Abstract
Methanol extract of Aloe vera leaves (AE) as a green inhibitor for controlling corrosion of low-alloy steel (mild steel) in 0.5 m HCl solution was studied using gravimetric analysis, potentiostatic polarization measurements, electrochemical impedance spectroscopy and quantum analysis. The results obtained revealed that AE has fairly good inhibiting properties against corrosion of mild steel in 0.5 m HCl solution, with efficiency >97%. Also, the inhibition was mixed type that follows the Langmuir adsorption isotherm. The value of ΔG reveals that AE inhibits the corrosion rate via physical type adsorption on the mild steel surface which is further supported by Dubinin-Radushkevich isotherm.
1 Introduction
In HCl medium, the corrosion of low-alloy steel is a subject of practical implication and a lot of manufacturing relevance as it is a widely applicable material but, unluckily, highly corrosive too. Hydrochloric acid is generally used for the removal of undesirable scale and rust in several industrial processes. Thus, use of inhibitors is one of the most convenient methods for protection of mild steel (MS) against corrosion to avoid unexpected metal dissolution and acid consumption. Most of the familiar acid inhibitors are organic compounds containing hetero atoms such as N, S and O (Singh et al., 2012). The mainstream inhibitors are extremely toxic and hazardous to the environment, restricting their applications as corrosion inhibitors. Due to the unhealthy environmental consequences of these corrosion inhibitors, modern researchers are deviating towards the usage of green compounds as corrosion inhibitors. Natural products of plant origin, including different organic compounds (e.g. alkaloids, tannins, pigments, and organic and amino acids) as their effective components, are known to inhibit the corrosion reaction (Muthukrishnan et al., 2014). These include the category of compounds that are environment friendly, less polluting, cheap, and easily available and are obtained from natural products such as plant extracts. Extracts of natural products like Murraya koenigii (Quraishi et al., 2010), Emblica officinalis (Saratha & Vasudha, 2010), Phyllanthus amarus (Okafor et al., 2010), black pepper extract (Quraishi et al., 2009), and fenugreek seeds and leaves (Noor, 2008) have been tested as corrosion inhibitors for metals. Therefore, the present paper incorporates the descriptive study of inhibition effect of leaf extract of Aloe barbedensis miller (AE) on the corrosion reaction on MS surface in HCl medium.
Aloe barbadensis, a medicinal plant, is one of the 500 species of Aloe vera, belongs to the family Asphodelaceae (Liliaceae) (Sharrif & Verma, 2011). The gel, which constitutes the bulk of the leaf substance, serves as the water storage organ for the plant containing more than 200 different substances (Sharrif & Verma, 2011). Some of these are polysaccharides, glycoproteins, vitamins, minerals, and enzymes. AE obtained from the plant eaves was used as green corrosion inhibitor in the present study. It is one of the natural inhibitors that have an inhibitive action on the corrosion of metals (Singh et al., 2016).
2 Materials and methods
2.1 Preparation of Aloe extract
Plant material (Aloe barbedensis) in natural condition was shade dried then kept in oven for 4 h at 50°C to remove the moisture content. Five grams of the powdered and dried material, characterized by Fourier transform infrared spectroscopy (FTIR), was placed in 500 ml round bottom flask and sufficient quantity of methanol (100 ml) was added; then the methanolic solution was refluxed for 5 h. Thereafter, AE was filtered in order to remove any suspended impurities. The solvent of this filtrate was evaporated to get the dried extract (Hart & James, 2014). Further, the stock solution of the dried extract (AE) was prepared by adding 50 ml of 0.5 m HCl and kept in a clean corked bottle for further use. The Aloe leaves have a number of chemical constituents, of which aloe-emodin (A1) (C15H10O5) and aloetic acid (A2) (C15H6N4O13) are major components (Sharrif & Verma, 2011), and, being electron rich as well, are expected to offer the inhibition properties (Hart & James, 2014). The chemical structures of A1 and A2 are presented in Figure 1A and B, respectively, proving that the compounds are rich in electron density also evidenced by the FTIR study (Kumar & Mathur, 2013; Sharrif & Verma, 2011; Tuaweri et al., 2015).

FTIR of Aloe vera leaf powder.
Structures of (A) aloe-emodin and (B) aloetic acid.
2.1.1 Fourier transform infrared spectroscopy study
The FTIR of fine powder of Aloe vera leaf was recorded by Perkin-Elmer FT-IR/RZX. The spectrum ranges from 4000 cm-1 to 400 cm-1. This spectrum shows the presence of phenolic –OH (corresponds to 3412.44 cm-1). The peak at 1605.90 cm-1 corresponds to >CO group, and a peak at 1419.11 cm-1 indicates the presence of C=C bond, whereas –COC corresponds to 1075.51 cm-1, and the absorption bands at almost 1300 cm-1 and 1390 cm-1 show the presence of –NO2 group. The weak absorption band at 620.04 cm-1 may be due to C-H bending, which further indicates the presence of polymeric compounds present in Aloe vera powder. The FTIR peaks present accord well with the infrared spectrum obtained from other researchers (Hamman, 2008; Kumar & Mathur, 2013; Ray & Aswatha, 2013).
2.2 Corrosive solutions
Test solutions of 0.5 m HCl were prepared after dilution of AR grade 35% HCl (Qualichem) with double-distilled water. The concentration of the inhibitor’s stock solution was further diluted to obtain different concentrations of AE solution. The concentration range of AE used for the corrosion studies was 0.5%, 1.0%, 1.5%, 2.0%, and 2.5% v/v.
2.3 Mild steel coupons
Gravimetric analysis and electrochemical studies were performed on MS coupons of dimensions 3 cm×1.5 cm× 0.028 cm and 1 cm2, respectively. For preparation, test coupons were polished successively, using grades of emery paper of 400, 800, 1000, and 1200.
2.4 Gravimetric analysis
The MS specimens used had a rectangular shape of 3 cm× 1.5 cm×0.028 cm were abraded with a series of emery papers (400, 800, 1000, and 1200 grades) and then washed with distilled water and finally with acetone. After weighing accurately (citizen scale CX230), the specimens were immersed in conical flask containing 200 ml of 0.5 m HCl in the absence and presence of different concentrations of the inhibitor. Each experiment was performed in duplicate in order to get reproducible results, and the temperature was thermostatically controlled at 303 K in thermostat for 4 h, using freshly prepared solution. Then, the specimens were taken out, washed carefully to remove the corrosion product, dried, and weighed again accurately.
2.5 Electrochemical polarization
The electrochemical analysis was carried out in a conventional three-electrode cell using Autolab Potentiostat Galvanostat (PGSTAT204), Netherlands. A carbon electrode was used as counter electrode along with a saturated Ag/AgCl electrode coupled to a fine Luggin capillary as reference electrode, and the exposed area of the working electrode was 1 cm2 during polarisation and for corrosion attack. Tafel polarization curves were obtained by changing the electrode potential automatically from -500 to +500 mV at open circuit potential with a scan rate of 1 mV s-1. Stern-Geary method (El-Etre et al., 2005) was used for determining corrosion current by extrapolation of anodic and cathodic Tafel lines to a point which gives log icorr and the corresponding corrosion potential (Ecorr) for acidic medium.
2.6 Electrochemical impedance spectroscopy technique
The electrochemical impedance spectroscopy EIS study was performed on the same cell setup as used for polarization technique. The parameters were observed on the analyzer in a frequency range of 1.0×105 to 1.0 Hz with the AC signal 5 mV peak to peak at open circuit potential, stabilized at 303±1 K for 4 h. The main parameter deduced from the analysis of Nyquist diagram is the resistance of charge transfer Rct (diameter of high frequency loop) which is then calculated to give the double layer capacitance Cdl. The fmax values are derived as a result of EIS technique.
2.7 Quantum chemical analysis
This technique is a theoretical means of determining the effectiveness of any inhibitor. AM1 (Austin Model 1), a semiempirical method based on the neglect of differential diatomic overlap approximation, was used for the quantum chemical studies. The quantum parameters were calculated using HyperChem professional 8.0 package (Hypercube Inc., USA). The technique was studied to correlate the various parameters and their results with the studied experimental data.
3 Results and discussion
3.1 Gravimetric analysis
The values of weight loss, inhibition efficiency (% IE), and surface coverage (θ) was obtained from gravimetric (weight loss) measurements at different concentrations (Eddy & Ebenso, 2010) of AE in 0.5 m HCl solution at 303 K temperature, which are summarized in Table 1.
Gravimetric data of AE at 303 K at different concentrations.
| Concentration (% v/v) | ΔW (Wo-Wi) (mg/cm2 h) | % IE | θ |
|---|---|---|---|
| 0 | 30.4 | – | – |
| 0.5 | 3.51 | 88.3 | 0.883 |
| 1.0 | 2.85 | 90.5 | 0.905 |
| 1.5 | 2.34 | 92.2 | 0.922 |
| 2.0 | 1.98 | 93.4 | 0.934 |
| 2.5 | 1.44 | 95.2 | 0.952 |
| 3.0 | 1.42 | 95.3 | 0.953 |
The coupons were weighed before and after immersion in the test solution, where Wo and Wi are the values of corrosion weight loss (mg/cm2 h) of MS in uninhibited and inhibited solutions, respectively. The increase in inhibition efficiency was observed with the inhibitor concentration. Maximum inhibition efficiency was showed at 2.5% AE. The next higher concentration is tabulated in Table 1 showing optimization of the inhibition effect by the inhibitor, and it was found that after 2.5% v/v, the inhibitor showed almost constancy in the inhibition behavior.
3.2 Electrochemical polarization
From the anodic and cathodic polarization curves of the Tafel plot (Bentiss et al., 2009) presented in Figure 2, various electrochemical parameters like corrosion current density (icorr), corrosion potential (Ecorr), and cathodic and anodic Tafel slopes (βc and βa, respectively) are listed in Table 2 with their computed inhibition efficiency (% IE) using the equation below.
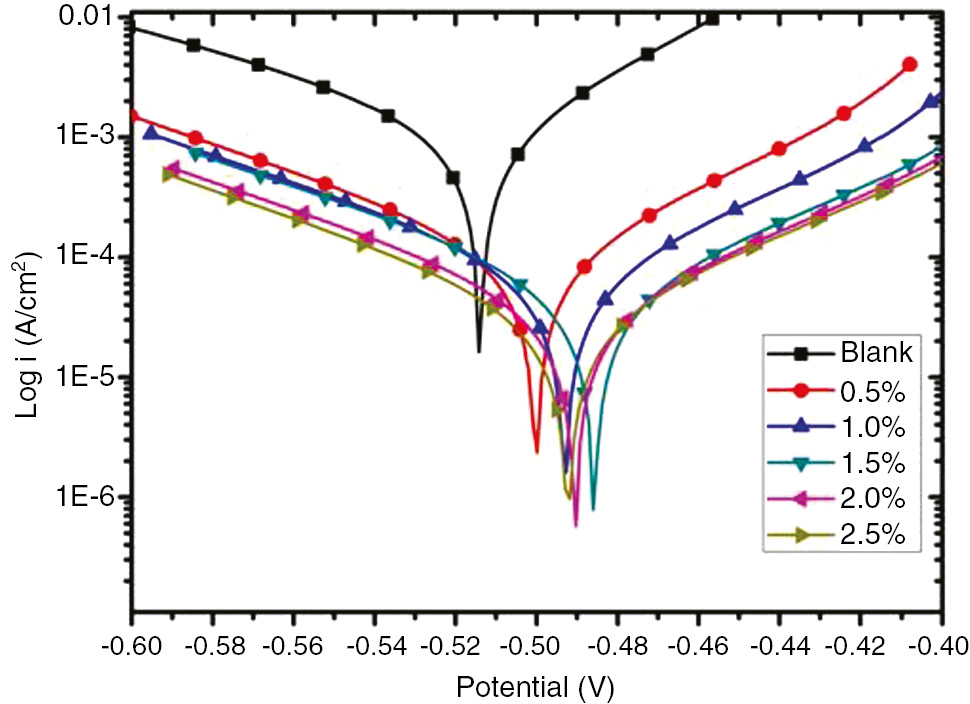
Tafel plot of AE at different concentrations on MS in 0.5 m HCl.
Electrochemical polarization parameters of AE at different concentrations on MS in 0.5 m HCl.
| Concentration (% v/v) | Ecorr (-mV) | βa (V/dec) | βc (V/dec) | CR (mmpy) | icorr (μA) | % IE | Θ |
|---|---|---|---|---|---|---|---|
| 0 | 504 | 111.3 | 98.56 | 15.41 | 1320.0 | – | – |
| 0.5 | 500 | 84.96 | 69.10 | 1.242 | 106.87 | 91.90 | 0.9190 |
| 1.0 | 493 | 92.03 | 78.10 | 0.913 | 78.54 | 94.05 | 0.9405 |
| 1.5 | 485 | 83.66 | 80.49 | 0.644 | 55.40 | 95.80 | 0.9580 |
| 2.0 | 490 | 92.82 | 89.98 | 0.517 | 44.54 | 96.62 | 0.9662 |
| 2.5 | 493 | 91.50 | 85.99 | 0.437 | 37.66 | 97.14 | 0.9714 |
The iocorr is the current density in the absence of AE and icorr in the presence of AE, and it was observed that the Ecorr values were shifted slightly to the anodic region in comparison to the Ecorr value obtained without inhibitor. However, both the anodic and cathodic Tafel slope values decreased with the increase in the concentration of the inhibitor, indicating the mixed mode of inhibition (Ahamad & Quraishi, 2010). This reveals that the green inhibitor also reduces anodic dissolution along with the reduction of cathodic hydrogen evolution.
3.3 Electrochemical impedance spectroscopy
From the Nyquist plots for MS in acid media as in Figure 3A, the plots are not perfect semicircles, attributing to the non-homogeneity of the surface and roughness of the metal (Bouklah et al., 2006). Also, these plots show the increase in impedance response on MS with addition of AE in the medium by their increasing diameters, corresponding to charge transfer resistance (Rct) values (Musa et al., 2012). The double-layer capacitance (Cdl) values were calculated using equation given, by fmax, maximum frequency.

Nyquist plots.
(A) Nyquist plots of AE at different concentrations and (B) equivalent circuit diagram for the Nyquist plot of the system.
Table 3 shows that the Rct values increased with the increase of the concentration of AE, which shows protection of MS surface by the inhibitor, while the values of Cdl decreased with the increase in the thickness of protective layer at higher concentrations. The inhibition efficiency (% IE) and surface coverage, calculated from the values of Rct, were both found to be maximum at a concentration of 2.5% v/v of AE, using the equation (Musa et al., 2012) below.
EIS parameters of AE at different concentrations on MS in 0.5 m HCl.
| Concentration (% v/v) | Rct (Ohm cm2) | Cdl (mF cm2) | % IE | θ |
|---|---|---|---|---|
| 0 | 14.17 | 2.14 | – | – |
| 0.5 | 111.2 | 0.0339 | 87.2 | 0.872 |
| 1.0 | 209.8 | 0.0095 | 93.2 | 0.932 |
| 1.5 | 263.9 | 0.0066 | 94.6 | 0.946 |
| 2.0 | 355.1 | 0.0031 | 96.0 | 0.960 |
| 2.5 | 473.3 | 0.0018 | 96.9 | 0.969 |
The findings of electrochemical studies (polarization and EIS) were in fair agreement with those of gravimetric studies along with slight deviations, tabulated in Figure 4. This deviation is due to the fact that the gravimetric study is an average technique of calculating the % IE (Bouklah et al., 2006), while an instantaneous efficiency can be calculated by the electrochemical studies performed.
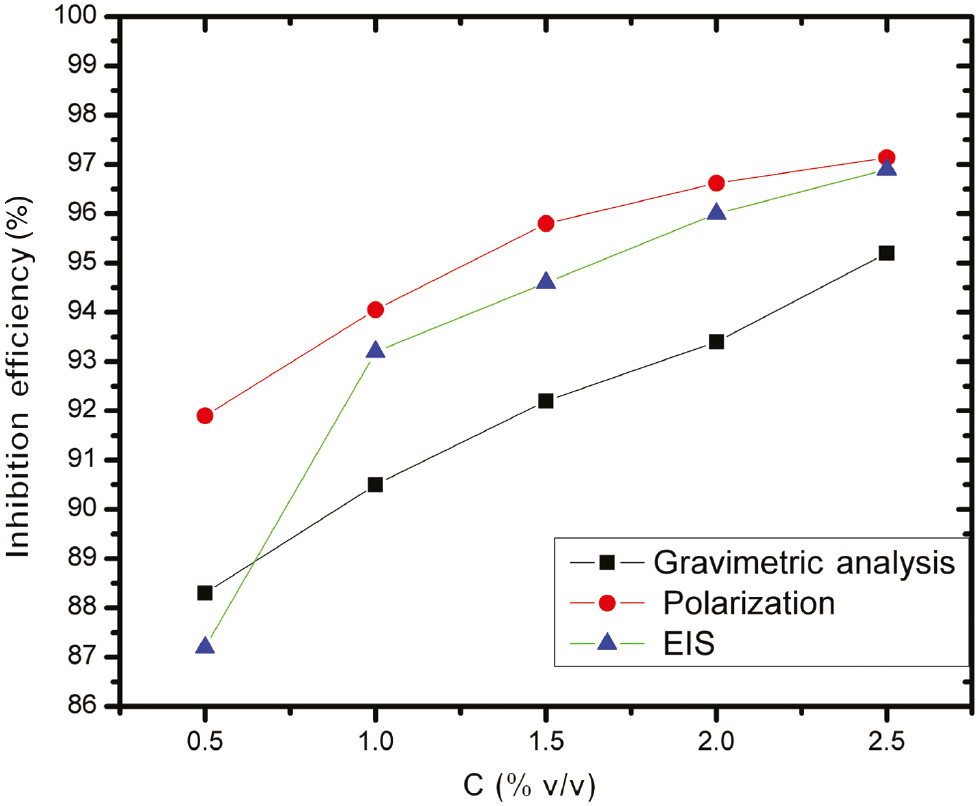
Comparative percent inhibition efficiency of AE on MS in 0.5 m HCl using different techniques.
3.4 Quantum chemistry
Adsorption of these organic inhibitors is also attained from interactions between metal (lowest unoccupied molecular orbitals – LUMO) and non-participant electrons of heteroatoms or π electrons of the inhibiting compound (highest occupied molecular orbitals – HOMO) (Sastri & Perumareddi, 1997). The energy gap between the two frontier orbitals (LUMO and HOMO) facilitates the extent of interaction between the metal and the inhibitor and hence a good adsorption.
The quantum chemical parameters (Lukovits et al., 2001), namely, the frontier molecular orbitals, EHOMO and ELUMO (HOMO and LUMO, respectively), the energy difference (ΔE) between EHOMO and ELUMO, dipole moment (μ), electron affinity (A), ionization potential (I), the absolute electronegativity (χ), absolute hardness (η), softness (σ), and the fraction of electrons (ΔN) transferred from inhibitor components, were determined and correlated with the experimental data, given in Table 4. Values of χinh, σinh, and ηinh were calculated by using the values of I and A obtained from quantum chemical calculation, where I=-EHOMO and A=-ELUMO
Quantum parameters of A1 and A2 components of AE on MS using Hyperchem 8.0.
| Quantum parameters | Aloe-emodin (A1) | Aloetic acid (A2) |
|---|---|---|
| EHOMO (eV) | -9.435 | -11.177 |
| ELUMO (eV) | -1.603 | -3.159 |
| ΔE, Energy gap (eV) | 7.832 | 8.018 |
| μ, Dipole moment (Debye) | 1.68 | 5.692 |
| χ, Absolute electronegativity (eV/mol) | 5.515 | 7.168 |
| η, Hardness (eV/mol) | 3.91 | 4.009 |
| ΔN, Fraction of electrons | 0.189 | 0.021 |
and ΔN, the fraction of electrons transferred from inhibitor to the iron molecule, was calculated, using a theoretical χFe value of 7 eV/mol and ηFe value of 0 eV/mol for iron atom in the equation as below:
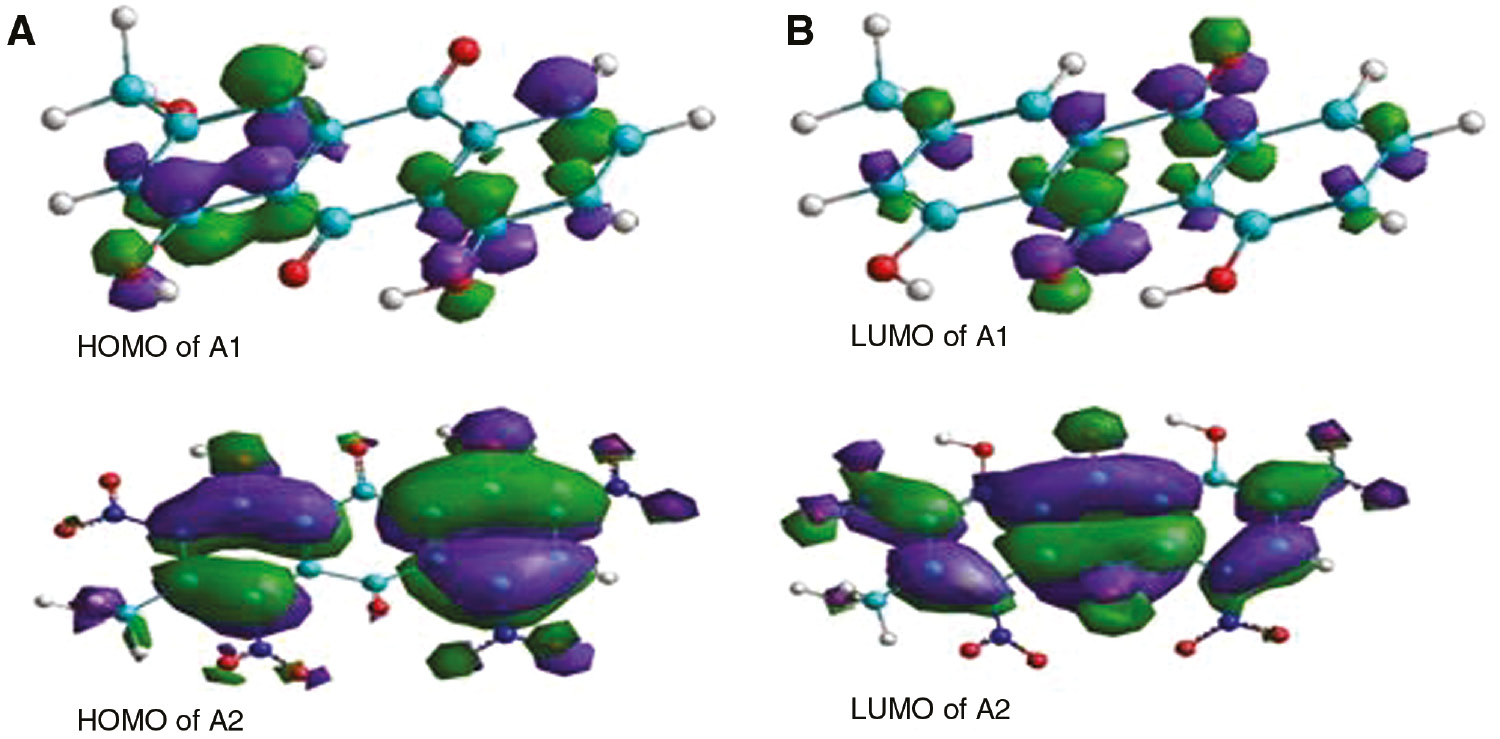
(A) HOMO and (B) LUMO of A1 and A2 components of AE.
The quantum analysis (Figure 5) predicts adsorption centers of the inhibitor molecules responsible for the interaction with metal atoms (Fang & Li, 2002). The study provides the spatial distribution of electronic density of the molecules on formation of chelating centers. The structure of the adsorbing components is influenced by the electron density on the active centers of the inhibitor. The components A1 and A2 have greater electron density on O and N heteroatoms present on the planar conjugated ring system of the molecules. These atoms are expected to contribute to the interaction with the MS surface. Any compound playing a role of outstanding corrosion inhibitor not only offers electrons to the unoccupied 3D orbitals of Fe but also accepts freely available electrons present on the metal resulting in back-bonding (Fang & Li, 2002; Sastri & Perumareddi, 1997). This back-bonding may also take place in the present study advocating its high degree of efficiency.
3.5 Adsorption isotherm
The interactions of the inhibitor and the MS surface can be examined by the adsorption isotherm. The degree of surface coverage (θ) values for various concentrations (c) of the inhibitors in the solution have been estimated from the polarization measurements. A straight line with linear correlation coefficient (R2) is >0.9999 and suggests that the adsorption is following the isotherm studied. The AE is found to follow Langmuir isotherm (Aisha et al., 2013) based upon the equation below:
The plot (Figure 6A) is drawn between C/θ vs. C with R2=1.000, suggesting that the adsorption of AE on MS is monolayer.

(A) Langmuir adsorption isotherm and (B) Dubinin-Radushkevich (D-R) isotherm of AE on MS.
Further from the isotherm, Gibbs free energy (ΔG) can be deduced using the equation
The value of ΔG is calculated as -18.05 kJ/mol, indicating that the adsorption is physical and confirming the experimental details. Supplementary to the Langmuir adsorption, the Dubinin-Radushkevich (D-R) isotherm (Amer et al., 2015) was also studied, using equation (11)
where ε can be correlated as
The value of E ascertains the kind of adsorption as physical adsorption when its value lies below 8 kJ/mol and chemical adsorption when this value is found to be in the range of 8–16 kJ/mol (Amer et al., 2015). The value of E from the present study is 0.267 kJ/mol, proving the adsorption as physical in nature.
4 Proposed mechanism of inhibition
Corrosion inhibition mechanism depends upon the adsorption of inhibitor compound over the MS surface in 0.5 m HCl. The acidic medium used does clean surfaces from rust, scales, etc., even in the presence of the inhibitor. In fact, the additional use of inhibitors protects the surface from supplementary acid attack on metal surface during its usage.
The proficiency of inhibitor depends on a number of factors, such as
the number of adsorption sites and their charge density,
molecular size,
heat of adsorption,
mode of interaction with the metal surface,
extent of formation of metal complexes.
The charge of the MS surface is determined by potential of zero charge (pzc) on correlative scale by the following equation (Shukla & Quraishi, 2010):
where Eq=0 is pzc with the correlation value (φc) as positive, showing that the MS surface has acquired the positive charge. The inhibitor molecule is either a neutral or a protonated species in the HCl medium. Since the surface is positively charged, the Cl- ions get adsorbed onto the surface making the metal surface negatively charged, and due to electrostatic forces of attraction between the protonated inhibitor, chloride ions and charged metal surface (complexation) gets adsorbed on MS surface. The Langmuir adsorption isotherm and D-R isotherm also support the finding that the adsorption of AE is quite physical in nature. The A1 and A2 components might get adsorbed on the MS surface through their planar conjugated system. The heteroatoms O and N, with higher electron density, may also enhance the direct π orbital donation by the inhibitor resulting into its molecular interaction over the positively charged Fe surface.
The quantum analysis also supports the inhibition by the molecule showing adsorption of AE components. The A2 component with high dipole value tends to donate its electron density towards the Fe surface more easily and efficiently making it a good inhibitor (Amin et al., 2010). Secondly, as the literature suggests (Bahrami et al., 2010), the energy gap between the HOMO and LUMO might also take part in this kind of donation which is true for A1 component along with larger fraction of electrons transferred. Hence, both the components co-operate with each other during the adsorption process.
5 Conclusion
The study has the following findings regarding the inhibition:
The AE acts as an excellent inhibitor for MS in 0.5 m HCl solution, and the extent of inhibition efficiency is directly proportional to the concentration of the inhibitor.
Tafel polarization curves showed that the compound has a mixed mode of inhibition.
The impressive performance of the inhibitor has been confirmed from Nyquist plots where the enhancing adsorption of the inhibitor gradually retards Cdl at the expense of Rct.
The adsorption model obeys Langmuir adsorption isotherm with ΔG°ads and Kads justifying the monolayer physical nature of adsorption on MS surface, and D-R isotherm supports the physiosorption phenomenon.
Quantum chemical calculations of the two major components of the extract substantiate the inhibition efficiencies obtained from experimental results.
Acknowledgments
The authors are grateful to the Department of Science and Technology (DST), Haryana, India, for providing financial assistance for completion of the research work under the HSCST-Fellowship 2013/1 scheme.
References
Ahamad I, Quraishi MA. Mebendazole: new and efficient corrosion inhibitor for mild steel in acid medium. Corros Sci 2010; 52: 651–656.10.1016/j.corsci.2009.10.012Suche in Google Scholar
Al-Turkustani AM, Al-Sawat RM, Al-Hassani RH, Al-Ghamdi NS, Al-Harbi EM, Al-Gamdi MA, Al-Solmi SA. Corrosion behaviour of mild steel in acidic solution using the aqueous seed extract of Phoenix dactylifera L (Date seeds). J Chem Acta 2013; 2: 253–261.Suche in Google Scholar
Amer MW, Ahmad RA, Awwad AM. Biosorption of Cu(II), Ni(II), Zn(II) and Pb(II) ions from aqueous solution by Sophora japonica pods powder. Int J Ind Chem 2015; 6: 67–75.10.1007/s40090-014-0030-8Suche in Google Scholar
Amin MA, Khaled KF, Fadl-Allah SA. Testing validity of Tafel extrapolation method for monitoring corrosion of cold rolled steel in HCl solutions – experimental and theoretical studies. Corros Sci 2010; 52: 140–151.10.1016/j.corsci.2009.08.055Suche in Google Scholar
Bahrami MJ, Hosseini SMA, Pilvar P. Experimental and theoretical investigation of organic compounds as inhibitors for mild steel corrosion in sulfuric acid medium. Corros Sci 2010; 52: 2793–2803.10.1016/j.corsci.2010.04.024Suche in Google Scholar
Bentiss F, Lebrini M, Vezin H, Chai F, Traisnel M, Lagrené M. Enhanced corrosion resistance of carbon steel in normal sulfuric acid medium by some macrocyclic polyether compounds containing a 1,3,4-thiadiazole moiety: AC impedance and computational studies. Corros Sci 2009; 51: 2165–2173.10.1016/j.corsci.2009.05.049Suche in Google Scholar
Bouklah M, Hammouti B, Lagrenée M, Bentiss F. Thermodynamic properties of 2,5-bis(4-methoxyphenyl)-1,3,4-oxadiazole as a corrosion inhibitor for mild steel in normal sulfuric acid medium. Corros Sci 2006; 48: 2831–2842.10.1016/j.corsci.2005.08.019Suche in Google Scholar
Eddy NO, Ebenso EE. Corrosion inhibition and adsorption characteristics of tarivid on mild steel in H2SO4. E-J Chem 2010; 7: S442–S448.10.1155/2010/594743Suche in Google Scholar
El-Etre AY, Abdallah M, El-Tantawy ZE. Corrosion inhibition of some metals using lawsonia extract. Corros Sci 2005; 47: 385–395.10.1016/j.corsci.2004.06.006Suche in Google Scholar
Fang J, Li J. Quantum chemistry study on the relationship between molecular structure and corrosion inhibition efficiency of amides. J Mol Struct: THEOCHEM 2002; 593: 179–185.10.1016/S0166-1280(02)00316-0Suche in Google Scholar
Hamman JH. Composition and application of Aloe vera leaf gel. Molecules 2008; 13: 1599–1616.10.3390/molecules13081599Suche in Google Scholar PubMed PubMed Central
Hart K, James AO. The inhibitive effect of Aloe vera barbadensis gel on copper in hydrochloric acid medium. J Emerg Trends Eng Appl Sci (JETEAS) 2014; 5: 24–29.Suche in Google Scholar
Kumar S, Mathur SP. Corrosion inhibition and adsorption properties of ethanolic extract of Calotropis for corrosion of aluminium in acidic media. ISRN Corrosion 2013; 2013: Article ID 476170; http://dx.doi.org/10.1155/2013/476170.10.1155/2013/476170Suche in Google Scholar
Lukovits I, Kálmán E, Zucchi F. Corrosion inhibitors – correlation between electronic structure and efficiency. Corrosion 2001; 57: 3–8.10.5006/1.3290328Suche in Google Scholar
Musa AY, Jalgham RTT, Mohamad AB. Molecular dynamic and quantum chemical calculations for phthalazine derivatives as corrosion inhibitors of mild steel in 1 M HCl. Corros Sci 2012; 56: 176–183.10.1016/j.corsci.2011.12.005Suche in Google Scholar
Muthukrishnan P, Saravana K, Jeyaprabha B, Prakash P. Anticorrosive activity of Kigelia pinnata leaves extract on mild steel in acidic media. Metall Mater Trans A 2014; 45: 4510–4524.10.1007/s11661-014-2366-2Suche in Google Scholar
Noor EA. Comparative study on the corrosion inhibition of mild steel by aqueous extract of Fenugreek seeds and leaves in acidic solutions. J Eng Appl Sci 2008; 3: 23–30.Suche in Google Scholar
Okafor V, Ikpi ME, Uwah IE, Ebenso EE, Ekpe UJ, Umoren SA. Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media. Corros Sci 2010; 50: 2310–2317.10.1016/j.corsci.2008.05.009Suche in Google Scholar
Quraishi MA, Yadav DK, Ahamad I. Green approach to corrosion inhibitor by black pepper extract in HCl solution. Open Corros J 2009; 2: 56–60.10.2174/1876503300902010056Suche in Google Scholar
Quraishi MA, Singh A, Singh VK, Yadav DK, Singh AK. Green approach to corrosion inhibition of mild steel in hydrochloric acid and sulphuric acid solutions by the extract of Murraya koenigii leaves. Mater Chem Phys 2010; 122: 114–122.10.1016/j.matchemphys.2010.02.066Suche in Google Scholar
Ray A, Aswatha SM. An analysis of the influence of growth periods on physical appearance, and acemannan and elemental distribution of Aloe vera L. gel. Ind Crop Prod 2013; 48: 36–42.10.1016/j.indcrop.2013.03.024Suche in Google Scholar
Saratha R, Vasudha VG. Emblica officinalis (Indian gooseberry) leaves extract as corrosion inhibitor for mild steel in 1N HCl. E-J Chem 2010; 7: 677–684.10.1155/2010/162375Suche in Google Scholar
Sastri VS, Perumareddi JR. Molecular orbital theoretical studies of some organic corrosion inhibitors. Corrosion 1997; 53: 617–622.10.5006/1.3290294Suche in Google Scholar
Sharrif MM, Verma SK. Aloe vera their chemicals composition and applications: a review. Int J Biol Med Res 2011; 2: 466–471.Suche in Google Scholar
Shukla SK, Quraishi MA. The effects of pharmaceutically active compound doxycycline on the corrosion of mild steel in hydrochloric acid solution. Corros Sci 2010; 52: 314–321.10.1016/j.corsci.2009.09.017Suche in Google Scholar
Singh A, Ebenso EE, Quraishi MA. Corrosion inhibition of carbon steel in HCl solution by some plant extracts. Int J Corrosion 2012; 2012: Article ID 897430; 10.1155/2012/897430.Suche in Google Scholar
Singh AK, Mohapatra S, Pani B. Corrosion inhibition effect of Aloe vera gel: gravimetric and electrochemical study. J Ind Eng Chem 2016; 33: 288–297.10.1016/j.jiec.2015.10.014Suche in Google Scholar
Tuaweri TJ, Ogbonnaya EA, Onyemaobi OO. Corrosion inhibition of heat treated mild steel with Neem leave extract in a chloride medium. IJRET 2015; 4: 404–409.10.15623/ijret.2015.0406069Suche in Google Scholar
©2016 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- In this issue
- Review
- On the performance of commercially available corrosion-resistant nickel alloys: a review
- Original articles
- Mechanical modeling of damage accumulation and life evaluation for stress corrosion cracking
- Resistance to chemical attack of cement composites impregnated with a special polymer sulfur composite
- Influence of 4 wt.% Cr addition on the corrosion-wear synergistic effect for Al2O3/Fe(Al) composites
- A descriptive study for corrosion control of low-alloy steel by Aloe vera extract in acidic medium
Artikel in diesem Heft
- Frontmatter
- In this issue
- Review
- On the performance of commercially available corrosion-resistant nickel alloys: a review
- Original articles
- Mechanical modeling of damage accumulation and life evaluation for stress corrosion cracking
- Resistance to chemical attack of cement composites impregnated with a special polymer sulfur composite
- Influence of 4 wt.% Cr addition on the corrosion-wear synergistic effect for Al2O3/Fe(Al) composites
- A descriptive study for corrosion control of low-alloy steel by Aloe vera extract in acidic medium

