Effect of rust layer stabilization on dry and wet cyclic corrosion behavior of bridge weathering steel Q345qNH in simulated industrial atmospheric medium
Abstract
It takes 3–5 years for the stable rust layer to form when the bridge weathering steel is used in the industrial atmosphere, and the loose rust layer appearing in the early stage will pollute the atmospheric environment. Therefore, a method for the rapid formation of stable rust layer is developed in this paper, and the influence of rust layer stabilizing treatment on corrosion behavior of bridge weathering steel Q345qNH in simulated industrial atmospheric medium is studied. The results showed that in simulated industrial atmospheric medium, the stabilizing treatment shortened the formation time of stable rust layer and significantly reduced the corrosion rate, which is 47.9 % lower than that of bare steel after 480 h of corrosion. During the whole corrosion process, the Iα-FeOOH/Iγ-FeOOH peak intensity ratio of the stabilizing treatment steel was always higher than that of the bare steel, and was 1.26 times that of the bare steel after 480 h corrosion. After the stabilizing treatment, the self-corrosion potential of the rust layer increases, the self-corrosion current density decreases, and the resistance of the rust layer after 480 h corrosion was 1.57 times that of the bare steel sample, and the protection of the rust layer was significantly improved.
1 Introduction
Weathering steel is a high-strength low-carbon low-alloy structural steel. It has excellent corrosion resistance (about 2–8 times than that of the ordinary carbon steel) and good economic and environmental benefits. Therefore, it is widely used in roads, railways, bridges, towers and other building structures exposed to the atmospheric conditions (Guo et al. 2019; Song et al. 2020; Wu et al. 2017; Yan et al. 2020). It is found that the excellent corrosion resistance characteristics of weathering steel are due to the presence of alloying elements Cu, Cr, and Ni at the interface between rust layer and matrix and rust layer defects which promotes the formation of a stable and dense inner rust layer on the surface of weathering steel and enhances the corrosion resistance (Cui et al. 2012; Liu et al. 2020; Zhang et al. 2014).
The most outstanding advantage of weathering steel is that it can be used without painting, thus avoiding the pollution caused by painting to the environment, and reducing the maintenance cost in the later period. The principle of using the weathering steel without coating is “rust prevents rust” that is a dense protective rust layer is formed on the surface of the weathering steel to prevent further corrosion (Guo et al. 2019). However, the study (Sei et al. 1999) found that it takes quite a long time (3–8 years) to form a dense protective rust layer under atmospheric natural conditions when the weathering steel is used without coating. In the initial stage of use, the weathering steel, like ordinary carbon steel, loose rust is often washed down by rain when it rains, thereby polluting the environment. When the concentration of the corrosive pollutants (such as Cl− and SO2) in the atmosphere is high, it takes longer or even more difficult to form a stable protective rust layer on the surface of weathering steel that limits their use and development to a great extent.
Rust layer stabilization is a technology developed in recent years that can short the formation time of a stable rust layer on the surface of weathering steel. Its principle is that through early chemical treatment on the surface of weathering steel, it quickly forms a protective rust layer, which can not only avoid the rust liquid flow and splash but also prevent the further erosion of corrosive ions in the natural working environment. In addition, alloying elements are enriched in the inner rust layer and metal element ions and replace the iron ions to repair the defects of the rust layer, promote the transformation of the rust layer from γ-FeOOH to α-FeOOH, and make the rust layer cationic selective forming a stable rust layer in a short time period (Gao et al. 2019; Tomita and Yukio 1998; Zhang et al. 2001). However, the stabilization technology and its related research are currently applied on the rural and marine atmospheric environments. For example, Yamashita et al. (2007) studied the formation mechanism of the stabilized rust layer in the mountain and marine atmospheric media and found that the stabilization treatment could promote the formation of the α-FeOOH stable phase while inhibit the crystal growth of γ-FeOOH. Komori et al. (2014) studied the influence of the stabilization treatment and alloy design on the corrosion behavior of weathering steel in the marine atmospheric environment and found that the stabilization treatment could effectively inhibit Cl− infiltration in the initial stage of corrosion, but this effect would gradually disappear under long-term exposure. Some data like the stabilization treatment applied to the industrial atmospheric environment dominated by SO2, and the differences between the initial stabilized rust layer and the unstabilized sample in the nucleation mechanism, rust layer evolution mechanism, and corrosion type during the corrosion in the industrial atmosphere are still unclear. At present, there is no mature rust layer stabilization treatment technology that can be applied to the industrial atmospheric environment in China. Therefore, it is of great engineering value and theoretical significance to develop a stabilization technology of rust layer formation for the weathering steel in the industrial atmospheric environment and study the mechanism of this stabilization technology for the application of coating-free weathering steel in our country.
This paper considers the weathering steel Q345qNH, as a research object, and develops a rapid generation technology of a protective rust layer on its surface for its application in the industrial atmospheric environment. The corrosion behavior of the stabilizing treatment steel in the simulated industrial atmospheric environment was studied by the dry-wet alternate corrosion experiment. Both the corrosion characteristics and the corrosion mechanism of the stabilizing treatment steel were studied by comparing with bare steel which provided the application basis for coating-free weathering steel application.
2 Materials and methods
2.1 Material preparation
The material used in this experiment is bridge weathering steel Q345qNH dedicated for highway and railway produced by a large steel mill. Its specific chemical composition is shown in Table 1. The sample was processed into two sizes of 40 × 40 × 8 mm and 10 × 10 × 8 mm by using the line cutting machine. The large sample, of 40 × 40 × 8 mm, was used for the macroscopic corrosion morphology observation and the corrosion weight gain measurement (three parallel samples). The sample sizes of 10 × 10 × 8 mm were used for microscopic corrosion morphology observation, phase analysis and electrochemical testing, etc. The surface oxide was removed by sandblasting machine, and then the samples surface were polished step by step with 400#, 600#, and 800# sandpaper until becoming smooth and flat, finally washed with alcohol and put into a vacuum drying oven for use.
The chemical composition of the used Q345qNH weathering steel.
| Element | C | Si | Mn | P | S | Cu | Cr | Ni | Al | V | Nb | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 0.076 | 0.269 | 1.498 | 0.010 | 0.001 | 0.307 | 0.484 | 0.335 | 0.027 | 0.031 | 0.004 | Bal |
2.2 The stabilization treatment
The stabilizing treatment process included immersion stabilizer treatment and water treatment. The purpose of water treatment is to provide an environment for the formation of a stable rust layer.
The formula of the stabilizer is shown in Table 2. Deionized water was used for water treatment. The specific operation process was as follows:
The main components and functions of the stabilizer.
| The composition | Wt./% | The effect |
|---|---|---|
| CuSO4 | 0.5–3.5 | Provides Cu2+ ions, and changes the characteristics of the rust layer |
| Cr2(SO4)3 | 1.0–4.0 | Provides Cr3+ ions, accelerates the formation of α-FeOOH, and inhibits the pitting |
| Accelerator (NaHSO3) | 0.3–1.2 | Promotes the formation of stable and dense rust layer |
| Inducer/toner (Fe3O4) | 0.1–1.0 | Induces the transformation of the rust layer, and improves the color of the rust layer |
| Deionized water | Allowance | Solvent |
Stabilizer treatment for 30 min → drying (2.5–3h) → water treatment for 5min → drying (2.5–3 h). Stabilizer treatment and water treatment were repeated twice a day for 7 days.
2.3 Corrosion of dry/wet alternate
The dry/wet alternate corrosion experiment was used to simulate the rain and dry conditions that the weathering steel surfaces can be exposed to them in the natural environment. According to the national standard TB/T 2375-1993, the corrosion medium adopts a 0.01 mol/L NaHSO3 solution (simulating the industrial atmospheric environment). The experiment was carried out in a constant temperature and humidity test chamber with a temperature of 30 ± 2 °C and relative humidity of 60 ± 5 %.
The dry/wet alternate corrosion test took 12 h as a cycle, and a total of 40 cycles were carried out. In each cycle, a pipette gun was used to evenly drop the corrosive medium onto the surface of the sample with a dose of 40 μL/cm−2. Samples were taken for observation at 24, 72, 144, 216, 288, 384, and 480 h intervals, respectively. The differences in the corrosion resistance between the surface-treated sample and the bare samples were compared and analyzed.
2.4 Characterization of the rust layer
Rigaku Ultima IV X-ray diffractometer (XRD) was used to detect the composition of the rust layer in different corrosion stages applying a Cu target under 40 kV, 150 mA, and a 10–90° 2θ range at a scanning speed of 4°/min. The macroscopic corrosion morphology of the rust layer was observed by a digital camera. FEG-450 cold field emission scanning electron microscope (SEM) and its attached energy dispersive spectrometer (EDS) were used to observe the microstructure and section morphology of the rust layer and the distribution of alloying elements at the section. After scraping the inner rust layer for ultrasonic dispersion, the JEM-F200 transmission electron microscope (TEM) was used to analyze its microstructure, while its phase structure was analyzed by the selected area diffraction.
The polarization curve and AC impedance were tested using a standard three-electrode system with a saturated calomel electrode as a reference electrode, Pt sheet as an auxiliary electrode, and the sample as the working electrode. The electrolyte solution was 0.1 mol/L Na2SO4. The scanning rate of the polarization curve was 0.01 V/s, the scanning range was −1.5 to 1 V, while the scanning frequency of the AC impedance was 0.01–100 kHz.
3 Results and discussion
3.1 Corrosion kinetics analysis
Figure 1 shows the corrosion kinetics curves and corrosion rate curves of Q345qNH steel of stabilizing treatment and bare after corrosion in a dry and wet cycle of 0.01 mol/L NaHSO3 solution. The corrosion kinetics curves of the two steels conform to the parabola law, but the unit corrosion gain of the stabilizing treatment steel is significantly lower than that of the bare steel. It can be seen from the corrosion rate curve that the corrosion rate of the two steels is the maximum in the initial corrosion stage, and the corrosion rate decreases with the extension of corrosion time, but the time to reach stable corrosion is different. After 288 h of corrosion, the corrosion rate of the bare steel decreases significantly and enters the steady corrosion stage, with the corrosion rate remaining at about 0.048 mg cm−2 h−1. After 144 h of corrosion, the stabilizing treatment steel enters the steady state corrosion stage, and the corrosion rate remained basically unchanged at around 0.025 mg cm−2 h−1. After 480 h of corrosion, the corrosion rate of the stabilizing treatment steel decreased about 47.9 % compared to the bare steel, indicating that the stabilizing treatment shortened the time for the formation of stable rust layer and greatly reduced the corrosion rate.

The corrosion kinetics curves and corrosion rate curves of stabilizing treatment steel and bare steel.
According to the empirical formula D = Atn, that is used to fit the corrosion kinetics, the values of A, n, and R2 corresponding to the two samples can be obtained.
Where R2 is the correlation coefficient. Both A and n are constants, while the value of A is related to the properties of the material and the environment. The value of n reflects the corrosion kinetic characteristics. When n > 1, the kinetics corresponds to the process of increasing the corrosion rate; when n < 1, the kinetics corresponds to the process of decreasing the corrosion rate (Dong and Ke 2013). It can be seen from the fitting results in Figure 1 that in the whole corrosion process n values for the two steels are less than 1 indicating that the corrosion of Q345qNH bare steel and stabilizing treatment steel in the NaHSO3 medium environment is a deceleration process. However, the n value of the stabilizing treatment steel is higher than that of the bare samples, which corresponds to the stage of rapid formation of the rust layer. The n value of the stabilizing treatment steel is smaller than that of the bare steels, indicating that the corrosion tendency is smaller and the rust layer is more protective.
3.2 Analysis of the phase composition of the rust layer
Figure 2 shows the XRD patterns of stabilizing treatment steel and bare steel after corrosion in NaHSO3 medium for different periods. It can be seen from Figure 2a and 2b that the products of the two steels contain α-FeOOH, γ-FeOOH, Fe3O4, and Fe2O3 phases after 480 h of corrosion exposure. The difference is that the diffraction peak of the product of the rust layer after stabilizing treatment is obviously widened (Figure 2a), indicating that the grain size of the rust layer is obviously refined. In addition, the product of the rust layer of the stabilizing treatment steel contains a large amount of Cu, which is due to the replacement reaction between the stabilizer and the steel matrix during the stabilization process:

XRD patterns of the stabilizing treatment steel and bare steel after corrosion for different times: (a) stabilizing treatment steel, (b) bare steel, (c) trend of peak strength ratio variation of Iα-FeOOH/Iγ-FeOOH.
The substituted Cu is enriched on the surface of the matrix forming Cu deposition, slowing down the corrosion, improving the passivation ability of steel, and inhibiting the anodic dissolution process (Misawa et al. 1974). With extending of the corrosion time, the thickness of the rust layer gradually increases and the characteristic peak of Cu gradually weakens. There is a peak of α-Fe (Figure 2a) when the bare steel is corroded for 24 h, which is due to the thin rust layer.
Figure 2c shows the changing trend of the peak strength ratio of α-FeOOH and γ-FeOOH. Although Iα-FeOOH/Iγ-FeOOH values for both samples increase during the entire corrosion process, Iα-FeOOH/Iγ-FeOOH values of the stabilizing treatment steel are higher than those of bare steel and this ratio difference is obvious especially in the middle and late stages of corrosion. The Iα-FeOOH/Iγ-FeOOH value of the stabilizing treatment steel after 480 h corrosion is 1.26 times that of the bare steel. α-FeOOH is the thermodynamically stable phase which is the most stable and dense crystal structure in the rust layer. The more α-FeOOH phase content is, the better protection of the rust layer is. γ-FeOOH has very low chemical stability and a certain degree of reducibility which can be easily used as an active cathode to form corrosion galvanic cells with the steel matrix. The ratio of Iα-FeOOH/Iγ-FeOOH of the stabilizing treatment steel is larger than that of the bare steel indicating that the stabilizing treatment can promote the formation of the α-FeOOH phase and short the formation period of stabilized rust layer.
3.3 Morphology and structure analysis of the rust layer
3.3.1 Morphology analysis of the outer rust layer
3.3.1.1 Macroscopic morphology analysis
Figure 3 shows the macroscopic morphologies of the two steels after corrosion at different times. It can be seen that with extending of the corrosion time, the rust layer color of the stabilizing treatment steel gradually deepens from light yellow to reddish–brown as in Figure 3a–e. At the initial stage of corrosion (24 h), the rust layer is thin and does not completely cover the matrix (Figure 3a). As the corrosion time increases, the rust layer gradually becomes uniform, and has a smooth surface and good compactness. The rust layer of the bare steel’ changes from brick red to black–brown and its growth is not uniform accompanied by a large number of rust cells. In the middle and late stages of corrosion, there are fine rust particles on the surface which are loose, easy to fall off, and have poor protection (Figure 3f–j). Compared with the stabilized samples, the corrosion of the bare steel is more serious and the rust layer is thicker which is consistent with the results of corrosion kinetics.

Macroscopic morphology of the stabilizing treatment and bare steel after corrosion exposure for different times: (a–e) stabilizing treatment steel, (f–j) bare steel; (a, f) 24 h, (b, g) 72 h, (c, h) 144 h, (d, i) 288 h, (e, j) 480 h corrosion times.
3.3.1.2 Microscopic morphology analysis of outer rust layer
Figure 4 shows the microstructure of the outer rust layer of the stabilizing treatment steel and bare steel at different corrosion times applying the SEM technique. After 144 h of corrosion, the surface of the rust layer of the stabilizing treatment steel is a fine granular corrosion product (Figure 4a) which is α-FeOOH (Qian et al. 2013; Ma et al. 2009) accompanied by a certain number of cracks. At the middle stage of corrosion (288 h), the cracks decrease and the rust layer becomes dense (Figure 4b). In the late stage of corrosion (480 h), the continuity and density of the rust layer are greatly improved while the surface defects are reduced. There are basically no cracks, and the surface of the rust layer is smooth. At the same time, the corrosion products are more uniform (Figure 4c) and the protection of the rust layer is greatly enhanced. The surface of the bare steel after 144 h corrosion is granular (α-FeOOH) and massive (γ-FeOOH) corrosion products. In addition to the cracks, there is also an uneven surface with large fluctuations of the rust layer caused by the accumulation of corrosion products (Figure 4d). In the middle stage of corrosion (288 h), the rust layer is obviously stratified and the interior is relatively dense, but there are still large cracks, unevenness, and rust layer falling off caused by rust layer accumulation (Figure 4e). In the late stage of corrosion (480 h), there are still easily detached flake like corrosion products on the surface of the rust layer (Figure 4f), and the rust particles are also much larger than the stabilizing treatment steel (Figure 4f).

The microstructure of the rust layer of the stabilized and bare steel at different corrosion stages: (a–c) stabilizing treatment steel, (d–f) bare steel; (a, d) 144 h, (b, e) 288 h, (c, f) 480 h corrosion times.
As a whole, the rust layer of the stabilizing treatment steel reaches density at 144 h and becomes more and more dense, with fewer defects. The surface defects of the bare steel are more serious, and the rust layer is loose and easy to fall off, which is consistent with the results of the macroscopic corrosion morphology, indicating that the stabilizing treatment has a good protection effect on the weathering steel.
3.3.2 Morphology, composition, and structure analysis of the inner rust layer
Figure 5 shows the microscopic morphology and composition of the inner rust layer of the two (stabilized and bare) samples after the corrosion for 480 h. The outer rust layer of the sample is scraped off with a blade, and the remaining rust layer that is tightly bound to the matrix is the inner rust layer. It can be seen by SEM observation that the inner rust layer of the stabilized steel (Figure 5a) is still denser and more uniform whose corrosion products are smaller than that of the bare steel (Figure 5b) which is consistent with the morphology of outer rust layer. Microscopic regional point scanning is carried out on the surface of the rust layer for the two steels ‘respectively’, and the substituted Cu element (Spectrum 1, the bright white particles in Figure 5a) is found in the stabilized steel, which is in good agreement with the XRD results. At the same time, the content of the Cr element is also significantly higher than that of the bare samples. This is due to Cr substituting a portion of the Fe atoms of α-FeOOH to form α-(Fe1−XCrX)OOH phase.

Microstructure and composition of the inner rust layer after 480 h corrosion of (a, c, d) the stabilizing treatment steel and (b, e, f) the bare steel.
Figure 6 shows the microstructure of the inner rust layer and the selected electron diffraction pattern of the two (stabilized and bare) steels after 480 h corrosion using the TEM technique. After the ultrasonic dispersion, it was found that the untreated sample is easy to disperse (Figure 6b), while the stabilizing treatment steel is in a flocculent state and it is not easy to disperse (Figure 6a). After the selective diffraction analysis, it is found that the grains of the two steels were very fine, but the bare steel are connected into rings in the form of fine diffraction spots, while the stabilized steel directly form diffraction rings indicating that their corrosion products exist in the form of nanocrystals. After calibrating the diffraction patterns of the two samples, it is found that the fine corrosion products are mainly α-FeOOH (Figure 6a) and γ-FeOOH (Figure 6b).

TEM micrographs of the microstructure and the selected electron diffraction pattern of the rust layer of (a) the stabilizing treatment steel and (b) the bare steel after 480 h corrosion time.
3.3.3 Analysis of the section morphology
Figure 7 shows the section morphology of the rust layer of the two (stabilized and untreated) samples at different corrosion stages. As can be seen from the figure, after the corrosion time of the stabilizing treatment steel increases from 144 to 288 h, the corrosion thickness dose not increases significantly (Figure 7a and b); after 480 h of corrosion (Figure 7c), the corrosion thickness increases somewhat, but the increase is not significant, indicating that the corrosion time of the stabilizing treatment steel at 144 h has become stable. However, the thickness of the rust layer of the bare steel after corrosion for 288 h is significantly higher than that for 144 h (Figure 7d and e), and the increase of the rust layer thickness decreases after corrosion time increased from 288 to 480 h (Figure 7f), indicating that the bare steel tend to be stable after corrosion 288 h, which is consistent with the kinetic curve. The thickness of the rust layer of the bare steel is obviously greater than that of the stabilizing treatment steel at the same corrosion time, indicating that the stabilized treatment can promote the formation of stable rust layer and reduce the corrosion rate.

Section morphology of the rust layer in different corrosion stages for the (a–c) stabilizing treatment steels, (d–f) bare steel; (a, d) 144 h, (b, e) 288 h, (c, f) 480 h corrosion times.
The rust layer of the two steels is stratified, the outer rust layer is relatively loose, the inner rust layer is relatively dense. After 144 h corrosion of the stabilizing treatment steel, the inner rust layer and the matrix are tightly bonded, and there are no obvious holes and cracks and other defects, and the rust layer has good density. After 144 h corrosion of bare steel, the inner and outer rust layers are not dense. After 288 h corrosion of the bare steel, the rust layer becomes dense, but the inner rust layer has obvious long cracks throughout the entire section, and there are void defects at the joint of the rust layer and the matrix. After corrosion to 480 h, in addition to transverse cracks, large longitudinal holes and gaps along the rust layer appear on the surface of the bare sample, providing a channel for the entry of water, oxygen, and other media.
Figure 8a and b shows the distribution of the alloying elements in the rust layer sections of the stabilized and bare steel ‘respectively’ after corrosion for 480 h. The alloying elements Cu and Cr in the stabilized steels are enriched at the interface between the rust layer and the substrate and inside the rust layer obviously (Figure 8a). Cr enriched in the rust layer will displaces part of the Fe in the α-FeOOH during the subsequent corrosion to form a more stable and dense α-(Fe1−XCrX)OOH. α-(Fe1−XCrX)OOH film is cationic selective which can effectively prevent the invasion of corrosive anions (Wang et al. 2013). The presence of Cr also inhibits the pitting and improves the corrosion resistance of steel (Liu et al. 2002; Yamashita et al. 2011). In addition, the content of S in the outer rust layer of the untreated sample is significantly higher than that of the stabilized ones (Figure 8b). This is because the stabilizing treatment makes the rust layer cationic selective preventing SO42− infiltration. The existence of the alloying elements can change the structure and organization of the rust layer refining the grains of the rust layer and playing an important role in improving the protection level of the rust layer.

Elements distribution in the rust layer section of (a) the stabilized steel, (b) the bare steel after 480 h corrosion time.
3.3.4 Electrochemical analysis
3.3.4.1 Polarization curve analysis
Figure 9 shows the polarization curves of the two (stabilized and bare) steels in 0.1 mol/L Na2SO4 electrolyte after corrosion at different times. The self-corrosion potential of the two steels increases and the self-corrosion current decreases with extending the corrosion time. This indicates that the corrosion tendency of the two steels decreases, the corrosion rate reduces, and the electrochemical stability of the rust layer for both steels improves and the corrosion resistance increases. Table 3 reveals the parameters table of the polarization curve. It can be seen from the table that the self-corrosion potential of the two steels moves forward and the self-corrosion current density decreases. The self-corrosion potential of the stabilizing treatment steels is always greater than that of the bare steel while its self-corrosion current density is always less than that of the bare steel. The results show that both the corrosion tendency and corrosion rate of the steel after the stabilization treatment are lower than those of the bare steel, and the electrochemical protection performance of the rust layer is improved which is in good agreement with the results of corrosion kinetics.
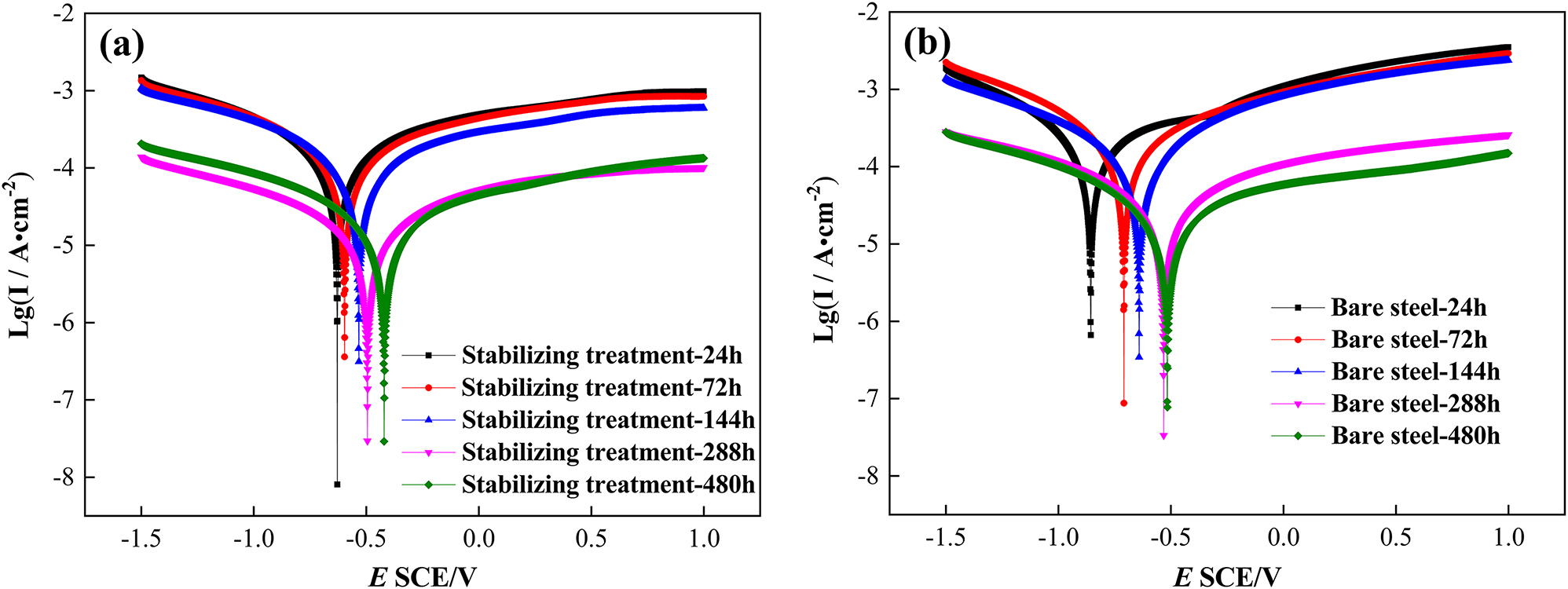
The polarization curves of (a) the stabilizing treatment steel, (b) the bare steel in 0.1 mol/L Na2SO4 electrolyte after corrosion for different times.
Parameters of the polarization curves.
| Type of steel | Electrochemical parameter | Corrosion time | ||||
|---|---|---|---|---|---|---|
| 24 h | 72 h | 144 h | 288 h | 480 h | ||
| Stabilizing treated | E corr/V | −0.6334 | −0.5961 | −0.5382 | −0.5009 | −0.4218 |
| I corr/mA cm−2 | 0.3663 | 0.3503 | 0.2776 | 0.0386 | 0.0474 | |
| Bare | E corr/V | −0.8593 | −0.7096 | −0.6454 | −0.5353 | −0.5199 |
| I corr/mA cm−2 | 0.5858 | 0.5242 | 0.3595 | 0.0805 | 0.0601 | |
The two samples are controlled by both the anodic and cathodic reactions in the electrochemical corrosion process. The anodic part of the steel types has no obvious passivation characteristic indicating that the anodic process is mainly controlled by the dissolution of steel. The reduction in the anode dissolution current indicates that the dissolution rate of the rust layer decreases, and the protection of the rust layer enhances which can inhibit the anodic reaction on the substrate to a certain extent. The cathodic reaction process is mainly controlled by the limited diffusion of oxygen dissolution in the early stage and the charge transfer of the reduction of corrosion products (γ-FeOOH) in the later stage. With extending of the corrosion time, the cathode current density decreases indicating that the cathode reduction process is inhibited which is related to the stabilization of the relative content of corrosion products. It can be seen from Figure 9a and b that during the whole corrosion process, the anodic and cathodic corrosion currents of the stabilizing treatment steel is always lower than that of the bare steel indicating that the rust layer formed after the stabilizing treatment is more protective and the corrosion rate is lower.
3.3.4.2 Impedance spectrum analysis
Figure 10a is the Nyquist diagram of the stabilizing treatment steel and bare steel in the 0.1 mol/L Na2SO4 electrolyte after corrosion at different times. Figure 10b is the equivalent circuit diagram that is used to simulate the corrosion of the two rusty steels in the electrolyte. It can be seen from the Figure 10a that the Nyquist diagrams of the two rusty steels contain a semi-circular capacitive reactance arc in the high-frequency region and a diffusion tail in the low-frequency region. The high-frequency capacitance loop is caused by charge transfer resistance at the interface between sample and solution, while the low-frequency capacitor circuit is caused by a small number of corrosion products initially that are formed on the surface of the steel. The larger the arc radius in the high-frequency region is, the better the corrosion resistance of the rust layer is.

Nyquist diagram of the stabilizing treatment steel and bare steel in 0.1 mol/L Na2SO4 electrolyte after corrosion for different times (a) and equivalent circuit diagram of the electrochemical impedance spectroscopy (b).
By observing Figure 10a, it can be found that with extending of the corrosion time, the radius of the capacitive reactance arc in the high-frequency region for both steels increases, and the protection of the rust layer develops better. However, the arc radius in the high-frequency region of the stabilizing treatment steel is much larger than that of the bare steel, which indicate that the rust layer generated on the surface of the weathering steel after the stabilizing treatment is dense and stable, and its corrosion resistance is better than that of the bare steel, which is consistent with the results of the morphology analysis of the rust layer.
In the equivalent circuit diagram of Figure 10b, where Rs represents the solution resistance, Rr and Rct represent the rust layer resistance and charge transfer resistance in the dissolution process of steel matrix ‘respectively’, ZW represents the impedance associated with the diffusion (Warburg impedance), Qr and Qd represent the double electric layer capacitance composed of the rust layer and bulk phase solution and double electric layer capacitance composed of steel matrix and infiltration electrolyte, respectively. The resistance of the rust layer (Rr) can reflect the density of the rust layer and the ability to resist the transmission of the corrosive media, so it can be used as an important indicator for evaluating the protection of the rust layer (Zhou et al. 2020).
The equivalent circuit model shown in Figure 10b can be used to fit the measured AC impedance data, and the rust resistance of the two steels at different corrosion cycles as shown in Figure 11. The standard deviation χ2 of the fitting process is all less than 10−4, and the results are in good agreement. It can be seen from Figure 11 that during the entire corrosion process, the resistance of the rust layer of the two steels increases with extending the corrosion time, but the rust layer resistance of the bare steel is always lower than that of the stabilizing treatment steel. The resistance of the rust layer of the stabilizing treatment steel (91.35 Ω cm2) is about 1.56 times that of the bare steel (58.55 Ω cm2) at the later stage of corrosion (480 h).

Resistance of rust layer of the stabilizing treatment steel and bare steel at different corrosion cycles.
3.4 Analysis and discussion
The atmospheric corrosion of steel can be regarded as the corrosion of iron matrix in the water film containing corrosive substances. The water in the atmosphere is easily adsorbed on the steel surface to form a liquid film, as well the corrosive ions that can be dissolved on the liquid film. In the industrial atmosphere, the SO2 is the main pollutant. SO2 is easily decomposed to an aqueous solution that combines with H2O to form H2SO3 which is further oxidized to form H2SO4. The SO42− in the H2SO4 easily reacts with Fe2+ generated by the anodic reaction to form an “acid regeneration cycle” mechanism (Morcillo et al. 2014) which further accelerates the dissolution of steel. The specific reaction processes are as follows:
At the initial stage of atmospheric corrosion, weathering steel forms a rust layer like ordinary carbon steel. But by the time, the rust layer close to the matrix can gradually become dense, and forms mainly of α-FeOOH enriched in Cu, Cr, Ni, and other alloying elements. However, this rust layer transformation process takes at least 3–5 years to complete as shown in Figure 12a.

The conversion process of the rust layer of weathering steel: (a) In the case of the bare steel, (b) in the case of the stabilizing treatment steel.
It can be seen that the corrosion rate of weathering steel greatly decreases after the stabilizing treatment (stabilizer treatment and water treatment), and the dense and uniform rust layer is rapidly formed on the surface of the steel. Compared with the bare steel, the electrochemical performance of the stabilizing treatment steel is significantly enhanced. This is because the Cu and Cr ions replaced by the reaction between the steel matrix and the stabilizer are enriched on the surface of the matrix in the process of stabilization which refines the microstructure, repairs the defects, such as cracks, in the inner rust layer, makes the rust layer denser, makes the steel matrix produce anodic passivation, improves the self-corrosion potential of the steel matrix and slows down the occurrence of corrosion. Cr can form an infinite solid solution in iron, so secondary distribution can occur in the corrosion process. Part of Cr forms α-(Fe1−XCrX)OOH phase in the rust layer to prevent the entry of the corrosive anions, such as SO42−, and part of it precipitates on the defects and the grain boundaries. In addition, Cr also inhibits the occurrence of pitting corrosion and makes the rust layer more protective. The NaHSO3 in the stabilizer will promote the dissolution of γ-FeOOH and accelerate the transformation of γ-FeOOH to α-FeOOH (Díaz et al. 2018). Water treatment after stabilizer treatment provides a environment for the formation of stable rust layer. During stabilizer treatment, a surface oxide layer containing chromium ions and elemental copper is formed. Through the water treatment process, the main phase of the rust hydroxyl oxide can be formed, and at the same time, these alloying element ions can be effectively solidly dissolved into the main phase of the rust hydroxyl oxide, thus forming a stable and dense rust layer. At the same time, the process of water treatment makes the weathering steel in a dry-wet alternate state. The steel matrix serves as anode and the replaced Cu serves as the cathode forming a corrosion microcell, accelerating the formation process of the protective rust layer and shortening the stabilization cycle (7 days). The formation process of the rust layer is depicted in Figure 12b.
4 Conclusions
In the simulated industrial atmosphere medium, the kinetics of the corrosion process of both the stabilizing treatment and the bare bridge weathering steel conformed to the power function equation W = Atn, but the stabilizing treatment shortened the formation time of stable rust layer and significantly reduced the corrosion rate, which was 47.9 % lower than that of bare sample after 480 h of corrosion.
The rust layer products of the two weathering steels were composed of α-FeOOH, γ-FeOOH, Fe3O4, and Fe2O3 phases, but the rust layer of the stabilizing treatment steel was rich in copper. In the whole corrosion process, the α-FeOOH/γ-FeOOH peak strength ratio of the two steels increased with the extension of corrosion time, but the peak strength ratio of the stabilizing treatment steel was always greater than that of the bare steel, and was 1.26 times that of the bare steel after 480 h corrosion.
After the stabilizing treatment, the electrochemical performance of the Q345qNH bridge weathering steel rust layer was improved. The self-corrosion potential increased, the self-corrosion current density reduced, and the resistance of the rust layer increased. After 480 h corrosion, the rust resistance of the stabilizing treatment steel was about 1.56 times that of the bare steel, and the corrosion resistance of the rust layer significantly improved.
After the stabilizing treatment, the alloying elements Cu and Cr in the stabilizer were enriched at the interface between the rust layer and the substrate and inside the rust layer. This made the rust layer has cationic selectivity preventing the infiltration of SO42−, refining the grain of the rust layer, and repairing the cracks and other defects in the rust layer. The rust layer had fewer defects and it was denser and well combined with the matrix. At the same time, the formation of the stable phase α-(Fe1−xCrx)OOH was promoted, the formation time of the stabilized rust layer was shortened, and the protection of the rust layer was enhanced.
Funding source: National Natural Science Fundation of China
Award Identifier / Grant number: No. 52161007
Funding source: Natural Science Foundation of Gansu Province
Award Identifier / Grant number: No. 20JR10RA170
Funding source: Science and technology project of Gansu Provincial Department of Transportation
Award Identifier / Grant number: No. 202102
Acknowledgments
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
-
Research ethics: Not applicable.
-
Author contributions: The authors participated in the characterization of corrosion experiments.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: The investigation is supported by National Natural Science Foundation of China (no. 52161007). The investigation is supported by Science and technology project of Gansu Provincial Department of Transportation (Project name: Research on key technology and industrial application of composite steel-concrete girder cable-stayed bridge in high seismic area, no. 202102). The investigation is also supported by Natural Science Foundation of Gansu Province (no. 20JR10RA170).
-
Data availability: Not applicable.
References
Cui, W., Shao, C., and Liu, C. (2012). Corrosion behavior of new weathering steel in the environment simulating coastal industrial atmosphere. Adv. Mater. Res. 479–481: 322–326, https://doi.org/10.4028/www.scientific.net/amr.479-481.322.Suche in Google Scholar
Díaz, I., Heidis, C., David, C., Belen, C., Daniel, D., and Manuel, M. (2018). Atmospheric corrosion of ASTM A-242 and ASTM A-588 weathering steels in different types of atmosphere. Corros. Eng. Sci. Technol. 53: 449–459, https://doi.org/10.1080/1478422x.2018.1500978.Suche in Google Scholar
Dong, J. and Ke, W. (2013). Fitting and evolution of atmospheric corrosion of low alloy steels under wet/dry cyclic corrosion test. John Wiley & Sons, Ltd., Hawaii, USA, pp. 639–644.10.1002/9781118792148.ch79Suche in Google Scholar
Gao, L., Yang, J., Zhang, X., Liu, K., Cao, J., and Wang, S. (2019). Study on surface rust stabilizers and corrosion resisting properties for weathering steels. Met. Mater. Metall. Eng. 47: 21–25.Suche in Google Scholar
Guo, X., Kang, J., Zhu, J., and Duan, M. (2019). Corrosion behavior and mechanical property degradation of weathering steel in marine atmosphere. J. Mater. Civ. Eng. 31: 1–16, https://doi.org/10.1061/(asce)mt.1943-5533.0002814.Suche in Google Scholar
Komori, T., Kage, I., Mitao, S., and Nakano, H. (2014). Effects of stabilizing treatment of protective rust layer and alloy design on corrosion behavior of weathering steels under high airborne salt condition. Corros. Eng. 63: 575–582, https://doi.org/10.3323/jcorr.63.575.Suche in Google Scholar
Liu, L., Qi, H., Lu, Y., and Li, X. (2002). Corrosion properties and surface rust scale stabilizing treatments of weathering steels. Corrs. Prot. 23: 515–518.Suche in Google Scholar
Liu, Z., Lian, X., Liu, T., Yang, Y., and Dong, H. (2020). Effects of rare earth elements on corrosion behaviors of low-carbon steels and weathering steels. Mater. Corros. 71: 258–266, https://doi.org/10.1002/maco.201911150.Suche in Google Scholar
Ma, Y., Li, Y., and Wang, F. (2009). Corrosion of low carbon steel in atmospheric environments of different chloride content. Corros. Sci. 51: 997, https://doi.org/10.1016/j.corsci.2009.02.009.Suche in Google Scholar
Misawa, T., Asami, K., Hashimoto, K., and Shimodaira, S. (1974). The mechanism of atmospheric rusting and the protective amorphous rust on low alloy steel. Corros. Sci. 14: 279–289, https://doi.org/10.1016/s0010-938x(74)80037-5.Suche in Google Scholar
Morcillo, M., Diaz, I., Chico, B., Cano, H., and Fuente, D. (2014). Weathering steels: from empirical development to scientific design. A review. Corros. Sci. 83: 6–31, https://doi.org/10.1016/j.corsci.2014.03.006.Suche in Google Scholar
Qian, Y., Ma, C., Niu, D., Niu, D., and Li, M. (2013). Influence of alloyed chromium on the atmospheric corrosion resistance of weathering steels. Corros. Sci. 74: 424, https://doi.org/10.1016/j.corsci.2013.05.008.Suche in Google Scholar
Sei, J., Cook, D., and Townsend, H. (1999). Atmospheric corrosion of different steels in marine, rural and industrial environments. Corros. Sci. 41: 1687–1702, https://doi.org/10.1016/s0010-938x(99)00005-0.Suche in Google Scholar
Song, Z., Guo, T., Zhang, Y., Nan, X., Xu, X., and Dong, Z. (2020). The corrosion behavior of weathering bridge steel with oxide scale in different corrosion environments. Surf. Topogr.: Metrol. Prop. 8: 1–9, https://doi.org/10.1088/2051-672x/ab8502.Suche in Google Scholar
Tomita and Yukio. (1998). Newly developed high performance structural steels for long span bridge construction. Zairyo to Kankyo 47: 684–690, https://doi.org/10.3323/jcorr1991.47.684.Suche in Google Scholar
Wang, Z., Liu, J., Wu, L., Han, R., and Sun, Y. (2013). Study of the corrosion behavior of weathering steels in atmospheric environments. Corros. Sci. 67: 1–10, https://doi.org/10.1016/j.corsci.2012.09.020.Suche in Google Scholar
Wu, W., Zeng, Z., Cheng, X., Li, X., and Liu, B. (2017). Atmospheric corrosion behavior and mechanism of a Ni-advanced weathering steel in simulated tropical marine environment. J. Mater. Eng. Perform. 26: 6075–6086, https://doi.org/10.1007/s11665-017-3043-6.Suche in Google Scholar
Yamashita, M., Hara, S., Kamimura, T., Miyuki, H., and Sato, M. (2007). X-ray diffraction analysis of rust layer on a weathering steel bridge with surface treatment using synchrotron radiation. Mater. Trans. 48: 579–583, https://doi.org/10.2320/matertrans.48.579.Suche in Google Scholar
Yamashita, M., Misawa, T., Oh, S., Balasubramanian, R., and Cook, D. (2011). Mossbauer spectroscopic study on X-ray amorphous substance in rust layer of weathering steel subjected to long-term exposure in North America. Zairyo to Kankyo 49: 82–87, https://doi.org/10.3323/jcorr1991.49.82.Suche in Google Scholar
Yan, X., Yu, Q., Guo, K., Sun, R., Gao, Y., and Wang, Q. (2020). Experimental design of Cu content in weathering resistance steel for industrial atmosphere application. Mater. Res. Express 7: 1–17, https://doi.org/10.1088/2053-1591/ab71c9.Suche in Google Scholar
Zhang, Q., Wang, J., Wu, J., Zheng, W., and Cheng, J. (2001). Effect of ion selective property on protective ability of rust layer formed on weathering steel exposed in the marine atmosphere. Acta Metall. Sinica 37: 193–196.10.1088/2053-1591/ab71c9Suche in Google Scholar
Zhang, X., Yang, S., Guo, H., and He, X. (2014). Atmospheric corrosion behavior of weathering steel in periodically changed environment. ISIJ Int. 54: 909–915, https://doi.org/10.2355/isijinternational.54.909.Suche in Google Scholar
Zhou, L., Yang, S., Dong, Y., Zhang, W., Ding, J., Liu, G., Shang, C., and Raja, D. (2020). Characterization of compactness of rust layers on weathering steels by an adsorption/dehydration test of ethanol. Acta Metall. Sinica 33: 846–856, https://doi.org/10.1007/s40195-020-01008-0.Suche in Google Scholar
© 2024 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Reviews
- Corrosion of stainless steels and corrosion protection strategies in the semiconductor manufacturing industry: a review
- Dopamine functionalized coatings for corrosion protection of metallic implants and advanced drug delivery: a review
- Anticorrosive action of eco-friendly plant extracts on mild steel in different concentrations of hydrochloric acid
- Corrosion mechanism and research progress of metal pipeline corrosion under magnetic field and SRB conditions: a review
- Original Articles
- Decision support system to evaluate a vandalized and deteriorated oil pipeline transportation system using artificial intelligence techniques. Part 2: analysis of the operational and economic risk
- Molecular-level investigation of the adsorption mechanisms of thiazolidinediones on Cu2O(111) surface: a first-principles DFT study
- Effect of rust layer stabilization on dry and wet cyclic corrosion behavior of bridge weathering steel Q345qNH in simulated industrial atmospheric medium
Artikel in diesem Heft
- Frontmatter
- Reviews
- Corrosion of stainless steels and corrosion protection strategies in the semiconductor manufacturing industry: a review
- Dopamine functionalized coatings for corrosion protection of metallic implants and advanced drug delivery: a review
- Anticorrosive action of eco-friendly plant extracts on mild steel in different concentrations of hydrochloric acid
- Corrosion mechanism and research progress of metal pipeline corrosion under magnetic field and SRB conditions: a review
- Original Articles
- Decision support system to evaluate a vandalized and deteriorated oil pipeline transportation system using artificial intelligence techniques. Part 2: analysis of the operational and economic risk
- Molecular-level investigation of the adsorption mechanisms of thiazolidinediones on Cu2O(111) surface: a first-principles DFT study
- Effect of rust layer stabilization on dry and wet cyclic corrosion behavior of bridge weathering steel Q345qNH in simulated industrial atmospheric medium


