Abstract
In the current study, we report an excellent high temperature oxidation resistance of AlCoCrFeNi high entropy alloy (HEA) following surface modification. The surface properties of HEA were tailored through a severe surface deformation technique. The as cast HEA exhibited coarse grain B2/BCC microstructure. In contrast, processed specimen showed significant grain refinement along with B2/BCC to FCC phase-transition. The processed specimen demonstrated 11–67% reduction in the oxidation kinetics. Cr2O3 and Al2O3 were the predominant oxides formed in all the oxidized specimens. In addition, Cr, Fe and Co rich spinels were also found in the as cast oxidized specimens. The superior oxidation resistance of the processed specimen is attributed to the microstructural refinement resulting in the formation of protective dense chromia layer.
Abbreviations
- 15min_1800
-
specimen processed at 1800 rpm for 15 min
- 15min_400
-
specimen processed at 400 rpm for 15 min
- 5min_1800
-
specimen processed at 388 rpm for 5 min
- 5min_400
-
specimen processed at 400 rpm for 5 min
- DOR
-
depth of refinement
- EDM
-
electric discharge machining
- HAGB
-
high angle grain boundary
- HEA
-
high entropy alloy
- IOZ
-
internal oxidation zone
- MEA
-
medium entropy alloy
- SFP
-
stationary friction processing
- TBC
-
thermal barrier coating
- TGO
-
thermally grown oxide
- TMAZ
-
thermo-mechanical affected zone
1 Introduction
Advanced structural materials with superior high temperature oxidation resistance are indispensable for a wide range of industrial applications including aerospace, power generation, transportation, material production and processing etc (Muktinutalapati 2011; Williams and Stardske 2003). In the pursuit to achieve higher system efficiency, the operating temperatures in most of these sectors have substantially increased over a period of time. In contrast, the research and development of advanced materials for efficient working at high temperatures has been rather sluggish (Meetham and Van de Voorde 2000). Super-alloys are currently the most widely used high temperature materials. The peak temperatures up to which these materials can be effectively utilized are usually limited to nearly 800–900 °C. To widen the temperature range, thermal barrier coatings (TBCs) are typically used on superalloy components (Chen et al. 2017, 2015). The TBCs form a persistent, adherent and gradually growing scale between the top and the bond coat which offers high oxidation resistance and interface adherence at elevated temperatures (Evans et al. 2001b; Padture et al. 2002). However, a large number of studies (Evans 2011; Ranjbar-Far et al. 2010, Shillington and Clarke 1999; Tzimas et al. 2000; Zhou and Hashida 2002) reported on premature failure of TBCs at or near the TGO/bond coat interface. Typically, the segregation of impurities at the interface/grain boundaries (Evans et al. 2001a) and the development of interfacial imperfections with the increase in TGO thickness result in failure of TBCs. The application of superalloys without TBC layer is limited due to substantial deprivation in their mechanical properties and microstructural instability. Thus, the development of advanced materials exhibiting high strength at elevated temperatures with high oxidation resistance at elevated temperatures can be transformative in the field of high temperature application.
Recently developed high entropy alloys (HEAs) are considered potential candidates for high temperature applications. This is attributed to their excellent high temperature strength (Chen et al. 2016; Munitz et al. 2016; Wang et al. 2009; Wu et al. 2014), enhanced fatigue resistance (Hemphill et al. 2012), good thermal stability (Karati et al. 2019; Shivam et al. 2018; Zhao et al. 2018), and good fracture toughness (Wang et al. 2015, 2018). There are large number of studies on the oxidation behavior of HEAs (Adomako et al. 2018; Butler et al. 2015; Butler and Weaver 2016; Dąbrowa et al. 2017; Garg et al. 2021; Lu et al. 2020a, 2020b, 2019). Adomako et al. (2018) report on the oxidation behavior of medium entropy alloys (CoCrNi and CoCrNiMn) and high entropy alloy (CoCrNiMnFe). Oxidation resistance of CoCrNi was reported to be the highest, whereas CoCrNiMn showed the least oxidation resistance while CoCrNiMnFe showed intermediate resistance. Mass gain of CoCrNiMnFe HEA was reported to be 4 mg/cm2 at 950 °C for 24 h of isothermal oxidation. Lu et al. (2019) reported the influence of yttrium addition on the oxidation behavior of MCrAlY (Ni, Co) alloy. It was found that the uniform yttrium distribution could: (1) slow down the kinetics of TGO; (2) decrease the TGO thermal stresses; and (3) prevent the segregation of sulfur content and the formation of interfacial imperfections (e.g. oxide intrusions, pores). The AlCoCrFeNi system is one of the most widely explored HEA owing to its excellent mechanical properties and structural stability (Chen et al. 2016; Hemphill et al. 2012; Karati et al. 2019; Munitz et al. 2016; Shivam et al. 2018; Wang et al. 2009, 2018). Butler et al. (2015) reported the oxidation behavior of Al10Co22.5Cr22.5Ni22.5Fe22.5, Al15Co10Cr35Ni35Si5 and Al20Co25Cr25Ni25Si5 alloys. Al10Co22.5Cr22.5Ni22.5Fe22.5 showed a mass gain of 1.87 mg/cm2 owing to the formation of thick Cr2O3 and internal precipitates, while alloys Al15Co10Cr35Ni35Si5 and Al20Co25Cr25Ni25Si5 showed mass gain of 0.47 mg/cm2 and 0.11 mg/cm2 respectively at 1050 °C for 100 h of testing. The superior oxidation resistance of these alloys was attributed to the formation of continuous Al2O3 with no internal oxidation. In addition, Butler and Weaver (2016) investigated the oxidation behavior of a series of arc melted Alx(NiCoCrFe)100-x HEAs where x = 8, 10, 12, 15, 20 and 30 at 1050 °C. The study reported highest oxidation resistance for Al30(NiCoCrFe)70 HEA owing to increased Al content, which promotes continuous protective Al2O3 scale. For AlxCoCrFeNi (x = 0.7, 1, 1.3) composition, the parabolic rate constant of the Al2O3 scale decreased with the increment in the Al content (Lu et al. 2020a). Further, Dąbrowa et al. (2017) studied the effect of copper content on the oxidation behavior of AlCoCrCuxFeNi high-entropy alloys (x = 0; 0.5; 1). It was reported that the oxidation rate declined with the increase in Cu content.
The major focus of prior studies has been on the development of new alloy systems for limiting the oxidation. In contrast, there are only a few reports on the surface modification of alloy systems for limiting oxidation which includes investigating the influence of grit blasting (Ni et al. 2011), shot peening (Ni et al. 2011), microwave processing (Garg et al. 2021) and ultrasonic treatment (Liu et al. 2017) on the high temperature oxidation kinetics and oxide layer morphology. However, there is no understanding on the effect of grain refinement on the oxidation behavior, kinetics and oxidation mechanisms. In the current study, we report on remarkable enhancement in the high temperature oxidation behavior of AlCoCrFeNi HEA through severe surface deformation. Stationary friction processing (SFP), an adaptation of well-known friction stir processing, was utilized for ensuring elemental homogenization of the as cast microstructure and microstructural refinement (Kumar et al. 2021). Following surface deformation, the HEA specimen showed extremely small mass gain and the reported values are one of the lowest in the literature. The outstanding high temperature oxidation behavior of processed HEA was attributed to an increase in the activation energy for oxygen diffusion as a result of grain refinement that limits the oxide-scale thickness resulting in enhanced oxidation resistance. The current study is the first report on the influence of severe surface deformation on the oxidation behaviour of HEAs. The proposed approach is highly generic and can be easily applied to a wide range of material systems to achieve excellent oxidation resistance.
2 Materials and methods
The elemental micro-powders of Al, Co, Cr, Fe and Ni were taken with 99.99% purity to pulverize and mix in equimolar composition. The powder mixture was molded into a button shape pellet of 12 mm diameter and 9 mm thick by using hydraulic press. The pellets were melted inside the chamber of an arc melting furnace in an argon environment. The arc melting furnace was equipped with a vacuum pump and an external chilling unit. The specimen was flipped and re-melted up to five times to homogenize the elemental distribution. The specimen was sectioned in a dimension of 20 × 15 × 2 mm3 by using electric discharge machining (EDM) and the EDM affected layer was removed by grinding and polishing. To perform stationary friction processing (SFP henceforth), a customized fixture was prepared to hold this small specimen on the machine table of universal milling machine as shown in Figure 1. The surface properties of the as cast specimen were tailored using SFP. A cylindrical shaped WC pin-less tool was used to perform the SFP. After the rotating tool plunges nearly 0.25–0.3 mm into the workpiece, it was held at the same location for a certain time period. The SFP was performed at two different rotational speeds viz. 400 and 1800 rpm for two different time durations viz. 5 and 15 min. A schematic representation of the SFP process is shown in Figure 1. The processed circular section (12 mm dia.) of the specimen was cut off using EDM. Both the as cast and processed circular specimens were polished down to 2000 grit SiC polishing paper. Following paper polishing, the specimens were cleaned ultrasonically in ethanol. Also, the cross section of the processed specimens was polished and etched with the aqua-regia solution for 20 s and optical micrographs of processed specimens were acquired using Leica DM750 M optical microscope (Germany). EBSD measurements were performed using FEI Quanta 3D FEG with step size of 0.1 μm. To investigate the microstructure of the HEA specimens in detail, thin foils of the specimens were prepared using focused ion beam (FIB) and analyzed using transmission electron microscopy (TEM-Thermo Scientific, Themis 300 G3) supplemented by energy dispersive X-ray spectroscopy (EDS). The initial mass of all the specimens was measured using precision weighing scale having 0.01 mg least count. The surface area of each specimen was calculated to be ∼ 3 cm2. The long term oxidation experiments were performed in laboratory air at elevated temperatures viz. 850 °C/950 °C/1050 °C of a tubular furnace. The mass gain of the specimens was taken after every 1 h during the first 10 h followed by every 5 h until the 100 h. X-ray diffraction (XRD-Bruker, D8 Discover) spectra was acquired after oxidation by using Cu-Kα source of radiation (λ = 1.54 Å). The oxide layer morphology was characterized by field emission scanning electron microscopy (FESEM-JEOL JSM-7610F Plus) equipped with backscattered secondary electron detector and energy dispersive X-ray spectroscopy (EDAX AMETEK). FactsageEdu73 software was used to calculate the values of standard free energy of the oxides at a particular temperature.

Schematic diagram for stationary friction processing (SFP).
3 Results and discussion
3.1 Oxidation kinetics
Figure 2 compares the oxidation kinetics of the as cast and processed specimens exposed at elevated temperature i.e. 850 °C/950 °C/1050 °C during cyclic thermal oxidation up to 100 h. During the initial 5–10 h, all the specimens showed transient oxidation stage, characterized by the rapid increase in cumulative mass gain and subsequently the curves enter into a steady state oxidation regime with significantly reduced oxidation kinetics. During the steady state oxidation, ionic diffusion governs the kinetics of oxide layer growth. All the processed specimens showed significantly lower mass gain compared to as cast specimen at all the three elevated temperatures. The cumulative mass gain for as cast, 5min_400, 15min_400, 5min_1800 and 15min_1800 at 850 °C after 100 h is 0.33, 0.27, 0.23, 0.17 and 0.11 mg/cm2, respectively. The cumulative mass gain for the same specimens at 950 °C is 0.6, 0.48, 0.40, 0.37 and 0.29 mg/cm2. Similarly, at 1050 °C, the mass gain values are found to be 0.92, 0.81, 0.71, 0.57 and 0.39 mg/cm2, respectively. Thus, the oxidation kinetics of the specimens processed at 400 rpm is reduced by 11–32% compared to as cast specimen at temperatures 850 °C–1050 °C. Further, there was remarkable reduction (by 37–67%) in the oxidation kinetics of the specimen processed at 1800 rpm compared to as cast specimen at temperatures 850 °C–1050 °C.

(A) Cumulative mass gain curves and (B) parabolic mass gain curves for as cast and all the processed AlCoCrFeNi HEA specimens at three temperatures: 850 °C, 950 °C and 1050 °C.
Cumulative mass gain curves follow linear rate law during the initial transient period, followed by parabolic rate law. Hence, the slope of the curve during transient period was used for determining the values of linear rate constant as shown in Table 1. Wagner’s parabolic rate law describes the reaction kinetics of as cast and processed specimens as: (Δm)2=kpt+C, where, Δm is cumulative mass gain per unit surface area (mg/cm2), kp is parabolic rate constant (mg2/cm4· h), t is the oxidation time and C is the constant of integration. The time dependence of the cumulative mass gain of the oxide layer is plotted in the parabolic mass gain curves as shown in Figure 2B. The slope of the curve yields the value of parabolic rate constant within the steady state oxidation region. The parabolic rate constant for the specimen processed at 400 rpm is marginally lower than as cast specimen while the specimens processed at 1800 rpm showed substantial reduction in parabolic rate constant highlighting excellent oxidation resistance of latter at elevated temperatures.
Oxidation rate constants calculated from the curves shown in Figure 2.
| Alloys | t1 (h) | t2 (h) | kl/kp | T = 850 °C | T = 950 °C | T = 1050 °C |
|---|---|---|---|---|---|---|
| As cast | 1 | 10 | kl [g·cm2·s−1] | 3.00 × 10−9 | 5.01 × 10−9 | 6.61 × 10−9 |
| 10 | 100 | kp [g2·cm4·s−1] | 2.77 × 10−13 | 10.38 × 10−13 | 23.25 × 10−13 | |
| 5min_400 | 1 | 10 | kl [g·cm2·s−1] | 3.18 × 10−9 | 6.32 × 10−9 | 8.42 × 10−9 |
| 10 | 100 | kp [g2·cm4·s−1] | 1.86 × 10−13 | 5.83 × 10−13 | 18.36 × 10−13 | |
| 15min_400 | 1 | 10 | kl [g·cm2·s−1] | 1.37 × 10−9 | 2.30 × 10−9 | 7.74 × 10−9 |
| 10 | 100 | kp [g2·cm4·s−1] | 1.44 × 10−13 | 4.53 × 10−13 | 13.52 × 10−13 | |
| 5min_1800 | 1 | 10 | kl [g·cm2·s−1] | 1.37 × 10−9 | 2.64 × 10−9 | 6.25 × 10−9 |
| 10 | 100 | kp [g2·cm4·s−1] | 0.74 × 10−13 | 3.88 × 10−13 | 8.16 × 10−13 | |
| 15min_1800 | 1 | 10 | kl [g·cm2·s−1] | 0.71 × 10−9 | 2.10 × 10−9 | 3.12 × 10−9 |
| 10 | 100 | kp [g2·cm4·s−1] | 0.30 × 10−13 | 2.46 × 10−13 | 4.28 × 10−13 |
The strain rate during processing was computed using the following equation (Garg et al. 2022a):
High temperature oxidation behavior is primarily related to the chemical reaction of oxygen with the metal elements. The peak temperature is one of the most prominent factors that effects the kinetics of this reaction. The reaction kinetics can be fairly estimated from the activation energy of the oxidation, which can be well approximated using the method purposed by Kofstad (1957). This involves a long term thermal oxidation run in approximate temperature regions and the obtained data is used to construct a plot of natural log of parabolic rate constant with respect to reciprocal of temperature. Supplementary Figure S2A shows the temperature dependence of the parabolic rate constant of the oxide layer for this study. Parabolic rate constants can be utilized to fit a linear regression model according to Arrhenius equation, in a diffusion controlled phenomenon: kp = ko· exp(−Ea/RT), where ko is a pre-exponential coefficient, R is universal gas constant, T is temperature of oxidation process and Ea is the activation energy. The slope of the regression model corresponds to (−Ea/R) in the temperature range of 850 °C–1050 °C which was used to compute the values of activation energy (Ea) for all the tested specimens. It can be seen that the trend of activation energy (Supplementary Figure S2B) is inverse of mass-gain i.e. Ea is lowest for the as cast specimen while the processed specimens showed higher values. Further, Ea was found be highest for 15min_1800 specimen indicating its higher resistance to oxidation. The possible reason for the higher activation energy for the processed specimens is the highly stable nature of the thin oxide layer formed on these specimens.
A comparison of parabolic rate constant of some of the commonly investigated HEAs and medium entropy alloys (MEAs) for high temperature oxidation is shown in Table 2. It can be observed that the alloys without Al show high value of parabolic rate constant indicating steep oxidation kinetics. In contrast, Al containing alloys show significantly slow oxidation kinetics as indicated by their lower kp values. Amongst all, AlCoCrFeNi showed lowest kp indicating its superior oxidation behavior. Further, the kp value got further reduced significantly after processing.
Comparison of parabolic rate constant with a common multiple (kp = 10−13 g2cm−4s−1).
| Alloys | Parabolic rate constants (g2·cm−4·s−1) | Reference | |||
|---|---|---|---|---|---|
| Temperature (°C) | |||||
| 800 | 900 | 1000 | 1050 | ||
| AlCoCrFeNi (15min_1800) | 4.28 | Present work | |||
| AlCoCrFeNi | 9.4 | Dąbrowa et al. (2017) | |||
| FeCoNiCrCu | 13.0 | 64.5 | 3840 | Kai et al. (2005) | |
| FeCoCrNi | 1.95 | 17.7 | 40.8 | Kai et al. (2005) | |
| FeCoNi | 2310 | 1020 | 36,000 | Kai et al. (2005) | |
| CoCrNiMnFe | 56.9 | 212 | 1920 | Adomako et al. (2018) | |
| CoCrNiMn | 245 | 683 | 3810 | Adomako et al. (2018) | |
| CoCrNi | 0.04 | 2.52 | 47.8 | Adomako et al. (2018) | |
In addition, Supplementary Figure S3 illustrates a comparative plot showing the mass gain analysis from various studies. Amongst all, the oxidation resistance of the 15min_1800 specimen is highest as compared to other structural materials such as Inconel 740 (Zhao et al. 2004) and numerous other HEAs (Butler and Weaver 2016; Chen et al. 2018b; Nong et al. 2018), emphasizing the efficacy of SFP. It is noteworthy that the oxidation mass gain obtained for processed AlCoCrFeNi in the current study is better or at least comparable to Al30(CoCrFeNi)70 composition which has significantly higher aluminium content. The major limitation with high aluminium fraction is the extreme brittleness of the alloy system which renders it useless for engineering applications. However, the exceptional oxidation resistance of highly ductile equimolar AlCoCrFeNi composition obtained in this study is of significant importance for high temperature engineering applications.
3.2 Microstructure
The results showed that 15min_1800 specimen showed remarkable oxidation resistance. To understand the possible reason for the same, its detailed microstructural characterization along with the as cast specimen was carried out. In addition, the microstructure of the as cast specimen along with cross section of 5min_400, 15min_400, 5min_1800 and 15min_1800 are shown in Supplementary Figure S4. As cast specimen exhibits a coarse grain microstructure while the grains of the processed specimens are significantly refined. The depth of refinement (DOR) varies from 300 to 600 µm for the specimen processed at 400 rpm for 5 and 15 min, respectively, while it varies from 650 to 800 µm for the specimen processed at 1800 rpm for 5 and 15 min, respectively, as shown in Supplementary Figure S5A. Thus, the DOR increases with rotational speed and processing time, likely due to extension of plastic deformation zone deeper into the specimen. Underneath the refined zone, the grains are elongated which corresponds to the thermo-mechanical affected zone (TMAZ) ranging up to few millimeters. The coarse elongated grains in the TMAZ are attributed to the effect of shearing action of the SFP tool. The interface between the nugget zone and the TMAZ is very sharp and distinct (Supplementary Figure S4). The temperature profiles obtained under both the processing conditions are shown in Supplementary Figure S5B. The average temperature at 400 rpm was around 280 °C while it reached nearly 760 °C at 1800 rpm. Higher temperature at 1800 rpm facilitates the material flow through thermal softening and resulted in larger DOR. Thus, SFP is highly efficient surface deformation technique to tailor the microstructure, the depth of which depends on the processing parameters and the time.
The grain structure for as cast and the processed specimens was obtained from EBSD analysis. Figure 3 shows the EBSD micrographs, misorientation angle chart and grain size distribution of the as cast and 15min_1800 specimen. The as cast specimen exhibits a wide range of grain size varying from 1 to 120 µm with an average grain size of nearly 90 µm. In contrast, the processed specimen showed highly refined grain structure with grain size varying between 0.4 and 7 µm and having a log normal distribution. The average grain size of the processed specimen is nearly 2 µm. In addition, the fraction of high angle grain boundaries (HAGB > 15°) in the as cast specimen was found to be high (∼88%) with a large statistical distribution (σ = 0.0412). The fraction of HAGBs got reduced to nearly 79% in the processed specimen with lower statistical distribution (σ = 0.0226). The refinement in grain size as well as reduced deviation in the fraction of HAGBs is likely attributed to the result of dynamic recrystallization manifested through the dislocation rearrangement into sub-grains and grain boundary migration (Garg et al. 2022b). Zener Holloman parameter (Z) quantifies the combined effect of strain rate and peak temperature during a plastic deformation process such as SFP through the relation:

EBSD map for (A) as cast and (B) 15min_1800 processed AlCoCrFeNi HEA specimen; mis-orientation angle chart for (C) as cast and (D) 15min_1800 processed AlCoCrFeNi HEA specimen; grain size distribution of (E) as cast and (F) 15min_1800 processed AlCoCrFeNi HEA specimen.
XRD spectra of the as cast and the 15min_1800 processed specimen is shown in Supplementary Figure S6. The as cast alloy is comprised of BCC and B2 as the primary phase which has been found in the earlier studies as well (Butler and Weaver 2016). In contrast, 15min_1800 processed specimen exhibits the combination of BCC, B2 and FCC phases, likely formed due to BCC/B2 to FCC phase transformation during processing. The key for strain induced phase transformation is the significant surge in the defect densities during deformation. The increase in grain boundary density, high dislocation and point defects density during deformation raises the system energy that favors the phase transformation. Strain induced phase transformation is a common phenomenon and has been widely reported in prior studies including pure iron (Nguyen et al. 2018), stainless steel (Gotawala et al. 2020), and high entropy alloys (Bahramyan et al. 2020; Ojha and Sehitoglu 2016; Zhang et al. 2020). The BCC-FCC transformation energy profiles associated with the Bain deformation (a combination of homogeneous lattice deformations), were obtained in FeMnAlNi alloy system (Ojha and Sehitoglu 2016). During the Bain deformation a Bravais lattice is converted into another by the coordinated shift of atoms within a unit cell which may change the crystal lattice. Extreme phase transformation from BCC to FCC is also observed during the tensile deformation of AlxCrCoFeCuNi HEA (x = 0.5, 1.5). During deformation, the dislocation density of the alloy increases with the increase in straining up to a threshold point where the FCC phase fraction was found to be maximum (Bahramyan et al. 2020). Thus, it can be realized that multiplication of defect density during the time of deformation is likely responsible for the BCC/B2 to FCC phase transformation. The energy barrier required to transform BCC to FCC phase is provided via severe thermoplastic deformation and a local stress field is formed owing to lattice distortion effect of HEA which restrict the reverse phase transformation (FCC to BCC/B2).
In addition, the elemental distribution in the intra-granular regions was investigated with high-angle annular dark-field transmission electron microscopy (HAADF-TEM) and energy-dispersive X-ray spectroscopy (EDS) mappings (Figures 4–5). The HAADF-TEM image (Figure 4) shows the apparent solute partitioning within the matrix regions in the as cast specimen which indicates high atomic number contrast region, labeled P, interspersed with medium atomic number contrast regions, labeled Q. Two kinds of phases were observed, namely, the Al-Ni-rich phase (region Q) and the Co-Cr-Fe-rich phase (region P). Selected area electron diffraction (SAED) pattern (Figure 4A) was obtained for bright field (BF) TEM image (Figure 4B) which confirmed the presence of B2 and BCC phase as evident from the XRD spectrum as well. Shiratori et al. (2016) reported the similar microstructure in the AlCoCrFeNi HEA and revealed the formation of Al-Ni-rich and Co-Cr-Fe-rich phase correspond to the B2 phase and BCC phases, respectively. On the contrary, SFP specimen clearly shows a significantly different microstructure from that of the as cast specimen. Ni intermixes with the Co-Cr-Fe rich region after SFP, due to the effect of high strain and temperature field, indicated by the high atomic number contrast, whereas Al precipitates (AlN) remain isolated, shown by the low atomic number contrast region. Careful investigation of two different regions (labeled R and labeled S, Figure 5C) of high atomic number contrasts allowed identifying the phases of the corresponding regions using SAED patterns. Labeled region R exhibits BCC phase while region S represents FCC phase microstructure.

(A) Selected area electron diffraction (SAED) pattern of (B) TEM bright-field (BF) image, (C) high angle annular dark field (HAADF) image and corresponding elemental maps for as cast AlCoCrFeNi HEA specimen.

(A) HAADF image and its corresponding elemental maps (B, D) selected area electron diffraction (SAED) pattern for two different contrast regions of (C) TEM-BF image for 15min_1800 AlCoCrFeNi HEA specimen.
3.3 Identification of oxide phases
The XRD analysis was performed on the oxidized surface of the as cast and processed specimens at 850 °C/950 °C/1050 °C as shown in Figure 6. The XRD results reveal the presence of Cr2O3 and Al2O3 as the predominant oxide phase in all the specimens oxidized at three temperatures. The presence of ternary oxides FeCr2O4, CoCr2O4 and CoFe2O4 (spinels) is also observed in the as cast specimens oxidized at 850 °C and 950 °C. In addition, a low-intensity peak of Fe2O3 is also detected in the as cast specimen oxidized at 850 °C, while it is absent at temperature 950 °C and 1050 °C. As cast specimen oxidized at 1050 °C exhibits spinel phase oxides FeCr2O4, CoCr2O4 and FeCo2O4. In contrast, the oxidation products of processed specimen primarily consist of Al2O3 and Cr2O3 in a temperature range of 850–1050 °C. In addition, a weak peak of CoFe2O4/CoCr2O4 is found in the processed specimen oxidized at 950 °C whereas a peak of FeCo2O4 is observed in the processed specimen oxidized at 1050 °C.

XRD analysis of as cast and 15min_1800 processed AlCoCrFeNi HEA specimen after oxidation at temperature (A) 850 °C, (B) 950 °C and (C) 1050 °C.
3.4 Temporal evolution of surface morphology
The changes in surface morphologies at different temperatures were investigated as a function of oxidation time. The SEM images of the as cast and 15min_1800 specimen oxidized at 850 °C for 1 h, 5 h, 10 h, 20 h, 40 h and 100 h are shown in Figures 7 and 8, respectively. It can be seen that during the initial stage of oxidation, the surface of as cast specimen got preferentially enriched with Cr rich oxides, particularly along the grain boundaries. In contrast, the surface of processed specimen was nearly homogeneously covered with both Al2O3 and Cr2O3. It is quite evident that the high surface free energy and fine grain stricture of the processed specimen favoured the formation of uniform oxide layer on the surface which is not the case for the as cast specimen. Following 5 h of oxidation, the as cast specimen shows a formation of defective oxide layer with large number of fine pores. It is likely due to the pinning of oxide layer at the grain boundaries and since the grain size is large, it fails to grow uniformly across the specimen and resulted in pores formation. As the oxidation proceeds, the pores were subsequently filled with metal oxides as seen for 10 h oxidized specimen. Further, Al2O3 can be seen in the oxide layer of as cast specimen after 10th hour. The oxide layer on the as cast specimen become highly heterogeneous with the further increase in the oxidation time. The surface showed isolated patches of Al2O3 and Cr2O3 after 20 h of oxidation which continues to grow later on. After 100 h, the surface was filled with coarse precipitates of Cr2O3 while Al2O3 layer forms in the backdrop. On the contrary, processed specimen shows an intact and highly uniform oxide layer morphology (after 5 h) which continues to grow for subsequent time durations as well (Figure 8).

Surface morphology of the oxide layer in the as cast AlCoCrFeNi HEA specimen after 1 h, 5 h, 10 h, 20 h, 40 h, 80 and 100 h of oxidation at temperature 850 °C.

Surface morphology of the oxide layer in the 15min_1800 processed AlCoCrFeNi HEA specimen after 1 h, 5 h, 10 h, 20 h, 40 h and 100 h of oxidation at temperature 850 °C.
Typically, grain boundaries are prone to oxygen attack at elevated temperatures owing to the defects and high energy sites. The grain boundaries act as diffusion channels for the outward flux of Fe, Ni and Cr. The standard free energy of the Cr2O3 is more negative as compare Ni and Fe rich oxides. Thus, Cr2O3 primarily forms along the grain boundaries and progresses with time. In addition, porous oxide layer detected after 5th hour of oxidation is also responsible for the poor oxidation behaviour of the as cast specimen. This porous oxide scale promotes the migration of cations (M+) and anions (O2−) towards each other and favors the reaction to proceed further. After 10 h of oxidation, Co, Fe, Ni and Al rich oxides are observed. These oxides form patches on the surface of as cast specimen at several locations. In contrast, high surface free energy of the processed HEA favors the formation of a uniformly distributed oxide scale during the initial 5 h only as shown in Figure 8. The initially formed scale acts as a rate controlling step which limits the attack of oxygen ions for the progress of reaction and the substrate material is protected via passivation. Thus, processed specimen attains a steady state during the initial 1–5 h of oxidation which continues to be same for many subsequent hours. Fine grain structure is known to promote stability of the passive oxide layer by the micro-pegging effect which results in a better oxide/substrate adhesion (Gao et al. 2012).
3.5 Cross section morphology
The cross section of the oxidized specimens is examined through FESEM and EDS analysis after 100 h of exposure at 850 °C, 950 °C and 1050 °C as shown in Figures 9, 10 and 11, respectively. In line with the temporal studies discussed above, the elemental distribution suggests the formation of non-homogeneous mixed oxides layer in the as cast specimen oxidized at temperature 850 °C–1050 °C owing to the low value of diffusion coefficient of elements through grain boundaries. The diffusion coefficient of elements increases with the decrease in grain size. The finer grain size results in high number of grain boundaries which provides the appropriate diffusion paths for the M+ cations and O2− anions and hence increases the diffusion coefficient with the decrease in grain size. A detailed discussion on the diffusivity is given in the forthcoming section. As cast specimen oxidized at 850 °C exhibits a mixture of Cr, Fe, and Co rich oxides at the top surface with Al2O3 at the bottom region (Figure 9A). In contrast, the 15min_1800 specimen shows a thin and highly intact oxide layer of Cr2O3 (Figure 9B). In addition, Al rich oxides are also observed as the inner oxides which are evident as Al2O3 from XRD results. Chromia forms on the surface of the alloy at elevated temperature through outward diffusion of Cr cations through grain boundary diffusion while alumina grows primarily by inward diffusion of O2− anions along oxide grain boundaries (Birks et al. 2006). Similar to 850 °C, an alumina rich layer at the bottom and high fraction of Cr, Fe and Co rich oxides on the top portion of the scale are observed in the as cast specimens oxidized at 950 °C and 1050 °C (Figures 10A and 11A). The XRD result confirms the presence of Cr, Co and Fe rich oxide phase as the Cr2O3 along with spinels FeCr2O4, CoCr2O4 and CoFe2O4. Spinel FeCo2O4 is also detected in the as cast specimen oxidized at temperature 1050 °C. These brittle spinels are detrimental as it reduces the protectiveness of the oxide scale and undermines the interfacial adhesion (Chen et al. 2018a; Lu et al. 2021). Further, some pores are apparent in the BSE image of as cast specimen at 950 °C. At 1050 °C, a broken oxide layer at numerous places with several cracks propagated within the layer are observed in the as cast specimen (Figure 11A). These pores (Figure 10A) and cracks (Figure 11A) in oxide layer favors the O2− anions to penetrate through the defects resulting in severe oxide growth. On the contrary, a densely packed and well adherent oxide scale is observed for the processed specimens oxidized at 950 °C and 1050 °C. No spinel was detected at the scale surface of the processed specimen, except for the formation of top Cr2O3 oxide layer. It is likely that the fine grain structure of the processed specimen suppressed the spinel formation. Further, EDS line-scan profiles were also extracted from the corresponding BSE images of all the specimens oxidized at three different temperatures as shown in Figure 12. It can be seen that results of EDS line-scan are in line with the elemental mapping which clearly shows change in signals intensity at the oxide/substrate interface. Processed specimens evidently shows Cr rich upper region with Al rich black inner oxides. According to selective oxidation theory, fine grain microstructure offers a high number of grain boundaries, which can considerably increase the diffusion channels for the outward flux of Cr cations and thus accelerates the Cr flux reaching specimen surface to form Cr2O3. A significant internal oxidation can be seen in the BSE images of processed specimen oxidized at 950 °C and 1050 °C. Further, EDS results reveal the Al rich oxides in the internal oxidation zone (IOZ). Thus, the accelerated flux of Cr and Al ions in the processed specimen favors the formation of protective chromia and alumina scales rather than brittle and un-protective spinel formation as is the case for the as cast alloy. Further, the development of layered oxide structure observed in the processed specimen can be explained based on the thermodynamic thrust of metal oxides which in-turn is governed by the standard (Gibb’s) free energy of formation at a particular temperature. Owing to the highest standard free energy of formation i.e. −903.88 kJ/mol of O2 at 850 °C, Al2O3 primarily grows as the bottommost oxide layer while Cr2O3 forms at the top surface of the processed specimens due to its least value of standard free energy i.e. −574.26 kJ/mol of O2 at 850 °C. It can also be noted that the processed specimen showed significant internal oxidation while it was not observed for the as cast specimen at all the investigated temperatures. The internal oxidation is also believed to hinder the ionic transport, control the oxide layer thickness and thus enhance the oxidation resistance of the specimen (Wang et al. 2020) (Figures 9B, 10B and 11B). The internal oxidation in the processed specimen is likely attributed to the high defect density wherein dislocations and vacancies present in the severely surface deformed specimens provide short-circuit diffusion channels (Peng et al. 2005). These channels help for the inward flux of O2− anions to react with the metallic ions and form the internal oxides.

BSE images with EDS analysis of (A) as cast and (B) 15min_1800 processed AlCoCrFeNi HEA specimen after oxidation at 850 °C.

BSE images with EDS analysis of (A) as cast and (B) 15min_1800 processed AlCoCrFeNi HEA specimen after oxidation at 950 °C.
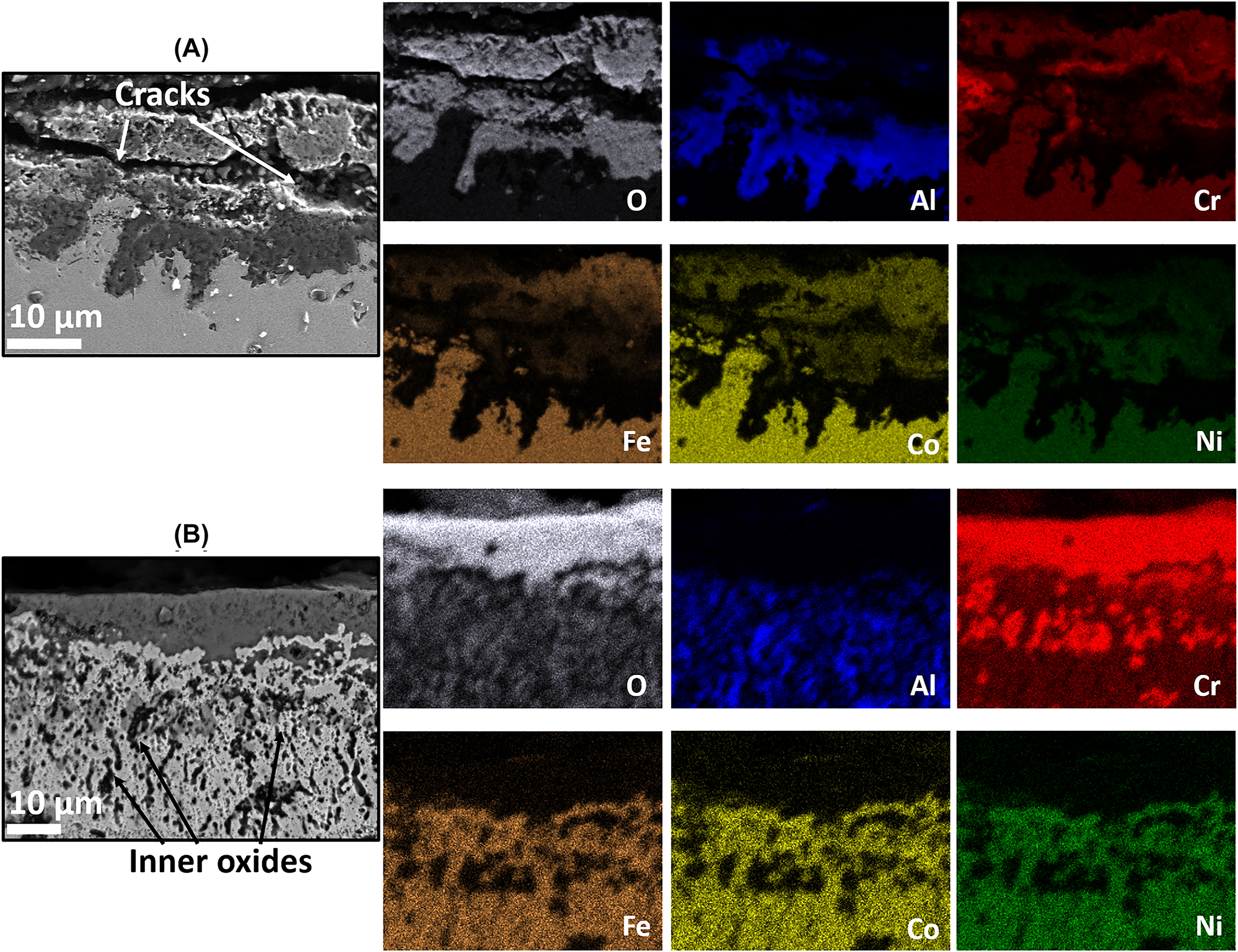
BSE images with EDS analysis of (A) as cast and (B) 15min_1800 processed AlCoCrFeNi HEA specimen after oxidation at 1050 °C.

EDS line-scan for as cast AlCoCrFeNi HEA specimen oxidized at (A) 850 °C, (C) 950 °C, (E) 1050 °C and processed AlCoCrFeNi HEA specimen oxidized at (B) 850 °C (D) 950 °C, (F) 1050 °C.
As mentioned before, the refined grain size of processed specimen is significantly smaller than as cast specimen. The increase grain-boundary density significantly influences the diffusion flux, which subsequently affects the oxidation kinetics. Typically, the diffusion coefficient is expressed in terms of grain-boundary diffusion and lattice diffusion as (Wang et al. 2020):
where, f denotes the grain boundary area fraction, Dgb, Dl and D describes the grain boundary diffusion, lattice diffusion and effective diffusion coefficient. The value of Dgb is typically 5 to 6 orders of magnitude greater than Dl, i.e. Dgb≫Dl. If δ is the breadth of grain boundary, d is the grain size, the grain boundary area fraction can be written as:
If the effective diffusion coefficient D∗ for coarse grains (d = 90 µm) is used as a reference, the ratio
A mechanism of oxide scale formation for the as cast and processed specimen is shown in Figure 13. Processed specimen exhibits the formation of a dense chromia layer and the Al rich inner oxides while as cast specimen consists of mixture of Cr, Fe and Co rich spinel oxides with an inner continuous alumina layer. In addition, as cast specimen has a number of pores and cracks. The presence of pores, cracks and detrimental spinel phases promotes the oxidation reaction to proceed further while the dense chromia layer in the processed specimen prevents the reaction to proceed, providing it high oxidation resistance.

Schematic illustration of the oxide scale formation through cross section of (A) as cast and (B) 15min_1800 processed AlCoCrFeNi HEA specimen.
4 Summary and conclusions
The as cast specimen was found to have a coarse grain microstructure with B2/BCC as the predominant phase. SFP refines the grain structure from 90 µm for the as cast alloy to the as low as 2 µm for the 15min_1800 processed specimen along with significant reduction in the fraction of HAGBs. Also, SFP resulted in BCC to FCC phase transformation. Specimens processed at 400 rpm for the 5 and 15 min duration demonstrates 11–18.6% and 22.5–31.7% reduction in the oxidation kinetics, respectively. Similarly, the specimens processed at 1800 rpm for the 5 and 15 min duration shows a 37.2–48% and 50.7–66.5% reduction in the oxidation kinetics, respectively. Temporal evolution of the surface morphology demonstrated an early transition of the processed specimen to the steady state oxidation regime compared to the as cast specimen. The primary oxide phases detected in the as cast and processed specimens were Al2O3 and Cr2O3 protective scales while Cr, Fe and Co rich detrimental spinel oxides were also observed in the as cast specimen after cyclic thermal oxidation at elevated temperatures. High activation energies of the processed specimens are attributed to the small thickness of oxide scale. Small parabolic rate constants and large activation energies clearly indicate the high oxidation resistance of the processed specimens as compared to as cast specimen. The presence of more number of grain boundaries in processed specimen favored the outward diffusion of Cr during the initial hours of oxidation, forming an intact/dense uniformly distributed oxide scale which acted as a rate controlling step and the substrate is protected via passivation.
-
Author contributions: MG: investigation, formal analysis, data curation, original draft; writing; HSG: supervision, validation, writing–review & editing; RS: formal analysis, writing–review and editing; HSA: conceptualization, methodology, supervision, writing–review and editing.
-
Research funding: None declared.
-
Conflicts of interest: The authors declare no conflicts of interest regarding this article.
References
Adomako, N.K., Kim, J.H., and Hyun, Y.T. (2018). High-temperature oxidation behaviour of low-entropy alloy to medium-and high-entropy alloys. J. Therm. Anal. Calorim. 133: 13–26, https://doi.org/10.1007/s10973-018-6963-y.Search in Google Scholar
Bahramyan, M., Mousavian, R.T., and Brabazon, D. (2020). Study of the plastic deformation mechanism of TRIP–TWIP high entropy alloys at the atomic level. Int. J. Plast. 127: 102649, https://doi.org/10.1016/j.ijplas.2019.102649.Search in Google Scholar
Birks, N., Meier, G.H., and Pettit, F.S. (2006). Introduction to the high temperature oxidation of metals, 2nd ed. Cambridge University Press, Cambridge.10.1017/CBO9781139163903Search in Google Scholar
Butler, T., Alfano, J., Martens, R., and Weaver, M. (2015). High-temperature oxidation behavior of Al-Co-Cr-Ni-(Fe or Si) multicomponent high-entropy alloys. J. Occup. Med. 67: 246–259, https://doi.org/10.1007/s11837-014-1185-7.Search in Google Scholar
Butler, T.M. and Weaver, M.L. (2016). Oxidation behavior of arc melted AlCoCrFeNi multi-component high-entropy alloys. J. Alloys Compd. 674: 229–244, https://doi.org/10.1016/j.jallcom.2016.02.257.Search in Google Scholar
Chang, C., Lee, C., and Huang, J. (2004). Relationship between grain size and Zener–Holloman parameter during friction stir processing in AZ31 Mg alloys. Scripta Mater. 51: 509–514, https://doi.org/10.1016/j.scriptamat.2004.05.043.Search in Google Scholar
Chen, J., Niu, P., Liu, Y., Lu, Y., Wang, X., Peng, Y., and Liu, J. (2016). Effect of Zr content on microstructure and mechanical properties of AlCoCrFeNi high entropy alloy. Mater. Des. 94: 39–44, https://doi.org/10.1016/j.matdes.2016.01.033.Search in Google Scholar
Chen, Y., Zhao, X., and Xiao, P. (2018a). Effect of microstructure on early oxidation of MCrAlY coatings. Acta Mater. 159: 150–162, https://doi.org/10.1016/j.actamat.2018.08.018.Search in Google Scholar
Chen, L., Zhou, Z., Tan, Z., He, D., Bobzin, K., Zhao, L., Öte, M., and Königstein, T. (2018b). High temperature oxidation behavior of Al0. 6CrFeCoNi and Al0. 6CrFeCoNiSi0. 3 high entropy alloys. J. Alloys Compd. 764: 845–852, https://doi.org/10.1016/j.jallcom.2018.06.036.Search in Google Scholar
Chen, Y., Zhao, X., Dang, Y., Xiao, P., Curry, N., Markocsan, N., and Nylen, P. (2015). Characterization and understanding of residual stresses in a NiCoCrAlY bond coat for thermal barrier coating application. Acta Mater. 94: 1–14, https://doi.org/10.1016/j.actamat.2015.04.053.Search in Google Scholar
Chen, Y., Zhao, X., Bai, M., Yang, L., Li, C., Wang, L., Carr, J., and Xiao, P. (2017). A mechanistic understanding on rumpling of a NiCoCrAlY bond coat for thermal barrier coating applications. Acta Mater. 128: 31–42, https://doi.org/10.1016/j.actamat.2017.02.003.Search in Google Scholar
Dąbrowa, J., Cieślak, G., Stygar, M., Mroczka, K., Berent, K., Kulik, T., and Danielewski, M. (2017). Influence of Cu content on high temperature oxidation behavior of AlCoCrCuxFeNi high entropy alloys (x= 0; 0.5; 1). Intermetallics 84: 52–61.10.1016/j.intermet.2016.12.015Search in Google Scholar
Evans, A., He, M., and Hutchinson, J. (2001a). Mechanics-based scaling laws for the durability of thermal barrier coatings. Prog. Mater. Sci. 46: 249–271, https://doi.org/10.1016/s0079-6425(00)00007-4.Search in Google Scholar
Evans, A., Mumm, D., Hutchinson, J., Meier, G., and Pettit, F. (2001b). Mechanisms controlling the durability of thermal barrier coatings. Prog. Mater. Sci. 46: 505–553, https://doi.org/10.1016/s0079-6425(00)00020-7.Search in Google Scholar
Evans, H. (2011). Oxidation failure of TBC systems: an assessment of mechanisms. Surf. Coat. Technol. 206: 1512–1521, https://doi.org/10.1016/j.surfcoat.2011.05.053.Search in Google Scholar
Gao, W., Li, Z., and He, Y. (2012). High temperature oxidation protection using nanocrystalline coatings. In: Saji, V. S. and Cook, R. (Eds.), Corrosion protection and control using nanomaterials. Woodhead Publishing, Sawston, pp. 146–166.10.1533/9780857095800.2.146Search in Google Scholar
Garg, M., Grewal, H., and Arora, H. (2021). Effect of microwave processing on the oxidation behavior of refractory high entropy alloy. Mater. Chem. Phys. 262: 124256, https://doi.org/10.1016/j.matchemphys.2021.124256.Search in Google Scholar
Garg, M., Grewal, H.S., Sharma, R.K., and Arora, H.S. (2022a). Enhanced oxidation resistance of ultrafine-grain microstructure AlCoCrFeNi high entropy alloy. ACS Omega 7: 12589–12600, https://doi.org/10.1021/acsomega.1c06014.Search in Google Scholar PubMed PubMed Central
Garg, M., Grewal, H.S., Sharma, R.K., Gwalani, B., and Arora, H.S. (2022b). High oxidation resistance of AlCoCrFeNi high entropy alloy through severe shear deformation processing. J. Alloys Compd. 914: 165385, https://doi.org/10.1016/j.jallcom.2022.165385.Search in Google Scholar
Gotawala, N., Wadighare, A., and Shrivastava, A. (2020). Phase transformation during friction stir processing of dual-phase 600 steel. J. Mater. Sci. 55: 4464–4477, https://doi.org/10.1007/s10853-019-04270-5.Search in Google Scholar
Hemphill, M.A., Yuan, T., Wang, G., Yeh, J., Tsai, C., Chuang, A., and Liaw, P. (2012). Fatigue behavior of Al0. 5CoCrCuFeNi high entropy alloys. Acta Mater. 60: 5723–5734, https://doi.org/10.1016/j.actamat.2012.06.046.Search in Google Scholar
Kai, W., Jang, W., Huang, R., Lee, C., Hsieh, H., and Du, C. (2005). Air oxidation of FeCoNi-base equi-molar alloys at 800–1000° C. Oxid. Metals 63: 169–192, https://doi.org/10.1007/s11085-004-3198-z.Search in Google Scholar
Karati, A., Guruvidyathri, K., Hariharan, V., and Murty, B. (2019). Thermal stability of AlCoFeMnNi high-entropy alloy. Scripta Mater. 162: 465–467, https://doi.org/10.1016/j.scriptamat.2018.12.017.Search in Google Scholar
Kofstad, P. (1957). Oxidation of metals: determination of activation energies. Nature 179: 1362–1363, https://doi.org/10.1038/1791362a0.Search in Google Scholar
Kumar, A., Thomas, A., Gupta, A., Garg, M., Singh, J., Perumal, G., Sujithkrishnan, E., Elumalai, P., and Arora, H.S. (2021). Facile synthesis of MnO2-Cu composite electrode for high performance supercapacitor. J. Energy Storage 42: 103100, https://doi.org/10.1016/j.est.2021.103100.Search in Google Scholar
Liu, J., Suslov, S., Li, S., Qin, H., Ren, Z., Ma, C., Wang, G.-X., Doll, G.L., Cong, H., and Dong, Y. (2017). Effects of ultrasonic nanocrystal surface modification on the thermal oxidation behavior of Ti6Al4V. Surf. Coat. Technol. 325: 289–298, https://doi.org/10.1016/j.surfcoat.2017.04.051.Search in Google Scholar
Lu, J., Chen, Y., Zhao, C., Zhang, H., Luo, L., Xu, B., Zhao, X., Guo, F., and Xiao, P. (2019). Significantly improving the oxidation and spallation resistance of a MCrAlY alloy by controlling the distribution of yttrium. Corrosion Sci. 153: 178–190, https://doi.org/10.1016/j.corsci.2019.03.051.Search in Google Scholar
Lu, J., Chen, Y., Zhang, H., Li, L., Fu, L., Zhao, X., Guo, F., and Xiao, P. (2020a). Effect of Al content on the oxidation behavior of Y/Hf-doped AlCoCrFeNi high-entropy alloy. Corrosion Sci. 170: 108691, https://doi.org/10.1016/j.corsci.2020.108691.Search in Google Scholar
Lu, J., Chen, Y., Zhang, H., Ni, N., Li, L., He, L., Mu, R., Zhao, X., and Guo, F. (2020b). Y/Hf-doped AlCoCrFeNi high-entropy alloy with ultra oxidation and spallation resistance. Corrosion Sci. 166: 108426, https://doi.org/10.1016/j.corsci.2019.108426.Search in Google Scholar
Lu, J., Li, L., Chen, Y., Liu, X., Zhao, X., Guo, F., and Xiao, P. (2021). Y-Hf co-doped AlCoCrFeNi high-entropy alloy coating with superior oxidation and spallation resistance at 1100° C. Corrosion Sci. 182: 109267, https://doi.org/10.1016/j.corsci.2021.109267.Search in Google Scholar
Meetham, G.W. and Van de Voorde, M.H. (2000). Materials for high temperature engineering applications. Springer Science & Business Media, Berlin.10.1007/978-3-642-56938-8Search in Google Scholar
Muktinutalapati, N.R. (2011). Materials for gas turbines–an overview. J. Eng. Gas Turbines Power 23.Search in Google Scholar
Munitz, A., Salhov, S., Hayun, S., and Frage, N. (2016). Heat treatment impacts the micro-structure and mechanical properties of AlCoCrFeNi high entropy alloy. J. Alloys Compd. 683: 221–230, https://doi.org/10.1016/j.jallcom.2016.05.034.Search in Google Scholar
Nguyen, T.Q., Sato, K., and Shibutani, Y. (2018). First-principles study of BCC/FCC phase transition promoted by interstitial carbon in iron. Mater. Trans. 59: 870–875, https://doi.org/10.2320/matertrans.m2018014.Search in Google Scholar
Ni, L.-y., Wu, Z.-l., and Zhou, C.-g. (2011). Effects of surface modification on isothermal oxidation behavior of HVOF-sprayed NiCrAlY coatings. Prog. Nat. Sci.: Mater. Int. 21: 173–179, https://doi.org/10.1016/s1002-0071(12)60052-5.Search in Google Scholar
Nong, Z.-S., Lei, Y.-N., and Zhu, J.-C. (2018). Wear and oxidation resistances of AlCrFeNiTi-based high entropy alloys. Intermetallics 101: 144–151, https://doi.org/10.1016/j.intermet.2018.07.017.Search in Google Scholar
Ojha, A. and Sehitoglu, H. (2016). Transformation stress modeling in new FeMnAlNi shape memory alloy. Int. J. Plast. 86: 93–111, https://doi.org/10.1016/j.ijplas.2016.08.003.Search in Google Scholar
Padture, N.P., Gell, M., and Jordan, E.H. (2002). Thermal barrier coatings for gas-turbine engine applications. Science 296: 280–284, https://doi.org/10.1126/science.1068609.Search in Google Scholar PubMed
Peng, X., Yan, J., Zhou, Y., and Wang, F. (2005). Effect of grain refinement on the resistance of 304 stainless steel to breakaway oxidation in wet air. Acta Mater. 53: 5079–5088, https://doi.org/10.1016/j.actamat.2005.07.019.Search in Google Scholar
Ranjbar-Far, M., Absi, J., Mariaux, G., and Shahidi, S. (2010). Effect of residual stresses and prediction of possible failure mechanisms on thermal barrier coating system by finite element method. J. Therm. Spray Technol. 19: 1054–1061, https://doi.org/10.1007/s11666-010-9512-1.Search in Google Scholar
Shillington, E. and Clarke, D. (1999). Spalling failure of a thermal barrier coating associated with aluminum depletion in the bond-coat. Acta Mater. 47: 1297–1305, https://doi.org/10.1016/s1359-6454(98)00407-8.Search in Google Scholar
Shiratori, H., Fujieda, T., Yamanaka, K., Koizumi, Y., Kuwabara, K., Kato, T., and Chiba, A. (2016). Relationship between the microstructure and mechanical properties of an equiatomic AlCoCrFeNi high-entropy alloy fabricated by selective electron beam melting. Mater. Sci. Eng., A 656: 39–46, https://doi.org/10.1016/j.msea.2016.01.019.Search in Google Scholar
Shivam, V., Basu, J., Pandey, V.K., Shadangi, Y., and Mukhopadhyay, N. (2018). Alloying behaviour, thermal stability and phase evolution in quinary AlCoCrFeNi high entropy alloy. Adv. Powder Technol. 29: 2221–2230, https://doi.org/10.1016/j.apt.2018.06.006.Search in Google Scholar
Tzimas, E., Müllejans, H., Peteves, S., Bressers, J., and Stamm, W. (2000). Failure of thermal barrier coating systems under cyclic thermomechanical loading. Acta Mater. 48: 4699–4707, https://doi.org/10.1016/s1359-6454(00)00260-3.Search in Google Scholar
Wang, F., Zhang, Y., Chen, G., and Davies, H. (2009). Cooling rate and size effect on the microstructure and mechanical properties of AlCoCrFeNi high entropy alloy. J. Eng. Mater. Technol. 131, https://doi.org/10.1115/1.3120387.Search in Google Scholar
Wang, Y., Yang, Y., Yang, H., Zhang, M., Ma, S., and Qiao, J. (2018). Microstructure and wear properties of nitrided AlCoCrFeNi high-entropy alloy. Mater. Chem. Phys. 210: 233–239, https://doi.org/10.1016/j.matchemphys.2017.05.029.Search in Google Scholar
Wang, Y., Zhang, M., Jin, J., Gong, P., and Wang, X. (2020). Oxidation behavior of CoCrFeMnNi high entropy alloy after plastic deformation. Corrosion Sci. 163: 108285.10.1016/j.corsci.2019.108285Search in Google Scholar
Wang, Z., Qiu, W., Yang, Y., and Liu, C. (2015). Atomic-size and lattice-distortion effects in newly developed high-entropy alloys with multiple principal elements. Intermetallics 64: 63–69, https://doi.org/10.1016/j.intermet.2015.04.014.Search in Google Scholar
Williams, J.C. and Starke, E.A.Jr (2003). Progress in structural materials for aerospace systems. Acta Mater. 51: 5775–5799, https://doi.org/10.1016/j.actamat.2003.08.023.Search in Google Scholar
Wu, Z., Bei, H., Pharr, G.M., and George, E.P. (2014). Temperature dependence of the mechanical properties of equiatomic solid solution alloys with face-centered cubic crystal structures. Acta Mater. 81: 428–441.10.1016/j.actamat.2014.08.026Search in Google Scholar
Zhang, T., Zhao, R., Wu, F., Lin, S., Jiang, S., Huang, Y., Chen, S., and Eckert, J. (2020). Transformation-enhanced strength and ductility in a FeCoCrNiMn dual phase high-entropy alloy. Mater. Sci. Eng., A 780: 139182, https://doi.org/10.1016/j.msea.2020.139182.Search in Google Scholar
Zhao, S., Xie, X., and Smith, G.D. (2004). The oxidation behavior of the new nickel-based superalloy Inconel 740 with and without Na2SO4 deposit. Surf. Coat. Technol. 185: 178–183, https://doi.org/10.1016/j.surfcoat.2003.12.003.Search in Google Scholar
Zhao, Y., Chen, H., Lu, Z., and Nieh, T. (2018). Thermal stability and coarsening of coherent particles in a precipitation-hardened (NiCoFeCr) 94Ti2Al4 high-entropy alloy. Acta Mater. 147: 184–194, https://doi.org/10.1016/j.actamat.2018.01.049.Search in Google Scholar
Zhou, Y. and Hashida, T. (2002). Thermal fatigue failure induced by delamination in thermal barrier coating. Int. J. Fatig. 24: 407–417, https://doi.org/10.1016/s0142-1123(01)00096-2.Search in Google Scholar
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/corrrev-2022-0011).
© 2022 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Publisher’s Note
- Editorial changes at Corrosion Reviews
- Editor’s Note
- An editorial transition
- Reviews
- Research progress on the corrosion behavior of titanium alloys
- The new trends in corrosion control using superhydrophobic surfaces: a review
- Original Articles
- Improving the high temperature oxidation resistance of high entropy alloy by surface modification
- Notch sensitivity and short cracks tolerance in a super 13Cr stainless steel under sulfide stress corrosion cracking conditions
- Influence of Fe3+ on titanium corrosion in H2SO4 solutions without and with F−
- Five decades spatial hazard maps of atmospheric corrosion predict the rate of deterioration of steel beams in different environments of India
- A sampling of environmental data, and its presentation, from a multi-role U.S. coast guard aircraft
- Annual Reviewer Acknowledgement
- Reviewer acknowledgement Corrosion Reviews volume 40 (2022)
Articles in the same Issue
- Frontmatter
- Publisher’s Note
- Editorial changes at Corrosion Reviews
- Editor’s Note
- An editorial transition
- Reviews
- Research progress on the corrosion behavior of titanium alloys
- The new trends in corrosion control using superhydrophobic surfaces: a review
- Original Articles
- Improving the high temperature oxidation resistance of high entropy alloy by surface modification
- Notch sensitivity and short cracks tolerance in a super 13Cr stainless steel under sulfide stress corrosion cracking conditions
- Influence of Fe3+ on titanium corrosion in H2SO4 solutions without and with F−
- Five decades spatial hazard maps of atmospheric corrosion predict the rate of deterioration of steel beams in different environments of India
- A sampling of environmental data, and its presentation, from a multi-role U.S. coast guard aircraft
- Annual Reviewer Acknowledgement
- Reviewer acknowledgement Corrosion Reviews volume 40 (2022)

