Abstract
Objectives
Amniotic band syndrome and osteogenesis imperfecta are two distinct diseases that develop due to structural defects of the collagen protein. In our paper, we report the concurrence of these two diseases rarely seen in the newborn period.
Case presentation
A female infant born at 33rd gestational week was found to have constrictive bands in her right lower extremity and flexion contractures in distal joints of lower and upper extremities due to amniotic bands in postnatal physical examination. While being treated for respiratory difficulty, she was diagnosed with osteogenesis imperfecta and treated with bisphosphonates upon being found to suffer bilateral humeral fractures on the sixth day of life. She received respiratory support with mechanical ventilation due to respiratory tract complications related to osteogenesis imperfecta and died on the 384th day of life.
Conclusions
One should bear in mind that other collagen tissue diseases may accompany the amniotic band syndrome; this possibility should be definitely pursued if clinical suspicion exists.
Introduction
Amniotic band syndrome (ABS) is a rare cluster of congenital deformities characterized by extremity amputations, constriction bands, and syndactyly [1]. Its incidence ranges between 1:1,200 and 1:15,000 live births and has an equal sex distribution [2]. Amniotic band syndrome occurs sporadically, with its prognosis being dependent on the severity of anomalies and the degree of organ involvement [3]. There are limb anomalies such as clubfoot and craniofacial anomalies such as cleft lip and cleft palate in a third of all cases. Visceral abnormalities such as omphalocele and gastroschisis have also been related to ABS. Dermatological disorders can also be seen, such as the alteration of dermal pattern, absence of normal hair curl, abnormal manual wrinkles, and alopecia at band attachment sites [4, 5].
The incidence of concurrent ABS and osteogenesis imperfecta is even lower. As such, only three cases of concurrent osteogenesis imperfecta and ABS have been reported so far in the literature [6], [7], [8]. Spontaneous respiration could not be achieved in one of the reported cases and another patient survived only 7 days. Only one of the reported patients survived long-term [6]. Our patient is the fourth case of concurrent osteogenesis imperfecta and ABS.
Case presentation
A female infant was born by cesarean section at 33rd gestational week with a birth weight of 1,535 g as the fourth living child of the fourth pregnancy of a 27-year-old mother who did not attend regular antenatal follow-up visits. The patient was admitted to the neonatal intensive care unit with low birth weight, respiratory distress, and structural limb anomalies. In postnatal physical examination, she was found to have hypoactive primitive reflexes, hypotonia, a dysmorphic face appearance, a protruding forehead, short neck, low ear and hairlines, micrognathia, and bluish discoloration of sclerae in addition to the signs of respiratory distress. She had flexion contractures in the distal joints of both upper limbs, developmental dysplasia and flexion contracture in the hip joint, and bilateral pes equinovarus deformity, which are suggesting those are caused by intrauterine amniotic bands. There were two constrictive rings in the distal part of her right lower limb that were caused by an amniotic band in the intrauterine life; she also had an increased skin thickness, edema, and restricted mobility in the dorsum of the right foot. When the patient was evaluated multidisciplinary by orthopedics, radiology and plastic and reconstructive surgery, they were of the opinion that there was no deterioration in blood circulation and there was sufficient blood flow with Doppler ultrasound was performed, that is why there was no need to surgery. In the subsequent clinical follow-ups, it was observed that his circulation became more relieved, and no undesirable events developed. There was thoracic convex scoliosis to the right in the vertebral column. The cystic structure found in the umbilical cord was an umbilical cord cyst. She was free of any limb fracture in her initial examination (Figure 1). She had no familial history of consanguineous marriage, and her father, mother, and the other children of the family were free of any known structural or genetic disease.

Constructive rings and structural anomalies due to amniotic band syndrome and osteogenesis imperfecta.
On the sixth day of the treatment a plain radiogram was taken for edema on both humeri and showed diaphyseal fractures and radiolucency in both humeri (Figure 2). For this reason, she was evaluated by the pediatric endocrinology department according to the current clinical findings, and the patient was diagnosed with osteogenesis imperfecta, and bisphosphonate treatment was started. Pamidronate was administered intravenously at a dose of 0.5 mg per kilogram for three consecutive days, and the same treatment was repeated three months later. She was consulted with the departments of plastic and reconstructive surgery and orthopedics. The lower extremities were then fixed with splints and bandages.
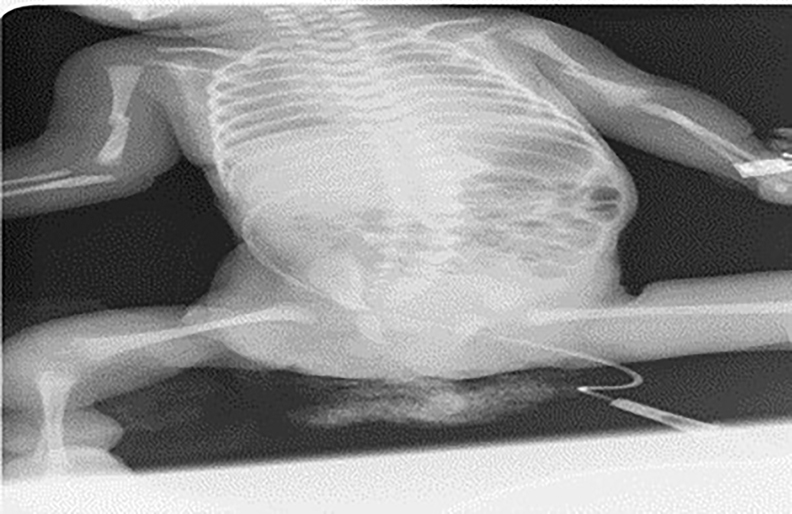
Diaphyseal fractures of humerus bilaterally show up 6th day of treatment.
The patient was evaluated for other possible accompanying anomalies. No vascular anomaly was found on arterial and venous color Doppler examinations of four limbs; an abdominal ultrasonographic examination found grade 1 pelvicaliectasis in the right kidney. In the follow-ups of the patient, it was observed that the pelvicaliectasis regressed and returned to normal. Transfontanelle ultrasound imaging showed a cephalohematoma with a size of 7 mm in the left occipital region. An echocardiogram revealed a patent foramen ovale. A magnetic resonance imaging study performed to examine accompanying vertebral anomalies was found a convex rotoscoliosis to the right. The patient could not pass the auditory test performed with the otoacoustic emission technique, and the acoustic brainstem response (ABR) test could not be performed since her clinical signs were not yet stabilized. Thus, she was deemed to have congenital hearing loss.
Her biochemical and hematological parameters showed no meaningful abnormality. In her genetic studies, a chromosome analysis from peripheral blood revealed a karyotype of 46, XX. Mutation analyses for osteogenesis imperfecta failed to show any mutation in the COLA1 and COL1A2 genes. SMN1 and SMN2 analyses to investigate hypotonia and weak deep tendon reflexes showed normal phenotypes of the said genes.
Discussion
Amniotic band syndrome is a rare structural collagen disorder [9]. It usually involves distal parts of the extremities. Its common signs are constriction rings, equinovarus deformity, amputation, and acrosyndactylia. It has also been shown to have non-classical signs such as cleft lip, cleft palate, and imperforate anus [10]. Osteogenesis imperfecta is a genetic disorder secondary to the structural and quantitative deficiency of type I collagen, which causes fragility in bones. It is known by a triad of blue sclerae, fragile bones, and deafness [11]. The presence of fragile bones is sufficient to suspect the diagnosis [12]. The spectrum of clinical signs ranges from a mild phenotype to the forms that are fatal in the perinatal period. Blue sclerae, generalized osteoporosis, and tooth abnormalities are the major criteria. Bone deformities, ligamentous laxity, deafness, easy bruising, and excessive sweating are the nonspecific minor criteria [13]. Our patient had long bone fractures that became clinically manifest with minor trauma, bone and joint deformities in the intrauterine life, and hearing loss. She was diagnosed with severe Sillence Type 3 osteogenesis imperfecta due to the absence of intrauterine fractures which first appeared on the postnatal 6th day in the long bones together with joint and bone deformities, blue sclerae, and hearing loss [14]. Her humeral fractures healed by splinting and bandaging in addition to bisphosphonate treatment.
There are mutations of the COL1A1 and COL1A2 genes in the majority of patients with osteogenesis imperfecta; however, it has also been shown that many different genetic mutations may cause the disease affecting collagen structure [14]. Our patient was free of mutations in the gene sequence analyses of the COL1A1 and COL1A2 genes; we, therefore, believe that it developed secondary to a rarer genetic mutation in our patient.
The concurrence of the ABS with other collagen tissue diseases such as Ehlers-Danlos syndrome and fatal osteogenesis imperfecta has been previously reported in the literature [6], [7], [8]. The concurrence of the ABS and osteogenesis imperfecta has been reported extremely rarely, with two of the three cases reported in the literature having the fatal phenotype and being deceased in the newborn period [6], [7], [8]. Our patient is the fourth reported case of this coexistence and survived 384 days. The patient was deceased to respiratory complications of osteogenesis imperfecta.
Conclusions
It should be kept in mind that other collagen tissue diseases such as osteogenesis imperfecta may accompany ABS; this possibility should be definitely pursued if clinical suspicion exists. Further studies are needed to better characterize the relationship between these two diseases. Families with such babies should be provided with genetic counseling and regular antenatal follow-up visits should be attended by pregnant women in order to make a prenatal diagnosis.
-
Research funding: None declared.
-
Author contributions: SMD conceived of the article and participated in design and coordination. SE supervised the study. STY participated in literature review. EU carried out genetic studies and participated in design of article. IY drafted the manuscript. ID participated in collecting data. All authors read and approved the final manuscript. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: The local Institutional Review Board deemed the study exempt from review.
References
1. Ray, M, Hendrick, SJ, Raimer, SS, Blackwell, SJ. Amniotic band syndrome. Int J Dermatol 1988;27:312–4. https://doi.org/10.1111/j.1365-4362.1988.tb02359.x.Search in Google Scholar PubMed
2. Burgess, RC. Brachydactyly in acrosyndactyly. J Hand Surg Am 1991;16:125–6. https://doi.org/10.1016/s0363-5023(10)80026-7.Search in Google Scholar PubMed
3. da Silva, CSM, e Silva, AJF, Mariano, SCR. Amniotic band syndrome with double encephalocele: a case report. Surg Neurol Int 2020;11:448. https://doi.org/10.25259/sni_454_2020.Search in Google Scholar PubMed PubMed Central
4. Patterson, TJ. Congenital ring-constrictions. Br J Plast Surg 1961;14:1–31. https://doi.org/10.1016/s0007-1226(61)80002-7.Search in Google Scholar PubMed
5. Higginbottom, MC, Jones, KL, Hall, BD, Smith, DW. The amniotic band disruption complex: timing of amniotic rupture and variable spectra of consequent defects. J Pediatr 1979;95:544–9. https://doi.org/10.1016/s0022-3476(79)80759-3.Search in Google Scholar PubMed
6. Shah, KH, Shah, H. A rare combination of amniotic constriction band with osteogenesis imperfecta [Online]. BMJ Case Rep 2015. https://doi.org/10.1136/bcr-2015-212400.Search in Google Scholar PubMed PubMed Central
7. van der Rest, M, Hayes, A, Marie, P, Desbarats, M, Kaplan, P, Glorieux, FH, et al.. Lethal osteogenesis imperfecta with amniotic band lesions: collagen studies. Am J Med Genet 1986;24:433–46. https://doi.org/10.1002/ajmg.1320240306.Search in Google Scholar PubMed
8. Young, ID, Lindenbaum, RH, Thompson, EM, Pembrey, ME. Amniotic bands in connective tissue disorders. Arch Dis Child 1985;60:1061–3. https://doi.org/10.1136/adc.60.11.1061.Search in Google Scholar PubMed PubMed Central
9. Goldfarb, CA, Sathienkijkanchai, A, Robin, NH. Amniotic constriction band: a multidisciplinary assessment of etiology and clinical presentation. J Bone Joint Surg Am 2009;91:68–75. https://doi.org/10.2106/jbjs.i.00339.Search in Google Scholar PubMed
10. Walter, JH, Goss, LR, Lazzara, AT. Amniotic band syndrome. J Foot Ankle Surg 1998:325–33. https://doi.org/10.1016/s1067-2516(98)80070-7.Search in Google Scholar PubMed
11. Kliegman, R, editor. Nelson textbook of pediatrics. 21. bs. Philadelphia: Elsevier; 2020.Search in Google Scholar
12. Falvo, KA, Root, L, Bullough, PG. Osteogenesis imperfecta: clinical evaluation and management. J Bone Joint Surg Am 1974;56:783–93. https://doi.org/10.2106/00004623-197456040-00012.Search in Google Scholar
13. Harrington, J, Sochett, E, Howard, A. Update on the evaluation and treatment of osteogenesis imperfecta. Pediatr Clin North Am 2014;61:1243–57. https://doi.org/10.1016/j.pcl.2014.08.010.Search in Google Scholar PubMed
14. Van Dijk, F, Sillence, D. Osteogenesis imperfecta: clinical diagnosis, nomenclature and severity assessment. Am J Med Genet A 2014;164:1470–81. https://doi.org/10.1002/ajmg.a.36545.Search in Google Scholar PubMed PubMed Central
© 2021 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Editorial
- The journal Case Reports in Perinatal Medicine starts with open access
- Case Reports – Obstetrics
- Myomectomy scar pregnancy ‒ a serious, but scarcely reported entity: literature review and an instructive case
- Postpartum ovarian vein thrombosis
- Management of a patient in the state of total occlusion of aorta due to Takayasu arteritis in preconceptional and pregnancy period
- Stress degree demonstrated in mothers with phenylketonuria or hyperphenylalaninemia infant when requested for total or partial breastfeeding replacement
- Successful pregnancy outcome in patient with cardiac transplantation
- Further insights into unusual acrania-exencephaly-anencephaly sequence caused by amniotic band – first trimester fetoscopic correlation with two- and three-dimensional ultrasound
- Elevated fetal middle cerebral artery peak systolic velocity in diabetes type 1 patient: a case report
- Postpartum fibroid degeneration associated with elevated procalcitonin levels
- Case report: The first COVID-19 case among pregnant women at 21-week in Vietnam
- Posterior urethral valves (PUVs): prenatal ultrasound diagnosis and management difficulties: a review of three cases
- Premature fetal closure of the ductus arteriosus of unknown cause – could it be influenced by maternal consumption of large quantities of herbal chamomile tea – a case report?
- Spontaneous resolution of fetal ascites secondary to gastrointestinal abnormality
- A case of severe SARS-CoV-2 infection with negative nasopharyngeal PCR in pregnancy
- Respiratory decompensation due to COVID-19 requiring postpartum extracorporeal membrane oxygenation
- Obstetrical history of a family with combined oxidative phosphorylation deficiency 3 and methylenetetrahydrofolate reductase polymorphisms
- A case of newly diagnosed autoimmune diabetes in pregnancy presenting after acute onset of diabetic ketoacidosis
- Mother and child with osteogenesis imperfecta type III. Pregnancy management, delivery, and outcome
- Early detection of Emanuel syndrome: a case report
- Case Reports – Newborn
- Neonatal cervical lymphatic malformation involving the fetal airway the setting of emergency caesarean section
- Rothia dentocariosa bacteremia in the newborn: causative pathogen or contaminant?
- Severe hypocalcemia and seizures after normalization of pCO2 in a patient with severe bronchopulmonary dysplasia and permissive hypercapnia
- Infrequent association of two rare diseases: amniotic band syndrome and osteogenesis imperfecta
- Transient congenital Horner syndrome and multiple peripheral nerve injury: a scarcely reported combination in birth trauma
- No footprint too small: case of intrauterine herpes simplex virus infection
- Liver laceration presented as intraabdominal bleeding in a newborn with hypoxic-ischemic encephalopathy
- Extremely preterm infant with persistent peeling skin: X-linked ichthyosis imitates prematurity
- Thrombospondin domain1-related congenital chylothorax in an infant with maple syrup urine disease: a challenging case
- Parenteral nutrition extravasation into the abdominal wall mimicking an abscess
- Subcutaneous fat necrosis of the newborn and nephrolithiasis
- Fetal MRI assessment of head & neck vascular malformation in predicting outcome of EXIT-to-airway procedure
- Scimitar syndrome – a case report
- Asymptomatic severe laryngotracheoesophageal cleft (LTEC) in a preterm newborn
- Transient generalized proximal tubular dysfunction in an infant with a urinary tract infection: the effect of maternal infliximab therapy?
- Congenital Lobular Capillary Hemangioma in a 48 hours old neonate: a case report and a literature review
- Neonate born with ischemic limb to a COVID-19 positive mother: management and review of literature
Articles in the same Issue
- Editorial
- The journal Case Reports in Perinatal Medicine starts with open access
- Case Reports – Obstetrics
- Myomectomy scar pregnancy ‒ a serious, but scarcely reported entity: literature review and an instructive case
- Postpartum ovarian vein thrombosis
- Management of a patient in the state of total occlusion of aorta due to Takayasu arteritis in preconceptional and pregnancy period
- Stress degree demonstrated in mothers with phenylketonuria or hyperphenylalaninemia infant when requested for total or partial breastfeeding replacement
- Successful pregnancy outcome in patient with cardiac transplantation
- Further insights into unusual acrania-exencephaly-anencephaly sequence caused by amniotic band – first trimester fetoscopic correlation with two- and three-dimensional ultrasound
- Elevated fetal middle cerebral artery peak systolic velocity in diabetes type 1 patient: a case report
- Postpartum fibroid degeneration associated with elevated procalcitonin levels
- Case report: The first COVID-19 case among pregnant women at 21-week in Vietnam
- Posterior urethral valves (PUVs): prenatal ultrasound diagnosis and management difficulties: a review of three cases
- Premature fetal closure of the ductus arteriosus of unknown cause – could it be influenced by maternal consumption of large quantities of herbal chamomile tea – a case report?
- Spontaneous resolution of fetal ascites secondary to gastrointestinal abnormality
- A case of severe SARS-CoV-2 infection with negative nasopharyngeal PCR in pregnancy
- Respiratory decompensation due to COVID-19 requiring postpartum extracorporeal membrane oxygenation
- Obstetrical history of a family with combined oxidative phosphorylation deficiency 3 and methylenetetrahydrofolate reductase polymorphisms
- A case of newly diagnosed autoimmune diabetes in pregnancy presenting after acute onset of diabetic ketoacidosis
- Mother and child with osteogenesis imperfecta type III. Pregnancy management, delivery, and outcome
- Early detection of Emanuel syndrome: a case report
- Case Reports – Newborn
- Neonatal cervical lymphatic malformation involving the fetal airway the setting of emergency caesarean section
- Rothia dentocariosa bacteremia in the newborn: causative pathogen or contaminant?
- Severe hypocalcemia and seizures after normalization of pCO2 in a patient with severe bronchopulmonary dysplasia and permissive hypercapnia
- Infrequent association of two rare diseases: amniotic band syndrome and osteogenesis imperfecta
- Transient congenital Horner syndrome and multiple peripheral nerve injury: a scarcely reported combination in birth trauma
- No footprint too small: case of intrauterine herpes simplex virus infection
- Liver laceration presented as intraabdominal bleeding in a newborn with hypoxic-ischemic encephalopathy
- Extremely preterm infant with persistent peeling skin: X-linked ichthyosis imitates prematurity
- Thrombospondin domain1-related congenital chylothorax in an infant with maple syrup urine disease: a challenging case
- Parenteral nutrition extravasation into the abdominal wall mimicking an abscess
- Subcutaneous fat necrosis of the newborn and nephrolithiasis
- Fetal MRI assessment of head & neck vascular malformation in predicting outcome of EXIT-to-airway procedure
- Scimitar syndrome – a case report
- Asymptomatic severe laryngotracheoesophageal cleft (LTEC) in a preterm newborn
- Transient generalized proximal tubular dysfunction in an infant with a urinary tract infection: the effect of maternal infliximab therapy?
- Congenital Lobular Capillary Hemangioma in a 48 hours old neonate: a case report and a literature review
- Neonate born with ischemic limb to a COVID-19 positive mother: management and review of literature

