Sustained SARS-CoV-2 nucleocapsid antibody levels in nonsevere COVID-19: a population-based study
-
Anna Schaffner
To the Editor,
Antibodies against the receptor-binding domain (RBD) of SARS-CoV-2 spike (S) protein are known to decay rapidly in patients with mild COVID-19 [1]. Titers of these antibodies have a strong correlation with virus neutralization titer [2]. Serological testing of SARS-CoV-2 antibodies cannot reliably recognize COVID-19 in the acute phase [3]. Therefore, antibody testing in the clinical and epidemiological setting is currently and primarily performed to evaluate whether a patient had COVID-19 in the past rather than to determine whether an individual with antibodies has protective immunity against future COVID-19 infection [2], [, 4]. Commercially available tests detect antibodies of different antibody isotypes (i.e., total antibodies, IgG, IgA, or IgM) directed against various antigenic targets (i.e., spike protein (S) and nucleocapsid antigen (N)) [4]. Specific IgM have been shown to increase from day 0 to 17 and then gradually decrease from day 18 to 62 [4], [, 5]. However, it is not known whether rapid decay of the SARS-CoV-2 antibodies directed against the RBD of the S protein can be extrapolated to other assays and beyond 90 days of symptom onset [1], [6], [7].
In the present analysis, we aimed to investigate the longitudinal course of antibody levels of various isotypes against S protein or N antigen in a population-based cohort, which included all 95 confirmed COVID-19 cases diagnosed during the first wave of the pandemic in the Principality of Liechtenstein, a European country with 38,749 inhabitants [8]. The first case was diagnosed in Liechtenstein on March 2, 2020, and after the last patient of the first wave (April 23, 2020), there were no further cases for almost 10 weeks (i.e., July 3) despite an extensive use of PCR tests with the results rapidly and reliably available within 24 h. The first follow-up serological test at a median of 48 days (interquartile range, IQR [43, 52]) after the symptom onset was performed in 89 patients. A second follow-up serological test was performed in 83/95 patients at a median of 140 days (IQR [133, 144]) after the symptom onset. Clinically, patients were contacted on average every 48 h until resolution of the symptoms. A detailed description of the first wave of the pandemic in Liechtenstein is provided in reference [8]. A single 94-year-old patient died two weeks after the symptom onset; eight out of 95 patients did not provide consent for one or two serological follow-up tests, and four patients were lost after the first serological follow-up test leaving a total of 82 patients included in the present analysis (86% of all COVID-19 national cases diagnosed during the first wave in the whole country). The study protocol was verified by the Kantonale Ethikkommission Zürich (BASEC-Number 2020-00676), and the study participants provided written informed consent. Conduct of the study complied with the World Medical Association Declaration of Helsinki. The following laboratory tests were used: anti-N SARS-CoV-2 total antibody electrochemiluminescence immunoassay (ECLIA, Elecsys) using a COBAS 6000 instrument (Roche Diagnostics, Rotkreuz, Switzerland; manufacturer’s cutoff index, COI, for positive result ≥1), anti-N SARS-CoV-2 IgG chemiluminescent microparticle immunoassay (CMIA) using an Architect i2000 analyzer (Abbott Diagnostics, Baar, Switzerland; manufacturer’s signal to cutoff ratio, S/C, for positive result ≥1.4), and anti-S-SARS-CoV-2 IgG and IgA enzyme-linked immunosorbent assay (ELISA; Euroimmun AG, Luzern, Switzerland; manufacturer’s signal to cutoff ratio, S/C, for positive result ≥1.1) using a Dynex DSX platform (Dynex Technologies, Denkendorf, Germany). The interseries coefficients of variation (CVs) were 7.1% for the anti-SARS-CoV-2 total antibody ECLIA (with mean COI of 26.6), 1.1 % for the anti-N SARS-CoV-2 IgG CMIA (with mean S/C of 4.7), 7.8% for the anti-SARS-CoV-2 IgG ELISA (with mean S/C of 2.67), and 8.6% for the anti-SARS-CoV-2 IgA ELISA (with mean S/C of 2.54). Wilcoxon signed rank test was used to evaluate antibody kinetics between the first and second antibody tests. Proportions were compared by chi-square test. Statistical analysis was performed using Medcalc version 18.3.11 (Medcalc software bvba, Ostend, Belgium).
Supplementary Table 1 illustrates the clinical characteristics of the 82 enrolled patients, COVID-19 was confirmed by RT-PCR in all patients. Figure 1 demonstrates the kinetics of antibody levels determined by various antibody tests. In agreement with the data of Ibarrondo et al., the SARS-CoV-2 S-protein IgG titers were significantly decreased after the first follow-up (median signal to cutoff ratio, S/C, 3.2 IQR [1.8, 4.8] to 2.2 IQR [1.4, 3.4], p<0.0001) [1]; a similar pattern was detected for SARS-CoV-2 S-protein IgA (S/C, 2.2 IQR [1.2, 3.7] to 1.5 IQR [0.8, 2.6]; p<0.001) and for SARS-CoV-2 N-antigen IgG (S/C 4.2 IQR [2.9, 7.4] to 1.8 IQR [1, 3.9], p<0.0001). In contrast, the SARS-CoV-2 N-antigen total antibody levels did not change significantly between the first and second follow-up median cutoff index, (COI, 46.2 IQR [20, 88.8] to 41.5 IQR [19, 99.2], p=0.32).
Sensitivities of for SARS-CoV-2 N antigen total antibody, SARS-CoV-2 N-antigen IgG, SARS-CoV-2 S protein IgG, and SARS-CoV-2 S protein IgA at median 48 and 140 days after symptom onset is shown. The difference in sensitivity is significant for SARS-CoV-2 N-antigen IgG (p=0.002).
| N-antigen total antibody | N-antigen IgG | S-antigen IgG | S-antigen IgA | |||||
|---|---|---|---|---|---|---|---|---|
| 48 days | 140 days | 48 days | 140 days | 48 days | 140 days | 48 days | 140 days | |
| Sensitivity | ||||||||
| Percent [95% CI] | 98% [92, 99] | 95% [88, 98] | 91 [83, 96] | 63 [53, 73] | 87 [78, 92] | 83 [73, 90] | 78 [64, 82] | 68 [58, 77] |
| n positive/N total | 80/82 | 78/82 | 75/82 | 52/82 | 71/82 | 68/82 | 64/82 | 56/82 |
CI, confidence interval; IgG, immunglobulin G; IgA, immunoglobulin A.
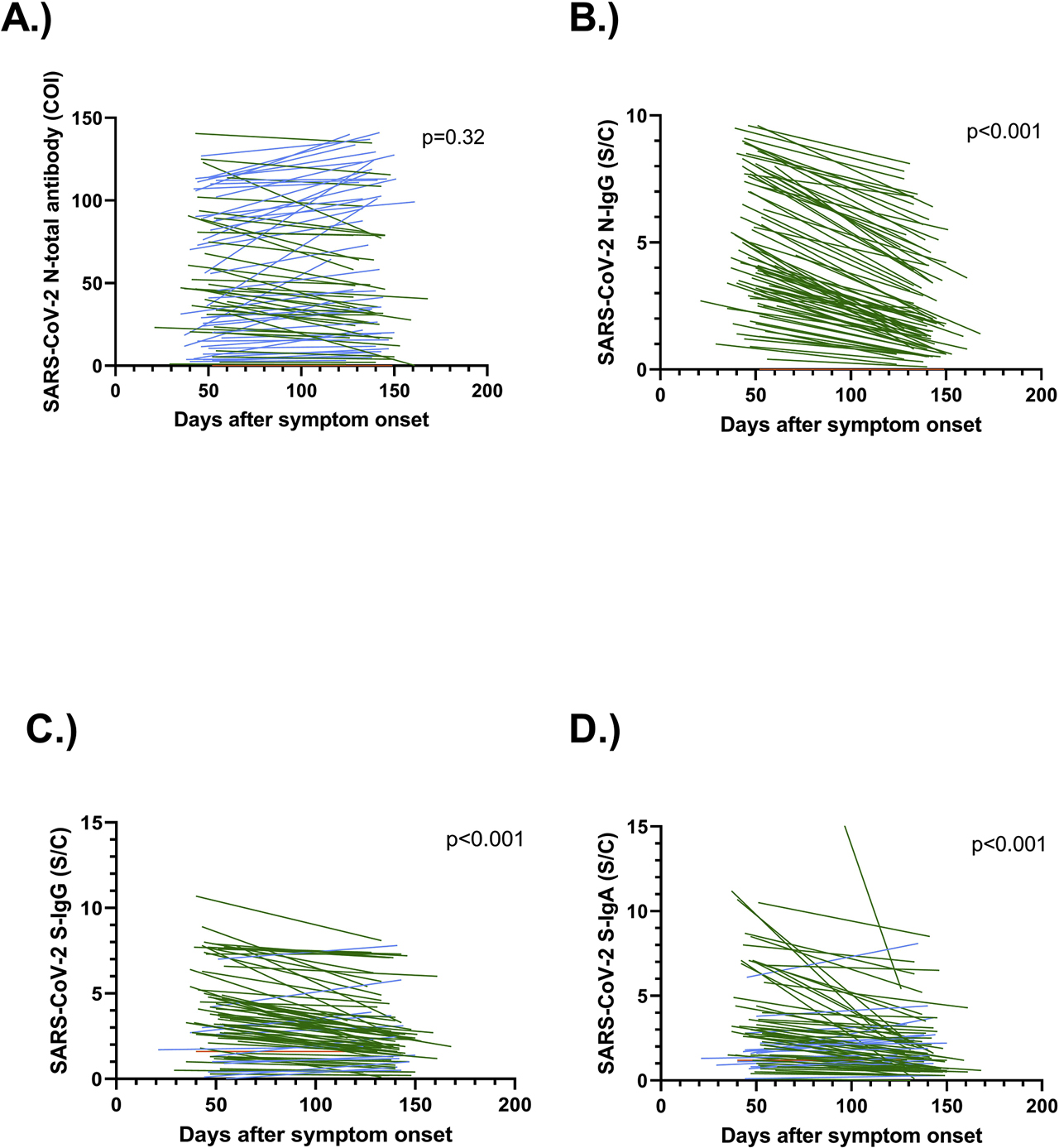
Longitudinal assessment of anti-SARS-CoV-2 antibodies using four various assays.
Patients with increasing antibody levels are shown in blue, decreasing antibody levels are given in green, whereas unchanged antibody levels are shown in orange color. (A) SARS-CoV-2 N-antigen total antibody. (B) SARS-CoV-2 N-antigen IgG. (C) SARS-CoV-2 S-protein IgG. (D) SARS-CoV-2 S-protein IgA. The data in panels (B, C and D) show significant kinetics (p<0.0001) in contrast to the data of panel (A). In panel (D) a datapoint taken on day 43 after symptom onset with an S/C-value of 32.4 is not shown.
The proportion of positive samples at first and second follow-up was calculated in the case of various antibody tests. This proportion corresponds to diagnostic sensitivity of various antibodies to indicate previous COVID-19 infection. The sensitivity of SARS-CoV-2 N antigen total antibody at first follow up after median 48 days and second follow-up at 140 days at the manufacturers’ cut-offs of the different antibodies is shown in Table 1. There is no statistically significant difference in sensitivity at the two timepoints for SARS-CoV-2 N antigen total antibody, SARS-CoV-2 S protein IgG, and SARS-CoV-2 S protein IgA. The sensitivities of the SARS-CoV-2 N-antigen IgG, however, differ between the two timepoints (p=0.002). If lower cut-offs than those provided by the manufacturers are used, higher sensitivities of the isotype specific assays could be expected and the time dependency of test performance could be less pronounced [9].
Overall, different patterns of antibody kinetics depend on the target antigen and antibody isotypes. IgA and IgG against the SARS-CoV-2 S-protein show a decrease; however, the total antibody levels directed against the SARS-CoV-2 N-antigen remain stable despite a decrease in SARS-CoV-2 N-antigen IgG. The levels of SARS-CoV-2 N-antigen total antibody may be due to sustained response of non-IgG antibody isotypes, which requires confirmation in additional studies. When looking in detail at the antibody level, a non-significant decline can be seen. Alternatively, the non-significant SARS-CoV-2 decline of N-antigen total antibody levels may thus also be explained with a type II error due to limited sample size or a slower decline of total antibody levels. In conclusion, the levels of SARS-CoV-2 N-antigen total antibody, unlike SARS-CoV-2 S-protein IgG and IgA and SARS-CoV-2 N-antigen IgG levels, are suitable for reliable detection of past COVID-19 infection of mild to moderate severity for up to five months after the symptom onset. Patients with severe COVID-19 and asymptomatic SARS-CoV-2 positive persons were not investigated in the present study. Currently, it is not known whether the sustained SARS-CoV-2 N-antigen total antibody response persists in asymptomatic SARS-CoV-2 positive persons, beyond 140 days and whether these antibody levels indicate protective immunity or only provide evidence for past recovery from COVID-19.
Funding source: Government of the Principality of Liechtenstein
Research funding: The research project was funded by a grant from the Government of the Principality of Liechtenstein.
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
Informed consent: Informed consent was obtained from all individuals included in this study.
Ethical approval: The study protocol was verified by the Kantonale Ethikkommission Zürich (BASEC-Number 2020-00676).
References
1. Ibarrondo, FJ, Fulcher, JA, Goodman-Meza, D, Elliott, J, Hofmann, C, Hausner, MA, et al.. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med 2020;383:1085–7. https://doi.org/10.1056/nejmc2025179.Search in Google Scholar PubMed PubMed Central
2. To, KK, Tsang, OT, Leung, WS, Tam, AR, Wu, TC, Lung, DC, et al.. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020;20:565–74. https://doi.org/10.1016/s1473-3099(20)30196-1.Search in Google Scholar
3. Long, QX, Liu, BZ, Deng, HJ, Wu, GC, Deng, K, Chen, YK, et al.. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 2020;26:845–8. https://doi.org/10.1038/s41591-020-0897-1.Search in Google Scholar PubMed
4. Ghaffari, A, Meurant, R, Ardakani, A. COVID-19 serological tests: how well do they actually perform? Diagnostics (Basel) 2020;10:453. https://doi.org/10.3390/diagnostics10070453.Search in Google Scholar PubMed PubMed Central
5. Mairesse, A, Favresse, J, Eucher, C, Elsen, M, Tre-Hardy, M, Haventith, C, et al.. High clinical performance and quantitative assessment of antibody kinetics using a dual recognition assay for the detection of SARS-CoV-2 IgM and IgG antibodies. Clin Biochem 2020. https://doi.org/10.1016/j.clinbiochem.2020.08.009. In press.Search in Google Scholar PubMed PubMed Central
6. Patel, MM, Thornburg, NJ, Stubblefield, WB, Talbot, HK, Coughlin, MM, Feldstein, LR, et al.. Change in antibodies to SARS-CoV-2 over 60 days among health care personnel in Nashville, Tennessee. J Am Med Assoc 2020;324:1781–2. https://doi.org/10.1001/jama.2020.18796.Search in Google Scholar PubMed PubMed Central
7. Beaudoin-Bussieres, G, Laumaea, A, Anand, SP, Prevost, J, Gasser, R, Goyette, G, et al.. Decline of humoral responses against SARS-CoV-2 spike in convalescent individuals. mBio 2020;11:e02590-20. https://doi.org/10.1128/mbio.02590-20.Search in Google Scholar PubMed PubMed Central
8. Thiel, S, Weber, MC, Risch, L, Wohlwend, N, Lung, T, Hillmann, D, et al.. Flattening the curve in 52 days: characterization of the COVID-19 pandemic in the Principality of Liechtenstein. Swiss Med Wkly 2020;150:w20361. https://doi.org/10.4414/smw.2020.20361.Search in Google Scholar PubMed
9. Favresse, J, Eucher, C, Elsen, M, Tre-Hardy, M, Dogne, JM, Douxfils, J. Clinical performance of the Elecsys electrochemiluminescent immunoassay for the detection of SARS-CoV-2 total antibodies. Clin Chem 2020;66:1104–6. https://doi.org/10.1093/clinchem/hvaa131.Search in Google Scholar PubMed PubMed Central
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/cclm-2020-1347).
© 2020 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- Assuring the quality of examinations using faecal immunochemical tests for haemoglobin (FIT)
- Review
- Capillary electrophoresis based on nucleic acid analysis for diagnosing inherited diseases
- Mini Reviews
- Negative hair test result after long-term drug use. About a case involving morphine and literature review
- Prorenin and active renin levels in paediatrics: a bioanalytical review
- Opinion Paper
- From therapeutic drug monitoring to total drug monitoring and drug-omics
- Perspectives
- The internal quality control in the traceability era
- Genetics and Molecular Diagnostics
- External quality assessment (EQA) and alternative assessment procedures (AAPs) in molecular diagnostics: findings of an international survey
- General Clinical Chemistry and Laboratory Medicine
- An evaluation of ten external quality assurance scheme (EQAS) materials for the faecal immunochemical test (FIT) for haemoglobin
- Optimizing hepcidin measurement with a proficiency test framework and standardization improvement
- Development of a certified reference material for anti-β2-glycoprotein I IgG – commutability studies
- Influence of patients’ clinical features at intensive care unit admission on performance of cell cycle arrest biomarkers in predicting acute kidney injury
- Efficacy of weekly administration of cholecalciferol on parathyroid hormone in stable kidney-transplanted patients with CKD stage 1–3
- Plasma metanephrines and prospective prediction of tumor location, size and mutation type in patients with pheochromocytoma and paraganglioma
- Emicizumab, the factor VIII mimetic bi-specific monoclonal antibody and its measurement in plasma
- Reference Values and Biological Variations
- Age appropriate reference intervals for eight kidney function and injury markers in infants, children and adolescents
- Cardiovascular Diseases
- Comparison of acetylsalicylic acid and clopidogrel non-responsiveness assessed by light transmittance aggregometry and PFA-100® in patients undergoing neuroendovascular procedures
- Trimethylamine-N-oxide (TMAO) predicts short- and long-term mortality and poor neurological outcome in out-of-hospital cardiac arrest patients
- Non-lactate strong ion difference and cardiovascular, cancer and all-cause mortality
- Infectious Diseases
- Performance of three automated SARS-CoV-2 antibody assays and relevance of orthogonal testing algorithms
- Development, evaluation, and validation of machine learning models for COVID-19 detection based on routine blood tests
- Searching for a role of procalcitonin determination in COVID-19: a study on a selected cohort of hospitalized patients
- Association of kidney function with effectiveness of procalcitonin-guided antibiotic treatment: a patient-level meta-analysis from randomized controlled trials
- Erratum
- Corrigendum to: The role of hyperhomocysteinemia as well as folate, vitamin B6 and B12 deficiencies in osteoporosis – a systematic review
- Letter to the Editors
- Harmonization of antineutrophil cytoplasmic antibodies (ANCA) testing by reporting test result-specific likelihood ratios: position paper
- Independent internal quality control (IQC) for faecal immunochemical tests (FIT) for haemoglobin: use of FIT manufacturers’ IQC for other FIT systems
- Parallel testing of 241 clinical nasopharyngeal swabs for the detection of SARS-CoV-2 virus on the Cepheid Xpert Xpress SARS-CoV-2 and the Roche cobas SARS-CoV-2 assays
- Sustained SARS-CoV-2 nucleocapsid antibody levels in nonsevere COVID-19: a population-based study
- Evidence for increased circulating procoagulant phospholipids in patients with COVID-19 pneumonia and their prognostic role
- Complete blood counts and cell population data from Sysmex XN analyser in the detection of SARS-CoV-2 infection
- Macro creatine kinase in an asymptomatic patient: a case report
- Implementation of pre-labelled barcode tubes and the GeT-System in a general hospital for the exact documentation of the time of venous blood sample and improvement of sample quality
- Determination of free light chains: assay-dependent differences in interpretation
- Association between SOX17, Wif-1 and RASSF1A promoter methylation status and response to chemotherapy in patients with metastatic gastric cancer
- Effect of two organizational interventions on the frequency of haemoglobin A1c and erythrocyte sedimentation rate testing
- Haemoglobin A1c determination from dried blood spots prepared with HemaSpot™ blood collection devices: comparison with fresh capillary blood
- Stability of catecholamines in whole blood: influence of time between collection and centrifugation
Articles in the same Issue
- Frontmatter
- Editorial
- Assuring the quality of examinations using faecal immunochemical tests for haemoglobin (FIT)
- Review
- Capillary electrophoresis based on nucleic acid analysis for diagnosing inherited diseases
- Mini Reviews
- Negative hair test result after long-term drug use. About a case involving morphine and literature review
- Prorenin and active renin levels in paediatrics: a bioanalytical review
- Opinion Paper
- From therapeutic drug monitoring to total drug monitoring and drug-omics
- Perspectives
- The internal quality control in the traceability era
- Genetics and Molecular Diagnostics
- External quality assessment (EQA) and alternative assessment procedures (AAPs) in molecular diagnostics: findings of an international survey
- General Clinical Chemistry and Laboratory Medicine
- An evaluation of ten external quality assurance scheme (EQAS) materials for the faecal immunochemical test (FIT) for haemoglobin
- Optimizing hepcidin measurement with a proficiency test framework and standardization improvement
- Development of a certified reference material for anti-β2-glycoprotein I IgG – commutability studies
- Influence of patients’ clinical features at intensive care unit admission on performance of cell cycle arrest biomarkers in predicting acute kidney injury
- Efficacy of weekly administration of cholecalciferol on parathyroid hormone in stable kidney-transplanted patients with CKD stage 1–3
- Plasma metanephrines and prospective prediction of tumor location, size and mutation type in patients with pheochromocytoma and paraganglioma
- Emicizumab, the factor VIII mimetic bi-specific monoclonal antibody and its measurement in plasma
- Reference Values and Biological Variations
- Age appropriate reference intervals for eight kidney function and injury markers in infants, children and adolescents
- Cardiovascular Diseases
- Comparison of acetylsalicylic acid and clopidogrel non-responsiveness assessed by light transmittance aggregometry and PFA-100® in patients undergoing neuroendovascular procedures
- Trimethylamine-N-oxide (TMAO) predicts short- and long-term mortality and poor neurological outcome in out-of-hospital cardiac arrest patients
- Non-lactate strong ion difference and cardiovascular, cancer and all-cause mortality
- Infectious Diseases
- Performance of three automated SARS-CoV-2 antibody assays and relevance of orthogonal testing algorithms
- Development, evaluation, and validation of machine learning models for COVID-19 detection based on routine blood tests
- Searching for a role of procalcitonin determination in COVID-19: a study on a selected cohort of hospitalized patients
- Association of kidney function with effectiveness of procalcitonin-guided antibiotic treatment: a patient-level meta-analysis from randomized controlled trials
- Erratum
- Corrigendum to: The role of hyperhomocysteinemia as well as folate, vitamin B6 and B12 deficiencies in osteoporosis – a systematic review
- Letter to the Editors
- Harmonization of antineutrophil cytoplasmic antibodies (ANCA) testing by reporting test result-specific likelihood ratios: position paper
- Independent internal quality control (IQC) for faecal immunochemical tests (FIT) for haemoglobin: use of FIT manufacturers’ IQC for other FIT systems
- Parallel testing of 241 clinical nasopharyngeal swabs for the detection of SARS-CoV-2 virus on the Cepheid Xpert Xpress SARS-CoV-2 and the Roche cobas SARS-CoV-2 assays
- Sustained SARS-CoV-2 nucleocapsid antibody levels in nonsevere COVID-19: a population-based study
- Evidence for increased circulating procoagulant phospholipids in patients with COVID-19 pneumonia and their prognostic role
- Complete blood counts and cell population data from Sysmex XN analyser in the detection of SARS-CoV-2 infection
- Macro creatine kinase in an asymptomatic patient: a case report
- Implementation of pre-labelled barcode tubes and the GeT-System in a general hospital for the exact documentation of the time of venous blood sample and improvement of sample quality
- Determination of free light chains: assay-dependent differences in interpretation
- Association between SOX17, Wif-1 and RASSF1A promoter methylation status and response to chemotherapy in patients with metastatic gastric cancer
- Effect of two organizational interventions on the frequency of haemoglobin A1c and erythrocyte sedimentation rate testing
- Haemoglobin A1c determination from dried blood spots prepared with HemaSpot™ blood collection devices: comparison with fresh capillary blood
- Stability of catecholamines in whole blood: influence of time between collection and centrifugation

