To the Editor,
In the first weeks of life, many preterm infants fail to mount an appropriate cortisol response for the degree of illness or stress, known as relative adrenal insufficiency (RAI) [1]. This may manifest as refractory hypotension, defined as hypotension that is unresponsive to volume replacement and inotropes, but responds readily to systemic corticosteroids [1]. RAI may predispose to a pro-inflammatory state [2], but it is unknown at which cortisol level RAI is likely to manifest [3]. Studies in preterm infants with refractory hypotension have shown that serum cortisol level was unable to predict hydrocortisone treatment response [4]. Therefore, clinical practice guidelines do not recommend determination of cortisol before initiating corticosteroid treatment in hypotensive infants.
Low activity of immature adrenal cortex enzymes, mainly 11β-hydroxylase, is considered one of the mechanisms behind RAI [5]. This leads not only to a low cortisol by also to a relative abundance of the precursor corticosteroids 17-hydroxyprogesterone (17-OHP) and 11-deoxycortisol. Furthermore, in preterm infants the cortisol-cortisone shuttlefavors cortisone, reflecting the developmental timing of 11β-hydroxysteroid steroid isoenzyme expression similar to what is found in fetal tissues [1, 6]. Cortisol is often measured using immunoassays, which are prone to cross-reactivity. Studies have demonstrated that in some conditions cortisol levels were overestimated by immunoassays [7, 8]. In patients with congenital adrenal hyperplasia (CAH) due to 21alpha-hydroxylase deficiency, this was caused by cross-reactivity with elevated precursor corticosteroids [7]. Such cross-reactivity does not occur with liquid chromatography-tandem mass spectrometry (LC-MS/MS), which is highly specific and is therefore considered superior for steroid hormone analysis [7].
We hypothesized that immunoassays may overestimate cortisol levels in preterm infants, considering that their precursor corticosteroid levels may be elevated like in patients with CAH. To our knowledge, evidence of cross-reactivity in cortisol measurement has not been previously investigated in preterm populations. The aim of this study was to assess whether two widely employed immunoassays, i.e., the Cobas assay and the Alinity assay, are able to determine cortisol levels as accurately as LC-MS/MS in preterm infants.
We collected serum samples for measurement of cortisol and other corticosteroids in preterm infants born <30 weeks gestational age, who participated in the PRIDICT-BPD (PulmonaRy Inflammation and glucocorticoiD sensitivity for the prediCTion of BronchoPulmonary Dysplasia) study. This feasibility study aimed to test the role of various biomarkers for the prediction of BPD. The serum samples were collected directly after birth from cord blood, and at days 3, 7, 14 and 28 from capillary or arterial blood. For each time point four pools of samples were formed, each of which had an equal amount of leftover serum samples from six infants, resulting in a total number of 20 pools. Serum samples from infants who received postnatal corticosteroids were excluded for this specific study. The PRIDICT-BPD study was approved by the Medical Research Ethics Committee of the Vrije Universiteit Amsterdam (protocol number 2019.371).
Cortisol concentrations were measured using two immunoassays, i.e., the Cobas Cortisol II Assay (Roche Diagnostics, Rotkreuz, Switzerland), and the Alinity Cortisol Assay (Abbott Diagnostics, Chicago, IL, USA), and with our in-house developed LC-MS/MS method (as described earlier [9]). Our LC-MS/MS method has a lower limit of quantitation of 0.5 nmol/L, an intra-assay coefficient of variation (CV) of <5 %, and an inter-assay CV of 4.5 and 3.9 % at cortisol levels of 125 and 650 nmol/L, respectively. This method is well comparable to other LC-MS/MS methods [9]. All analyses were performed at the Endocrine Laboratory of the Amsterdam UMC (ISO15189 accredited).
We calculated the absolute difference in measured cortisol level of each immunoassay relative to the LC-MS/MS method. Agreement across the measurement range was tested using Passing and Bablok regression analysis, and correlation was tested using Pearson correlation.
Figure 1 shows the absolute difference in measured cortisol levels of each immunoassay relative to the LC-MS/MS method for each pool separately. For the Alinity assay, as compared to the LC-MS/MS method, the absolute difference in cortisol level was <50 nmol/L at all time points. For the Cobas assay, as compared with the LC-MS/MS method, the absolute difference in cortisol level was largest at day 3, with a maximum difference of 131 nmol/L (Cobas 394 vs. LC-MS/MS 263 nmol/L), and at day 7, with a maximum difference of 155 nmol/L (Cobas 491 vs. LC-MS/MS 336 nmol/L).
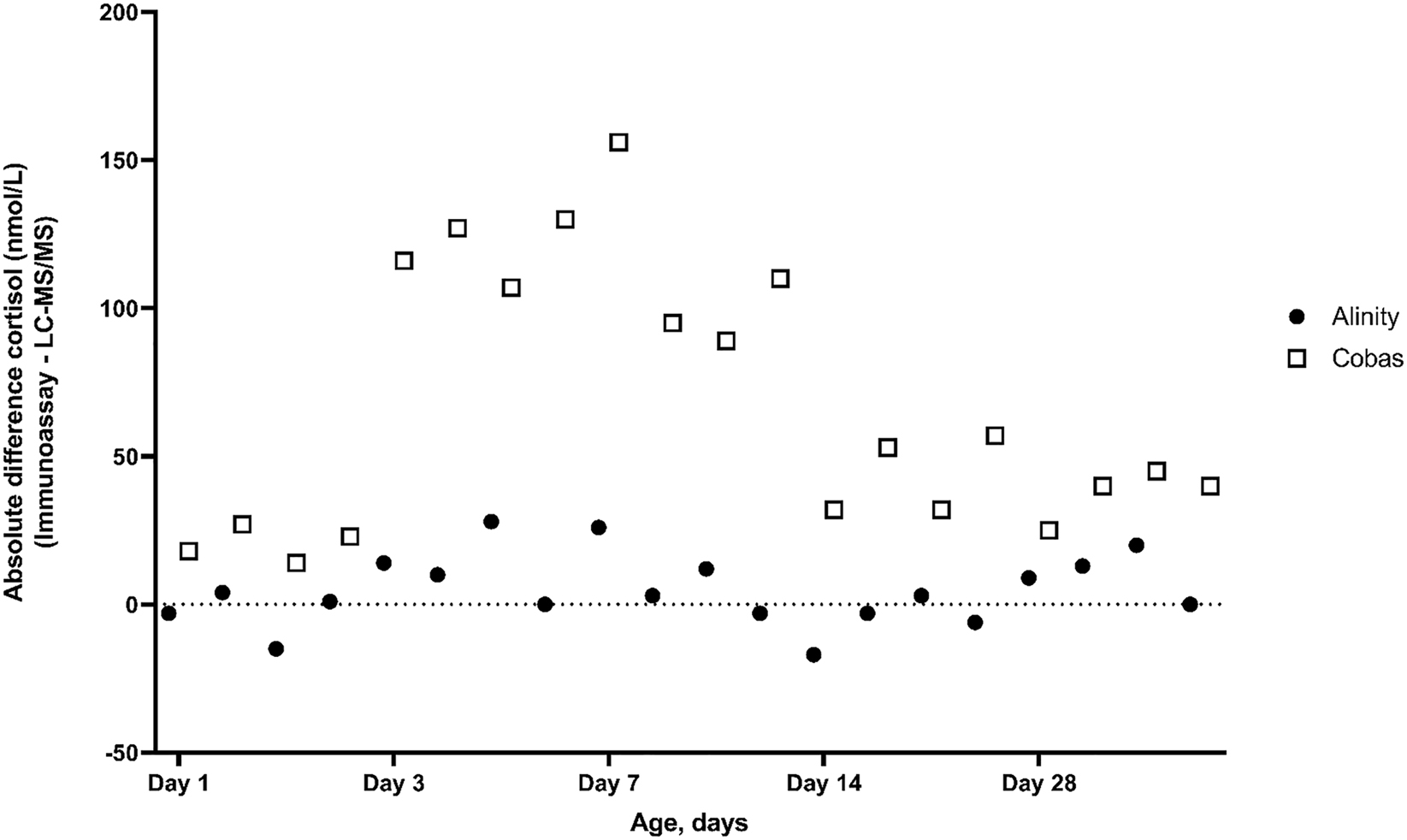
Absolute difference in cortisol level (nmol/L) of each immunoassay relative to the LC-MS/MS method by postnatal age in pooled samples of preterm infants. Cortisol levels were measured with two immunoassays (Alinity and Cobas) and with the LC-MS/MS method in preterm infants during the first four weeks of life. The zero line indicates no difference in cortisol levels between assay methods.
Passing and Bablok regression analysis showed a slope of 1.01 (95 % CI 0.95–1.08) and an intercept of 0.79 (95 % CI −9.1 to 15) nmol/L for the Alinity assay, indicating good agreement with the LC-MS/MS method (Figure 2A). For the Cobas assay, analysis showed poor agreement with the LC-MS/MS method, as shown by a slope of 1.39 (95 % CI 1.21–1.55), and an intercept of −3.13 (95 % CI −26.5 to 17.6) (Figure 2B). Pearson correlation coefficients were 0.984 and 0.947 for the Alinity assay and the Cobas assay, respectively.

Passing and Bablok regression analyses for serum cortisol measurements in pooled samples of preterm infants. On the x-axis, the cortisol concentrations measured using the LC-MS/MS method and on the y-axis the cortisol concentrations measured using the respective immunoassays are shown. (A) Alinity assay; (B) Cobas assay.
At last, Table 1 shows, in the same pooled samples, the results of also by LC-MS/MS measured levels of corticosteroids that could possibly lead to cross-reactivity. Concentrations varied between 143 and 188 nmol/L for cortisone, between 0.4 and 2.4 nmol/L for 11-deoxycortisol and between 8 and 41 nmol/L for corticosterone.
Mean corticosteroid concentrations (nmol/L), as assessed by LC-MS/MS, by postnatal age in pooled samples of preterm infants.
| Mean cortisol concentration, nmol/L | Mean cortisone concentration, nmol/L | Mean 11-deoxycortisol concentration, nmol/L | Mean corticosterone concentration, nmol/L | |
|---|---|---|---|---|
| Day 1 | 53 | 188 | 2.4 | 8 |
| Day 3 | 213 | 154 | 0.4 | 23 |
| Day 7 | 279 | 150 | 0.5 | 41 |
| Day 14 | 207 | 147 | 0.6 | 25 |
| Day 28 | 120 | 143 | 0.5 | 9 |
Our study shows that the Cobas assay, unlike the Alinity assay, severely overestimates cortisol levels in preterm infants. As such, it confirms previous observations demonstrating that in conditions characterized by elevated levels of precursor corticosteroids, immunoassays may be prone to cross-reactivity with hormones that share the general structure of the hormone of interest [8]. An implication of our findings is that previous studies among preterm infants addressing associations between serum cortisol levels and clinical outcomes should be interpreted cautiously, because of the possible risk of overestimated cortisol levels.
In healthy adults without medication use, cross-reactivity is unlikely to play a major role in immunoassays for cortisol. This was corroborated by analyses at our laboratory, demonstrating good agreement of both immunoassays with our LC/MS-MS method, with slopes of 0.94 (95 % CI 0.88–0.98) and 0.96 (95 % CI 0.93–0.98), intercepts of −5.9 (95 % CI −16 to 8.1) and 1.3 (95 % CI −4.9 to 8.1) and correlation coefficients of 0.986 and 0.996 for the Alinity assay and the Cobas assay, respectively [10].
The adrenocortical function of preterm infants is, similar to CAH, characterized by an abundance of corticosteroid precursors relative to cortisol, and by high cortisone levels. Although this biochemical pattern likely offers an explanation for overestimated cortisol levels by use of immunoassays, it is unclear why the degree of cross-reactivity differs between immunoassays, in spite of absence of evidence for substantial cross-reactivity according to both kit inserts. According to the kit inserts, cross-reactivity was estimated at 4.9 and 1.9 % for 11-deoxycortisol, at 0.08 and 0.6 % for 17-OHP, at 6.6 and 2.7 % for cortisone, and at 2.5 and 0.9 % for corticosterone in the Cobas assay (after addition of 10 μg/mL and the Alinity assay (after addition of 100 μg/dL 11-deoxycortisol and 1,000 μg/dL 17-OHP and cortisone), respectively. Levels of cortisone, 11-deoxycortisol and corticosterone measured by LC-MS/MS, only marginally contributed to cross-reactivity in cortisol measurement (see Table 1). However, other steroid hormones could contribute to cross-reactivity too. Another mechanism which could lead to falsely elevated results in the cortisol immunoassay are differences in the serum protein composition between preterm infants and term infants or adults. Among these differences is the concentration of cortisol-binding globulin (CBG), which is lower in preterm infants [11, 12]. Low CBG levels were previously shown to overestimate cortisol levels in certain immunoassays [13]. Nonetheless, it should be acknowledged that reported cross-reactivity may vary between patient populations, and, therefore, we would like to call upon manufacturers to improve their cortisol immunoassays for patient populations with a different composition of precursor corticosteroids and/or corticosteroid metabolites than healthy adults.
One of the limitations of this study is that due to the relatively large serum volume required for immunoassays in relation to the limited volume available, we had to pool samples from multiple infants, and we were unable to test other cortisol immunoassays being used in clinical practice. However, it can be expected that more cortisol immunoassays are susceptible to overestimation in preterm infants, encouraging further testing.
In conclusion, some immunoassays may overestimate serum cortisol levels in preterm infants in their first weeks of life, necessitating the use of a LC-MS/MS method or an immunoassay that has proved to be accurate in preterm infants.
Funding source: Amsterdam Reproduction and Development Research Institute
-
Research funding: This research was supported by the Amsterdam Reproduction and Development Research Institute.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: Research involving human subjects complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as revised in 2013), and has been approved by the authors’ institutional review board (Medical Research Ethics Committee of the Vrije Universiteit Amsterdam, protocol number 2019.371).
References
1. Finken, MJJ, van der Voorn, B, Hollanders, JJ, Ruys, CA, de Waard, M, van Goudoever, JB, et al.. Programming of the hypothalamus-pituitary-adrenal axis by very preterm birth. Ann Nutr Metab 2017;70:170–4. https://doi.org/10.1159/000456040.Search in Google Scholar PubMed PubMed Central
2. Watterberg, KL, Scott, SM, Backstrom, C, Gifford, KL, Cook, KL. Links between early adrenal function and respiratory outcome in preterm infants: airway inflammation and patent ductus arteriosus. Pediatrics 2000;105:320–4. https://doi.org/10.1542/peds.105.2.320.Search in Google Scholar PubMed
3. Ng, PC, Wong, SP, Chan, IH, Lam, HS, Lee, CH, Lam, CW. A prospective longitudinal study to estimate the “adjusted cortisol percentile” in preterm infants. Pediatr Res 2011;69:511–6. https://doi.org/10.1203/pdr.0b013e31821764b1.Search in Google Scholar PubMed
4. Baker, CF, Barks, JD, Engmann, C, Vazquez, DM, Neal, CRJr, Schumacher, RE, et al.. Hydrocortisone administration for the treatment of refractory hypotension in critically ill newborns. J Perinatol 2008;28:412–9. https://doi.org/10.1038/jp.2008.16.Search in Google Scholar PubMed
5. Kamrath, C, Hartmann, MF, Boettcher, C, Wudy, SA. Reduced activity of 11beta-hydroxylase accounts for elevated 17alpha-hydroxyprogesterone in preterms. J Pediatr 2014;165:280–4. https://doi.org/10.1016/j.jpeds.2014.04.011.Search in Google Scholar PubMed
6. Stewart, PM, Murry, BA, Mason, JI. Type 2 11 beta-hydroxysteroid dehydrogenase in human fetal tissues. J Clin Endocrinol Metab 1994;78:1529–32. https://doi.org/10.1210/jcem.78.6.8200959.Search in Google Scholar PubMed
7. Agrawal, N, Chakraborty, PP, Sinha, A, Maiti, A. False elevation of serum cortisol in chemiluminescence immunoassay by Siemens Advia Centaur XP system in 21-hydroxylase deficiency: an ‘endocrine laboma’. BMJ Case Rep 2020;13:e235450. https://doi.org/10.1136/bcr-2020-235450.Search in Google Scholar PubMed PubMed Central
8. Krasowski, MD, Drees, D, Morris, CS, Maakestad, J, Blau, JL, Ekins, S. Cross-reactivity of steroid hormone immunoassays: clinical significance and two-dimensional molecular similarity prediction. BMC Clin Pathol 2014;14:33. https://doi.org/10.1186/1472-6890-14-33.Search in Google Scholar PubMed PubMed Central
9. Fanelli, F, Cantu, M, Temchenko, A, Mezzullo, M, Lindner, JM, Peitzsch, M, et al.. Report from the HarmoSter study: impact of calibration on comparability of LC-MS/MS measurement of circulating cortisol, 17OH-progesterone and aldosterone. Clin Chem Lab Med 2022;60:726–39. https://doi.org/10.1515/cclm-2021-1028.Search in Google Scholar PubMed
10. Jansen, HI, van Herwaarden, AE, Huijgen, HJ, Vervloet, MG, Hillebrand, JJ, Boelen, A, et al.. Lower accuracy of testosterone, cortisol, and free T4 measurements using automated immunoassays in people undergoing hemodialysis. Clin Chem Lab Med 2023;61:1436--45. https://doi.org/10.1515/cclm-2022-1133.Search in Google Scholar PubMed
11. Hanna, CE, Jett, PL, Laird, MR, Mandel, SH, LaFranchi, SH, Reynolds, JW. Corticosteroid binding globulin, total serum cortisol, and stress in extremely low-birth-weight infants. Am J Perinatol 1997;14:201–4. https://doi.org/10.1055/s-2007-994127.Search in Google Scholar PubMed
12. Scott, SM, Wells, L. Corticosteroid-binding globulin in preterm infants in an intensive care unit. Horm Res 1995;44:218–21. https://doi.org/10.1159/000184629.Search in Google Scholar PubMed
13. Vos, MJ, Bisschop, PH, Deckers, MML, Endert, E. The cortisol-CBG ratio affects cortisol immunoassay bias at elevated CBG concentrations. Clin Chem Lab Med 2017;55:e262–4. https://doi.org/10.1515/cclm-2017-0095.Search in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/cclm-2023-0123).
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Laboratory Medicine: from just testing to saving lives
- Reviews
- Serum biomarkers of remodeling in severe asthma with fixed airway obstruction and the potential role of KL-6
- Safety monitoring of drug-induced muscle injury and rhabdomyolysis: a biomarker-guided approach for clinical practice and drug trials
- Mini Review
- Concise review on the combined use of immunocapture, mass spectrometry and liquid chromatography for clinical applications
- Opinion Paper
- Recommendation for the design of stability studies on clinical specimens
- General Clinical Chemistry and Laboratory Medicine
- Assessment of WHO 07/202 reference material and human serum pools for commutability and for the potential to reduce variability among soluble transferrin receptor assays
- veRification: an R Shiny application for laboratory method verification and validation
- Impact of storage temperature and time before analysis on electrolytes (Na+, K+, Ca2+), lactate, glucose, blood gases (pH, pO2, pCO2), tHb, O2Hb, COHb and MetHb results
- The stability of blood gases and CO-oximetry under slushed ice and room temperature conditions
- Elevated levels of renal function tests conferred increased risks of developing various pregnancy complications and adverse perinatal outcomes: insights from a population-based cohort study
- Poor comparability of plasma renin activity measurement in determining patient samples: the status quo and recommendations for harmonization
- Salivary cortisol and cortisone in diagnosis of Cushing’s syndrome – a comparison of six different analytical methods
- Improved diagnostics of purine and pyrimidine metabolism disorders using LC-MS/MS and its clinical application
- Analytical evaluation of a GAD65 antibodies chemiluminescence immunoassay for CSF in neurological syndromes
- Reference Values and Biological Variations
- Evaluation of low-density lipoprotein cholesterol equations by cross-platform assessment of accuracy-based EQA data against SI-traceable reference value
- Highly sensitive tandem mass spectrometric measurement of serum estradiol without derivatization and pediatric reference intervals in children and adolescents
- Cancer Diagnostics
- Practical delta check limits for tumour markers in different clinical settings
- Comparison between free β subunit of human chorionic gonadotropin (hCG) and total hCG assays in adults with testicular cancer
- Hematology and Coagulation
- Value of monocyte distribution width for predicting severe cholecystitis: a retrospective cohort study
- Performance of digital morphology analyzer Medica EasyCell assistant
- Validation of non-invasive point of care blood content analysis using the TensorTip™ MTX device: a method comparison study
- Infectious Diseases
- Kinetics and ability of binding antibody and surrogate virus neutralization tests to predict neutralizing antibodies against the SARS-CoV-2 Omicron variant following BNT162b2 booster administration
- Letters to the Editor
- The first case of VEXAS syndrome in Austria
- Acetylcholine receptor and muscle-specific tyrosine kinase antibodies detection: is it time for a change?
- Performance of the 2009 CKDEPI, 2021 CKDEPI, and EKFC equations among high-risk patients in Denmark
- Biotin interference in immunoassays: water under the bridge?
- The new synthetic benzimidazole opioid etonitazepipne: an emerging fatal harm and a challenge for laboratory medicine
- Unexplained increase of serum carcinoembryonic antigen: don’t forget the thyroid!
- Falsely elevated cortisol serum levels in preterm infants due to use of immunoassay
- Misdiagnosis of Hb Bart’s disease: prenatal screening and diagnosis of thalassemia in special population
Articles in the same Issue
- Frontmatter
- Editorial
- Laboratory Medicine: from just testing to saving lives
- Reviews
- Serum biomarkers of remodeling in severe asthma with fixed airway obstruction and the potential role of KL-6
- Safety monitoring of drug-induced muscle injury and rhabdomyolysis: a biomarker-guided approach for clinical practice and drug trials
- Mini Review
- Concise review on the combined use of immunocapture, mass spectrometry and liquid chromatography for clinical applications
- Opinion Paper
- Recommendation for the design of stability studies on clinical specimens
- General Clinical Chemistry and Laboratory Medicine
- Assessment of WHO 07/202 reference material and human serum pools for commutability and for the potential to reduce variability among soluble transferrin receptor assays
- veRification: an R Shiny application for laboratory method verification and validation
- Impact of storage temperature and time before analysis on electrolytes (Na+, K+, Ca2+), lactate, glucose, blood gases (pH, pO2, pCO2), tHb, O2Hb, COHb and MetHb results
- The stability of blood gases and CO-oximetry under slushed ice and room temperature conditions
- Elevated levels of renal function tests conferred increased risks of developing various pregnancy complications and adverse perinatal outcomes: insights from a population-based cohort study
- Poor comparability of plasma renin activity measurement in determining patient samples: the status quo and recommendations for harmonization
- Salivary cortisol and cortisone in diagnosis of Cushing’s syndrome – a comparison of six different analytical methods
- Improved diagnostics of purine and pyrimidine metabolism disorders using LC-MS/MS and its clinical application
- Analytical evaluation of a GAD65 antibodies chemiluminescence immunoassay for CSF in neurological syndromes
- Reference Values and Biological Variations
- Evaluation of low-density lipoprotein cholesterol equations by cross-platform assessment of accuracy-based EQA data against SI-traceable reference value
- Highly sensitive tandem mass spectrometric measurement of serum estradiol without derivatization and pediatric reference intervals in children and adolescents
- Cancer Diagnostics
- Practical delta check limits for tumour markers in different clinical settings
- Comparison between free β subunit of human chorionic gonadotropin (hCG) and total hCG assays in adults with testicular cancer
- Hematology and Coagulation
- Value of monocyte distribution width for predicting severe cholecystitis: a retrospective cohort study
- Performance of digital morphology analyzer Medica EasyCell assistant
- Validation of non-invasive point of care blood content analysis using the TensorTip™ MTX device: a method comparison study
- Infectious Diseases
- Kinetics and ability of binding antibody and surrogate virus neutralization tests to predict neutralizing antibodies against the SARS-CoV-2 Omicron variant following BNT162b2 booster administration
- Letters to the Editor
- The first case of VEXAS syndrome in Austria
- Acetylcholine receptor and muscle-specific tyrosine kinase antibodies detection: is it time for a change?
- Performance of the 2009 CKDEPI, 2021 CKDEPI, and EKFC equations among high-risk patients in Denmark
- Biotin interference in immunoassays: water under the bridge?
- The new synthetic benzimidazole opioid etonitazepipne: an emerging fatal harm and a challenge for laboratory medicine
- Unexplained increase of serum carcinoembryonic antigen: don’t forget the thyroid!
- Falsely elevated cortisol serum levels in preterm infants due to use of immunoassay
- Misdiagnosis of Hb Bart’s disease: prenatal screening and diagnosis of thalassemia in special population

