| Lesson 1 | Amount of sugar on soft drinks | Solutions, solubility |
| Precipitation by solution mixture | Solutions; solubility; precipitation | |
| Coke foam | Solutions; solubility: physical states | |
| Instantaneous ice | Physical states | |
| Dissolving styrofoam | Physical states | |
| Hot ice | Solutions; solubility, physical states | |
|
|
||
| Lesson 2 | Enlightening fountain | Electromagnetic radiation, Visible electromagnetic spectrum, optical fibres |
| Dispersion Slits | Electromagnetic radiation, Visible electromagnetic spectrum | |
| Absorption of different molecules | Emission and absorption spectra | |
| Radiation of the black body | Electromagnetic radiation, Visible electromagnetic spectrum, Emission and absorption spectra | |
|
|
||
| Lesson 3 | Flames colors | Spectroscopy |
| Shinning water | Fluorescence | |
| Photochemical reaction | Energy quantization; Ionization energy | |
|
|
||
| Lesson 4 | Tying water filaments | Polarity |
| Pencil trick | Polarity; surface tension | |
| Magical balloon inflation | Chemical reaction; chemical bond; pressure; moles | |
| Firelighters production | Solutions; concentration; dilutions | |
|
|
||
| Lesson 5 | Lava lamp | Density |
| Bottle egg | Pressure; Moles | |
| Floating egg | Density | |
| Rainbow floating and sinking | Density | |
| Submarine volcano | Density | |
|
|
||
| Lesson 6 | Pharaoh’s snake | Chemical reactions |
| Jumping grapes | Chemical reactions | |
| Invisible flaming tissue | Chemical reactions | |
Lesson number one focused on the Chemical Elements and the Periodic Table, in order to celebrate the 150th anniversary of the Periodic Table of Chemical Elements and also the “International Year of the Periodic Table of Chemical Elements (IYPT2019)” declared by the United Nations General Assembly and UNESCO. The development of the Periodic Table of Chemical Elements is one of the most significant achievements in science and a consolidating scientific concept with broad implications in chemistry, physics, biology and other sciences (Imberti & Sadler, 2020; Semenova, Tarasov, & Goodilin, 2019). Consolidate, deepen and expand knowledge by understanding concepts related to the Periodic Table provides the students a solid base of knowledge of chemistry and science values. This theme is part of the content: Chemical Elements and their organization. The class given incorporated three different sections: (1) from matter to atom; (2) distribution of electrons in the atom and their respective energies; (3) organization of chemical elements in the Periodic Table, based on the properties of atoms. In the first two sections, knowledge about matter and chemical elements was consolidated. In the latter section, the recognition of the Periodic Table as a mean to organize information about chemical elements and their elementary substances, and to understand that the electronic structure of atoms determines their properties was widely addressed. The main contributions to the evolution of the Periodic Table, from Döbereiner (Law of triads) to Newlands (Law of octaves), passing by Moseley, through Meyer and Mendeleev, and advancing to the present day, were underlined. It was highlighted and discussed the reasoning of the proposals that emerged showing that the Periodic Table is a document open to the incorporation of new chemical elements and new knowledge. This approach allowed us to show how science has been evolving in the last centuries. One of the applications that was discussed, concerning the properties of different periodic table elements and their interrelationships, was energy. Some authors have focussed on the key energy requirements for fuels, overlaying those with the evident chemical and physical trends across the periodic table (Yao et al., 2020).
The main topics of the second lesson were Radiation, Energy and Spectra. These topics aimed to introduce concepts of modern and quantum physics and particle physics regarding the radiations used in medicine. The modern physics part encompassed the origins and conceptual development of black body radiation, photons, wave-particle duality. The quantum physics unit of the lesson focused on the concept of molecular orbitals, quantum numbers and light absorption. The particle physics part was focused on the nature and properties of main nuclear radiations used in medicine. In addition, a discussion of light as classical electromagnetic waves (wave properties such as diffraction) and photons (particle properties: photoelectric effect) took place, as well as the processes of pair production and annihilation of radiation, introducing the concept of anti-particle and the utility of positron emission in medicine. In the experimental/demonstration part, the students were allowed to measure spectra of the sun, light candle, cell phone light, etc…; comparing the black body radiation with that of the sun and candle light. They also were able to play with a device to produce diffraction of light in different shapes and geometries. More generic experiments were made explaining the properties of different waves and their nature: electromagnetic or sound, transverse or longitudinal.
The third lesson was centred on Electron Energy on Atoms. The class revolved around the answer to the question of how to describe a Chemical Element. Inspired by the example of the spaceprobes Pioneer, launched in 1972, and Voyager, launched in 1976, students were asked to graphically represent the hydrogen atom in such a way that this representation would be understood by … an alien. About half of the students used the symbol H in their description. 35% showed drawings similar to Rutherford and Bohr representations of hydrogen. 25% used more complex drawings meant to represent simplicity, abundance, relation with the sun, water and hydrocarbons, and one-line spectrum. The class continued around two perspectives on how to describe an element (and the associated energy): from a conceptual and mathematical and from an experimental point of view. We focused on where in the atom electrons can be found, the dimensions of atoms, wavelike properties of a particle and demystifying Schrödinger equation. We endeavoured on connecting hydrogen emission spectra and Schrödinger equation. Methodologically, this class was developed from the answers given by the students to questions being proposed, with the speaker guiding the students to the correct answer. Lively examples of recent research by the speaker were given whenever possible, as for example the particle-like nature of light (Schaberle, Reis, Serpa, & Arnaut, 2019).
Chemical Bonding was the main topic of the lesson number four. This lesson was designed to address the topic learned in Secondary School, but using an approach that is more similar to University level, promoting creative thinking and interaction during the lesson. More specifically, we started by defining the meaning of chemical bond and the history behind the definition. Acknowledging Gilbert Lewis was the next logical step, introducing Lewis representations as a useful tool to understand chemistry and how molecules exist. Special emphasis was given to the formation of covalent bonds, including its formation, required energies, lengths, angles, geometries and most commonly available bonds in nature and everyday life materials/molecules. Intermolecular bonding was also addressed, from the weakest bond to the strongest one, particularly the hydrogen bond/bridge. In a relaxed manner, we have also provided several examples of organic and biological molecules and some of their characteristics, focusing on some research carried out at the university, namely in the synthesis/application of tetrapyrrolic macrocycles (Calvete, Piccirillo, Vinagreiro, & Pereira, 2019; Maldonado-Carmona et al., 2020; Pinto et al., 2019), of which molecules such as hemoglobin and chlorophyll are distinguished members.
Regarding lesson number five, the main target was Gases and dispersions being the main goal of this module to recognize that most of the available materials are in the form of dispersions. The student should be able to understand how to characterize these dispersions, either by their qualitative or quantitative composition, or by their classification as a solution, colloid or suspension according to the size of the constituent particles. A deeper approach to quantitative composition was made, focusing on different ways of expressing concentrations as well as the preparation of diluted solutions from more concentrated ones. As a means of showing the reality of a chemical analysis laboratory in a pharmaceutical industry, a practical exercise of analysis of the content of an active pharmaceutical ingredient (API) in tablets of an antidepressant medicine was presented (Gonsalves, Pineiro, Martins, Barata, & Menezesc, 2010). This exercise addressed the preparation of solutions, standards and samples with consecutive presentation of results for the necessary calculation. The main goal was to comprehend the importance of a correct execution of an entire analytical method used to control the final product and guarantee its quality.
Finally, the sixth and last class was related to Chemical Transformations and Chemistry of the Atmosphere. The main objective of the lesson was to show how different chemical processes could be applied in the modelling of the atmosphere and daily environmental applications such as pollution forecast. One of the most difficult concepts for chemistry students is the chemical bond and bond rupture, in particular noting that the process of bond-breaking should be rationalized as an electronic excitation effect. The misleading concept of bond as a line (or multiple) connecting two atoms is very present on undergraduate students. To replace this static concept of molecules, gather together at rigid conformations, simple electronic structure calculations have been carried out together with the students to analyse molecular orbitals and their role in the bond-breaking process. Other concepts have been highlighted such as solar radiation with the quiz “what is the colour of the sun?” to introduce the radiation interaction with molecules in the upper atmosphere. The simple chemistry involved in the ozone layer has been shown with new reactions involving highly vibrational species that are abundant in those regions (Caridade, Horta, & Varandas, 2013). The second part of the lesson was to show how new Earth Observation satellite constellations could help environmental analysis. Using Copernicus satellite imagery and services, it has been shown how new experiments are conducted in deep space, in particular using Sentinel 5P and CAMS to quantify NOx, O3 and PM2.5,10 at troposphere. Artificial intelligence methods have been presented showing how transdisciplinary approaches combining chemistry, mathematics and physics produce forecasts of major pollutants at street level, helping people with airborne diseases to reduce exposure to harsh environments.
The experimental activities developed in the laboratory were complementary to the contents that were lectured during the theoretical session of the corresponding morning, employing both the chemical concepts taught previously and some of their practical applications. The standard names of the different experiments and the corresponding topics carried out are summarized in Table 2. A representative protocol of an experiment conducted in one of the classes is reported in the Supplementary Material (Roesky and Möckel, 1996). During these activities, the students were divided into groups of five or six elements. They ran the experiments by themselves, following an experimental procedure prepared by the PhD students of Molecular JE. In each procedure, different questions were provided to the students. They were encouraged to answer these questions not only at the end of the experiment, but also during the laboratory work, in order to assimilate and elucidate the chemical phenomena they were observing. In this first edition, and since the majority of the students were from the 10th grade, it was our intention to apply the second group of the laboratory work teaching methodologies described above. There is a general agreement that the methodology where the students design and perform the experiment is the best, regarding their motivation and learning achievements, we chose to follow the methodology involving the execution of laboratory instructions performed step-by-step, due to the inexperience of the students at the laboratory environment.
Students evaluation
An inquiry was performed during the last session of Molecular School, to analyse the opinion of the students on the first edition of this project.
The first part of the inquiry referred to the theoretical part of each session. The students were asked about the clarity and interest of each class, if the teacher gave examples to connect the topic to daily life problems and if they were interested to learn more about that topic. A scale of 1 (Strongly Disagree) to 5 (Strongly agree) was chosen to answer each question (see Supplementary Material). Whenever a student was not present in a class, his answers were marked as NA (not available). The second part of the inquiry concerned Molecular School experimental sessions. The students evaluated the mentors that helped them with the experiments in a scale from 1 (Strongly Disagree) to 5 (Strongly agree). The students chose their favourite experiment and were asked if the group size allowed them to dynamically participate in every experiment. In the end of the inquiry, students were also asked to give suggestions to improve the next edition of Molecular School.
The questions asked in the first part of the inquiry are summarized in Figure 1. In Figure 1a, it is possible to see that most students agree or strongly agree that the subject was clearly explained in all classes. This experience allowed the students to understand better the diverse topics, from chemical bonds or transformations to radiation and energy, fundamental to understand most of the concepts in more advanced chemistry classes.
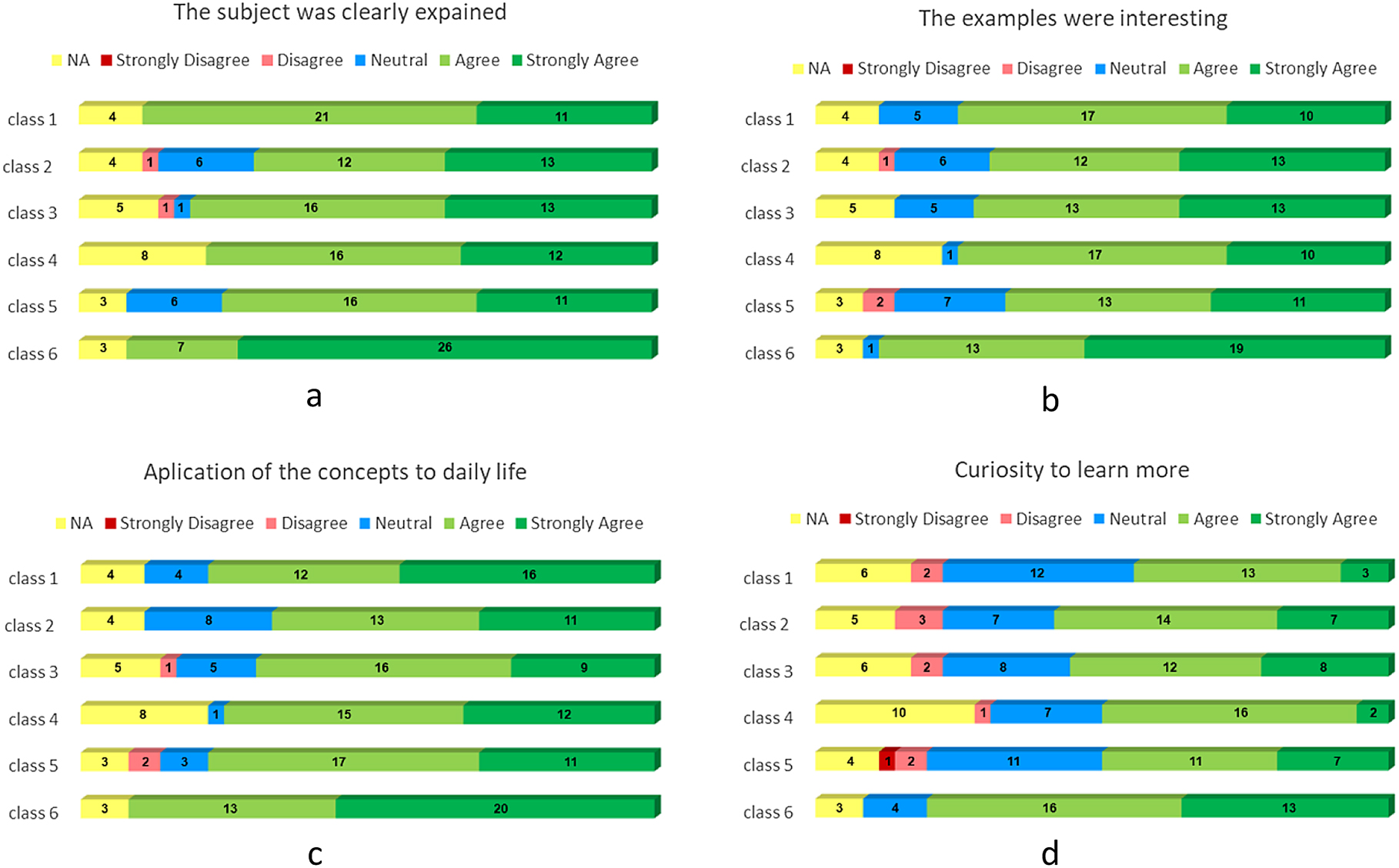
Graphical representation of the answers of the students to the inquiry hand over in each session: a) If the subjects were clearly explained; b) If the examples given were interesting; c) the interestingness of the applications of the taught concepts to daily life; d) their curiosity to learn more about the subjects taught.
Regarding the second question (Figure 1b), once again the students agree and strongly agree that the examples gave in classes were interesting and allowed them to understand the subject. In fact, this was one of the main differences between the classes the students have in their secondary school and the classes in Molecular School, where an effort to present more relevant examples was made. This prompt us to continue increasing the focus on teaching based on examples present on state-of-the-art research and innovation projects or that deal with topics which are found more interesting to the students age range.
With respect to the application of concepts taught to daily life, most students understood the utility of scientific concepts to interpret different events that occur every day (Figure 1c). Scientific concepts can be applied in the laboratory, but also in industry, from manufacturing to technology. The laboratory classes were also planned and developed based on the application of these examples. Thus, this point was the main link between the theoretical and the laboratory modules.
The curiosity of the students to learn more about chemistry is unquestionable, as we can see by the responses presented in Figure 1d. More than half of the students want to learn more about the different subjects taught in Molecular School. To arouse the curiosity of the students towards chemistry was one of the main ambitions of this program, which we consider that have been accomplished in all six classes.
Future perspectives
The second edition of the Molecular School was expectedly planned to be implemented during the first semester of 2020. Due to the current COVID-19 pandemic crisis, it was postponed to the beginning of 2021. The selection of the students for the 2020 edition was already complete, with 66 students from 10th, 11th and 12th grades accepted, based on the same criteria employed in the first edition. From the number of applications to the second edition of the Molecular School, it was possible to corroborate the success of the first edition. More than half of the students that participated in the first edition applied to participate again (18 students from 32 students that are still in the secondary school level).
Notwithstanding this postponement, the general plan for the second edition of Molecular School is already accomplished. There will be an individual program for each of the three secondary school years, with specific theoretical and laboratory programmes. The programme for the 10th grade will be maintained, with minor improvements, when comparing with the first edition. Such improvements may arise from the possibility of enhancing theoretical lessons, using even more appealing examples. This will be highly encouraged to each of the lecturers.
The 11th grade programme will be based on the chemistry part of the Portuguese physics and chemistry curricula for this year in the secondary school, in a similar process to the 10th grade (see Table S1). The theoretical part will also be divided in five different topics, from the quantitative aspects of chemical reactions to solutions and solubility equilibrium. Contrary to the theoretical part, the laboratory work will hold significant differences in its methodology. Unlike the approach used for the 10th grade students, where the main goal was to increase the familiarity with the laboratory practices and procedures, the 11th graders will experience the full range of chemical events. The students will design and complete a series of experiments in order to achieve different scientific goals, in different chemical areas. The supervision will be similar to the one given to the 10th graders, assisted by PhD, MSc or undergraduate students.
The scope of the 12th grade formation will be significantly different from the other two grades. Instead of a series of theoretical and experimental classes, the 12th graders will have the opportunity to attend one plenary talk in each session. These sessions will be developed by PhD scientists from different chemical fields that have one particular characteristic in common: they created a start-up or a company based on state-of-the-art chemical research. Therefore, the main goal is to demonstrate to the students the result of a chemical education complemented with entrepreneurship. The students who are motivated to pursue a chemistry-related university degree will also have the opportunity to participate in a research project in the Chemistry Department of the University of Coimbra during their summer holidays.
From the second edition on, based on the evaluation of the success of this second program, a final curriculum to enable this educational initiative to occur during numerous years will also be prepared.
Conclusions
The creation of Molecular School, the first pre-university chemistry school held in Portugal is herein reported. Specifically, the implementation of the pilot edition is described, consisting of a scientific programme prepared for students of the 10th grade and the planning and preparation for the second year of the Molecular School, designed also for 11th and 12th grade students. Molecular School comprises both theoretical and experimental modules. The former module was aimed to give the students the comprehension of the applicability of chemical concepts and the understanding of abstract theories based on daily examples. The experimental module was designed to help in the latter objective and to give the students the perception of how to work in a laboratory environment. The first year (pilot edition) of this school was a success (more than 50% of the students applied to participate in the second edition), which gave us the greatest appeal to continue for the second edition of Molecular School, aiming to increase the number of participant students to more than double.
Teaching chemistry using new non-formal approaches revealed to be a powerful tool for scientific literacy, given the enthusiasm shared by the students after every session of the school. Therefore, we consider that the methodology employed in this educational initiative can be replicated worldwide, with the necessary adaptations from region to region.
Funding source: Fundação para a Ciência e a Tecnologia
Award Identifier / Grant number: UIDB/00313/2020UIDP/QUI/0313/2020
Acknowledgments
The authors would like to thank Fábio A. Schaberle, PhD Researcher at the Department of Chemistry of the University of Coimbra, for his theoretical lecture and his help planning the laboratory work of the second session. We are also very thankful to all the PhD students who helped preparing the laboratory sessions and the master and undergraduate students that collaborated during those activities.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This research was funded by Faculty of Sciences and Technology of the University of Coimbra (FCTUC) and Portuguese Institute of Sports and Youth (IPDJ) for the financial support of the activity. The Coimbra Chemistry Centre (CQC) is supported by the Portuguese Science Foundation (“Fundação para a Ciência e a Tecnologia” – FCT), through the projects UIDP/QUI/0313/2020 and UIDB/00313/2020.
-
Conflict of interest statement: There are no conflicts of interest to declare.
Supplementary Material
The Supplementary Material is available on https://doi.org/10.1515/cti-2020-0013.
Questionnaire procedure; Representative Protocol of the experimental activities; Main topics, subtopics and contents of the different lessons for the 11th planned to the 2nd edition of Molecular School.
The face validity of the questionnaire was validated internally by the Molecular JE elements. Specifically, the questions were individually evaluated to verify if they capture the fulfilment of the participants regarding every component of the lessons. We have also performed an evaluation to find potential errors in terms of confusing, double-barrelled, and leading questions.
References
ACS Project SEED Program. American Chemical Society. https://www.acs.org/content/acs/en/education/students/highschool/seed.html [Accessed 22 Nov 2019].Suche in Google Scholar
Baptista, M., Martins, I., Conceição, T., & Reis, P. (2019). Multiple representations in the development of students’ cognitive structures about the saponification reaction. Chemistry Education: Research and Practice, 20(4), 760–771. https://doi.org/10.1039/c9rp00018f.Suche in Google Scholar
Bensaude-Vincent, B., & Simon, J. (2008). Chemistry and pollution. In Chemistry — The impure science (pp. 11–31). London: Imperial College Press.10.1142/9781848162266_0002Suche in Google Scholar
Calvete, M. J. F., Piccirillo, G., Vinagreiro, C. S., & Pereira, M. M. (2019). Hybrid materials for heterogeneous photocatalytic degradation of antibiotics. Coordination Chemistry Reviews, 395, 63–85. https://doi.org/10.1016/j.ccr.2019.05.004.Suche in Google Scholar
Caridade, P. J. S. B., Horta, J.-Z. J., & Varandas, A. J. C. (2013). Implications of the O + OH reaction in hydroxyl nightglow modeling. Atmospheric Chemistry and Physics, 13(1), 1–13. https://doi.org/10.5194/acp-13-1-2013.Suche in Google Scholar
Coe, R., Searle, J., Barmby, P., Jones, K., & Higgins, S. (2008). Relative difficulty of examinations in different subjects. Durham: CEM Center, Durham University.Suche in Google Scholar
Costa, N., Marques, L., & Kempa, R. (2000). Science teachers’ awareness of findings from education research. Research in Science & Technological Education, 18(1), 37–44. https://doi.org/10.1080/713694955.Suche in Google Scholar
Emsley, J. (1996). The consumer’s good chemical guide: Separating facts from fiction about everyday products. New York: Corgi.Suche in Google Scholar
Emsley, J. (1998). The elements. Oxford University Press.Suche in Google Scholar
Exstrom, C. L., & Mosher, M. D. (2000). A novel high school chemistry camp as an outreach model for regional colleges and universities. Journal of Chemical Education, 77(10), 1295–1297. https://doi.org/10.1021/ed077p1295.Suche in Google Scholar
Figueiredo, M., Esteves, L., Neves, J., & Vicente, H. (2016). A data mining approach to study the impact of the methodology followed in chemistry lab classes on the weight attributed by the students to the lab work on learning and motivation. Chemistry Education: Research and Practice, 17(1), 156–171. https://doi.org/10.1039/c5rp00144g.Suche in Google Scholar
Fiolhais, C., Festas, I., Damião, H., Portela, C., Ventura, G., Nogueira, R., & Rodrigues, S. (2014). Programa de Química e Física A 10o e 11o anos [Programme of Physics and Chemistry A, 10th and 11th grades]. Ministério da Educação e Cultura (MEC).Suche in Google Scholar
Gonsalves, A. R., Pineiro, M., Martins, J. M., Barata, P. A., & Menezesc, J. C. (2010). Identification of Alprazolam and its degradation products using LC-MS-MS. Arkivoc, 2010(5), 128–141.10.3998/ark.5550190.0011.513Suche in Google Scholar
Hartings, M. R., & Fahy, D. (2011). Communicating chemistry for public engagement. Nature Chemistry, 3(9), 674–677. https://doi.org/10.1038/nchem.1094.Suche in Google Scholar
Hofstein, A., & Lunetta, V. N. (2004). The laboratory in science education: Foundations for the twenty-first century. Science Education, 88(1), 28–54. https://doi.org/10.1002/sce.10106.Suche in Google Scholar
Imberti, C., & Sadler, P. J. (2020). Chapter One – 150 years of the periodic table: New medicines and diagnostic agents. In Advances in inorganic chemistry (pp. 3–56). Cambridge: Academic Press.10.1016/bs.adioch.2019.11.001Suche in Google Scholar
Johnstone, A. H. (1982). Macro-and micro-chemistry. School Science Review, 64, 377–379.Suche in Google Scholar
Johnstone, A. H. (1993). The development of chemistry teaching: A changing response to changing demand. Journal of Chemical Education, 70(9), 701. https://doi.org/10.1021/ed070p701.Suche in Google Scholar
Logar, A., & Ferk-Savec, V. (2011). Students’ hands-on experimental work vs lecture demonstration in teaching elementary school chemistry. Acta Chimica Slovenica, 58(4), 866–75.Suche in Google Scholar
Maldonado-Carmona, N., Ouk, T. S., Calvete, M. J. F., Pereira, M. M., Villandier, N., & Leroy-Lhez, S. (2020). Conjugating biomaterials with photosensitizers: Advances and perspectives for photodynamic antimicrobial chemotherapy. Photochemical and Photobiological Sciences, 19(4), 445–461. https://doi.org/10.1039/c9pp00398c.Suche in Google Scholar
Mernoff, B., Aldous, A. R., Wasio, N. A., Kritzer, J. A., Sykes, E. C. H., & O’Hagan, K. (2017). A reverse science fair that connects high school students with university researchers. Journal of Chemical Education, 94(2), 171–176. https://doi.org/10.1021/acs.jchemed.6b00111.Suche in Google Scholar
Millar, R. (2010). Practical work. In Good practice in science teaching - What research has to say (pp. 108–134). Maidenhead: McGraw-Hill Education.Suche in Google Scholar
Pinto, S. M., Tomé, V., Calvete, M. J. F., Castro, M. M. C. A., Tóth, É., & Geraldes, C. F. G. C. (2019). Metal-based redox-responsive MRI contrast agents. Coordination Chemistry Reviews, 390, 1–31. https://doi.org/10.1016/j.ccr.2019.03.014.Suche in Google Scholar
Prain, V., Tytler, R., & Peterson, S. (2009). Multiple representation in learning about evaporation. International Journal of Science Education, 31(6), 787–808. https://doi.org/10.1080/09500690701824249.Suche in Google Scholar
Ragsdale, R. O. (1982). A cooperative university-high school AP lab program. Journal of Chemical Education, 59(9), 770. https://doi.org/10.1021/ed059p770.Suche in Google Scholar
Robbins, M. E., & Schoenfisch, M. H. (2005). An interactive analytical chemistry summer camp for middle school girls. Journal of Chemical Education, 82(10), 1486–1488. https://doi.org/10.1021/ed082p1486.Suche in Google Scholar
Roesky, H. W., & Möckel, K. (1996). Chemical curiosities: Spectacular experiments and inspired quotes (pp. 20–22). VCH.Suche in Google Scholar
Saleh, R., Bearth, A., & Siegrist, M. (2019). “Chemophobia” today: Consumers’ knowledge and perceptions of chemicals. Risk Analysis, 39(12), 2668–2682. https://doi.org/10.1111/risa.13375.Suche in Google Scholar
Schaberle, F. A., Reis, L. A., Serpa, C., & Arnaut, L. G. (2019). Photon momentum transfer at water/air interfaces under total internal reflection. New Journal of Physics, 21(3), 033013. https://doi.org/10.1088/1367-2630/ab19d6.Suche in Google Scholar
Schwarz, G., Frenzel, W., Richter, W. M., Täuscher, L., & Kubsch, G. (2016). A multidisciplinary science summer camp for students with emphasis on environmental and analytical chemistry. Journal of Chemical Education, 93(4), 626–632. https://doi.org/10.1021/acs.jchemed.5b00211.Suche in Google Scholar
Semenova, A. A., Tarasov, A. B., & Goodilin, E. A. (2019). Periodic table of elements and nanotechnology. Mendeleev Communications, 29(5), 479–485. https://doi.org/10.1016/j.mencom.2019.09.001.Suche in Google Scholar
Siegrist, M., & Bearth, A. (2019). Chemophobia in Europe and reasons for biased risk perceptions. Nature Chemistry, 11(12), 1071–1072. https://doi.org/10.1038/s41557-019-0377-8.Suche in Google Scholar
Taber, K. S., & García-Franco, A. (2010). Learning processes in chemistry: Drawing upon cognitive resources to learn about the particulate structure of matter. The Journal of the Learning Sciences, 19(1), 99–142. https://doi.org/10.1080/10508400903452868.Suche in Google Scholar
Talanquer, V. (2011). Macro, Submicro, and Symbolic: The many faces of the chemistry “triplet”. International Journal of Science Education, 33(2), 179–195. https://doi.org/10.1080/09500690903386435.Suche in Google Scholar
Tarhan, L., & Sesen, B. A. (2010). Investigation the effectiveness of laboratory works related to “acids and bases” on learning achievements and attitudes toward laboratory. In Procedia – Social and behavioral sciences (pp. 2631–2636).10.1016/j.sbspro.2010.03.385Suche in Google Scholar
UNESCO (2001). Draft proposal and plan for a United Nations literacy decade. Paris: UNESCO.Suche in Google Scholar
Yao, B., Kuznetsov, V. L., Xiao, T., Jie, X., Gonzalez-Cortes, S., Dilworth, J. R., Edwards, P. P. (2020). Fuels, power and chemical periodicity. Philosophical Transactions of the Royal Society A Mathematical Physical and Engineering Sciences, 378(2180), 20190308. https://doi.org/10.1098/rsta.2019.0308.Suche in Google Scholar
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/cti-2020-0013).
© 2021 Bernardo A. Nogueira et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Good Practice Report
- Tracking down chemical phenomena with the usage of mobile phone slow-motion videos
- Research Article
- Student experiences of project-based learning in an analytical chemistry laboratory course in higher education
- Review Article
- Teaching chemistry with LEGO® bricks
- Research Articles
- Molecular School – a pre-university chemistry school
- Explicit teaching of problem categorization using concept mapping, and an exploratory study of its effect on student achievement and on conceptual understanding – the case of chemical equilibrium problems
- Good Practice Reports
- The production of less harmful and less toxic sparklers in an experiment for school students
- Design of a structure model set for inorganic compounds based on ping-pong balls linked with snap buttons
- Research Articles
- Why school-related content knowledge for pre-service chemistry teachers should include basic concepts in organic chemistry
- Application of stoichiometric hydrogen atoms for balancing organic combustion reactions
- Good Practice Report
- Teaching introductory chemistry through world cultural heritage history
Artikel in diesem Heft
- Frontmatter
- Good Practice Report
- Tracking down chemical phenomena with the usage of mobile phone slow-motion videos
- Research Article
- Student experiences of project-based learning in an analytical chemistry laboratory course in higher education
- Review Article
- Teaching chemistry with LEGO® bricks
- Research Articles
- Molecular School – a pre-university chemistry school
- Explicit teaching of problem categorization using concept mapping, and an exploratory study of its effect on student achievement and on conceptual understanding – the case of chemical equilibrium problems
- Good Practice Reports
- The production of less harmful and less toxic sparklers in an experiment for school students
- Design of a structure model set for inorganic compounds based on ping-pong balls linked with snap buttons
- Research Articles
- Why school-related content knowledge for pre-service chemistry teachers should include basic concepts in organic chemistry
- Application of stoichiometric hydrogen atoms for balancing organic combustion reactions
- Good Practice Report
- Teaching introductory chemistry through world cultural heritage history

