Abstract
Gel capsules of calcium alginate as a matrix stuffed with a non-ionic surfactant, namely 2-(2-(3,4-bis(2-methoxyethoxy)tetrahydrofuran-2-yl)-2-(2-methoxyethoxy)ethoxy)ethyl stearate, Tween-60, as an inhibitor was prepared by a piercing-solidifying method for corrosion protection of carbon steel tubes from produced water in acidizing oil wells. The Fourier transform infrared and thermal gravimetric analysis techniques were used to study the properties of the capsules. The release of the inhibitor from the solid capsules to the corrosive acidizing produced water was studied gradually using ultraviolet-visible spectroscopy. A heavy additive was used to promote the sinking of the capsules in the oil well downhole tubes. The inhibitive effect of the released inhibitor on carbon steel in the corrosive produced water in acidizing oil wells was investigated using weight loss measurement, potentiodynamic polarization, electrochemical impedance spectroscopy, and morphologically by scanning electron microscopy. It was found that the inhibition efficiency increases with the increase of inhibitor release. The increase in temperature leads to partial desorption of inhibitor molecules at the metal surface, which causes increase in the corrosion rate. The positive sign of the activation enthalpy (ΔHa) reflects the endothermic nature of the carbon steel dissolution process.
1 Introduction
Corrosion is worth investigating in oil-field applications because corrosion problems represent a large portion of the total costs for oil- and gas-producing companies every year worldwide (Konno et al., 2016; Saha et al., 2016; Fernandes et al., 2019; Verma et al., 2019). Corrosion in oil fields occurs at all stages from the downhole to the surface equipment and processing facilities. It appears as leaks in tanks, casings, tubing, pipelines, and other equipment (Panossian et al., 2012; Murmu et al., 2019). Many reservoirs of hydrocarbons (e.g. crude oil) are latent in limestone formations or carbonate-bearing sandstone rocks (Huizinga & Liek, 1994). In order to improve the productivity of this rock reservoir, the acidizing procedure is used (Quraishi & Jamal, 2000). During the acidizing process, the acids are forced under high pressure through the borehole into the pore spaces of the rock formation, where they react chemically with rocks (usually calcite, limestone, and dolomite) to dissolve them, which enlarges the existing flow channels and opens new ones to the wellbore. Acidizing is used in conjunction with hydraulic fracturing techniques and matrix acidizing techniques. In fracture acidizing treatments, one or more fractures are produced in the formation and acidic solution is introduced into the fracture to etch flow channels in the fracture face. The acid also enlarges the pore spaces in the fracture face and in the formation. The fractures are then filled with sand or other material in order to prevent them from closing and to allow the penetration of natural resources or water. Acids are often also used in pickling the well tubing (remove scale) and for the removal of drilling mud damage in newly drilled wells before being brought into production (Yadav et al., 2016). Scale removal treatments are usually done with 15% HCl at temperatures up to 60°C in order to remove iron oxides and carbonated minerals (Baddini et al., 2007). Acidizing steps are frequently repeated, which involve the injection of acids into the well steel tubes. During this acidizing process, metallic materials come into contact with high concentrations of acid solution. Sardar and Ali (2002) investigated acidizing inhibitors, namely dicinnamylidene acetone, disalicylidene acetone, and divanillidene acetone, for N-80 alloy corrosion in boiling, highly concentrated (15%) HCl. It was found that the disalicylidene acetone exhibited the best performance, giving an inhibition efficiency of 98.7%, and all three organic compounds were mixed-type inhibitors. They also reported that the three inhibitors exhibited synergistic effects with the addition of potassium iodide and adsorbed on N-80 alloy according to Temkin’s adsorption isotherm. Therefore, the acidizing process requires a high degree of corrosion protection for tubular materials and other equipment employed. To control the corrosion damage of well tubular materials and other metallic surfaces, acids need to be inhibited by the use of an effective corrosion inhibitor (Wang et al., 2019). Typically, effective corrosion inhibitors are organic compounds containing either N, S, or O atoms in their structures, or electronegative functional groups and π electrons in triple or conjugated double bonds. The inhibiting action of these organic compounds is through an adsorptive interaction between the highly electronic centers in the organic inhibitor structure and the metal substrate (Zhu et al., 2017). Non-ionic surfactants with their amphiphilic structure (where the polar head group is without charge) have been used as effective corrosion inhibitors in oil and gas fields due to their intrinsic ability to easily adsorb on surfaces and interfaces (Han et al., 2018). Migahed et al. (2011) studied some new synthesized non-ionic surfactants as corrosion inhibitors in pipelines in oil fields; they reported that the non-ionic surfactants exhibited a highly efficient corrosion inhibition even at low concentrations. In order to bring the corrosion inhibitors into the deep oil wells, many strategies can be used, such as squeeze job treatment and use of capillary injector tubes (Cabral et al., 2008). Due to the high pressure and high saltiness of the downhole oil wells, it is difficult for the corrosion inhibitor to reach the bottom of the downhole. Thus, corrosion inhibitors should be heavy enough to reach the bottom of the oil wells (You et al., 2011). Heavy solid containers encapsulated with corrosion inhibitors may be applied to solve these problems in oil wells. In this technique, the corrosion inhibitor (core) is surrounded by a container of polymeric material (shell) (Choi et al., 2012). Many degradable materials can be used as a container shell, such as alginate, chitosan, gelatin, and polylactic acid (Wikström et al., 2012). The most commonly used methods in encapsulation are physical and chemical methods. The physical methods include pan and air-suspension coatings, centrifugal extrusion, spray drying, piercing with solidifying, etc. (Guan et al., 2010; Zhu et al., 2012). Piercing-solidifying as a physical method is an efficient encapsulation method due to its simplicity and low cost. Herein, gel capsules of calcium alginate loaded with Tween-60 (T-60) (Figure 1) as an inhibitor were synthesized by a piercing-solidifying method. The properties of the capsules were investigated using Fourier transform infrared (FT-IR) and thermal gravimetric analysis (TGA) techniques. Ultraviolet (UV)-visible spectroscopy was used for studying the inhibitor release to the corrosive solution. The corrosion inhibition effect on carbon steel tubes in acidizing produced water was investigated using weight loss measurement, potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), and morphologically by scanning electron microscopy (SEM). The effect of temperature, the thermodynamic parameters, and the adsorption isotherm were studied and discussed.
Chemical structure of T-60.
2 Materials and methods
2.1 Materials and solutions
Polyoxyethylene sorbitan monostearate (T-60), sodium alginate, barium sulfate, and calcium chloride were purchased from Sigma-Aldrich. The composition (wt%) of carbon steel tubes used was 0.07% C, 1.35% Mn, 0.24% Si, 0.017% P, 0.16% Cr, 0.18% Ni, 0.005% S, 0.12% Mo, and the remainder being iron. The oil well produced water used in this study was obtained from the Egyptian Badr El-Din Petroleum Company (BAPETCO) with physical and chemical properties shown in Tables 1 and 2, respectively. The aggressive solution of acidizing produced water was prepared by dilution of analytical grade 37% HCl with filtered oil well-produced water to produce 1 mol l−1 HCl.
Physical properties of produced water in oil well.
| Property | Specific gravity | pH | Total dissolved solids | Hardness |
|---|---|---|---|---|
| Value | 1.08 | 5.1 | 99,175 mg L−1 | 13,528 mg L−1 |
Chemical composition of produced water in oil well.
| Constituents | Sodium | Potassium | Magnesium | Calcium | Strontium | Chloride | Bromide | Sulfate |
|---|---|---|---|---|---|---|---|---|
| Concentration (mg L−1) | 32,008 | 1075 | 857 | 4005 | 201 | 59,500 | 464 | 502 |
2.2 Preparation of gel capsules
The excess concentration of corrosion inhibitor (T-60) was mixed with 0.6 g sodium alginate and 1.2 g barium sulfate as a heavy additive under stirring at 70°C for 20 min until obtaining a homogeneous viscous mixture. Then, the capsules were formed with average diameter of 0.73 cm by injecting the viscous mixture into (2% wt) calcium chloride solution as presented in Figure 2. The formed capsules containing T-60 were collected and left to dry. The average diameter of dried capsules was 0.22 cm.

Prepared capsules loaded with T-60.
2.3 Weight loss measurements
Weight loss measurements were carried out according to the American Society for Testing and Materials (ASTM) standard method (ASTM International, 1999). The coupons with dimensions of 7 cm×2 cm×0.3 cm were immersed in aerated covered bottle containing acidizing produced water without and with a constant weight of gel capsules loaded with T-60, at different time intervals (3, 6, 12, 24, and 48 h) and different temperatures (298, 323, and 343 K). Then, the carbon steel tube coupons were taken out, carefully washed with distilled water, ultrasonically cleaned in acetone, dried, and then weighed again. The average loss in weight (ΔW) was calculated using the following equation (Farag, 2018):
where W1 and W2 are the weight values of coupons before and after immersion in the acidizing produced water, respectively. All experiments were repeated three times and average values were obtained.
2.4 Electrochemical measurements
A Volta Lab 40 (Tacussel-Radiometer PGZ301) potentiostat was used for the electrochemical measurements. The Volta Lab 250 ml corrosion cell with a conventional three-electrode system was used. The carbon steel electrode with 1 cm2 embedded in the specimen holder was used as a working electrode. The platinum wire and saturated calomel electrode (SCE) were used as an auxiliary and a reference electrode, respectively. All experiments were performed under aerated and unstirred conditions. Prior to all experiments, the working electrode was immersed in the test solution for 1 h to stabilize the potential and establish a steady-state open circuit potential (OCP). The polarization curves were obtained from −900 to −200 mV (vs. SCE) with a scan rate of 0.5 mV s−1 in the potentiodynamic polarization study. The extracted parameters, such as corrosion potential (Ecorr), corrosion current density (icorr), anodic Tafel slope (βa), and cathodic Tafel slope (βc), were analyzed using a Tacussel corrosion analysis software model (Voltamaster 4). In addition, EIS measurements were carried out in the frequency spectrum limit from 100 kHz to 0.03 Hz at OCP with an amplitude of 10 mV using alternating current sine wave. The obtained impedance data were fitted with Zsimpwin 3.6 software. All measurements were performed in triplicate under the same condition to obtain reproducibility of experiments.
2.5 Instrument
The prepared gel capsules without and with T-60 inhibitor were analyzed using Nicolet iS10 FT-IR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA), with measurement ranges from 4000 to 400 cm−1 with a 4 cm−1 resolution. A TGA SDT Q600 V20.5 Build 15 instrument (TA Instruments, New Castle, DE, USA) with a heating rate of 283 K/min and temperature range from 293 to 1123 K, under nitrogen atmosphere, was used to estimate the thermal stability of the gel capsules loaded without and with T-60 inhibitor. For UV-visible absorption, a Cary 50 Scan spectrophotometer (Palo Alto, CA, USA) was used to detect the absorbance of the released T-60 into the acidizing produced water at different times and temperatures to calibrate the inhibitor concentration. A JEOL 5410 scanning electron microscope (Tokyo, Japan) was used for studying the surface morphology of the carbon steel surface.
3 Results and discussion
3.1 Gel capsule characterization
The prepared gel capsules without and with T-60 inhibitor were analyzed using FT-IR spectroscopy, which is a great tool to reveal whether the T-60 inhibitor molecules were encapsulated into the alginate gel or not, as shown in Figure 3. The FT-IR absorption peak of gel capsules alone without T-60 showed a symmetric vibration peak of carboxylate (COO−) at 1431 cm−1 (Macher et al., 2015). The weak limited peak at 2925 cm−1 can be assigned to the asymmetric stretching vibration of C–H, due to the cross-linkage of alginate with calcium ions instead of sodium ions, which confirms the transformation of sodium alginate to calcium alginate after being injected in the calcium chloride solution (Taha et al., 2005). The broad peaks around stretching 3424 cm−1 and bending 1615 cm−1 of O–H vibrations suggest the presence of physically adsorbed water molecules in the gel capsule samples. The FT-IR spectrum of T-60 showed typical stretching bands of –C–O–C groups at 1110 cm−1 and 1735 cm−1 band from –C=O stretching of the ester bond from the hydrophilic portion of T-60. The asymmetric 1644 cm−1 and symmetric 1462 cm−1 vibrations were related to COO− groups. The much broader band around 3424 cm−1 likely included the contribution from the -OH groups of T-60 molecules. The FT-IR spectrum of gel capsules loaded with T-60 shows the characteristic bands of both the capsules and the T-60 inhibitor, which confirm that the T-60 inhibitor molecules have been successfully encapsulated into the gel capsules.
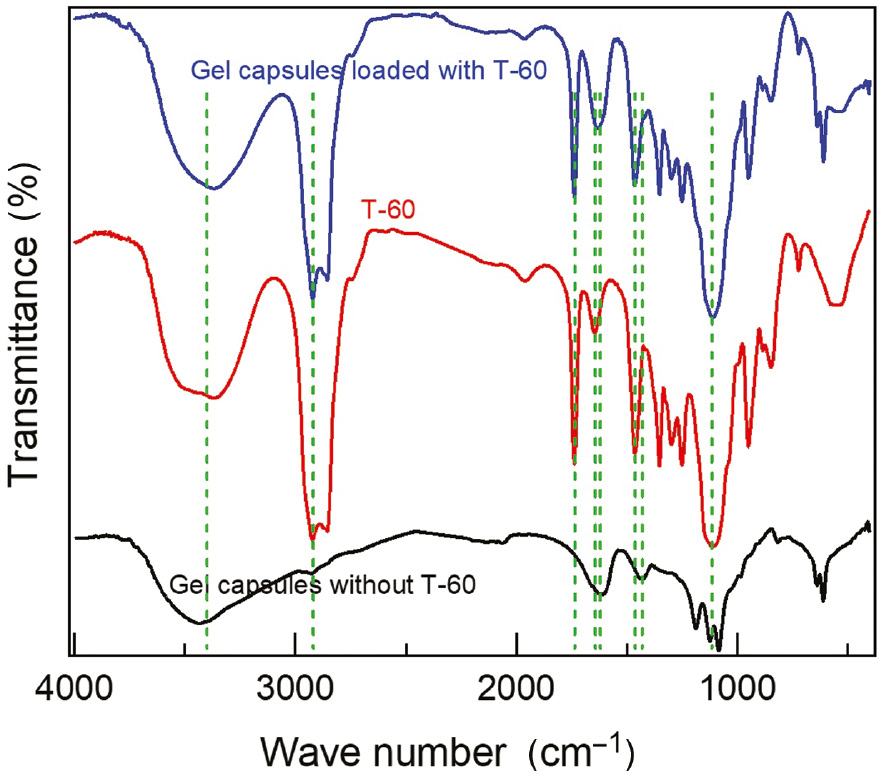
FT-IR chart of gel capsules without and with T-60.
Figure 4 shows the TGA curves of the gel capsules loaded without and with T-60. The weight loss that started for both samples at the temperature range of 303–393 K can be attributed to the desorption of physically adsorbed moisture (Chen et al., 2019). The TGA curves illustrate that the gel capsules without T-60 began to decompose at 453 K, and their complete decomposition occurred at approximately 542 K. The capsules loaded with T-60 showed a higher decomposition temperature and began to degrade at 650 K due to the blending of gel capsules with the T-60 molecules. This confirms that the T-60 inhibitor loaded in the gel capsules is stable at high temperatures and has a long duration time.
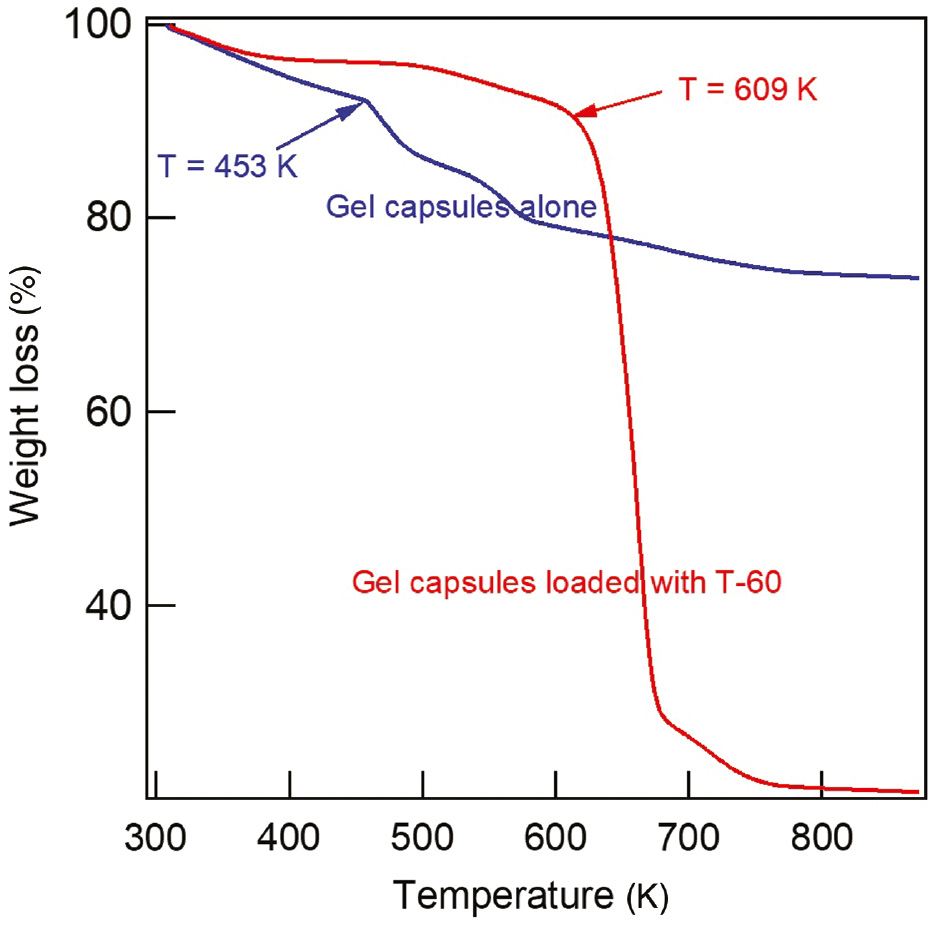
TGA curves of gel capsules loaded without and with T-60.
3.2 Release of T-60 to the acidizing produced water
The idea of the releasing experiments is to calibrate the released concentration of T-60 inhibitor molecules into the acidizing produced water solution at various times and temperatures. Figure 5 shows the releasing of the T-60 inhibitor from gel capsules into the acidizing produced water medium at different times (3, 6, 12, 24, and 48 h) and different temperatures (298, 323, and 343 K). The experiments were conducted using UV-visible measurements with related concentrations, and the data are listed in Table 3. It was found that the T-60 inhibitor molecules loaded in the gel capsules diffused faster at higher temperatures, whereas the capsules were harder to splinter at 298 K than that at 343 K. The experiments revealed that capsule swelling occurred in the acidizing produced water solution and release was induced by capsule splintering. Figure 6 shows a representative scheme of the acidizing process and the addition of inhibitor capsules for an oil well. First, the dry gel capsules became swollen because the acidizing produced water permeated into the interior channels and pores of the gel capsules. Therefore, the inhibitor molecules of T-60 were released rapidly at all temperatures in the initial time. This observation indicates that the inhibitor molecules in open pores at the gel capsule surface dissolve easily and leave quickly. Second, the interior pores and channels of the gel capsules broke due to the acidity and salinity effect of acidizing produced water and finally the capsules splintered, leading to the permeation of inhibitor molecules into solution, which increased more at high temperatures.
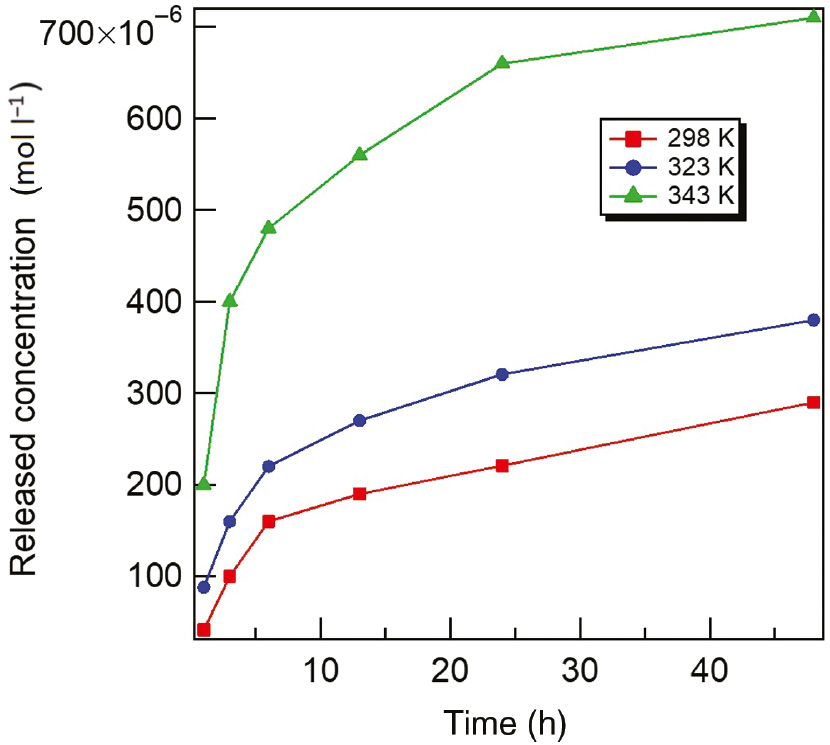
Release curves of T-60 in acidizing produced water at different temperatures.
Releasing rate of T-60 from gel capsules to acidizing produced water at different times and temperatures.
| Time (h) | At 298 K |
At 323 K |
At 343 K |
|||
|---|---|---|---|---|---|---|
| Absorbance | Released conc. (m) | Absorbance | Released conc. (m) | Absorbance | Released conc. (m) | |
| 0.5 | 1.81 | 0.00007 | 1.88 | 0.00009 | 3.02 | 0.00020 |
| 1 | 2.07 | 0.00010 | 2.60 | 0.00016 | 3.20 | 0.00023 |
| 3 | 2.61 | 0.00016 | 3.20 | 0.00022 | 3.73 | 0.00028 |
| 12 | 2.87 | 0.00019 | 3.70 | 0.00027 | 4.42 | 0.00036 |
| 24 | 3.07 | 0.00021 | 4.21 | 0.00032 | 5.21 | 0.00044 |
| 48 | 3.78 | 0.00029 | 4.80 | 0.00038 | 5.61 | 0.00047 |
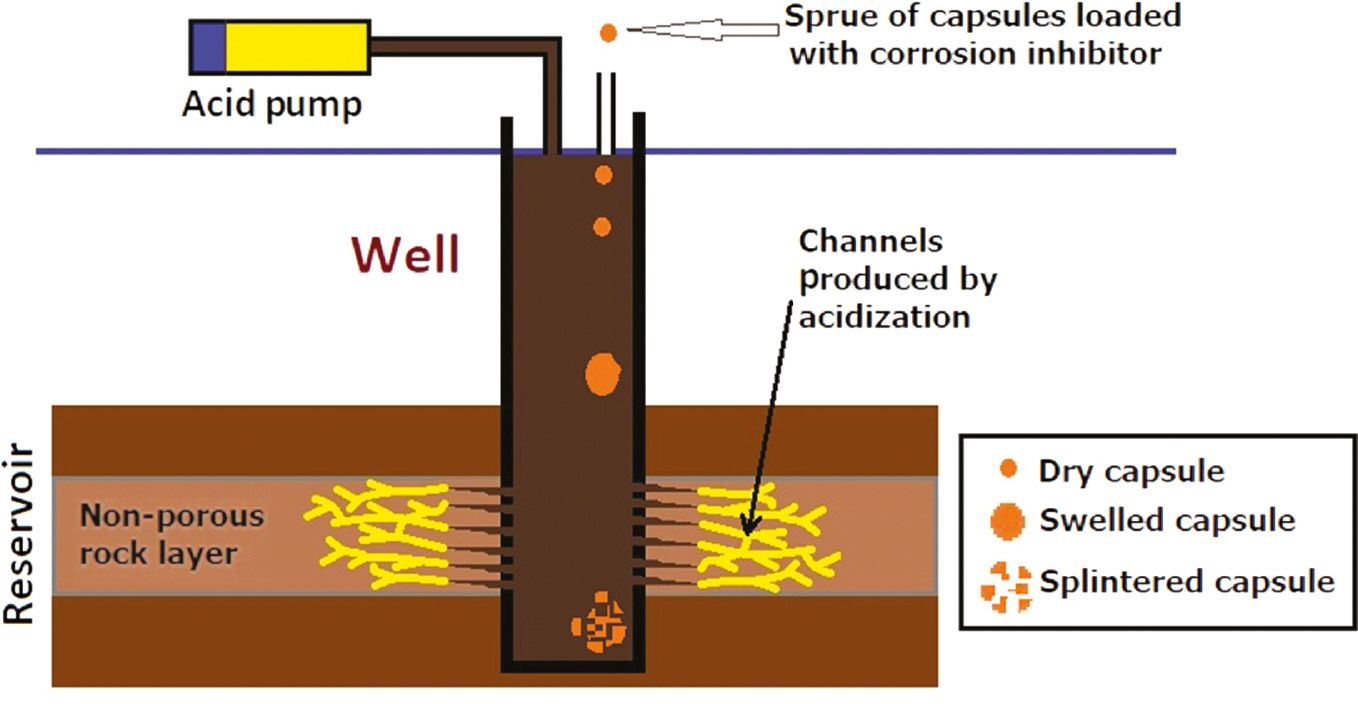
Scheme of acidizing process and addition of inhibitor capsules for an oil well.
3.3 Gravimetric study
3.3.1 Effect of concentration
The concentration effect of the released T-60 inhibitor on the corrosion rate of carbon steel in the acidizing produced water was investigated using weight loss measurements. The weight losses of steel coupons were used to calculate the corrosion rate (CR, mg cm−2 h−1), surface coverage (θ), and corrosion inhibition efficiency (η) from the following equations (Farag et al., 2015a,b):
where S is the surface area (cm2) of the steel coupon, t is the immersion time (h), and CRo and CR are the corrosion rate values without and with inhibitor, respectively.
The values of calculated parameters are listed in Table 4, and the decay of carbon steel corrosion rate with various released T-60 concentrations is presented in Figure 7. It can be seen that the value of corrosion rate in the presence of released T-60 is much smaller compared to only the acidizing produced water (blank). Obviously, the corrosion rate decreases with increasing released T-60 inhibitor into the acidizing produced water, which may be related to the adsorption of inhibitor molecules on the carbon steel surface and formation of a stable protective film. In addition, the inhibition efficiency increases with the increase of inhibitor releasing, which shows inhibition efficiencies of 64.0% and 87.9% at released T-60 concentration after 3 and 48 h of immersion, respectively. This increase in the inhibition efficiency after 48 h of immersion of the carbon steel in the acidizing produced water confirms that the releasing rate of T-60 inhibitor increases with time, which increases the surface coverage on the carbon steel. Moreover, the inhibition effect of T-60 can be related to its high molecular weight (648.919 g/mol), which covers a large size of the steel surface, as well as the high electronic density of 10 oxygen atoms (molecular formula: C35H68O10) in the inhibitor molecules that increase the adsorption centers on the steel surface.
Weight loss parameters obtained for carbon steel in acidizing produced water without and with various released T-60 at 298 K.
| Release time (h) | Released conc. (mol.) | ν (mg cm−2 h−1) | θ | η (%) |
|---|---|---|---|---|
| 0 | – | 1.0143 | – | – |
| 1 | 0.00010 | 0.3654 | 0.640 | 64.0 |
| 3 | 0.00016 | 0.2204 | 0.783 | 78.3 |
| 12 | 0.00019 | 0.1811 | 0.822 | 82.2 |
| 24 | 0.00021 | 0.1511 | 0.851 | 85.1 |
| 48 | 0.00029 | 0.1225 | 0.879 | 87.9 |
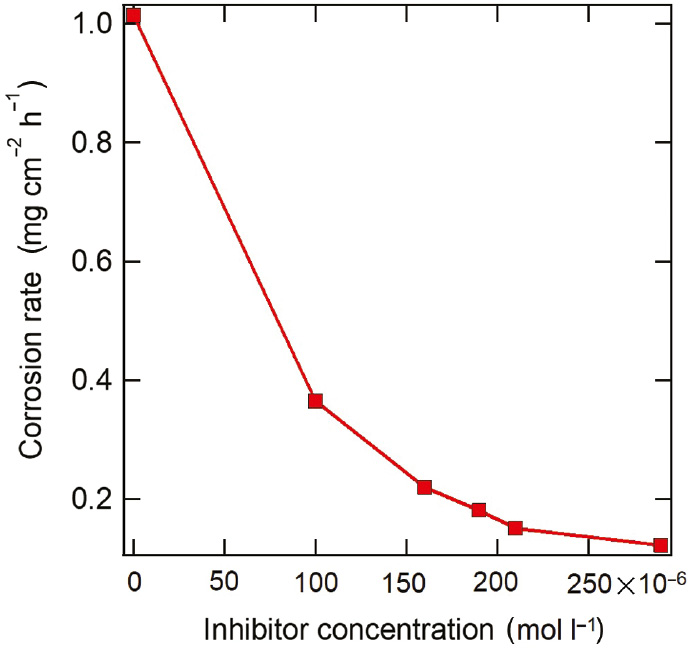
Decay of corrosion rate of carbon steel in acidizing produced water solution with different T-60 released at 298 K.
3.3.2 Adsorption isotherm
Different isotherm modules were employed to study the interactions between the released concentration of T-60 from the gel capsules and the carbon steel surface coverage (θ) in the acidizing produced water. Adsorption isotherms (Frumkin, Langmuir, and Temkin) have been applied to simulate the relationship between θ obtained from gravimetric measurements and the released T-60 concentration. The adsorption isotherms can be expressed in the following equations (Gürten et al., 2015):
where C is the inhibitor concentration, Kads is the equilibrium constant, and a is the molecular interaction parameter. According to the values of correlation coefficient (R2), as shown in Figure 8A–C, it can be concluded that the Langmuir isotherm (Figure 8B) was the best-fit model for the adsorption of T-60 molecules on the steel surface. The correlation coefficient (R2) was found close to unity, which indicates the existence of molecular adsorption within the adsorbed layer. The slope value in the Langmuir model was less than unity and indicates the existence of interactions between the adsorbed inhibitor molecules and the metal surface. The equilibrium constant Kads can be calculated from the intercept of Figure 8B. The higher value of Kads (16,666.7 mol−1) implies more efficient adsorption. The dimensionless separation factor, RL (Mall et al., 2005), can be calculated by Eq. (8), indicating a highly favorable adsorption process in the case of a smaller value (0<RL<1).
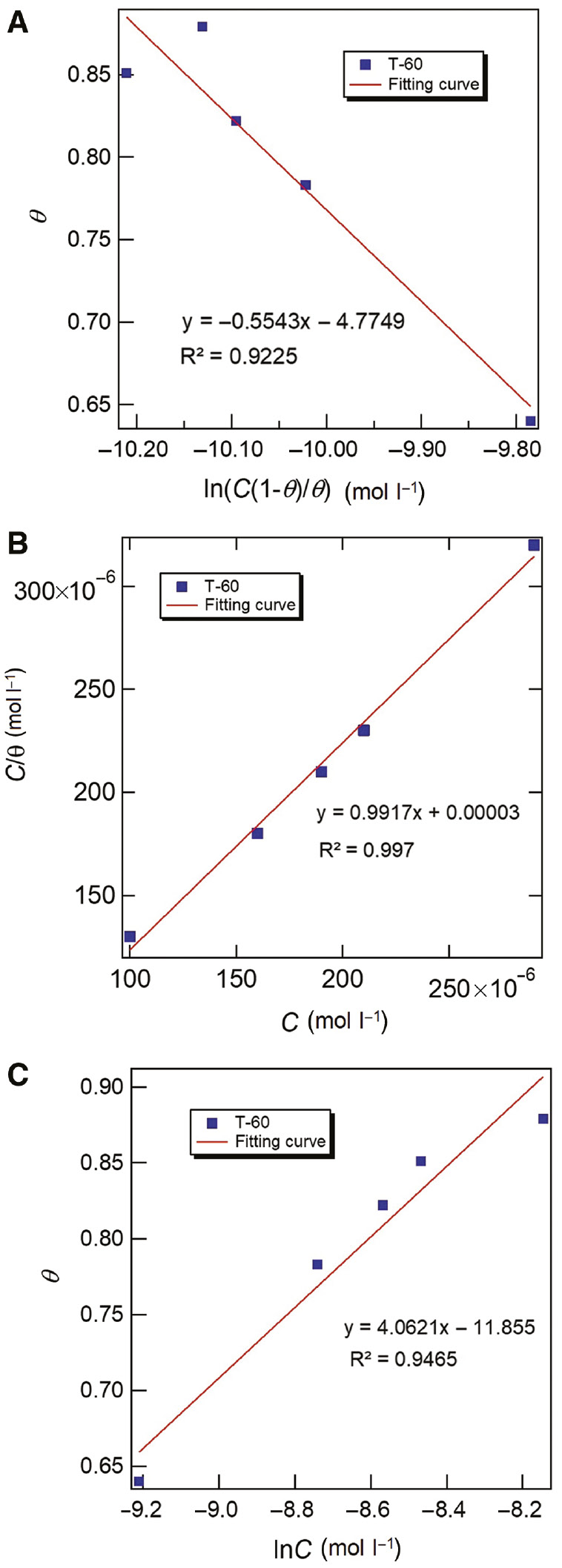
(A) Frumkin, (B) Langmuir, and (C) Temkin adsorption plots of carbon steel in acidizing produced water solution containing different T-60 released at 298 K.
The calculated RL for all concentrations of T-60 was found in the range 0.38–0.17, confirming a highly efficient adsorption (Dkhireche et al., 2010; Moschona et al., 2018). Meanwhile, the relationship between the equilibrium constant Kads and the standard free energy of adsorption
where T is the absolute temperature in K. The negative value of
3.3.3 Effect of temperature
The effect of temperature on the inhibition behavior of the released T-60 on carbon steel tube corrosion was also investigated based on gravimetric measurements. Table 5 presents the corrosion rates and the inhibition efficiencies for carbon steel in the acidizing produced water without and with various released T-60 concentrations at different temperatures of 298, 323, and 343 K. It is clear that the corrosion rate values increased with increasing temperature not only for the inhibited sample but also for the blank (acidizing produced water) sample. The increase in temperature usually accelerates the corrosion reaction, resulting in a higher dissolution rate of the carbon steel in the aggressive acidizing produced water solution. The increase in the corrosion rate with the temperature in the presence of the released T-60 inhibitor may be related to the partial desorption of inhibitor molecules at the metal surface (Cao et al., 2014). However, the released T-60 inhibitor exhibits a smaller value of CR compared with the blank uninhibited solution at the same temperature, which may be related to the strong adsorption of the inhibitor molecules on the metal surface. The apparent activation energy (Ea) for carbon steel dissolution in the acidizing produced water solution without and with released T-60 inhibitor can be calculated according to the Arrhenius equation [Eq. (10)] (Rugmini Ammal et al., 2018). The change in enthalpy and entropy of activation values (ΔHa, ΔSa) was calculated from the transition state theory equation [Eq. (11)]:
Weight loss parameters obtained for carbon steel in acidizing produced water without and with 0.00029 mol l−1 of T-60 at different temperatures.
| Medium | Temperature (K) | ν (mg cm−2 h−1) | ηg (%) |
|---|---|---|---|
| Blank | 298 | 1.0143 | ‒ |
| 323 | 3.1362 | ‒ | |
| 343 | 4.4345 | ‒ | |
| T-60 | 298 | 0.1225 | 87.9 |
| 323 | 0.8663 | 72.4 | |
| 343 | 1.3864 | 68.7 |
where T is absolute temperature and A is the pre-exponential factor. The Ea values are summarized in Table 6, which can be obtained from the straight-line slope of the relationship between ln(CR) vs. 1/T, as depicted in Figure 9. It was found that the calculated value of Ea in the presence of the released T-60 inhibitor was 47 kJ/mol, which was larger than the value of 28 kJ/mol in the absence of the inhibitor. In the previous study, the unchanged or lowered value of Ea compared to the blank uninhibited solution indicates strong adsorption of the inhibitor on the metal surface. This conclusion matches well with that inferred from the Langmuir adsorption isotherm that the released T-60 inhibitor predominantly chemically adsorbed on the carbon steel surface besides being physically absorbed. Further, using Eq. (11), plots of ln(CR/T) vs. 1/T gave straight lines as presented in Figure 10. The change values of the activation enthalpy (ΔHa) and the activation entropy (ΔSa) can be calculated through the slope (−ΔHa/R) and the intercept [ln (R/Nh) + ΔSa/R] of the ln(CR/T) vs. 1/T curve, respectively. The calculated values of ΔHa and ΔSa are listed in Table 6. The positive sign of the activation enthalpy (ΔHa) reflects the endothermic nature of the carbon steel dissolution process. The negative values of ΔSa indicate that the formation of the activated complex in the rate-determining step represents an association rather than a dissociation step, signifying that a decrease in disorder takes place during the transition from reactants to the activated complex (Fouda et al., 2014).
Thermodynamic parameters obtained for carbon steel in acidizing produced water without and with 0.00029 mol l−1 of T-60.
| Medium | E a | ΔH | ΔS |
|---|---|---|---|
| Blank | 28 | 26 | −237 |
| T-60 | 47 | 44 | −112 |
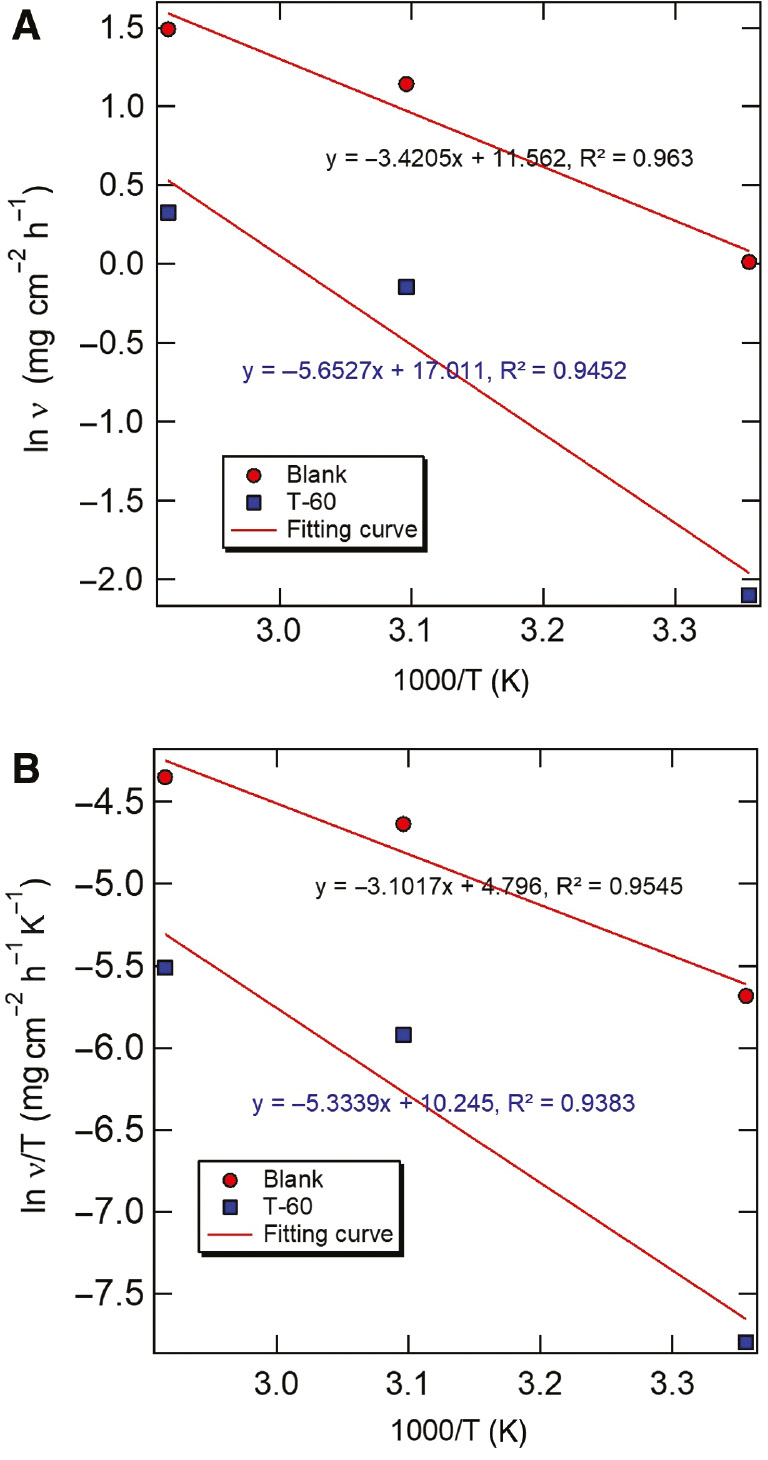
(A) Arrhenius and (B) alternative Arrhenius plots for the corrosion rate of steel in acidizing produced water solution in the absence and presence of released T-60.
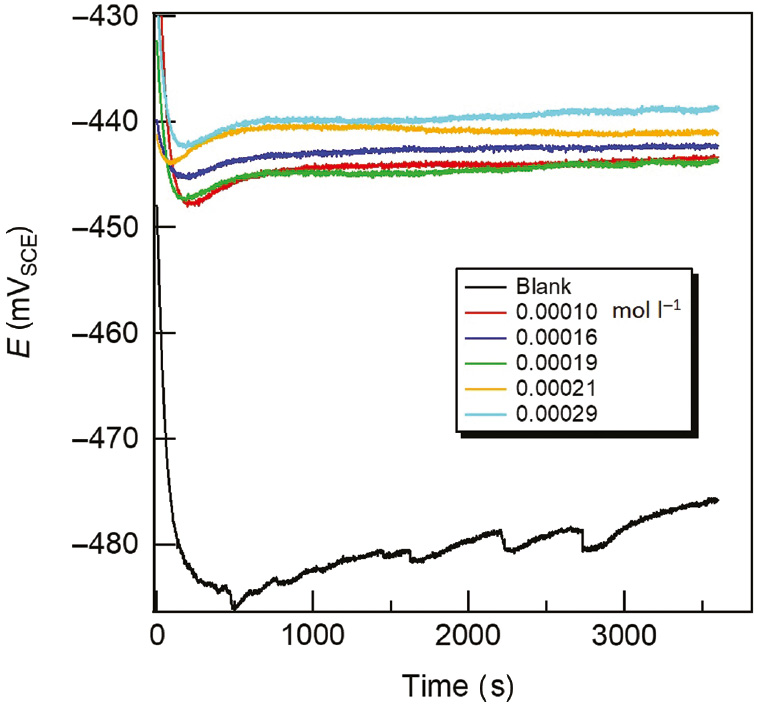
OCP curves of carbon steel in acidizing produced water solution without and with various released T-60 at 298 K.
3.4 OCP and potentiodynamic polarization testing
The time-dependent variation of the OCP of carbon steel immersed in acidizing produced water solution without and with different concentrations of released T-60 inhibitor is presented in Figure 10. It was observed that the OCP values for all uninhibited and inhibited solutions started with decreasing as a result of the dissolution of the oxide layer formed at the carbon steel sample surface (working electrodes) due to exposure to air after polishing. They potential gradually become stable with time. In the presence of released T-60, the OCP was found to shift to a more positive potential compared to that in its absence, suggesting the formation of a protecting layer due to adsorption of the inhibitor molecules on the metal surface. However, the shift in OCP curves between the absence and presence of the inhibitor was <85 mV (30–45 mV), which indicates that the investigated inhibitor is a mixed-type inhibitor as reported by Riggs and Nathan (1973).
The potentiodynamic polarization curves obtained for carbon steel after 1 h of immersion in the acidizing produced water solution without and with the released T-60 are shown in Figure 11. The values of corrosion potential (Ecorr), corrosion current density (icorr), inhibition efficiency (η), and cathodic (βc) and anodic (βa) Tafel slopes are listed in Table 7. Based on the corrosion current density values, the values of inhibition efficiency (η) were calculated as per the following relationship (Migahed et al., 2012):
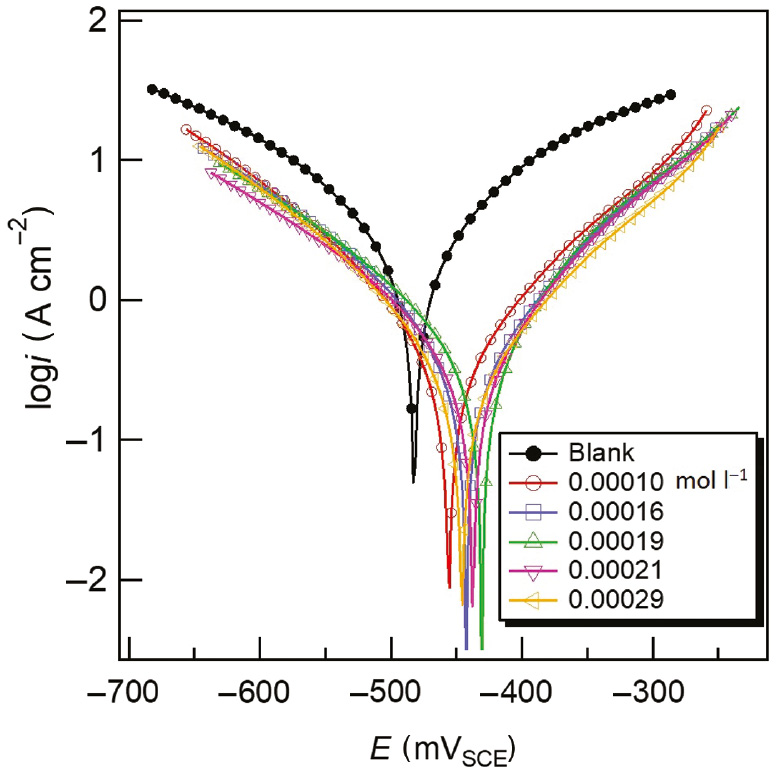
Tafel plots of carbon steel in acidizing produced water solution without and with various released T-60 at 298 K.
Potentiodynamic polarization parameters obtained for carbon steel in acidizing produced water without and with various released T-60 at 298 K.
| Release time (h) | Released conc. (mol l−1) | –E (mV) | i corr (mA cm−2) | βa (mV) | –βc (mV) | η (%) |
|---|---|---|---|---|---|---|
| 0 | – | 482 | 4.89 | 244 | 246 | – |
| 1 | 0.00010 | 451 | 1.15 | 140 | 135 | 76.5 |
| 3 | 0.00016 | 443 | 0.59 | 131 | 147 | 87.9 |
| 12 | 0.00019 | 431 | 0.52 | 121 | 160 | 89.4 |
| 24 | 0.00021 | 438 | 0.49 | 126 | 164 | 90.0 |
| 48 | 0.00029 | 446 | 0.43 | 128 | 165 | 91.2 |
Impedance parameters obtained for carbon steel in acidizing produced water without and with various released T-60 at 298 K.
| Release time (h) | Released conc. (mol l−1) | R s (ohm·cm2) | Q-Yo×104 (Ω−1 s−n cm−2) | n | R ct (ohm·cm2) | Chi-square×103 | η |
|---|---|---|---|---|---|---|---|
| 0 | – | 0.8 | 1.63 | 0.82 | 4.8 | 1.09 | – |
| 1 | 0.00010 | 1.2 | 1.15 | 0.83 | 21.2 | 2.41 | 77.4 |
| 3 | 0.00016 | 0.81 | 2.98 | 0.83 | 38.2 | 1.36 | 87.4 |
| 12 | 0.00019 | 0.82 | 2.51 | 0.8 | 43.6 | 9.78 | 89.0 |
| 24 | 0.00021 | 0.86 | 2.45 | 0.8 | 48.8 | 8.22 | 90.2 |
| 48 | 0.00029 | 1.01 | 4.31 | 0.82 | 54.2 | 1.59 | 91.1 |
where io and i are the corrosion current densities in the absence and presence of the released T-60 inhibitor. It is clear that the release of T-60 inhibitor molecules to the acidic solution of the produced water causes a remarkable decrease in corrosion current density (icorr) at all released inhibitor concentrations compared to the uninhibited blank solution. Also, the presence of T-60 in acidizing produced water solution affects the cathodic and anodic Tafel slopes. The differences observed for the values of cathodic and anodic Tafel slopes suggest that the inhibition corrosion mechanism takes place at the active metal surface due to the adsorption of the protective layer of T-60 inhibitor on the carbon steel surface. Moreover, the corrosion potential was shifted to positive values for inhibited solutions, indicating that the carbon steel became passive by forming a protective layer from the T-60 inhibitor on the carbon steel surface capable of providing corrosion protection. However, the displacement in the corrosion potential between the absence and presence of the inhibitor is not enough to decide the anodic type inhibitor; therefore, the inhibitor behaves as a mixed-type inhibitor, which agrees with the OCP measurements (Farag & Noor El-Din, 2012).
3.5 EIS measurements
EIS was performed to investigate the corrosion inhibition behavior of the carbon steel in the acidizing produced water solution in the absence and presence of different released concentrations of T-60, as shown in Figure 12. Nyquist and Bode plots are shown in Figure 12A and B, respectively. The Nyquist plots (Figure 12A) exhibit a semicircle that can be attributed to the charge transfer that takes place at the carbon steel electrode-solution interface, and this process controls the corrosion reaction. It is evident from these plots that the impedance response of the carbon steel in uninhibited acidizing produced water solution is significantly changed after releasing T-60 to the solution. It was found that the semicircle diameters increased with increasing released inhibitor concentrations, and hence increased the charge transfer resistance (Rct). The values of Rct can be calculated by the following equation (Farag & Hegazy, 2013):
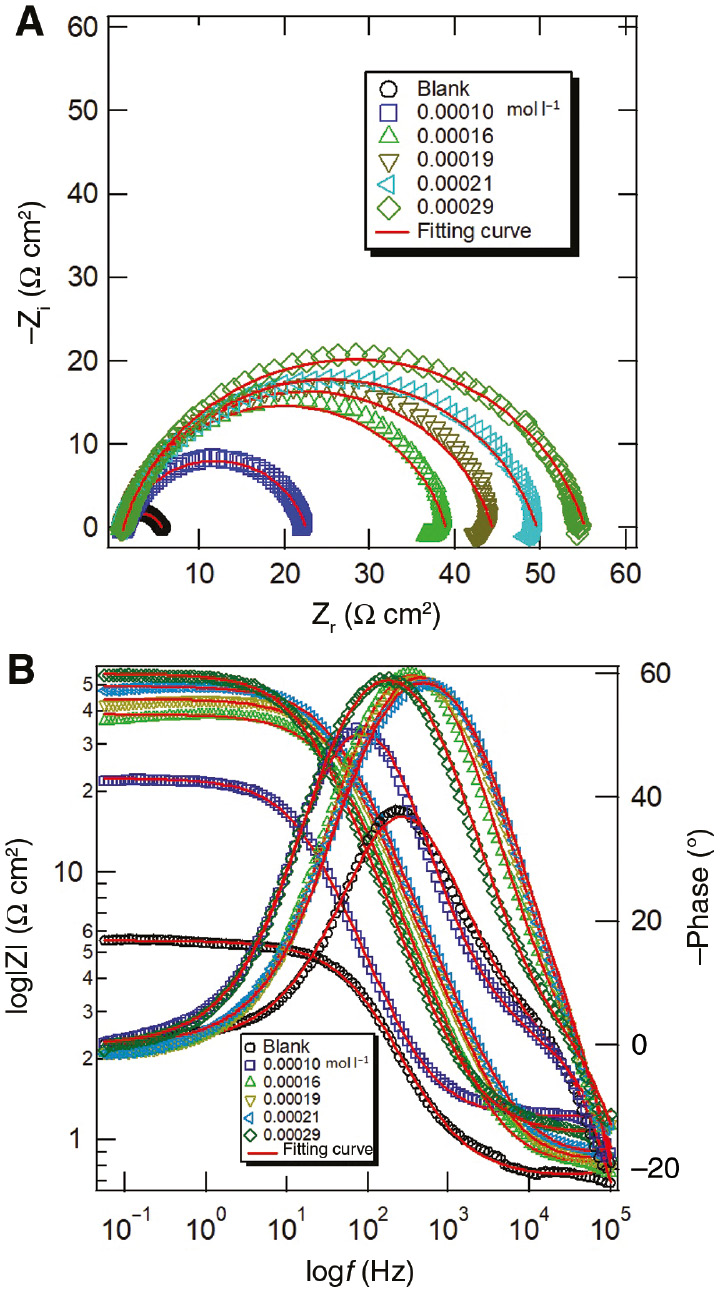
(A) Nyquist and (B) Bode plots of carbon steel in acidizing produced water solution without and with various released T-60 at 298 K.
where Zr(At low f) and Zr(At high f) are the low- and high-frequency impedance, respectively. Meanwhile, the values of Rct can be used to obtain the double-layer capacitance (Cdl) on the working electrode surface at the frequency fmax (at which the imaginary component of the impedance is maximal), through the following equation (Farag & Hegazy, 2013):
Moreover, some Nyquist plots are not perfect semicircles with their centers below the real axis, and this can be ascribed to surface inhomogeneity and roughness of the solid electrode (El Bakri et al., 2019). Therefore, the ideal capacity of the double-layer capacitance (Cdl) was substituted by a constant phase element (CPE) in the equivalent circuit model. The Nyquist impedance plots were analyzed by fitting the experimental data to a simple circuit model [R(QR)], which includes the solution resistance (Rs), the charge transfer resistance (Rct), and the constant phase element (Q), and the values are listed in Table 8. The impedance of CPE can be expressed as follows:
where Yo is the magnitude of ZCPE, j is the imaginary root, ω is the angular frequency, and n is the exponential term. The inhibition efficiency obtained from fitting impedance data was calculated using the following relationship (Farag et al., 2015a,b):
where Rct and
Meanwhile, it is apparent from Bode plots that the release of T-60 to the aggressive solution results in an increase in the impedance modulus, which further increases with increasing released inhibitor concentrations (Figure 12B). This can be attributed to the formation of a protective film of the released T-60 on the carbon steel surface, which inhibits the dissolution of iron. As shown in Figure 12B, there is only a single time constant for the corrosion process at the metal-solution interface in the phase-angle plots. The increase in the peak heights with increasing concentrations of T-60 implies a more capacitive response at the metal-solution interface owing to the adsorption of inhibitor molecules (Tang et al., 2013).
3.6 SEM analysis
To investigate the variation on the carbon steel surface images for the polished carbon steel before and after 48 h of immersion in the acidizing produced water solution without and with released inhibitor, the SEM technique was used. It can be observed from Figure 13A that the SEM image of the polished carbon steel surface is relatively smooth with some polished scratches before immersion in the acidizing produced water solution. In the case of immersed carbon steel in uninhibited blank acidizing produced water solution (Figure 13B), the carbon steel is highly corroded with an uneven surface due to the effect of acidity and salinity of the aggressive solution (Mobin et al., 2018). The inhibiting effect of the released T-60 inhibitor to the acidizing produced water is obvious in Figure 13C, where significant improvements of carbon steel, with a smoother surface compared to those in uninhibited blank solution, is shown. This observation clearly proves that the released T-60 inhibitor exhibits good inhibition effect on the corrosion of the carbon steel surface (Cui et al., 2018).
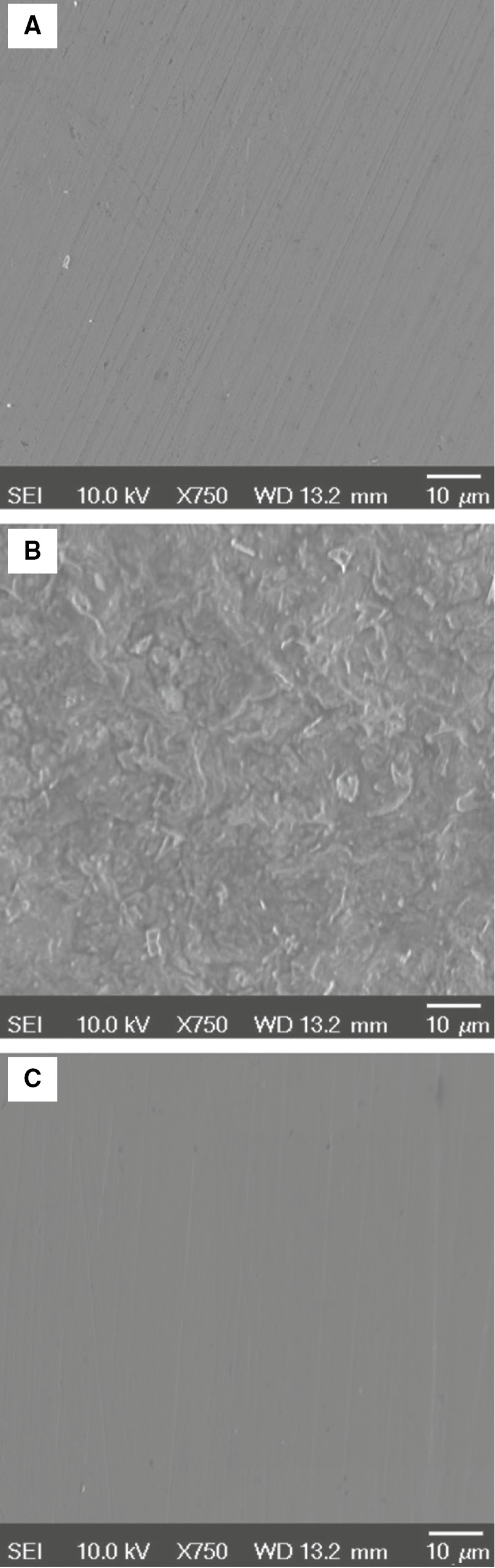
SEM images of (A) freshly polished carbon steel surface, (B) after 48 h immersion in acidizing produced water without inhibitor, and (C) with released T-60 inhibitor.
4 Conclusions
This paper introduces a new technology for the injection of corrosion inhibitors in oil fields, especially for oil wells. In this work, gel capsules loaded with T-60 inhibitor were prepared, characterized, and investigated for corrosion protection of carbon steel tubes from acidizing produced water in oil fields. When the capsules loaded with T-60 inhibitor were added into the acidizing produced water, the capsules tended to release the inhibitor gradually and protected carbon steel tubes from corrosion, especially the downhole, during its sinking to the bottom of the oil well. The high temperature is favorable for the release of the inhibitor. The OCP and the potentiodynamic polarization measurements indicated that T-60 behaves as a mixed-type inhibitor. The EIS results indicated that the Rct values increase when the T-60 inhibitor is released to the aggressive solution, confirming the adsorption of the inhibitor molecules on the carbon steel surface. The adsorption of T-60 inhibitor molecules on the carbon steel surface is a combined physisorption/chemisorption process.
-
Funding: The authors are greatly thankful to the Egyptian Petroleum Research Institute for funding and support.
References
ASTM International. Standard practice for preparing, cleaning, and evaluating corrosion test. Significance 1999; 90: 1–9 (reapproved 2011).Suche in Google Scholar
Baddini ALdQ, Cardoso SP, Hollauer E, Gomes JAdCP. Statistical analysis of a corrosion inhibitor family on three steel surfaces (duplex, super-13 and carbon) in hydrochloric acid solutions. Electrochim Acta 2007; 53: 434–446.10.1016/j.electacta.2007.06.050Suche in Google Scholar
El Bakri Y, Guo L, Anouar EH, Essassi EM. Electrochemical, DFT and MD simulation of newly synthesized triazolotriazepine derivatives as corrosion inhibitors for carbon steel in 1 M HCl. J Mol Liquids 2019; 274: 759–769.10.1016/j.molliq.2018.11.048Suche in Google Scholar
Cabral P, Gierega CR, Costanza P, Suriano AA. Production optimization by use of the capillary technology in the Loma la Lata field. SPE Prod Operat 2008; 23: 392–403.10.2118/107374-PASuche in Google Scholar
Cao Z, Tang Y, Cang H, Xu J, Lu G, Jing W. Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part II: Theoretical studies. Corros Sci 2014; 83: 292–298.10.1016/j.corsci.2014.02.025Suche in Google Scholar
Chen Z, Yang W, Xu B, Chen Y, Qian M, Su X, Li Z, Yin X, Liu Y. Corrosion protection of carbon steels by electrochemically synthesized V-TiO2/polypyrrole composite coatings in 0.1 M HCl solution. J Alloys Comp 2019; 771: 857–868.10.1016/j.jallcom.2018.09.003Suche in Google Scholar
Choi H, Song YK, Kim KY, Park JM. Encapsulation of triethanolamine as organic corrosion inhibitor into nanoparticles and its active corrosion protection for steel sheets. Surf Coat Technol 2012; 206: 2354–2362.10.1016/j.surfcoat.2011.10.030Suche in Google Scholar
Cui M, Ren S, Zhao H, Wang L, Xue Q. Novel nitrogen doped carbon dots for corrosion inhibition of carbon steel in 1 M HCl solution. Appl Surf Sci 2018; 443: 145–156.10.1016/j.apsusc.2018.02.255Suche in Google Scholar
Dkhireche N, Dahami A, Rochdi A, Hmimou J, Touir R, Ebn Touhami M, El Bakri M, El Hallaoui A, Anouar A, Takenouti H. Corrosion and scale inhibition of low carbon steel in cooling water system by 2-propargyl-5-o-hydroxyphenyltetrazole. Mater Chem Phys 2010; 122: 1–9.Suche in Google Scholar
Farag AA. Oil-in-water emulsion of a heterocyclic adduct as a novel inhibitor of API X52 steel corrosion in acidic solution. Corros Rev 2018; 36: 575–588.10.1515/corrrev-2018-0002Suche in Google Scholar
Farag AA, Ali TA. The enhancing of 2-pyrazinecarboxamide inhibition effect on the acid corrosion of carbon steel in presence of iodide ions. J Ind Eng Chem 2015; 21: 627–634.10.1016/j.jiec.2014.03.030Suche in Google Scholar
Farag AA, Hegazy MA. Synergistic inhibition effect of potassium iodide and novel Schiff bases on X65 steel corrosion in 0.5 M H2SO4. Corros Sci 2013; 74: 168–177.10.1016/j.corsci.2013.04.039Suche in Google Scholar
Farag AA, Noor El-Din MR. The adsorption and corrosion inhibition of some nonionic surfactants on API X65 steel surface in hydrochloric acid. Corros Sci 2012; 64: 174–183.10.1016/j.corsci.2012.07.016Suche in Google Scholar
Farag AA, Ismail AS, Migahed MA. Inhibition of carbon steel corrosion in acidic solution using some newly polyester derivatives. J Mol Liquids 2015a; 211: 915–923.10.1016/j.molliq.2015.08.033Suche in Google Scholar
Farag AA, Migahed MA, Al-Sabagh AM. Adsorption and inhibition behavior of a novel Schiff base on carbon steel corrosion in acid media. Egypt J Petrol 2015b; 24: 307–315.10.1016/j.ejpe.2015.07.001Suche in Google Scholar
Fernandes CM, Alvarez LX, dos Santos NE, Maldonado Barrios AC, Ponzio EA. Green synthesis of 1-benzyl-4-phenyl-1H-1,2,3-triazole, its application as corrosion inhibitor for mild steel in acidic medium and new approach of classical electrochemical analyses. Corros Sci 2019; 149: 185–194.10.1016/j.corsci.2019.01.019Suche in Google Scholar
Fouda AS, Shalabi K, Elewady GY, Merayyed HF. Chalcone derivatives as corrosion inhibitors for carbon steel in 1 M HCl solutions. Int J Electrochem Sci 2014; 9: 7038–7058.10.1016/S1452-3981(23)10950-3Suche in Google Scholar
Guan H, Fan L-Z, Zhang H, Qu X. Polyaniline nanofibers obtained by interfacial polymerization for high-rate supercapacitors. Electrochim Acta 2010; 56: 964–968.10.1016/j.electacta.2010.09.078Suche in Google Scholar
Gürten AA, Keleş H, Bayol E, Kandemirli F. The effect of temperature and concentration on the inhibition of acid corrosion of carbon steel by newly synthesized Schiff base. J Indust Eng Chem 2015; 27: 68–78.10.1016/j.jiec.2014.11.046Suche in Google Scholar
Han P, Chen C, Li W, Yu H, Xu Y, Ma L, Zheng Y. Synergistic effect of mixing cationic and nonionic surfactants on corrosion inhibition of mild steel in HCl: experimental and theoretical investigations. J Colloid Interface Sci 2018; 516: 398–406.10.1016/j.jcis.2018.01.088Suche in Google Scholar PubMed
Huizinga S, Liek WE. Corrosion behavior of 13% chromium steel in acid stimulations. Corrosion 1994; 50: 555–566.10.5006/1.3294357Suche in Google Scholar
Konno Y, Farag AA, Tsuji E, Aoki Y, Habazaki H. Formation of porous anodic films on carbon steels and their application to corrosion protection composite coatings formed with polypyrrole. J Electrochem Soc 2016; 163: C386–C393.10.1149/2.1451607jesSuche in Google Scholar
Quraishi MA, Jamal D. Technical note: CAHMT – a new and eco-friendly acidizing corrosion inhibitor. Corrosion 2000; 10: 983–985.10.5006/1.3294388Suche in Google Scholar
Macher T, Sherwood J, Xu Y, Lee M, Dennis G, Qin Y, Daly DT, Swatloski RP, Bao Y. Scalable production of iron oxide nanowhiskers. J Nanomater 2015; 16: 1–8.10.1155/2015/376579Suche in Google Scholar
Mall ID, Srivastava VC, Agarwal NK, Mishra IM. Adsorptive removal of malachite green dye from aqueous solution by bagasse fly ash and activated carbon-kinetic study and equilibrium isotherm analyses. Colloids Surf A Physicochem Eng Aspects 2005; 264: 17–28.10.1016/j.colsurfa.2005.03.027Suche in Google Scholar
Migahed MA, Farag AA, Elsaeed SM, Kamal R. Corrosion inhibition of carbon steel in formation water of oil wells by some Schiff base non ionic surfactants. In: European Corrosion Congress 2009, EUROCORR 2009, 2009. http://www.scopus.com/inward/record.url?eid=2-s2.0-77953707807&partnerID=MN8TOARS.Suche in Google Scholar
Migahed MA, Farag AA, Elsaed SM, Kamal R, Mostfa M, AbdEl-Bary H. Synthesis of a new family of Schiff base nonionic surfactants and evaluation of their corrosion inhibition effect on X-65 type tubing steel in deep oil wells formation water. Mater Chem Phys 2011; 125: 125–135.10.1016/j.matchemphys.2010.08.082Suche in Google Scholar
Migahed MA, Farag AA, Elsaeed SM, Kamal R, Abd El-Bary H. Corrosion inhibition of steel pipelines in oil well formation water by a new family of nonionic surfactants. Chem Eng Commun 2012; 199: 1335–1356.10.1080/00986445.2012.662922Suche in Google Scholar
Mobin M, Basik M, Aslam J. Boswellia serrata gum as highly efficient and sustainable corrosion inhibitor for low carbon steel in 1 M HCl solution: experimental and DFT studies. J Mol Liquids 2018; 263: 174–186.10.1016/j.molliq.2018.04.150Suche in Google Scholar
Moschona A, Plesu N, Mezei G, Thomas AG, Demadis KD. Corrosion protection of carbon steel by tetraphosphonates of systematically different molecular size. Corros Sci 2018; 145: 135–150.10.1016/j.corsci.2018.09.021Suche in Google Scholar
Murmu M, Saha SK, Murmu NC, Banerjee P. Effect of stereochemical conformation into the corrosion inhibitive behaviour of double azomethine based Schiff bases on mild steel surface in 1 mol L−1 HCl medium: an experimental, density functional theory and molecular dynamics simulation study. Corros Sci 2019; 146: 134–151.10.1016/j.corsci.2018.10.002Suche in Google Scholar
Panossian Z, Lira de Almeida N, Ferreira de Sousa RM, de Souza Pimenta G, Schmidt Marques LB. Corrosion of carbon steel pipes and tanks by concentrated sulfuric acid: a review. Corros Sci 2012; 58: 1–11.10.1016/j.corsci.2012.01.025Suche in Google Scholar
Rabizadeh T, Asl SK. Casein as a natural protein to inhibit the corrosion of mild steel in HCl solution. J Mol Liquids 2019; 276: 694–704.10.1016/j.molliq.2018.11.162Suche in Google Scholar
Riggs Jr OL, Nathan CC. Corrosion Inhibitors. Houston, TX: CC Nathan, 1973.Suche in Google Scholar
Rugmini Ammal P, Prasad AR, Joseph A. Comparative studies on the electrochemical and physicochemical behaviour of three different benzimidazole motifs as corrosion inhibitor for mild steel in hydrochloric acid. Egypt J Petrol 2018; 27: 1067–1076.10.1016/j.ejpe.2018.03.006Suche in Google Scholar
Saha SK, Murmu M, Murmu NC, Banerjee P. Evaluating electronic structure of quinazolinone and pyrimidinone molecules for its corrosion inhibition effectiveness on target specific mild steel in the acidic medium: a combined DFT and MD simulation study. J Mol Liquids 2016; 224: 629–638.10.1016/j.molliq.2016.09.110Suche in Google Scholar
Sardar N, Ali H. A study of some new acidizing inhibitors on corrosion of N-80 alloy in 15% boiling hydrochloric acid. Corrosion 2002; 58: 317–321.10.5006/1.3287679Suche in Google Scholar
Taha MO, Aiedeh KM, Al-Hiari Y, Al-Khatib H. Synthesis of zinc-crosslinked thiolated alginic acid beads and their in vitro evaluation as potential enteric delivery system with folic acid as model drug. Pharmazie 2005; 60: 736–742.Suche in Google Scholar
Tang Y, Zhang F, Hu S, Cao Z, Wu Z, Jing W. Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part I: gravimetric, electrochemical, SEM and XPS studies. Corros Sci 2013; 74: 271–282.10.1016/j.corsci.2013.04.053Suche in Google Scholar
Verma C, Haque J, Quraishi MA, Ebenso EE. Aqueous phase environmental friendly organic corrosion inhibitors derived from one step multicomponent reactions: a review. J Mol Liquids 2019; 275: 18–40.10.1016/j.molliq.2018.11.040Suche in Google Scholar
Wang ZZ, Li YY, Zhang GA. Inhibitive effects of inhibitors on the galvanic corrosion between N80 carbon steel and 13Cr stainless steel under dynamic supercritical CO2 conditions. Corros Sci 2019; 146: 121–133.10.1016/j.corsci.2018.10.028Suche in Google Scholar
Wikström J, Elomaa M, Nevala L, Räikkönen J, Heljo P, Urtti A, Yliperttula M. Viability of freeze dried microencapsulated human retinal pigment epithelial cells. Eur J Pharmaceut Sci 2012; 47: 520–526.10.1016/j.ejps.2012.06.014Suche in Google Scholar PubMed
Yadav M, Behera D, Sharma U. Nontoxic corrosion inhibitors for N80 steel in hydrochloric acid. Arab J Chem 2016; 9: S1487–S1495.10.1016/j.arabjc.2012.03.011Suche in Google Scholar
You Q, Tang Y, Wang B, Zhao F. Enhanced oil recovery and corrosion inhibition through a combined technology of gel treatment for water shutoff and corrosion inhibitor huff & puff in oil well. Proc Eng 2011; 18: 7–12.10.1016/j.proeng.2011.11.002Suche in Google Scholar
Zhu GY, Xiao ZB, Zhou RJ, Yi FP. Fragrance and flavor microencapsulation technology. Adv Mater Res 2012; 535 537: 440–445.10.4028/www.scientific.net/AMR.535-537.440Suche in Google Scholar
Zhu Y, Free ML, Woollam R, Durnie W. A review of surfactants as corrosion inhibitors and associated modeling. Progr Mater Sci 2017; 90: 159–223.10.1016/j.pmatsci.2017.07.006Suche in Google Scholar
©2020 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Review
- Corrosion protection of copper and copper alloys in different corrosive medium using environmentally friendly corrosion inhibitors
- Original articles
- Complementary methods for characterization of the corrosion products on the surface of Ag60Cu26Zn14 and Ag58.5Cu31.5Pd10 brazing alloys
- Influence of temperature and potential range on Zn-Ni deposition properties formed by cyclic voltammetry electrodeposition in chloride bath solution
- Evaluation of corrosion inhibition and adsorption behavior of Thymuszygis subsp. gracilis volatile compounds on mild steel surface in 1 m HCl
- Non-ionic surfactant loaded on gel capsules to protect downhole tubes from produced water in acidizing oil wells
- Towards a better understanding of the oxide film growth mechanism in E110 zirconium alloy under high-temperature oxidation in steam
- Environmentally assisted cracking of T91 ferritic-martensitic steel in heavy liquid metals
Artikel in diesem Heft
- Frontmatter
- Review
- Corrosion protection of copper and copper alloys in different corrosive medium using environmentally friendly corrosion inhibitors
- Original articles
- Complementary methods for characterization of the corrosion products on the surface of Ag60Cu26Zn14 and Ag58.5Cu31.5Pd10 brazing alloys
- Influence of temperature and potential range on Zn-Ni deposition properties formed by cyclic voltammetry electrodeposition in chloride bath solution
- Evaluation of corrosion inhibition and adsorption behavior of Thymuszygis subsp. gracilis volatile compounds on mild steel surface in 1 m HCl
- Non-ionic surfactant loaded on gel capsules to protect downhole tubes from produced water in acidizing oil wells
- Towards a better understanding of the oxide film growth mechanism in E110 zirconium alloy under high-temperature oxidation in steam
- Environmentally assisted cracking of T91 ferritic-martensitic steel in heavy liquid metals

