Abstract
Corrosion of reinforcing steel remains one of the most pressing durability challenges for concrete infrastructure, with substantial economic and safety implications. This review provides a systematic and critical synthesis of research on hot-dip galvanized (HDG) reinforcement, integrating electrochemical, structural, and field-based perspectives. The methodology involved comparative evaluation of laboratory studies, long-term monitoring data, and technological advances to identify consensus findings and unresolved controversies. The novelty of this review lies in its focused analysis of three critical debates: the influence of initial hydrogen evolution on interfacial bond strength, the conditional stability of the calcium hydroxyzincate passive layer in high-alkalinity environments, and the discrepancy between accelerated laboratory testing and field performance. The review also compares HDG with alternative reinforcement systems, particularly epoxy-coated and stainless steel rebars, to contextualize durability and cost trade-offs. Recent innovations, including continuous galvanized rebar (CGR), zinc-alloy modifications, non-destructive evaluation methods, and computational service-life modeling, are highlighted as transformative developments. By systematically bridging mechanistic insights with practical outcomes, this review advances current understanding of galvanized reinforcement and identifies critical directions for future research aimed at reliable service life prediction and optimized application in durable concrete structures.
1 Introduction
Reinforced concrete stands as the most ubiquitous construction material in the modern world, forming the backbone of global infrastructure, from bridges and buildings to marine structures and transportation networks. The composite nature of steel and concrete provides exceptional structural efficiency and design flexibility. However, the long-term durability of these structures is not guaranteed. A primary life-limiting factor is the corrosion of the embedded steel reinforcement, a pervasive and costly problem that compromises structural integrity and safety (Roberge 2000). The economic burden of corrosion-induced degradation is staggering, with annual costs for repair and rehabilitation estimated in the tens of billions of dollars in the United States alone, a figure that continues to grow as the existing infrastructure ages (Shu et al. 2021). The electrochemical degradation of steel reinforcement leads to the formation of voluminous iron oxides (rust), which exert immense internal pressure on the surrounding concrete (Ye et al. 2021). This pressure eventually exceeds the concrete’s tensile strength, resulting in cracking, delamination, and spalling, which not only diminishes the load-bearing capacity of the structure but also accelerates further degradation by creating pathways for aggressive species to reach the reinforcement (Aguirre and Mejía De Gutiérrez 2013).
To combat this pervasive threat and meet the increasing demand for infrastructure with design lives of 75–125 years, a spectrum of corrosion protection strategies has been developed and implemented. These strategies can be broadly categorized into three approaches: modifying the concrete environment, employing electrochemical methods, and modifying the reinforcement itself (Collins et al. 1993). Modifying the concrete includes using high-performance concrete with low permeability, incorporating supplementary cementitious materials (SCMs) like fly ash and silica fume, or adding chemical corrosion inhibitors to the mix (Goyal et al. 2018). Electrochemical methods, such as impressed current cathodic protection, are highly effective but are typically applied as a remedial action for existing corroded structures (Lal et al. 2022). The third approach, modifying the reinforcement, involves using materials with inherently greater corrosion resistance. This category is dominated by the use of coated bars, such as fusion-bonded epoxy-coated rebar (ECR), and corrosion-resistant alloys, most notably stainless steel and hot-dip galvanized (HDG) steel reinforcement (Kepler et al. 2000).
Among these options, hot-dip galvanized rebar has been employed as a corrosion protection solution for nearly a century, with its first documented uses dating back to the 1930s. The protection mechanism of the zinc coating is twofold: it provides a physical barrier that isolates the steel from the corrosive concrete pore solution, and it offers sacrificial (cathodic) protection, where the more active zinc corrodes preferentially to protect any small areas of exposed steel at cuts or points of damage (Patnaik 2024). This dual-action protection has made it a widely specified material for extending the service life of concrete structures in aggressive environments (Van Leeuwen et al. 2022). However, despite its long history and widespread application, the use of HDG rebar is not without significant scientific controversy. The literature is replete with conflicting findings and ongoing debates regarding the fundamental corrosion mechanisms, the long-term stability of its passive film in the highly alkaline concrete environment, and its ultimate effect on the critical bond strength with the surrounding concrete (Pokorný et al. 2017). These unresolved questions have created uncertainty for specifiers and asset owners, hindering a complete consensus on its performance envelope and optimal application. This paper aims to provide a critical and exhaustive review of the current state of understanding regarding the corrosion and performance of hot-dip galvanized rebar in concrete. Moving beyond foundational principles, this review will dissect the complex interplay between the material’s electrochemistry, its structural performance, and recent technological advancements. The objective is to synthesize the vast and often contradictory body of literature, presenting the core arguments and evidence from competing scientific viewpoints.
2 Electrochemical behavior and corrosion mechanisms of galvanized steel in concrete
The performance of hot-dip galvanized rebar is fundamentally governed by a series of complex electrochemical reactions that occur at the zinc–concrete interface. While the general principles are understood, the specific mechanisms, the stability of the resulting products, and their long-term implications remain subjects of intense scientific scrutiny and debate. This section critically examines the electrochemical behavior of galvanized steel, from its initial contact with fresh concrete to its eventual depassivation in the presence of aggressive agents.
2.1 Initial reactions in fresh concrete: passivation and the hydrogen evolution debate
When hot-dip galvanized rebar is first embedded in fresh concrete, its zinc coating is exposed to a highly alkaline pore solution, typically with a pH between 12.5 and 13.5, saturated with calcium hydroxide. As zinc is an amphoteric metal, it is reactive in such a strong alkaline environment, leading to a series of immediate chemical reactions that define the nature of the interface for the entire service life of the structure (Sistonen 2009). The initial anodic reaction involves the dissolution of zinc into zinc ions (Zn→Zn2++2e−), while the corresponding cathodic reaction is predominantly the reduction of water to produce hydrogen gas (2H2O+2e−→H2↑+2OH−), as the high pH suppresses other cathodic reactions like oxygen reduction (Yeomans 2004).
This initial period of active corrosion is transient. The dissolved zincate ions react with the abundant calcium ions (Ca2+) present in the cement pore solution to precipitate a dense, adherent layer of a complex salt known as calcium hydroxyzincate (Ca[Zn(OH)3]2⋅2H2O), often abbreviated as CHZ (Bellezze et al. 2021). The formation of this calcium hydroxyzincate (CHZ) layer is widely considered to be the key passivation mechanism for galvanized steel in concrete (Garnica-Rodríguez et al. 2022). Once the surface is covered by this stable, insoluble film, the vigorous initial corrosion and hydrogen evolution cease, and the zinc coating enters a passive state with a very low corrosion rate (Maeda et al. 2020). The formation of this layer typically consumes about 10 µm of the outer pure zinc (eta) layer of the galvanized coating (Mandal et al. 2009). The thickness of the zinc coating plays a critical role in this process. A thicker coating provides a larger reservoir of zinc available for CHZ precipitation, ensuring that the initial 10 µm consumption represents a smaller fraction of the total coating. This generally results in a denser and more continuous CHZ layer and prolongs the duration of passivation (Al-Negheimish et al. 2021; Mandal et al. 2009). However, excessively thick coatings, particularly on reactive steels, may promote the growth of brittle intermetallic phases, which could adversely affect bond performance (Baig and Ahmad 2022). Thus, the balance between adequate thickness for passivation and avoidance of brittleness remains an important consideration in practice. Figure 1 provides a schematic representation of these initial reactions at the zinc–concrete interface. This diagram illustrates the electrochemical processes occurring when galvanized rebar is first placed in wet concrete. It shows the zinc coating (anode) dissolving to form zincate ions (Zn2+), while the cathodic reaction on the zinc surface reduces water to evolve hydrogen gas (H2). The diagram depicts the subsequent reaction of zincate and calcium ions (Ca2+) from the pore solution to precipitate a protective, crystalline layer of calcium hydroxyzincate (Ca[Zn(OH)3]2⋅2H2O) on the rebar surface, which passivates the zinc and arrests the initial reaction.
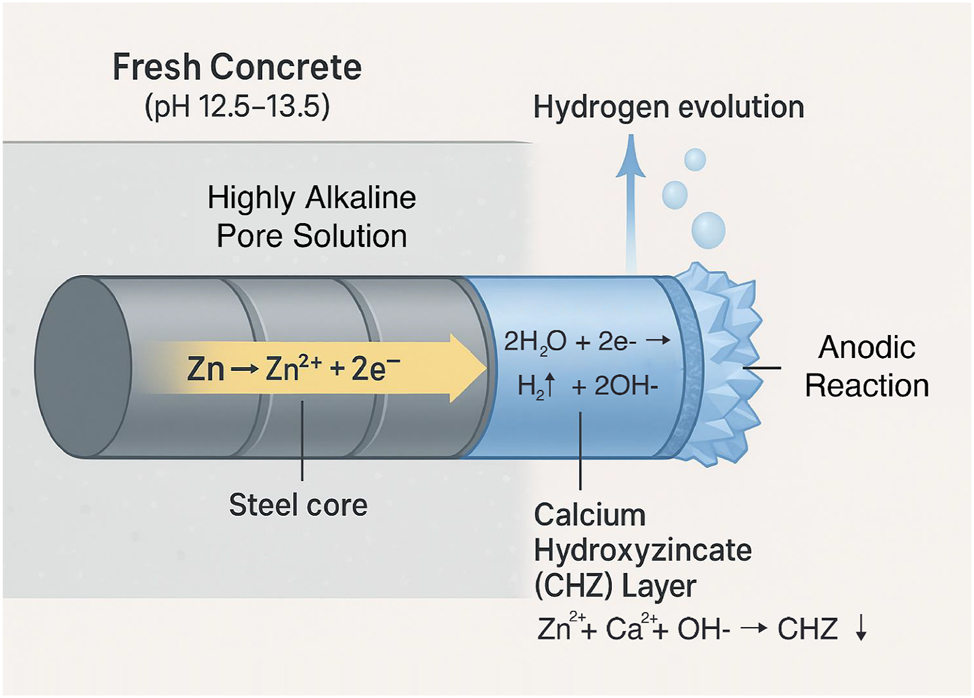
Schematic diagram of initial passivation of hot-dip galvanized rebar in fresh concrete (author-created).
The morphology of this critical passive layer has been studied using advanced microscopy. Figure 2 presents a representative scanning electron microscope (SEM) micrograph, illustrating the characteristic faceted morphology of the calcium hydroxyzincate layer formed on a galvanized surface.
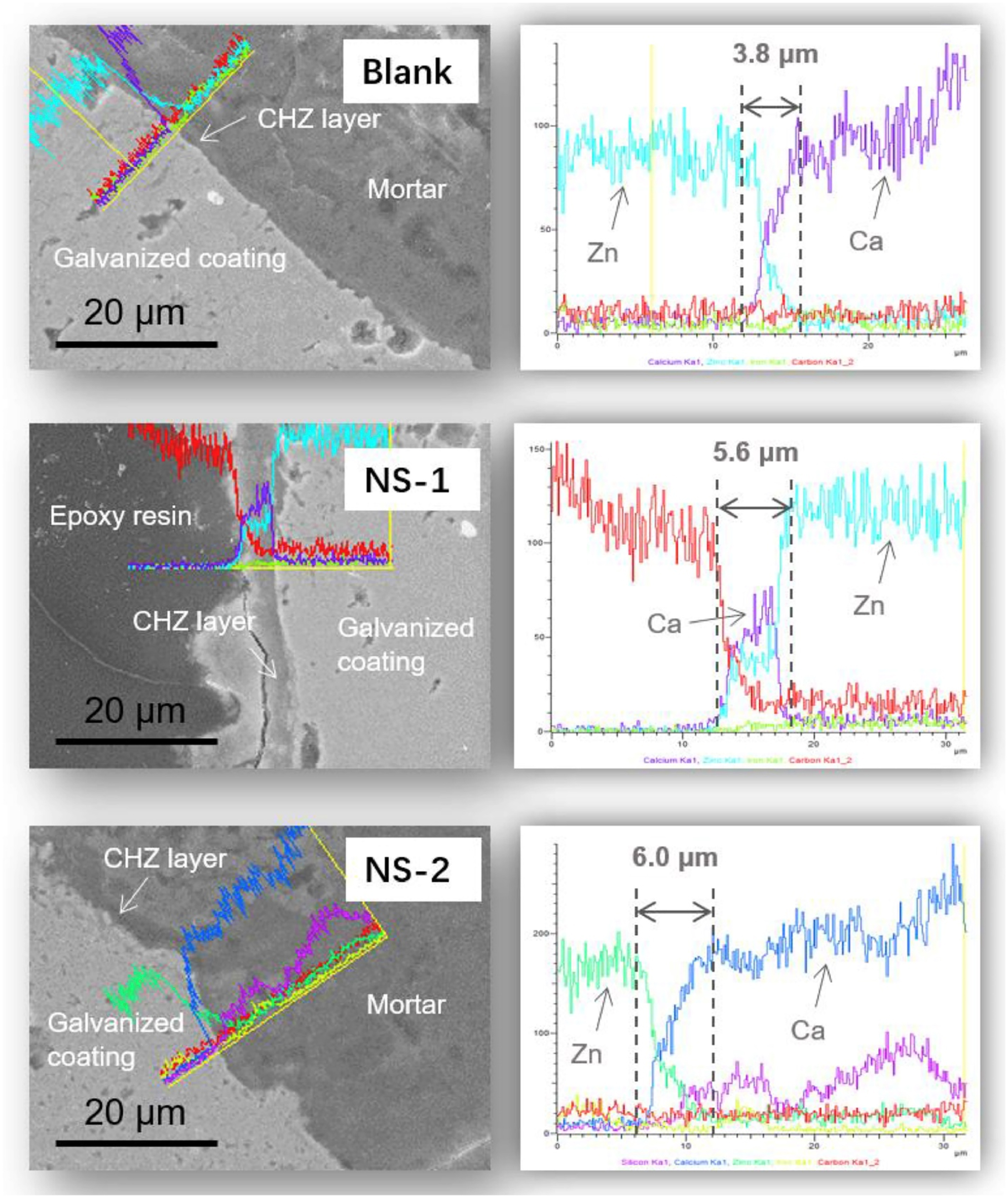
SEM micrograph of the CHZ passive film on galvanized steel (Zheng et al. 2020). Reproduced with permission from Elsevier.
While the formation of the CHZ layer is an accepted phenomenon, the consequences of the initial hydrogen evolution are a central point of scientific controversy, leading to two divergent viewpoints on its impact. It is important to note, however, that galvanized coatings are not applied to high-strength or prestressing steels, as hydrogen uptake during the early corrosion reactions can induce embrittlement of the steel matrix. This risk has led to prohibitions in standards and specifications for prestressing applications, confining the use of hot-dip galvanized reinforcement primarily to conventional reinforcing steels (mild or medium-strength grades) (Pernicova et al. 2017; Pokorný et al. 2024a, b). The first viewpoint, often promoted by industry associations and supported by a significant body of performance testing, treats the hydrogen evolution as a manageable and temporary side-effect. Proponents of this view argue that the reaction is short-lived, ceasing as soon as the concrete hardens and the CHZ layer passivates the surface. Laboratory studies are cited to show that the liberated hydrogen does not permeate the zinc coating to cause embrittlement of the underlying steel substrate. Furthermore, it is often suggested that any small voids or increased porosity at the interface created by the hydrogen bubbles are subsequently filled by the zinc corrosion products, negating any long-term detrimental effect on the bond. This perspective emphasizes that the reaction can be effectively controlled or suppressed through post-galvanizing passivation treatments. Historically, chromate-based methods (as required by ASTM A767) were widely adopted; however, the carcinogenicity of hexavalent chromium has led to their progressive replacement with environmentally safer alternatives. Recent developments include trivalent chromium (Cr(III)) passivation systems (e.g., ADL-3P, ADL-32), as well as organic and zinc-based coatings such as zinc phosphate, silicate, ceramic, or silane treatments, all of which provide effective protection against white rust and hydrogen evolution. Furthermore, passivating oils and molybdate/polyphosphate conversion processes have also been reported as viable non-chromate options for maintaining the integrity of galvanized reinforcement in concrete environments (Liu et al. 2012; Saravanan and Srikanth 2019).
In stark contrast, a more critical school of thought, supported by detailed mechanistic studies, posits that the initial hydrogen evolution is an inherently detrimental process that imparts a permanent weakness at the steel-concrete interface (Pernicova et al. 2017). The core of this argument is that the formation of hydrogen gas bubbles creates a zone of increased and irreversible porosity within the cement paste immediately adjacent to the rebar surface, known as the interfacial transition zone (ITZ) (Scrivener et al. 2004). Contrary to the first viewpoint, these researchers present evidence that this porosity is not sufficiently filled by corrosion products and thus remains as a permanent microstructural defect. This weakened, more porous ITZ is theorized to directly reduce the mechanical bond between the rebar and the concrete. Moreover, for high-strength steels or prestressing strands under tension, the hydrogen can cause embrittlement and micro-cracking of the zinc coating itself, compromising its barrier function from the very beginning. This debate is crucial, as it establishes a direct causal link between the chemistry of the first few hours of curing and the ultimate mechanical performance and long-term durability of the reinforced concrete element. The initial chemical reaction may therefore define the service life trajectory by creating a permanent microstructural feature that impacts both bond and the future ingress of aggressive ions.
2.2 The stability of the passive layer
The long-term performance of galvanized rebar hinges on the stability and protective quality of the CHZ passive layer (Al-Negheimish et al. 2021). The conventional understanding is that this layer, once formed, provides robust and lasting protection. It acts as a stable physical barrier, isolating the zinc from the concrete pore solution, and is particularly effective in the typical pH range of sound concrete (approximately pH 12.5 to 13.2) (Pokorný et al. 2022).
However, this foundational assumption has been challenged by recent, highly detailed research that suggests the passivity of galvanized steel in concrete is not an absolute state but rather a conditional and potentially precarious equilibrium. Work by Roventi et al. (2014) provides compelling evidence that the protective nature of the CHZ layer is highly dependent on both pH and moisture availability. Their electrochemical and microscopic analyses showed that while a protective film forms at a pH of 12.6, at slightly higher pH levels (13.0 and above), which can occur in some modern concrete formulations, the zinc coating remains in a state of active corrosion, continuing to produce hydrogen for extended periods. Their findings indicate that the CHZ layer formed at these higher pH levels is less compact and non-protective, and that the presence of calcium ions may actually destabilize the formation of more desirable passive films like zinc oxide (ZnO) or zinc hydroxide (Zn(OH)2) (Tan and Hansson 2008). This research raises the critical possibility that the apparent passivity observed in many older tests may not be true electrochemical stability, but rather a state of very slow corrosion dictated by the drying of the concrete and the consequent lack of a continuous electrolyte path. If the concrete becomes re-saturated with water, this “dormant” active corrosion could be re-initiated (Roventi et al. 2013). This nuanced perspective implies that in environments with persistent moisture and high alkalinity, the long-term protective performance of the CHZ layer may be significantly overestimated, suggesting its stability is conditional rather than absolute (Pokorný et al. 2024a, b).
2.3 Depassivation mechanisms
The protective passive layer on galvanized rebar, whether fully stable or conditionally so, can be broken down by two primary mechanisms: chloride ingress and carbonation.
2.3.1 Chloride-induced corrosion
The ingress of chloride ions, typically from de-icing salts or marine environments, is the most common cause of premature corrosion of reinforcement in concrete (Shi et al. 2023). Interestingly, galvanized rebar can maintain a protective passive film in pore solutions with pH values between approximately 12.0 and 13.5, but once localized pH declines below about 10.5, depassivation becomes increasingly likely (Pu et al. 2017). Environmental factors strongly modulate this process. Roventi et al. (2014) demonstrated that carbonation can degrade the original CHZ layer, and subsequent chloride-rich wet–dry cycles substantially reduce the chloride threshold for corrosion initiation, while wet–dry cycles in tap water alone have little accelerating effect. Furthermore, González and Andrade (1982) emphasized that ambient humidity and fluctuating moisture availability significantly affect the persistence of passivation. These findings highlight that both chemical (pH) and environmental (wet–dry cycling, humidity) conditions are decisive in governing the durability of galvanized reinforcement in chloride-contaminated concrete. The concentration of chlorides at the rebar surface required to initiate corrosion is known as the chloride threshold (CT). For black steel, this threshold is generally accepted to be around 0.4 % total chloride ion content by weight of cement. For galvanized steel, the literature consistently reports a much higher threshold, typically ranging from 2 to 5 times that of black steel, with some long-term field studies suggesting a tolerance up to 10 times higher. Reported values for the CT of galvanized rebar vary, but a value of 1.2 % by cement weight is often cited as a conservative average, with some studies finding initiation not occurring until chloride levels exceed 2.0 % (Conciatori et al. 2015). This elevated threshold significantly extends the corrosion initiation phase of the structure’s service life.
Once the chloride threshold is breached, the aggressive chloride ions locally disrupt the CHZ passive film. The exact mechanism is complex, but it is understood to involve the formation of soluble zinc chloride complexes, which leads to the breakdown of the passive layer and the precipitation of new, less protective corrosion products (Yu et al. 2020). The primary solid corrosion products identified in chloride-contaminated concrete are zinc oxide (ZnO) and a complex salt called zinc hydroxychloride, or simonkolleite (Zn5(OH)8Cl2⋅H2O) (Ghazaee et al. 2024). The formation of simonkolleite is of particular concern, as some studies suggest it is more voluminous than ZnO and could potentially generate expansive pressures, though this point is debated. Figure 3 illustrates the mechanism of chloride-induced depassivation. This diagram shows a cross-section of a galvanized rebar embedded in chloride-contaminated concrete. It illustrates chloride ions (Cl−) migrating through the concrete pores and attacking the protective CHZ layer. The schematic depicts the local breakdown of the CHZ film, the formation of new corrosion products like simonkolleite (Zn5(OH)8Cl2⋅H2O), and the subsequent active corrosion of the underlying zinc and zinc–iron alloy layers. The relative performance of different reinforcement types with respect to chloride tolerance is a critical factor for material selection in aggressive environments. Table 1 compiles typical chloride threshold values from the literature for a comparative overview.
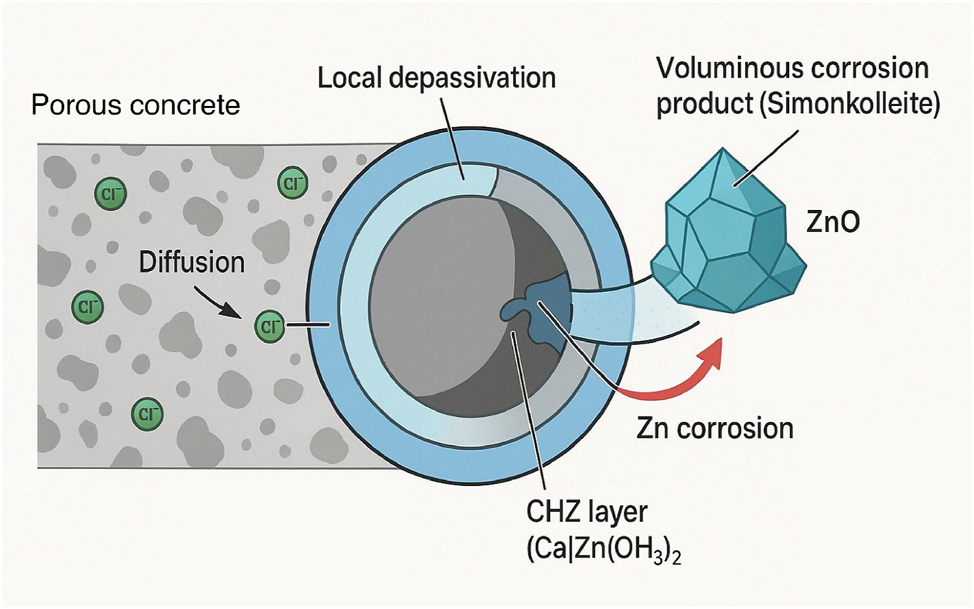
Schematic diagram of chloride-induced corrosion of galvanized rebar (author-created).
Comparative chloride thresholds for various reinforcement types in concrete.
| Reinforcement type | Typical chloride threshold (% Cl− by weight of cement) | Relative threshold (vs. black steel) | Key references |
|---|---|---|---|
| Black (carbon) steel | 0.2 %–0.4 % | 1x | Hurley and Scully (2006) |
| Epoxy-coated steel (ECR) | Similar to black steel if damaged; very high if intact | ∼1x (at defects) | López-Calvo et al. (2013) |
| HDG steel | 0.8 %–2.0 % (or higher) | 2x – 5x (or higher) | Kenny and Katz (2019) |
| Stainless steel (e.g., 316) | > 2.0 % (often much higher) | >5x – 24x | Darwin et al. (2007) |
2.3.2 Carbonation-induced corrosion
The second major depassivation mechanism is carbonation, a process where atmospheric carbon dioxide (CO2) dissolves in the concrete pore water to form carbonic acid, which then reacts with calcium hydroxide, neutralizing the concrete’s alkalinity and lowering its pH (Li and Wu 2022). Uncoated steel relies on a high pH (above ∼11.5) to maintain its passive film; once the carbonation front reaches the rebar and the pH drops below this level, corrosion initiates (Pu et al. 2017). Zinc, however, is passive over a much broader pH range, remaining stable down to a pH of approximately 9.5. This inherent chemical property makes galvanized rebar significantly more resistant to carbonation-induced corrosion than black steel, a point on which there is broad consensus in the scientific literature (González and Andrade 1982). This provides a substantial durability advantage in structures where carbonation is the primary degradation risk, such as buildings and infrastructure in urban or industrial environments with lower airborne chloride levels.
3 Methodology for measuring and classifying corrosion severity of hot-dip galvanized rebar
This section defines how corrosion severity for HDG reinforcement is measured and classified from safe to severe, and how those classes are used in service-life prediction. The classification is grounded in routinely applied diagnostics – electrochemical corrosion rate by LPR, chloride concentration at bar depth relative to a galvanized-specific threshold, remaining zinc-coating thickness, and qualified use of half-cell potential mapping.
Electrochemical corrosion current density (icorr) measured by LPR is interpreted using widely adopted on-site categories for reinforcement in concrete. Following Andrade and Alonso, values below 0.10 μA cm−2 indicate negligible corrosion, values between 0.10 and 0.50 μA cm−2 indicate low activity, values between 0.50 and 1.0 μA cm−2 indicate moderate activity, and values exceeding 1.0 μA cm−2 are characteristic of high corrosion. These ranges, long used for on-site appraisal of steel in concrete, provide a practical basis for classifying galvanized bars as well, when interpreted together with the complementary indicators below (Andrade and Alonso 2001).
Half-cell potential mapping is used primarily to localize anodic regions rather than to assign severity for zinc. ASTM C876 specifies probability thresholds for uncoated reinforcing steel; because zinc exhibits more negative potentials in alkaline pore solutions, those thresholds cannot be transferred directly to HDG without risking false positives. In this review, potential mapping guides where to perform targeted LPR/EIS measurements and material sampling, while severity classification is assigned from icorr, chemical exposure at depth, and coating condition (Astm 2015).
Chloride exposure is evaluated against a galvanized-specific critical chloride threshold (CTgalv). Darwin’s experimental program on galvanized reinforcement reported a mean threshold on the order of 2.6 l b yd−3 of concrete, higher than that for conventional steel (Darwin et al. 2007). In practice, the ratio of the measured chloride content at bar depth to CTgalv is used to judge proximity to initiation; ratios well below unity are considered safe, ratios approaching unity are considered moderate, and ratios exceeding unity indicate a severe condition likely to initiate corrosion of the zinc layer.
The state of the zinc coating is assessed by the remaining thickness relative to its original specification. Batch-galvanized rebar must satisfy minimum coating mass requirements in ASTM A767, while CGR is governed by ASTM A1094, for which commercial CGR products typically provide a pure-zinc layer of ≈ 50 µm or greater. Magnetic thickness measurements in the field, corroborated where necessary by metallographic sectioning, indicate depletion toward the intermetallic layers; pronounced depletion is treated as a marker of elevated severity even when short-term icorr remains modest (Yeomans 2023).
To illustrate the classification with published data, Jaśniok et al. (2020) reported that for galvanized steel embedded in chloride-bearing concrete, the corrosion current density decreased to ≈ 0.34 μA cm−2 after exposure, a level consistent with low-to-moderate activity when considered alongside the chloride content and coating condition. Such cases underscore that individual indicators should be read together: a moderate icorr coupled with chloride content below CTgalv and substantial remaining zinc thickness is classified overall as moderate, whereas the same icorr measured in concrete at or above CTgalv or with a heavily depleted coating would be classified as severe.
For service-life prediction, the initiation limit state is tied directly to these measurements. In line with performance-based durability design practice, time-to-initiation is reached when the chloride content at bar depth first attains CTgalv or when sustained icorr reaches the moderate-to-high boundary (≈0.5–1.0 μA cm−2) under stable exposure; the subsequent propagation phase is governed by the rate of zinc consumption and the approach to steel exposure. By parameterizing the initiation and propagation stages with measured icorr, chloride at bar depth, and coating depletion, the severity classes provide limit-state inputs that can be used consistently in probabilistic or deterministic service-life models such as those summarized by FHWA’s Service Life Design Reference Guide (Hartmann 2022).
4 Structural performance and long-term durability
Beyond the fundamental electrochemistry, the ultimate utility of hot-dip galvanized rebar is determined by its in-service structural performance and its ability to extend the durable life of concrete structures. This section evaluates these practical outcomes, focusing on the highly contentious topic of bond strength, the comparative performance against other protection systems, and the crucial evidence gathered from long-term field studies.
4.1 The controversy of bond strength: a critical analysis
The composite action of reinforced concrete is entirely dependent on the transfer of stress between the rebar and the surrounding concrete, a mechanism known as bond (Khaksefidi et al. 2021). Any corrosion protection system applied to the rebar must not compromise this critical property. For galvanized rebar, the literature presents a deeply divided and conflicting picture of its effect on bond strength, representing one of the most significant areas of debate in the field (Kobayashi et al. 2021).
One body of research, comprising numerous pull-out and beam-end tests, concludes that the bond strength of hot-dip galvanized rebar is equivalent to, or in some cases superior to, that of uncoated black steel (Mohammed and Roopa 2022). The primary mechanism proposed to explain this enhanced performance is the formation of the crystalline CHZ layer at the interface during concrete curing. It is theorized that these crystals grow into the cement paste, creating a strong chemical adhesion and enhancing the mechanical interlock between the rebar’s surface and the concrete matrix, thereby increasing the force required to cause slip (Khalaf et al. 2016). Studies supporting this view often find that while the initial hydrogen evolution reaction occurs, its effect on the final, fully cured bond strength is negligible.
Conversely, a compelling and growing body of evidence, often from studies employing more detailed microstructural analysis, reports a significant reduction in bond strength for galvanized rebar compared to black steel (Jaśniok et al. 2021). The principal mechanism cited for this reduction is the detrimental effect of hydrogen evolution during the initial 24–48 h of curing. As discussed in Section 2.1, the formation of hydrogen gas bubbles at the interface is argued to create a permanent zone of increased porosity in the adjacent cement paste (Pernicova et al. 2017). This porous and weaker ITZ provides a less effective medium for stress transfer, leading to a lower overall bond capacity. Some researchers also suggest that the formation of zinc corrosion products themselves can be non-adherent or voluminous, leading to local disintegration at the interface that further compromises the bond (Groshek 2017). Another contributing factor can be the physical nature of the coating itself; a very thick or non-uniform galvanized coating, particularly on reactive steels, can smooth the profile of the rebar’s ribs, reducing the mechanical bearing component of the bond (Baig et al. 2022). This fundamental disagreement in the literature highlights the complexity of the interfacial zone, where competing mechanisms – chemical adhesion from CHZ versus microstructural damage from hydrogen – are at play. The net effect likely depends on a sensitive balance of factors including cement chemistry, concrete mix design, curing conditions, and the quality of the galvanizing itself. Table 2 summarizes the key arguments and findings from this ongoing debate.
Summary of conflicting research findings on the bond strength of galvanized rebar.
| Key finding (relative to black steel) | Proposed mechanism | Representative test method(s) | Key references |
|---|---|---|---|
| Equivalent or superior bond strength | Formation of adhesive calcium hydroxyzincate (CHZ) crystals enhances chemical and mechanical interlock | Pull-out tests; beam-end splice tests | Mohammed and Roopa (2022) |
| Reduced bond strength | Hydrogen evolution during initial curing creates a permanent, porous, and weaker ITZ | Pull-out tests combined with microstructural analysis (porosimetry, SEM) | Pokorný et al. (2017) |
| Reduced bond strength | Thick or non-uniform zinc coating smooths rebar rib geometry, reducing mechanical bearing | Pull-out tests with geometric analysis of coated bars | Hussain and Kim (2023) |
4.2 Comparative performance against alternative systems
The decision to use galvanized rebar is often made in the context of other available corrosion protection systems, primarily epoxy-coated rebar (ECR) and stainless steel rebar (Gagné et al. 2020).
ECR has been a widely used alternative to black steel, relying on an organic coating to provide a purely physical barrier against moisture and chlorides (Yadav et al. 2022). When the epoxy coating is perfectly applied and remains undamaged, it can provide excellent corrosion protection. However, its primary weakness lies in its susceptibility to damage during transportation, handling, and installation on the construction site. Even minor nicks or scratches can create holidays in the coating, exposing the underlying steel. At these defect sites, corrosion can initiate and propagate aggressively underneath the coating, a failure mode known as under-film or filiform corrosion, which can cause localized debonding of the epoxy and intense pitting of the steel (Zhang et al. 2019). In contrast, the metallurgically bonded zinc coating of HDG rebar is significantly harder and more abrasion-resistant, making it more robust against construction site damage (Pinger et al. 2024). Crucially, if the HDG coating is damaged, the surrounding zinc provides sacrificial cathodic protection to the exposed steel, preventing the initiation of corrosion until the local zinc is consumed (Choe et al. 2019). Life-cycle cost analyses frequently conclude that while ECR may have a lower initial cost in some cases, HDG often proves more cost-effective over the life of the structure due to its greater durability, reduced need for field repairs, and superior performance when minor damage is considered (Ni et al. 2022).
Stainless steel reinforcement represents a premium corrosion protection solution, offering exceptionally high resistance to chloride-induced corrosion, with a chloride threshold many times higher than that of both black steel and galvanized steel. For structures in the most aggressive environments requiring a service life of 100 years or more, stainless steel is often considered the most reliable option (Rabi et al. 2022). However, this superior performance comes at a substantial cost premium, which can be prohibitive for many projects. Hot-dip galvanized rebar is therefore positioned as a highly effective intermediate solution. It provides a significant and well-documented extension of service life over uncoated steel at a much more moderate initial cost than stainless steel, offering a balanced approach to durability and economics for a wide range of applications (Coppola et al. 2022).
While laboratory tests provide mechanistic insights, the true measure of a corrosion protection system is its performance over decades in real-world structures. Field studies of aged structures containing galvanized rebar provide invaluable data on its long-term durability. One of the most widely cited and compelling case studies is the Athens Bridge in Pennsylvania, USA, constructed in 1973 using HDG rebar in its deck (Shear 1973). Inspections conducted 8, 18, and 28 years after construction consistently found the galvanized rebar to be in excellent condition with no signs of active corrosion, despite the presence of high chloride concentrations in the concrete (up to 7.9 lbs/yd3, or approximately 4.7 kg/m3) from the use of de-icing salts – levels that would have caused severe corrosion in black steel. Even after nearly three decades of service, the zinc coating thickness was still well in excess of the minimum requirements for new bars, suggesting many more decades of maintenance-free life. Similar positive long-term performance has been documented in other structures, including bridges in Bermuda and Florida, and various projects undertaken by Departments of Transportation. Table 3 summarizes the findings from several key field studies.
Key findings from long-term field performance studies of hot-dip galvanized rebar.
| Structure/location | Year built | Environment | Key findings from inspection | References |
|---|---|---|---|---|
| Athens bridge, Pennsylvania, USA | 1973 | De-icing salts | Inspected 1981, 1991, 2001. High chloride levels (up to 7.9 lbs/yd3). No active corrosion found. Significant coating thickness remained after 28 years | Xu et al. (2021) |
| Longbird bridge, Bermuda | 1953 | Severe marine | Inspected 1995 (42 years). High chloride levels. Galvanized coating thickness still met new rebar specifications | Allan (2004) |
| Boca Chica bridge, Florida, USA | 1970s | Marine splash zone | Long-term monitoring projects service life extension. Projected time to reach zinc’s chloride threshold is over 100 years | Misterly Grant et al. (2018) |
A critical aspect of this long-term performance is the behavior of the zinc corrosion products. Unlike the voluminous, expansive iron oxides that cause cracking and spalling, zinc corrosion products are less dense, more soluble in the alkaline pore water, and tend to migrate away from the rebar surface into the surrounding concrete matrix. This migration prevents the buildup of localized stress at the interface, meaning that even when the zinc coating does begin to corrode, it does not typically cause the destructive spalling associated with black steel corrosion (Karlsson 2014). There is also evidence that these migrating zinc compounds can fill the pores in the ITZ, leading to a densification of the concrete around the bar, which can further slow the ingress of aggressive species and may contribute to the observed good long-term bond strength. This fundamental difference in the failure mechanism is a key advantage of galvanized rebar (Belaı̈d et al. 2001). Figure 4 provides a schematic comparison of the failure modes.
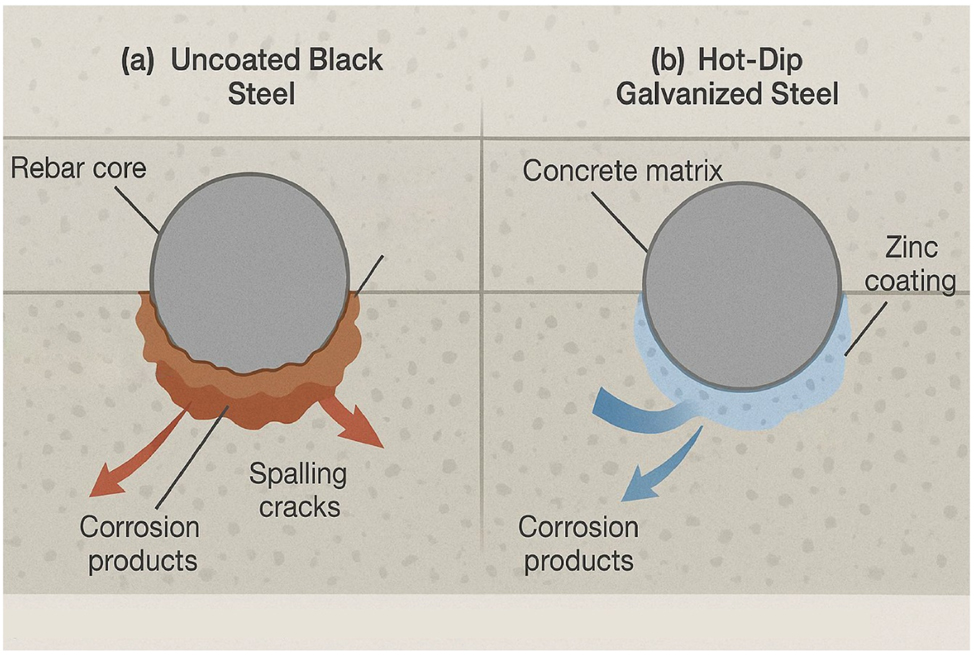
Schematic comparison of corrosion-induced failure mechanisms for black steel and hot-dip galvanized steel (author-created).
While the majority of long-term field data is positive, it is important to acknowledge reports of mixed or poor performance. These instances are often linked to specific, highly aggressive local conditions, poor quality or highly permeable concrete, or issues with the galvanizing process itself, such as the use of highly “reactive” steels (with specific silicon or phosphorus contents) that can result in overly thick and brittle zinc–iron alloy coatings that are prone to flaking. This highlights that the performance gap between controlled laboratory tests and real-world field observations is a significant source of the ongoing controversies. Accelerated lab tests can fail to replicate the slow, complex chemical evolution of the concrete environment and may accelerate incorrect failure mechanisms, leading to pessimistic predictions (Mao et al. 2025). Conversely, positive field results demonstrate the system’s potential under real service conditions. This underscores that the selection of a corrosion protection system is not merely a materials science decision but also a comprehensive risk management and economic calculation, balancing initial cost, construction practicalities, and desired service life against the specific environmental challenges of the project.
5 State-of-the-art advances in technology and assessment
The corrosion resistance of galvanized reinforcement is fundamentally linked to the galvanizing process, which metallurgically bonds zinc to steel through immersion in a molten zinc bath. In the traditional batch HDG process, cleaned and fluxed reinforcing bars are submerged for several minutes in molten zinc at approximately 450 °C. This immersion results in the formation of a coating composed of distinct iron–zinc intermetallic alloy layers (gamma, delta, and zeta phases) topped by an outer layer of pure eta-phase zinc. The coating thickness typically ranges from 85 µm or more, providing a substantial reservoir for both barrier and sacrificial protection (Marder 2000). By contrast, the more recent CGR process immerses pre-heated, straight rebar for only a few seconds while introducing a small amount of aluminum into the zinc bath. This approach significantly reduces the growth of brittle intermetallic phases, yielding a thinner but more uniform coating composed almost entirely of ductile eta-phase zinc with a very thin Fe–Al–Zn interfacial layer. This microstructure not only enhances formability but also provides an efficient reservoir of sacrificial zinc for passivation in concrete environments (Patnaik and Stroia 2019). Providing this metallurgical context is essential, as the subsequent discussion of recent technological advances – including CGR, zinc-alloy modifications, and improved performance assessment – relies heavily on the fundamental differences in coating structure and composition that arise directly from the galvanizing process.
5.1 Innovations in galvanizing processes: continuous galvanized rebar (CGR)
One of the most significant recent technological advancements is the development and commercialization of CGR (Liu and Stroia 2020). This technology represents a fundamental shift from the traditional batch HDG process. In the CGR process, blast-cleaned and pre-heated rebar is passed continuously through a molten zinc bath at high speeds (e.g., 10 m per minute), resulting in a very short immersion time of only a few seconds. This is in sharp contrast to the batch process, where bundles of fabricated rebar are immersed for several minutes.
A critical feature of the CGR process is the addition of a small amount of aluminum (typically around 0.2 %) to the molten zinc bath. The combination of the short immersion time and the presence of aluminum effectively retards the metallurgical reaction between the iron and zinc. This prevents the formation of the thick, relatively brittle iron-zinc intermetallic alloy layers (zeta and delta phases) that characterize traditional HDG coatings. Instead, the CGR process produces a coating that is typically thinner (around 50–60 µm) and consists almost entirely of a ductile, pure zinc (eta) phase over a very thin iron-aluminum-zinc bonding layer (Marder 2000). Figure 5 schematically compares the microstructures of the two coating types.
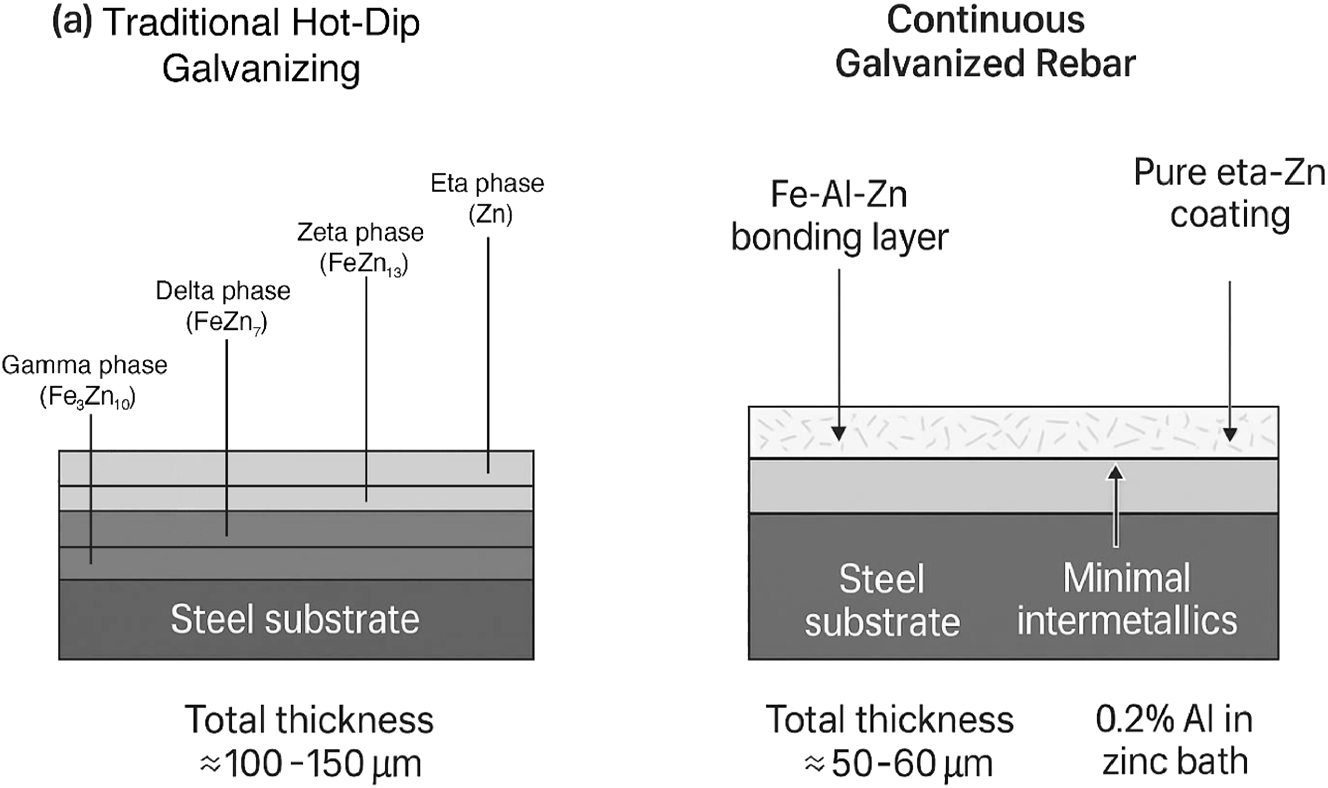
Schematic comparison of coating microstructures: Traditional HDG versus CGR. This diagram presents a side-by-side cross-sectional view of (a) a traditional HDG coating and (b) a CGR coating. Panel (a) shows the distinct, thick layers of the HDG coating: The gamma, delta, and zeta iron–zinc intermetallic alloys, topped with a layer of pure eta zinc. Panel (b) illustrates the CGR coating, highlighting its much thinner overall profile, the near-absence of thick intermetallic layers, and a composition that is almost entirely pure, ductile eta zinc over a very thin Fe–Al–Zn bonding layer (author-created).
This distinct microstructure endows CGR with significant performance advantages. The most notable is superior formability. The ductile, pure zinc coating allows the rebar to be bent and fabricated after galvanizing without the risk of cracking or flaking that can occur with the brittle intermetallic layers of traditional HDG bars (Andrade and Alonso 2001). This simplifies the supply chain and reduces the need for costly and time-consuming post-fabrication galvanizing or field repairs. From a corrosion perspective, the thicker layer of pure zinc is theoretically ideal for the passivation reaction in concrete and provides a larger, more uniform reservoir for sacrificial protection.
The CGR process also offers substantial economic and environmental benefits. It is a more energy-efficient, in-line process that uses significantly less zinc per ton of steel compared to batch galvanizing (typically reducing zinc consumption by about half), making it a more cost-effective and sustainable manufacturing method (Viklund-White 2000). A new standard, ASTM A1094/A1094 M, has been established to govern the properties of CGR, formalizing its place as a modern alternative to traditional HDG. The development of CGR can be seen as an implicit acknowledgment by the industry of the known limitations of traditional HDG, particularly its brittleness and fabrication constraints. CGR is a targeted technological solution designed to overcome these specific weaknesses, representing a significant evolutionary step. Table 4 provides a direct comparison of the key features of the two processes.
Comparison of traditional HDG and CGR.
| Property | HDG | CGR |
|---|---|---|
| Process | Batch immersion of fabricated rebar for several minutes | In-line, continuous passage of straight rebar for seconds |
| Coating thickness | Thicker, typically >85 µm | Thinner, typically 50–60 µm |
| Coating composition | Thick Fe–Zn intermetallic layers (gamma, delta, zeta) with an outer pure zinc (eta) layer | Almost entirely pure zinc (eta) with a very thin Fe–Al–Zn bonding layer |
| Formability | Brittle; bending after galvanizing can cause cracking/flaking. Typically fabricated before galvanizing | Ductile; can be bent and fabricated after galvanizing without significant coating damage |
| Corrosion protection | Barrier and sacrificial; performance depends on outer eta layer and slower corrosion of alloys | Barrier and sacrificial; large reservoir of pure zinc for passivation and protection |
| Governing standard | ASTM A767/A767 M | ASTM A1094/A1094 M |
5.2 Advances in zinc alloy coatings
Beyond the Al–Zn system used in CGR, research continues into other zinc alloy coatings to further enhance performance. For instance, studies on Zn–Al-Mischmetal coatings have demonstrated the potential for creating thinner, more ductile coatings with improved corrosion resistance in both simulated concrete pore solution and chloride environments (Wang et al. 2019). These investigations point toward an active field of materials development focused on tailoring the composition of the zinc bath to optimize the final coating’s mechanical properties and electrochemical behavior, offering pathways to even more durable and specialized reinforcement solutions.
The ability to accurately assess the condition of galvanized rebar in concrete and predict its remaining service life is crucial for infrastructure management. The field has seen significant advances in both laboratory testing and in-situ monitoring techniques. A variety of electrochemical methods are used to evaluate the corrosion performance of galvanized rebar in laboratory settings. The most common is the macrocell corrosion test outlined in ASTM G109, which measures the corrosion current between a top, chloride-exposed rebar and a bottom, passive rebar (Alonso et al. 2000). While widely used for comparative studies, the standard G109 test is often criticized for being slow and labor-intensive, leading many researchers to develop modified or accelerated versions (Darwin et al. 2007). Results from these tests generally show that galvanized rebar outperforms black steel, but performance can be marginal under certain conditions (Presuel-Moreno and Rourke 2009).
For assessing structures in the field, several non-destructive testing (NDT) techniques are employed. These include corrosion potential mapping (ASTM C876) to identify areas with a high probability of corrosion, linear polarization resistance (LPR) to measure the instantaneous corrosion rate, and ground-penetrating radar (GPR) to determine concrete cover and locate reinforcement (Zaki et al. 2018). However, the interpretation of data from these methods, which were primarily developed for black steel, can be more complex for galvanized rebar due to the different electrochemical nature of zinc and its corrosion products.
5.3 Computational modeling
In parallel with experimental work, computational modeling has emerged as a powerful tool for service life prediction. Finite element method (FEM) models are increasingly applied to simulate chloride ingress, carbonation, and the initiation of corrosion in reinforced concrete (Dominicini and Calmon 2017). These can be implemented in widely available software platforms. COMSOL Multiphysics has been frequently employed to solve coupled diffusion–migration–reaction equations, including zinc-specific electrochemical parameters, and allows flexible incorporation of environmental variables. Similarly, ANSYS and Abaqus are commonly used to couple transport models with mechanical response, enabling simulation of cracking caused by corrosion product expansion. For engineering practice, dedicated durability software such as Life-365 and the European DuraCrete model provide probabilistic frameworks for chloride ingress and carbonation, and although originally calibrated for black steel, these models have been adapted by researchers for galvanized reinforcement by inputting higher chloride thresholds and modified corrosion kinetics. The selection of software depends on the modeling objective: while Life-365 provides accessible and probabilistic service-life prediction for design, multi-physics packages such as COMSOL and ANSYS remain indispensable for fundamental research requiring coupled transport–mechanical–electrochemical analysis. This integration of numerical tools and experimental calibration represents a crucial pathway toward reliable full-service-life modeling of galvanized reinforcement.
These models are currently most reliable for predicting the initiation stage, i.e., the time to depassivation, defined either by exceeding a critical chloride threshold or by carbonation-induced pH reduction. Some recent models also attempt to represent the propagation stage, incorporating zinc consumption rates and the mechanical effects of corrosion products on concrete cracking (Jaśniok et al. 2020). However, such propagation modeling remains limited due to uncertainties in parameterizing zinc corrosion kinetics and product morphology. It should be noted that explicit modeling of the passivation stage – including the kinetics of CHZ film formation and stabilization – is still lacking, with current approaches treating passivation qualitatively rather than quantitatively (Angst 2018). Likewise, the so-called protection stage, in which sacrificial zinc continues to shield exposed steel areas, is often simplified or omitted from current frameworks. Future research should therefore develop multi-stage models that encompass passivation, initiation, protection, and propagation in a unified framework, calibrated against long-term field evidence (Roventi et al. 2014). Such integrated modeling would allow engineers to more reliably predict the full service life of galvanized reinforcement under diverse exposure conditions.
6 Synthesis and future research directions
This review has traversed the complex landscape of hot-dip galvanized rebar in concrete, from the fundamental electrochemical reactions at the steel-concrete interface to the long-term structural performance observed in the field and the latest technological innovations. The synthesis of this extensive body of literature reveals a technology that is both proven and effective, yet still subject to significant scientific debate and ongoing evolution. This concluding section summarizes the key findings, crystallizes the unresolved controversies, and identifies the critical knowledge gaps that must be addressed by future research to further advance the field.
6.1 Synthesis of key findings and unresolved controversies
The collective evidence overwhelmingly confirms that hot-dip galvanizing provides a substantial improvement in the durability of reinforced concrete compared to traditional uncoated steel. The established benefits are clear and significant. First, the zinc coating possesses a much higher chloride threshold for corrosion initiation, consistently tolerating chloride concentrations 2 to 5 times greater than black steel, which significantly prolongs the corrosion-free initiation phase of a structure’s service life (Yeomans 2019). Second, galvanized steel exhibits excellent resistance to carbonation-induced corrosion due to the inherent stability of zinc over a wider pH range (Jiang et al. 2018). Third, the failure mechanism of galvanized rebar is fundamentally more benign; its corrosion products are less voluminous and tend to migrate into the concrete matrix rather than generating the destructive expansive pressures that lead to spalling and cracking, a key failure mode for black steel (Xu et al. 2016).
Despite these established advantages, this review has highlighted several critical and persistent scientific controversies where the literature remains divided. These unresolved issues represent the frontier of knowledge in this field and can be synthesized as follows:
Nature and Stability of the Passive Layer. The true protective capacity of the CHZ layer remains debated. Some studies suggest it provides long-term passivity, while others indicate that its stability is conditional on moisture and alkalinity, with potential for reactivated corrosion under wet conditions (Pokorný et al. 2022).
Net Effect on Bond Strength. Conflicting findings persist on whether CHZ-induced adhesion enhances bond, or whether hydrogen evolution and associated porosity reduce bond strength. This divergence highlights the need for improved interfacial characterization (Pokorný et al. 2023; Pokorný et al. 2024a, b).
Predictive Power of Laboratory Testing. Accelerated laboratory tests often fail to reproduce field behavior, leading to discrepancies in service-life prediction. Laboratory protocols may accelerate unrepresentative mechanisms, making long-term field validation essential (Mao et al. 2025).
6.2 Future research directions and knowledge gaps
Addressing these controversies and advancing the technology requires targeted research in several key areas. Based on the knowledge gaps identified in this review, the following future research directions are proposed:
Long-term performance of CGR: CGR requires long-term field validation in marine, de-icing, and carbonation-prone environments. Studies should also clarify the Al–Zn passivation mechanism in modern cement chemistries.
Resolving bond strength debate: Advanced micro-analytical techniques, such as X-ray microtomography and nano-indentation, are necessary to distinguish between hydrogen-induced porosity and CHZ-induced adhesion at the steel–concrete interface.
Behavior in modern concrete binders: Galvanized rebar performance in sustainable concretes (high SCM content, geopolymers, alkali-activated systems) remains underexplored, despite their growing use in infrastructure.
Development of advanced service life models: Models must incorporate zinc-specific electrochemical parameters, variable chloride thresholds, and the non-expansive nature of zinc corrosion products. This requires better accelerated testing protocols validated against long-term field data.
6.3 Concluding remarks
HDG reinforcement has demonstrated, across decades of laboratory and field research, a quantifiable enhancement of durability compared to black steel. Long-term bridge surveys in the United States and Bermuda have shown galvanized rebar remaining corrosion-free even after 25–40 years in chloride-rich or marine environments where black steel would have failed. These findings confirm that galvanized reinforcement can sustain chloride concentrations 2–5 times higher than conventional steel before depassivation occurs. Similarly, galvanized rebar maintains passivity down to pH ≈ 9.5, offering superior protection against carbonation compared to black steel, which depassivates below pH 11.5.
A second distinguishing advantage lies in the benign nature of zinc corrosion products. Unlike expansive iron oxides that generate internal stresses and spalling, zinc corrosion products are less voluminous and can migrate into the cement matrix, sometimes even densifying the interfacial transition zone. This fundamentally different degradation mechanism explains why galvanized reinforcement often avoids the catastrophic cracking associated with black steel, even after significant zinc loss. At the same time, scientific controversies remain unresolved. Studies diverge on whether hydrogen evolution during early curing permanently weakens the steel–concrete interface or whether the formation of CHZ crystals provides compensatory adhesion. Likewise, while accelerated laboratory tests frequently predict shorter service lives, field studies often demonstrate far superior durability, raising questions about the representativeness of current testing protocols. These debates highlight the need for refined test methods and advanced microstructural characterization.
Finally, the field is rapidly evolving with new technologies. CGR with its ductile eta-phase zinc layer and superior formability offers both sustainability and performance advantages. Early results are promising, but multi-decade field validation is still required before it can fully replace traditional HDG in critical infrastructure. Service-life modeling tools, including FEM-based chloride ingress simulations and probabilistic durability frameworks, are being adapted to account for galvanized-specific parameters, yet they require better calibration against long-term evidence. In conclusion, galvanized reinforcement should be regarded as a proven and cost-effective mid-tier solution: more durable and reliable than epoxy-coated rebar, yet more economical than stainless steel. With continued research into interfacial chemistry, performance in sustainable concrete mixtures, and validated service-life prediction, HDG and CGR technologies can play a central role in achieving infrastructure lifespans exceeding 100 years. The balance of field evidence strongly supports their inclusion in modern durability design, provided specifications are tailored to environmental exposure and supported by ongoing scientific refinement.
Funding source: Key Project of Chongqing Municipal Education Commission’s Science and Technology Research Program
Award Identifier / Grant number: KJZD-K202503205
Funding source: Chongqing Municipal Education Commission’s Science and Technology Research Project
Award Identifier / Grant number: KJQN202403227
Acknowledgments
The author gratefully acknowledges the financial support from the Chongqing Municipal Education Commission’s Science and Technology Research Project (KJQN202403227) and the Key Project of Chongqing Municipal Education Commission’s Science and Technology Research Program (KJZD-K202503205).
-
Research ethics: Not applicable. This review article did not involve any human or animal subjects.
-
Informed consent: Not applicable. No human participants or personal data were involved.
-
Author contributions: J.W. conducted the literature review, analysis, and manuscript preparation.
-
Use of Large Language Models, AI and Machine Learning Tools: The author used Google’s Gemini large language model solely for language editing and polishing. No AI tools were involved in content generation, data analysis, or interpretation.
-
Conflict of interest: The author declares no conflicts of interest.
-
Research funding: This work was supported by the Chongqing Municipal Education Commission’s Science and Technology Research Project (KJQN202403227) and the Key Project of the Science and Technology Research Program (KJZD-K202503205).
-
Data availability: Not applicable. This article is based on previously published literature and does not include original datasets.
References
Aguirre, A.M. and Mejía De Gutiérrez, R. (2013). Durability of reinforced concrete exposed to aggressive conditions. Mater. Constr. 63: 7–38. https://doi.org/10.3989/mc.2013.00313.Suche in Google Scholar
Al-Negheimish, A., Hussain, R.R., Alhozaimy, A., and Singh, D.D.N. (2021). Corrosion performance of hot-dip galvanized zinc-aluminum coated steel rebars in comparison to the conventional pure zinc coated rebars in concrete environment. Constr. Build. Mater. 274: 121921, https://doi.org/10.1016/j.conbuildmat.2020.121921.Suche in Google Scholar
Allan, N.D. (2004). The Bermuda experience: leading the way on galvanized reinforcement. In: Yeomans, S.R. (Ed.). Galvanized steel reinforcement in concrete. Elsevier Science, Amsterdam, pp. 199–291.10.1016/B978-008044511-3/50022-0Suche in Google Scholar
Alonso, C., Andrade, C., Castellote, M., and Castro, P. (2000). Chloride threshold values to depassivate reinforcing bars embedded in a standardized OPC mortar. Cem. Concr. Res. 30: 1047–1055, https://doi.org/10.1016/s0008-8846(00)00265-9.Suche in Google Scholar
Andrade, C. and Alonso, C. (2001). On-site measurements of corrosion rate of reinforcements. Constr. Build. Mater. 15: 141–145, https://doi.org/10.1016/s0950-0618(00)00063-5.Suche in Google Scholar
Angst, U.M. (2018). Challenges and opportunities in corrosion of steel in concrete. Mater. Struct. 51: 4, https://doi.org/10.1617/s11527-017-1131-6.Suche in Google Scholar
ASTM C876 (2015). Standard test method for corrosion potentials of uncoated reinforcing steel in concrete. ASTM International, West Conshohocken, PA, USA.Suche in Google Scholar
Baig, M., Munir, Q., and Ahmad, B. (2022). Bond performance of zinc galvanized rebars in reinforced concrete and their effectiveness in corrosion prevention. Int. Res. J. Mod. Eng. Technol. Sci. 4.Suche in Google Scholar
Baig, M.M.H.U.H., Munir, Q., Zain-Ul-Abideen and Ahmed, B. (2022b). Bond performance of zinc galvanized rebars in reinforced concrete and their effectiveness in corrosion prevention. Int. Res. J. Mod. Eng. Technol. Sci. 4: 1479–1486.Suche in Google Scholar
Belaïd, F., Arliguie, G., and François, R. (2001). Porous structure of the ITZ around galvanized and ordinary steel reinforcements. Cem. Concr. Res. 31: 1561–1566, https://doi.org/10.1016/s0008-8846(01)00597-x.Suche in Google Scholar
Bellezze, T., Mobili, A., and Tittarelli, F. (2021). Corrosion behaviour of galvanized steel in cement- and geopolymer-based concrete: a review on scientific work at the Polytechnic University of Marche. In: Book of abstracts of EM4SS’21: engineered materials for sustainable structures. Online), Modena, pp. 91.Suche in Google Scholar
Choe, H., Nishio, Y., and Kanematsu, M. (2019). An investigation on the usability of acceleration test by impressed anodic current for evaluating corrosion behavior of hot-dip galvanized rebar in concrete. Materials 12: 3566, https://doi.org/10.3390/ma12213566.Suche in Google Scholar PubMed PubMed Central
Collins, W.D., Weyers, R.E., and Al-Qadi, I.L. (1993). Chemical treatment of corroding steel reinforcement after removal of chloride-contaminated concrete. Corrosion 49: 74–88, https://doi.org/10.5006/1.3316037.Suche in Google Scholar
Conciatori, D., Grégoire, É., Samson, É., Marchand, J., and Chouinard, L. (2015). Sensitivity of chloride ingress modelling in concrete to input parameter variability. Mater. Struct. 48: 3023–3036, https://doi.org/10.1617/s11527-014-0374-8.Suche in Google Scholar
Coppola, L., Beretta, S., Bignozzi, M.C., Bolzoni, F., Brenna, A., Cabrini, M., Candamano, S., Caputo, D., Carsana, M., Cioffi, R., et al. (2022). The improvement of durability of reinforced concretes for sustainable structures: a review on different approaches. Materials 15: 2728, https://doi.org/10.3390/ma15082728.Suche in Google Scholar PubMed PubMed Central
Darwin, D., Browning, J., O’Reilly, M., and Xing, L. (2007). Critical chloride corrosion threshold for galvanized reinforcing bars. University of Kansas Center for Research, Inc, Lawrence, Kansas.Suche in Google Scholar
Dominicini, W.K. and Calmon, J.L. (2017). Computational modeling for predicting corrosion initiation in reinforced concrete structures. Rev. IBRACON Estrut. Mater. 10: 1205–1244, https://doi.org/10.1590/s1983-41952017000600006.Suche in Google Scholar
Gagné, M., Van Leeuwen, M., and Goodwin, F.E. (2020). Modeling corrosion resistance of reinforced concrete structures. Mater. Perform. 59: 54–57, https://doi.org/10.5006/mp2020_59_3-54.Suche in Google Scholar
Garnica-Rodríguez, A., Montoya, R., Rodríguez-Gomez, F.J., Pérez-López, T., and Genesca, J. (2022). Effect of a fast potential change on the early stage of zinc passivation in a saturated calcium hydroxide solution. Front. Mater. 9: 877728, https://doi.org/10.3389/fmats.2022.877728.Suche in Google Scholar
Ghazaee, A., Pour-Ali, S., Mahdavi, S., Tavangar, R., and Khalili, M. (2024). Corrosion inhibition of steel rebar in chloride-contaminated concrete pore solution: ecofriendly glutamic acid inhibitor and its synergy with galvanized coating. Inorg. Chem. Commun. 167: 112832, https://doi.org/10.1016/j.inoche.2024.112832.Suche in Google Scholar
González, J.A. and Andrade, C. (1982). Effect of carbonation, chlorides and relative ambient humidity on the corrosion of galvanized rebars embedded in concrete. Br. Corros. J. 17: 21–28, https://doi.org/10.1179/000705982798274589.Suche in Google Scholar
Goyal, A., Pouya, H.S., Ganjian, E., and Claisse, P. (2018). A review of corrosion and protection of steel in concrete. Arab. J. Sci. Eng. 43: 5035–5055, https://doi.org/10.1007/s13369-018-3303-2.Suche in Google Scholar
Groshek, I.G. (2017). Corrosion behavior of ASTM A1010 stainless steel for applications in bridge components. Doctoral dissertation, Virginia Tech.Suche in Google Scholar
Hartmann, J. (2022). Service life design reference guide. In: FHWA-HIF-22-052. Federal Highway Administration, Washington, DC.Suche in Google Scholar
Hurley, M.F. and Scully, J.R. (2006). Threshold chloride concentrations of selected corrosion-resistant rebar materials compared to carbon steel. Corrosion 62: 892–904, https://doi.org/10.5006/1.3279899.Suche in Google Scholar
Hussain, H. and Kim, D.-K. (2023). Advancements and future prospects of buckling restrained braces for corrosive-environments: a comprehensive literature review. Buildings 13: 2156, https://doi.org/10.3390/buildings13092156.Suche in Google Scholar
Jaśniok, M., Kołodziej, J., and Gromysz, K. (2021). An 18-month analysis of bond strength of hot-dip galvanized reinforcing steel B500SP and S235JR+AR to chloride contaminated concrete. Materials 14: 747, https://doi.org/10.3390/ma14040747.Suche in Google Scholar PubMed PubMed Central
Jaśniok, M., Sozańska, M., Kołodziej, J., and Chmiela, B. (2020). A two-year evaluation of corrosion-induced damage to hot galvanized reinforcing steel B500SP in chloride contaminated concrete. Materials 13: 3315, https://doi.org/10.3390/ma13153315.Suche in Google Scholar PubMed PubMed Central
Jiang, J., Chu, H., Liu, Y., Wang, D., Guo, D., and Sun, W. (2018). Galvanic corrosion of duplex corrosion-resistant steel rebars under carbonated concrete conditions. RSC Adv. 8: 16626–16635, https://doi.org/10.1039/c8ra03320j.Suche in Google Scholar PubMed PubMed Central
Karlsson, J. (2014). Alternative reinforcement approaches: extended service life of exposed concrete structures. Chalmers University of Technology, Gothenburg, Sweden, Master thesis.Suche in Google Scholar
Kenny, A. and Katz, A. (2019). A statistical analysis of the distribution of the chloride threshold with relation to steel–concrete interface. Am. J. Constr. Build. Mater. 3: 16–22, https://doi.org/10.11648/j.ajcbm.20190301.13.Suche in Google Scholar
Kepler, J.L., Darwin, D., and Locke, C.E. (2000). Evaluation of corrosion protection methods for reinforced concrete highway structures. University of Kansas Center for Research, Inc., Lawrence, KS, SM Report No. 58.Suche in Google Scholar
Khaksefidi, S., Ghalehnovi, M., and De Brito, J. (2021). Bond behaviour of high-strength steel rebars in normal (NSC) and ultra-high performance concrete (UHPC). J. Build. Eng. 33: 101592, https://doi.org/10.1016/j.jobe.2020.101592.Suche in Google Scholar
Khalaf, J., Huang, Z., and Fan, M. (2016). Analysis of bond-slip between concrete and steel bar in fire. Comput. Struct. 162: 1–15, https://doi.org/10.1016/j.compstruc.2015.09.011.Suche in Google Scholar
Kobayashi, K., Suzuki, H., Nishio, Y., and Kanematsu, M. (2021). Evaluation of bond performance of reinforced concrete using hot-dip galvanized rebar by neutron diffraction. J. Struct. Constr. Eng. (Trans. AIJ) 86: 1026–1035, https://doi.org/10.3130/aijs.86.1026.Suche in Google Scholar
Lal, M., Chidambaram, R.S., and Karade, S.R. (2022). Critical review on the rehabilitation technique of corrosion-damaged reinforced concrete (RC) beams using composites. In: Proc. Int. Conf. novel innovations and sustainable development in civil engineering (NISDCE’22). Vel Tech Institute, Udaipur, India, p. 79.Suche in Google Scholar
Li, L. and Wu, M. (2022). An overview of utilizing CO2 for accelerated carbonation treatment in the concrete industry. J. CO2 Util. 60: 102000, https://doi.org/10.1016/j.jcou.2022.102000.Suche in Google Scholar
Liu, G., Liu, B. and Y, F. (2012). Chromium-free passivation method for galvanized steel sheet by using molybdate/polyphosphate composite system. [Unpublished report, October].Suche in Google Scholar
Liu, D. and Stroia, M. (2020). Continuous galvanized rebar at the cusp of a market breakthrough. Adv. Civ. Eng. Technol. 4: 1–3, https://doi.org/10.31031/acet.2020.04.000586.Suche in Google Scholar
López-Calvo, H.Z., Montes-Garcia, P., Kondratova, I., Bremner, T.W., and Thomas, M.D.A. (2013). Epoxy-coated bars as corrosion control in cracked reinforced concrete. Mater. Corros. 64: 599–608, https://doi.org/10.1002/maco.201106319.Suche in Google Scholar
Maeda, M., Li, X., Ooi, A., Tada, E., and Nishikata, A. (2020). Passivation mechanism of galvanized steel rebar in fresh concrete. ISIJ Int. 60: 337–345, https://doi.org/10.2355/isijinternational.isijint-2019-396.Suche in Google Scholar
Mandal, G.K., Mandal, D., Das, S.K., Balasubramaniam, R., and Mehrotra, S.P. (2009). Microstructural study of galvanized coatings formed in pure as well as commercial grade zinc baths. Trans. Indian Inst. Met. 62: 35–40, https://doi.org/10.1007/s12666-009-0005-1.Suche in Google Scholar
Mao, L., Li, L., Wang, Y., and Liu, Q. (2025). Physicochemical modelling in chlorides migration in concrete with account of multi-species coupling, reaction kinetic and pore evolution. Constr. Build. Mater. 460: 139707, https://doi.org/10.1016/j.conbuildmat.2024.139707.Suche in Google Scholar
Marder, A.R. (2000). The metallurgy of zinc-coated steel. Prog. Mater. Sci. 45: 191–271, https://doi.org/10.1016/s0079-6425(98)00006-1.Suche in Google Scholar
Misterly, G., Ellenberger, C. and Trigg, S. (2018). Hurricanes, conquistadors, and endangered species? The unique challenges of under-bridge pipe replacement on Florida’s historic coast. Pipelines 2018: 637–647, https://doi.org/10.1061/9780784481653.070.Suche in Google Scholar
Mohammed, M.S.H.S. and Roopa, V. (2022). Performance of galvanized steel rebars in concrete. Indian Concr. J. 96: 81–90.Suche in Google Scholar
Ni, W., Li, P., Zhu, Y., Di, Z., Guo, L., and Liu, Y. (2022). Comparative study of anti-corrosion properties and lifespan prediction model for inorganic zinc-rich coating and thermal-spray zinc coating. Coatings 12: 505, https://doi.org/10.3390/coatings12040505.Suche in Google Scholar
Patnaik, A. (2024). Structural and corrosion performance of continuous galvanized rebar (CGR). Curr. Trends Civ. Struct. Eng. 11: 1–10, https://doi.org/10.33552/ctcse.2024.11.000761.Suche in Google Scholar
Patnaik, A. and Stroia, M.M. (2019). Structural and corrosion performance of continuous galvanized rebar (CGR). In: GalvaBar, Catoosa, OK, USA.Suche in Google Scholar
Pernicova, R., Dobias, D., and Pokorny, P. (2017). Problems connected with use of hot-dip galvanized reinforcement in concrete elements. Procedia Eng. 172: 859–866, https://doi.org/10.1016/j.proeng.2017.02.086.Suche in Google Scholar
Pinger, T., Brand, M., Grothe, S., and Marginean, G. (2024). Abrasive wear behavior of batch hot-dip galvanized coatings. Materials 17: 1547, https://doi.org/10.3390/ma17071547.Suche in Google Scholar PubMed PubMed Central
Pokorný, P., Chobotský, T., Prodanovic, N., Steinerová, V., and Hurtig, K. (2024a). Bond strength and corrosion protection properties of hot-dip galvanized prestressing reinforcement in normal-strength concrete. J. Compos. Sci. 8: 407, https://doi.org/10.3390/jcs8100407.Suche in Google Scholar
Pokorný, P., Kostelecká, M., Prodanovic, N., and Sýkora, M. (2022). Effect of calcium hydroxyzincate on bond strength of hot-dip galvanized plain bars with normal strength concrete. Cem. Concr. Compos. 130: 104540, https://doi.org/10.1016/j.cemconcomp.2022.104540.Suche in Google Scholar
Pokorný, P., Prodanovic, N., Hurtig, K., Steinerová, V., Fojt, J., Janata, M., and Brožek, V. (2024b). Corrosion properties and bond strength in normal strength concrete of Al2O3 plasma-sprayed plain bars with ZrCC/organofunctional silane coating. Buildings 14: 1543, https://doi.org/10.3390/buildings14061543.Suche in Google Scholar
Pokorný, P., Tej, P., and Kouřil, M. (2017). Evaluation of the impact of corrosion of hot-dip galvanized reinforcement on bond strength with concrete – a review. Constr. Build. Mater. 132: 271–289, https://doi.org/10.1016/j.conbuildmat.2016.11.096.Suche in Google Scholar
Pokorný, P., Vacek, V., Prodanovic, N., Zabloudil, A., and Hurtig, K. (2023). The effect of addition potassium permanganate on bond strength of hot-dip galvanized plain bars with cement paste. Materials 16: 2556, https://doi.org/10.3390/ma16072556.Suche in Google Scholar PubMed PubMed Central
Presuel-Moreno, F.J. and Rourke, D. (2009). Review of galvanized rebar performance on G-109 specimens after 9 years. In: Corrosion 2009. NACE International, Atlanta, GA, pp. 1–26.10.5006/C2009-09209Suche in Google Scholar
Pu, Q., Yao, Y., Wang, L., Shi, X., Luo, J., and Xie, Y. (2017). The investigation of pH threshold value on the corrosion of steel reinforcement in concrete. Comput. Concr. 19: 257–262, https://doi.org/10.12989/cac.2017.19.3.257.Suche in Google Scholar
Rabi, M., Shamass, R., and Cashell, K.A. (2022). Structural performance of stainless steel reinforced concrete members: a review. Constr. Build. Mater. 325: 126673, https://doi.org/10.1016/j.conbuildmat.2022.126673.Suche in Google Scholar
Roberge, P.R. (2000). Handbook of corrosion engineering. McGraw-Hill, New York.10.5006/C2000-00478Suche in Google Scholar
Roventi, G., Bellezze, T., Barbaresi, E., and Fratesi, R. (2013). Effect of carbonation process on the passivating products of zinc in Ca(OH)2 saturated solution. Mater. Corros. 64: 1007–1014, https://doi.org/10.1002/maco.201206868.Suche in Google Scholar
Roventi, G., Bellezze, T., Giuliani, G., and Conti, C. (2014). Corrosion resistance of galvanized steel reinforcements in carbonated concrete: effect of wet –dry cycles in tap water and in chloride solution on the passivating layer. Cem. Concr. Res. 65: 76–84, https://doi.org/10.1016/j.cemconres.2014.07.014.Suche in Google Scholar
Saravanan, P. and Srikanth, S. (2019). Post treatment of hot dip galvanized steel sheet – chromating, phosphating and other alternative passivation technologies. J. Mater. Sci. Appl. 3: 1–22.Suche in Google Scholar
Scrivener, K.L., Crumbie, A.K., and Laugesen, P. (2004). The interfacial transition zone (ITZ) between cement paste and aggregate in concrete. Interface Sci 12: 411–421, https://doi.org/10.1023/b:ints.0000042339.92990.4c.10.1023/B:INTS.0000042339.92990.4cSuche in Google Scholar
Shear, T.L. (1973). The athenian agora: excavations of 1971. Hesperia 42: 121–179, https://doi.org/10.2307/147544.Suche in Google Scholar
Shi, R., Pan, Z., Lun, P., Zhan, Y., Nie, Z., Liu, Y., Mo, Z., and He, Z. (2023). Research on corrosion rate model of reinforcement in concrete under chloride ion environments. Buildings 13: 965, https://doi.org/10.3390/buildings13040965.Suche in Google Scholar
Shu, S., Dai, D., Chung, C.-Y., Yuan, Q., Wang, B., Hung, T.-T., Dai, W., Wei, Q., Fu, L., Yu, J., et al.. (2021). Significant enhancement of corrosion resistance of stainless steel with nanostructured carbon coatings by substrate-catalytic CVD. Appl. Nanosci. 11: 725–733, https://doi.org/10.1007/s13204-020-01621-6.Suche in Google Scholar
Sistonen, E. (2009). Service life of hot-dip galvanised reinforcement bars in carbonated and chloride-contaminated concrete. Teknillinen Korkeakoulu, Espoo, Finland.Suche in Google Scholar
Tan, Z.Q. and Hansson, C.M. (2008). Effect of surface condition on the initial corrosion of galvanized reinforcing steel embedded in concrete. Corros. Sci. 50: 2512–2522, https://doi.org/10.1016/j.corsci.2008.06.035.Suche in Google Scholar
Van Leeuwen, M.C., Lai, D., Kong, G., and Gagné, M. (2022). Continuous galvanized reinforcing steel in concrete structures. IABSE Congr. Rep. 22: 622–627, https://doi.org/10.2749/nanjing.2022.0622.Suche in Google Scholar
Viklund-White, C. (2000). The use of LCA for the environmental evaluation of the recycling of galvanised steel. ISIJ Int. 40: 292–299, https://doi.org/10.2355/isijinternational.40.292.Suche in Google Scholar
Wang, Y., Kong, G., and Che, C. (2019). Corrosion behavior of Zn-Al, Zn-Mg, and Zn-Mg-Al coatings in simulated concrete pore solution. Corrosion 75: 203–209, https://doi.org/10.5006/3029.Suche in Google Scholar
Xu, Y., Li, K., Liu, L., Yang, L., Wang, X., and Huang, Y. (2016). Experimental study on rebar corrosion using the galvanic sensor combined with the electronic resistance technique. Sensors 16: 1451, https://doi.org/10.3390/s16091451.Suche in Google Scholar PubMed PubMed Central
Xu, S., Zhou, Z., Feng, L., Cui, N., and Xie, N. (2021). Durability of pavement materials with exposure to various anti-icing strategies. Processes 9: 291, https://doi.org/10.3390/pr9020291.Suche in Google Scholar
Yadav, M., Dey, I., and Ghosh, S.K. (2022). A comparative study on the microstructure, hardness and corrosion resistance of epoxy coated and plain rebars. Mater. Res. Express 9: 56504, https://doi.org/10.1088/2053-1591/ac6857.Suche in Google Scholar
Ye, C., Zhu, Y., Sun, H., Chen, F., Sun, H., Dai, W., Wei, Q., Fu, L., Yu, A., Du, S., et al.. (2021). Layer-by-layer stacked graphene nanocoatings by marangoni self-assembly for corrosion protection of stainless steel. Chin. Chem. Lett. 32: 501–505, https://doi.org/10.1016/j.cclet.2020.03.013.Suche in Google Scholar
Yeomans, S. (2004). Galvanized steel reinforcement in concrete. Elsevier, Kensington, Australia.10.1016/B978-008044511-3/50016-5Suche in Google Scholar
Yeomans, S.R. (2019). Galvanized reinforcement in bridge and coastal construction. IABSE Rep 115: 1591–1597, https://doi.org/10.2749/newyork.2019.1591.Suche in Google Scholar
Yeomans, S.R. (2023). Galvanized steel reinforcement: recent developments and future opportunities. In: Poursaee, A. (Ed.). Corrosion of steel in concrete structures, 2nd ed.. Woodhead Publishing, pp. 161–186.10.1016/B978-0-12-821840-2.00005-5Suche in Google Scholar
Yu, Z., Hu, J., and Meng, H. (2020). A review of recent developments in coating systems for hot-dip galvanized steel. Front. Mater. 7: 74, https://doi.org/10.3389/fmats.2020.00074.Suche in Google Scholar
Zaki, A., Johari, M.A.M., Hussin, W.M.A.W., and Jusman, Y. (2018). Experimental assessment of rebar corrosion in concrete slab using ground penetrating radar (GPR). Int. J. Corros. 2018: 5389829.10.1155/2018/5389829Suche in Google Scholar
Zhang, J., Zhang, W., Wei, L., Pu, L., Liu, J., Liu, H., Li, Y., Fan, J., Ding, T., and Guo, Z. (2019). Alternating multilayer structural epoxy composite coating for corrosion protection of steel. Macromol. Mater. Eng. 304: 1900374, https://doi.org/10.1002/mame.201970035.Suche in Google Scholar
Zheng, H., Dai, J.-G., Hou, L., Meng, G., Poon, C.S., and Li, W. (2020). Enhanced passivation of galvanized steel bars in nano-silica modified cement mortars. Cem. Concr. Compos. 111: 103626, https://doi.org/10.1016/j.cemconcomp.2020.103626.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

