The aim of standardization and harmonization of laboratory diagnostics is to provide accurate and comparable test results for an individual patient at any time, any place. The process of standardization and harmonization should cover all phases of a diagnostic test: pre-analytical (test request, patient identification and sample collection), analytical, and post-analytical (interpretation of the test result) [1]. Although challenging, roadmaps on how to achieve standardization and harmonization have been drawn [2], [3], [4]. In general, these roadmaps apply to laboratory tests in any type of field. Antibody-specificity testing, however, adds an additional layer of complexity, as each individual person produces its own set of unique polymorphic antibodies. In the context of autoimmune disease, each patient produces a spectrum of polyclonal antibodies that are unique in structure, selectivity, affinity and avidity for the autoantigen [5].
In the immunology section of this special issue, several aspects on harmonization/standardization of autoimmune testing are covered: international initiatives, antinuclear antibody (ANA) testing, anti-neutrophil cytoplasmic antibody (ANCA) testing, rheumatoid factor (RF) testing and thyroid antibody testing. Before we go over each of these aspects, we will first embark on the basic question: what do we measure and what are the pitfalls?
Detection of autoantibodies: what do we measure?
Monogioudi et al. stress that standardization depends on reliable reference materials [6]. The calibration material should resemble the selectivity, affinity and avidity of the patient sample to ensure that it behaves in the same way with respect to the method used [6]. Reference materials and calibrators for autoimmune diagnostics can be obtained from a single patient (e.g. the certified reference material for PR3-ANCA and MPO-ANCA) [7] or can be obtained by pooling sera of large groups of patients with antibodies against a specific autoantigen. By definition, a polyclonal antibody pool will have an average selectivity, affinity and avidity for the autoantigen. Besides, each diagnostic method has its unique test characteristics. This heterogeneity in test characteristics depends, among others, on how the antigen is presented, assay kinetics and antibody detection methodology. In the end, these test characteristics define the performance of a test to detect autoantibodies with a certain affinity/avidity for the autoantigen. As a result, we observe strong heterogeneity in test results in autoimmune diagnostics. As long as there is no consensus on what is measured and how it is measured, discrepancies between assays will persist.
This is illustrated by Falkenburg et al., who compared four different commercially available assays to measure RF. One of the parameters by which these methods differ is the influence of the target antigen source (human or rabbit IgG). Falkenburg et al. show strong discrepancies of individual samples in RF status and level, with potentially important consequences for patient care [8]. Interestingly, a very good correlation is observed when the same method comparison is performed on pooled samples instead of individual samples (Figure 1). Does that make sense? Yes, it does. Because, contrary to individual samples, pooled samples resemble the pooled calibrator and/or the pooled reference materials that were used to calibrate the assay. Each pool has its own RF titer, but on average, its RF selectivity, affinity and avidity are similar to the pooled calibrators used in the various RF assays. These pools now become commutable and behave similarly with respect to the calibrators used in the various RF methods.
![Figure 1: Method comparison of two different assays to detect rheumatoid factor (RF).RF titers in 32 individual patient samples (indicated by green small dots) show substantial discrepancies and a poor correlation (R=0.56) in the method comparison of two commercial RF tests. No discrepancies and a good correlation (R=0.96) were observed when the method comparison was performed on pools of these samples (purple dots, the size of the dots reflects the number of sera pooled). AU, arbitrary units; Relares, Reference Laboratory for Rheumatoid Serology; RF, rheumatoid factor. Modified, with courtesy, from Dr. Cas Weykamp [8].](/document/doi/10.1515/cclm-2018-0807/asset/graphic/j_cclm-2018-0807_fig_001.jpg)
Method comparison of two different assays to detect rheumatoid factor (RF).
RF titers in 32 individual patient samples (indicated by green small dots) show substantial discrepancies and a poor correlation (R=0.56) in the method comparison of two commercial RF tests. No discrepancies and a good correlation (R=0.96) were observed when the method comparison was performed on pools of these samples (purple dots, the size of the dots reflects the number of sera pooled). AU, arbitrary units; Relares, Reference Laboratory for Rheumatoid Serology; RF, rheumatoid factor. Modified, with courtesy, from Dr. Cas Weykamp [8].
With the continued expanse of in vitro diagnostic methods to detect autoantibodies, it has proven extremely challenging to standardize and harmonize autoimmune diagnostics worldwide. Even within one and the same manufacturer, batch-to-batch differences can be observed, which have a significant impact in reported autoantibody concentration of an individual patient. That could have a direct negative effect on clinical decision making in case the autoantibody titer has prognostic value or the titer is important to monitor disease activity. Anti-double stranded DNA IgG (anti-dsDNA) are autoantibodies used in the classification criteria of systemic lupus erythemathosus and for monitoring response to treatment [9]. Batch-to-batch variation may go unnoticed in case the pooled kit control provided by the manufacturer is used as internal control. Over a 5-year period, we observed perfect on-target performance and high inter-assay precision using the kit control for anti-dsDNA measurements (data not shown). Over the same 5-year period, we additionally included a control from an individual patient obtained by plasmapheresis and frozen as single aliquots. The batch-to-batch variation now becomes strikingly apparent (Figure 2). This demonstrates that small changes in test reagents may have a profound effect on anti-dsDNA measurements in individual patients. We observed in a 5-year period that batch variation can result in significant differences in absolute quantification (up to 30% change) and inter-assay precision (up to 130% change). Most often, batch-to-batch changes occur gradually, however strong and significant changes in adjacent batches also occur (Figure 2). This demonstrates that individual patient controls provide a more sensitive tool to detect batch variation. However, its use is limited to the availability of a large volume of sample material and its stability as frozen aliquot.
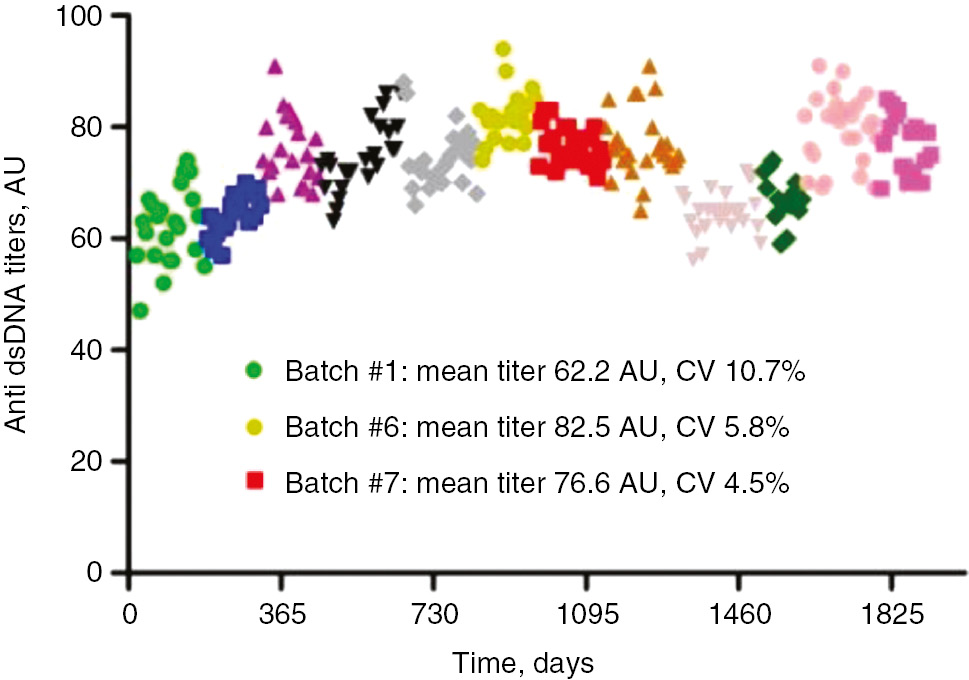
Anti-dsDNA titers of internal control derived from one individual donor.
Each symbol (n=272) represents one weekly anti dsDNA measurement on the same analyzer using reagents from the same manufacturer. The 12 different batches are each represented by a unique colored symbol. Highlighted in the legend are three batches with significant differences in terms of absolute quantification and inter-assay precision. Anti-dsDNA anti double stranded DNA IgG; AU, arbitrary units; CV, coefficient of variation.
International initiatives to harmonize/standardize autoantibody testing
The European Autoimmunity Standardization Initiative (EASI) aims to improve autoimmune diagnostics by encouraging the dialogue between laboratory professionals and clinicians. In this issue, Damoiseaux et al. give a historical overview of the activities and achievements of EASI [10].
The International Consensus on Antinuclear Antibody (ANA) Patterns (ICAP) aims to promote consensus on the morphological patterns observed in the indirect immunofluorescence (IIF) assay on HEp-2 cells for antinuclear antibodies (www.anapatterns.org) [11]. ICAP contributed to this special issue with two papers that are discussed in the section on ANA (below).
The IUIS (International Union of Immunology Societies) Autoantibody Standardization Committee is active in the standardization of autoantibodies in rheumatic and related diseases (www.AutoAb.org). They, for example, provide a collection of ANA reference standards from the Centers for Disease Control and Prevention (www.AutoAb.org). In this special issue, the IUIS Autoantibody Standardization Committee describes and characterizes new reference standards for the detection of anti-mitochondrial and anti-rod/rings autoantibodies [12]. Anti-mitochondrial antibodies are characteristic for primary biliary cholangitis and anti-rods/rings antibodies are linked to interferon-α and ribavirin treatment in hepatitis C infection. The standards are freely available to qualified laboratories through Plasma Services Group and can be used to calibrate or to establish internal reference material for immunodiagnostic assays [12].
A working group on the Harmonization of Autoantibody tests of the International Federation of Clinical Chemistry and Laboratory Medicine, together with the Joint Research Centre of the European Commission, has a mandate to develop and produce certified reference materials. Monogioudi et al. discuss and illustrate the advantages and limitations that are related to the production and correct use of certified reference materials [6].
Antinuclear antibodies
Traditionally, IIF is considered the reference method for ANA detection [13], [14]. In this issue, Meroni and Borghi question whether it is time to break the dogma of IIF as the golden standard [15]. They discuss the evolution of the new solid-phase assays and suggest that combining solid-phase assays with IIF might add diagnostic value, a concept for which there is already some evidence [16], [17], [18], [19], [20], [21]. They also put forward the concept of using disease-specific autoantibody profiles in the diagnosis of systemic rheumatic disease and illustrate this for rheumatoid arthritis and the anti-phospholipid syndrome [15].
Peréz et al. show that an addressable laser bead immunoassay (BioPlex ANA screen, BioRad) was more sensitive than IIF for ANA detection [22]. Interestingly, 312 out of the 411 patients that tested positive by BioPlex but negative by IIF became IIF positive after a 3-year follow-up period. Moreover, 87% of the 411 subjects that tested positive by BioPlex and negative by IIF were diagnosed with an autoimmune disease after a 3-year follow-up period. The most prevalent autoimmune disease was systemic lupus erythematosus. The positive predictive value for the diagnosis of an autoimmune disease was dependent on the autoantibody and varied between 65% (RNP-A) and 100% (Sm, Jo-1). The predictive value was very high when several antibodies were simultaneously present. This is an important study that shows that BioPlex detects autoantibodies earlier than IIF and that these antibodies may be an early predictor for the emergence of autoimmune disease [22].
Even though the position of IIF as reference method is being questioned [15], [23], IIF remains an important method to screen for ANA and international efforts to harmonize ANA nomenclature are being undertaken by ICAP (www.anapatterns.org). In this issue, ICAP (i) proposes an international consensus on negative ANA results and on reporting unidentified patterns [24] and (ii) defines the pattern associated with antibodies to DNA topoisomerase I [25].
Anti-neutrophil cytoplasmic antibodies
ANCAs are useful markers to support the diagnosis of ANCA-associated small vessel vasculitis. The EASI group initiated a questionnaire to collect information on methods and testing algorithms for ANCA in clinical laboratories from 12 countries. The outcome is reported by Damoiseaux et al. in this issue [26]. The survey revealed substantial variation with respect to the use of IIF in the algorithm, the definition of atypical ANCA by IIF, and the use of newer technologies [26]. In this respect, it is worth mentioning that, based on a large multicenter study on the performance of IIF and immunoassays for ANCA detection [27], [28], a revision of the international consensus on ANCA testing has been issued [29]. In this revised consensus statement, it is proposed that high-quality immunoassays can be used as the primary screening method for patients suspected of having the ANCA-associated vasculitis [29].
Even though certified reference materials for PR3- and MPO-ANCA have recently become available [7], full comparability of results will remain a challenge as not all assays for PR3- and MPO-ANCA correlate well [30].
An alternative way to harmonize PR3- and MPO-ANCA test results is to harmonize test result interpretation by reporting test result interval-specific likelihood ratios. Such ANCA test result interval-specific likelihood ratios have been established for eight different commercial assays [31]. Likelihood ratios are clinically useful, as they can be used to estimate post-test probability for disease.
Rheumatoid factor
RF and anti-citrullinated protein antibodies (ACPA) are included in the ACR-EULAR 2010 classification criteria for rheumatoid arthritis [32]. In this issue, Falkenburg et al. report on a comparison of RF measurements using four commercial assays [8]. They found substantial differences and conclude that this might have consequences for patient care. These conclusions are in line with the results of another recently published study in which six different RF and seven different ACPA assays were compared using samples from well characterized patients with rheumatoid arthritis and controls [33]. Poor agreement was observed between the different RF and ACPA assays with a large variation in sensitivity and specificity between assays (mainly for RF), which impacted classification of rheumatoid arthritis according to the 2010 ACR/EULAR criteria [33].
Thyroid-specific antibodies
Antibodies to thyroid peroxidase, thyroglobulin and TSH receptor are found in patients with autoimmune thyroid disease (Hashimoto and Graves disease). Tozzoli and Bizzaro discuss principal aspects related to pre-analytical, analytical and post-analytical steps of thyroid autoantibody testing (e.g. test requests, retesting, terminology, units, reference limits) [34].
Conclusions
Autoantibodies are complex analytes that can vary between patients and even within a patient during the course of disease. Since an exact definition of the measurand is a prerequisite for reliable design of diagnostic assays, absolute standardization of autoantibody testing is generally recognized as extremely challenging if not impossible. Fact remains that steps can be taken toward better standardization/harmonization within the field of autoimmune diagnostics. Contributions in the immunology section of this special issue demonstrate that many initiatives are in progress and sound results have been achieved in the total testing cycle of autoimmune diagnostics: standardization/harmonization is on the agenda.
Acknowledgments
The authors wish to thank Dr. Cas Weykamp for the access to the raw data to generate Figure 1 of this editorial and for the fruitful discussion.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: JFMJ is supported by a grant from the Dutch Cancer Society (KWF Kankerbestrijding, #10817).
Employment or leadership: None declared.
Honorarium: None declared.
References
1. Plebani M. Harmonization in laboratory medicine: the complete picture. Clin Chem Lab Med 2013;51:741–51.10.1515/cclm-2013-0075Search in Google Scholar PubMed
2. Aarsand AK, Sandberg S. How to achieve harmonisation of laboratory testing – the complete picture. Clin Chim Acta 2014;432:8–14.10.1016/j.cca.2013.12.005Search in Google Scholar PubMed
3. Tate JR, Johnson R, Barth J, Panteghini M. Harmonization of laboratory testing – current achievements and future strategies. Clin Chim Acta 2014;432:4–7.10.1016/j.cca.2013.08.021Search in Google Scholar PubMed
4. Plebani M. Harmonization in laboratory medicine: more than clinical chemistry? Clin Chem Lab Med 2018;56:1579–86.10.1515/cclm-2017-0865Search in Google Scholar PubMed
5. Meroni PL, Biggioggero M, Pierangeli SS, Sheldon J, Zegers I, Borghi MO. Standardization of autoantibody testing: a paradigm for serology in rheumatic diseases. Nat Rev Rheumatol 2014;10:35–43.10.1038/nrrheum.2013.180Search in Google Scholar PubMed
6. Monogioudi E, Martos G, Hutu DP, Schimmel H, Meroni PL, Sheldon J, et al. Standardization of autoimmune testing – is it feasible? Clin Chem Lab Med 2018;56:1734–42.10.1515/cclm-2017-1077Search in Google Scholar PubMed
7. Monogioudi E, Hutu DP, Martos G, Sheldon J, Schimmel H, Meroni PL, et al. Development of a certified reference material for myeloperoxidase-anti-neutrophil cytoplasmic autoantibodies (MPO-ANCA). Clin Chim Acta 2017;467:48–50.10.1016/j.cca.2016.05.031Search in Google Scholar PubMed
8. Falkenburg WJ, von Richthofen HJ, Koers J, Weykamp C, Schreurs MW, Bakker-Jonges LE, et al. Clinically relevant discrepancies between different rheumatoid factor assays. Clin Chem Lab Med 2018;56:1749–58.10.1136/annrheumdis-2018-eular.1477Search in Google Scholar
9. Yu C, Gershwin ME, Chang C. Diagnostic criteria for systemic lupus erythematosus: a critical review. J Autoimmun 2014; 48–49:10–3.10.1016/j.jaut.2014.01.004Search in Google Scholar PubMed
10. Damoiseaux J, Olschowka N, Shoenfeld Y. EASI – European Autoimmunity Standardisation Initiative: facing the challenges of diagnostics in autoimmunity. Clin Chem Lab Med 2018;56:1620–3.10.1515/cclm-2017-0826Search in Google Scholar PubMed
11. Chan EK, Damoiseaux J, Carballo OG, Conrad K, de Melo Cruvinel W, Francescantonio PL, et al. Report of the first international consensus on standardized nomenclature of antinuclear antibody HEp-2 cell patterns 2014–2015. Front Immunol 2015;6:412.10.3389/fimmu.2015.00412Search in Google Scholar PubMed PubMed Central
12. Calise SJ, Zheng B, Hasegawa T, Satoh M, Isailovic N, Ceribelli A, et al., the IUIS Autoantibody Standardization Committee. Reference standards for the detection of anti-mitochondrial and anti-rods/rings autoantibodies. Clin Chem Lab Med 2018;56:1789–98.10.1515/cclm-2017-1152Search in Google Scholar PubMed PubMed Central
13. Meroni PL, Schur PH. ANA screening: an old test with new recommendations. Ann Rheum Dis 2010;69:1420–2.10.1136/ard.2009.127100Search in Google Scholar PubMed
14. Agmon-Levin N, Damoiseaux J, Kallenberg C, Sack U, Witte T, Herold M, et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis 2014;73:17–23.10.1136/annrheumdis-2013-203863Search in Google Scholar PubMed
15. Meroni PL, Borghi MO. Diagnostic laboratory tests for systemic autoimmune rheumatic diseases: unmet needs towards harmonization. Clin Chem Lab Med 2018;56:1743–8.10.1515/cclm-2018-0066Search in Google Scholar PubMed
16. Bizzaro N, Brusca I, Previtali G, Alessio MG, Daves M, Platzgummer S, et al. The association of solid-phase assays to immunofluorescence increases the diagnostic accuracy for ANA screening in patients with autoimmune rheumatic diseases. Autoimmun Rev 2018;17:541–7.10.1016/j.autrev.2017.12.007Search in Google Scholar PubMed
17. Bossuyt X, Fieuws S. Detection of antinuclear antibodies: added value of solid phase assay? Ann Rheum Dis 2014;73:e10.10.1136/annrheumdis-2013-204793Search in Google Scholar PubMed
18. Claessens J, Belmondo T, De Langhe E, Westhovens R, Poesen K, Hue S, et al. Solid phase assays versus automated indirect immunofluorescence for detection of antinuclear antibodies. Autoimmun Rev 2018;17:533–40.10.1016/j.autrev.2018.03.002Search in Google Scholar PubMed
19. Robier C, Amouzadeh-Ghadikolai O, Stettin M, Reicht G. Comparison of the clinical utility of the Elia CTD Screen to indirect immunofluorescence on Hep-2 cells. Clin Chem Lab Med 2016;54:1365–70.10.1515/cclm-2015-1051Search in Google Scholar PubMed
20. Willems P, De Langhe E, Claessens J, Westhovens R, Van Hoeyveld E, Poesen K, et al. Screening for connective tissue disease-associated antibodies by automated immunoassay. Clin Chem Lab Med 2018;56:909–18.10.1515/cclm-2017-0905Search in Google Scholar PubMed
21. Willems P, De Langhe E, Westhovens R, Vanderschueren S, Blockmans D, Bossuyt X. Antinuclear antibody as entry criterion for classification of systemic lupus erythematosus: pitfalls and opportunities. Ann Rheum Dis 2018. doi: 10.1136/annrheumdis-2018-213821. [Epub ahead of print].10.1136/annrheumdis-2018-213821Search in Google Scholar PubMed
22. Pérez D, Gilburd B, Cabrera-Marante Ó, Martínez-Flores JA, Serrano M, Naranjo L, et al. Predictive autoimmunity using autoantibodies: screening for anti-nuclear antibodies. Clin Chem Lab Med 2018;56:1771–7.10.1515/cclm-2017-0241Search in Google Scholar PubMed
23. Meroni PL, Chan EK, Damoiseaux J, Andrade LE, Bossuyt X, Conrad K, et al. Unending story of the indirect immunofluorescence assay on HEp-2 cells: old problems and new solutions? Ann Rheum Dis 2018. doi: 10.1136/annrheumdis-2018-213440. [Epub ahead of print].10.1136/annrheumdis-2018-213440Search in Google Scholar PubMed
24. Herold M, Klotz W, Andrade LE, Conrad K, Cruvinel WM, Damoiseaux J, et al. International Consensus on Antinuclear Antibody Patterns: defining negative results and reporting unidentified patterns. Clin Chem Lab Med 2018;56:1799–802.10.1515/cclm-2018-0052Search in Google Scholar PubMed
25. Andrade LE, Klotz W, Herold M, Conrad K, Ronnelid J, Fritzler MJ, et al. International consensus on antinuclear antibody patterns: definition of the AC-29 pattern associated with antibodies to DNA topoisomerase I. Clin Chem Lab Med 2018;56:1783–8.10.1515/cclm-2018-0188Search in Google Scholar PubMed
26. Damoiseaux J, Heijnen I, Van Campenhout C, Eriksson C, Fabien N, Herold M, et al. An international survey on anti-neutrophil cytoplasmic antibodies (ANCA) testing in daily clinical practice. Clin Chem Lab Med 2018;56:1759–70.10.1515/cclm-2017-0306Search in Google Scholar PubMed
27. Damoiseaux J, Csernok E, Rasmussen N, Moosig F, van Paassen P, Baslund B, et al. Detection of antineutrophil cytoplasmic antibodies (ANCAs): a multicentre European Vasculitis Study Group (EUVAS) evaluation of the value of indirect immunofluorescence (IIF) versus antigen-specific immunoassays. Ann Rheum Dis 2017;76:647–53.10.1136/annrheumdis-2016-209507Search in Google Scholar PubMed
28. Csernok E, Damoiseaux J, Rasmussen N, Hellmich B, van Paassen P, Vermeersch P, et al. Evaluation of automated multi-parametric indirect immunofluorescence assays to detect anti-neutrophil cytoplasmic antibodies (ANCA) in granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). Autoimmun Rev 2016;15:736–41.10.1016/j.autrev.2016.03.010Search in Google Scholar PubMed
29. Bossuyt X, Cohen Tervaert JW, Arimura Y, Blockmans D, Flores-Suarez LF, Guillevin L, et al. Position paper: Revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol 2017;13:683–92.10.1038/nrrheum.2017.140Search in Google Scholar PubMed
30. Rasmussen N, Damoiseaux J, Csernok E, Heegaard NH, Hellmich B, Paassen PV, et al. Individual values of antineutrophil cytoplasmic antibodies do not correspond between antigen-specific assays. Clin Chem Lab Med 2018;56:e39–42.10.1515/cclm-2017-0362Search in Google Scholar PubMed
31. Bossuyt X, Rasmussen N, van Paassen P, Hellmich B, Baslund B, Vermeersch P, et al. A multicentre study to improve clinical interpretation of proteinase-3 and myeloperoxidase anti-neutrophil cytoplasmic antibodies. Rheumatology (Oxford) 2017;56:1533–41.10.1093/rheumatology/kex170Search in Google Scholar PubMed
32. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81.10.1002/art.27584Search in Google Scholar PubMed
33. Van Hoovels L, Jacobs J, Vander Cruyssen B, Van den Bremt S, Verschueren P, Bossuyt X. Performance characteristics of rheumatoid factor and anti-cyclic citrullinated peptide antibody assays may impact ACR/EULAR classification of rheumatoid arthritis. Ann Rheum Dis 2018;77:667–77.10.1136/annrheumdis-2017-212365Search in Google Scholar PubMed
34. Tozzoli R, Bizzaro N. Harmonization in autoimmune thyroid disease diagnostics. Clin Chem Lab Med 2018;56:1778–82.10.1515/cclm-2018-0037Search in Google Scholar PubMed
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorials
- Harmonization in laboratory medicine: Blowin’ in the wind
- Standardization and harmonization of autoimmune diagnostics
- On the complexity of hemostasis and the need for harmonization of test practice
- Harmonization of laboratory hematology: a long and winding journey
- Section 1: Current Harmonization Activities at Global Level
- Harmonization in laboratory medicine: more than clinical chemistry?
- Harmonization of External Quality Assessment Schemes and their role – clinical chemistry and beyond
- An overview of EFLM harmonization activities in Europe
- Metrological traceability and harmonization of medical tests: a quantum leap forward is needed to keep pace with globalization and stringent IVD-regulations in the 21st century!
- Assessment of bone turnover in osteoporosis: harmonization of the total testing process
- Recent initiatives in harmonization of hemostasis practice
- EASI – European Autoimmunity Standardisation Initiative: facing the challenges of diagnostics in autoimmunity
- Harmonization of microbiology processes and standards: work in progress
- Harmonization initiatives in the generation, reporting and application of biological variation data
- Harmonization of accreditation to ISO15189
- External quality assessment programs in the context of ISO 15189 accreditation
- Section 2: Pre-Pre and Pre-Analytical Phase
- Laboratory testing in the emergency department: an Italian Society of Clinical Biochemistry and Clinical Molecular Biology (SIBioC) and Academy of Emergency Medicine and Care (AcEMC) consensus report
- The EFLM strategy for harmonization of the preanalytical phase
- Section 3: The Analytical Phase
- The roadmap for harmonization: status of the International Consortium for Harmonization of Clinical Laboratory Results
- The quest for equivalence of test results: the pilgrimage of the Dutch Calibration 2.000 program for metrological traceability
- Current state and recommendations for harmonization of serum/plasma 17-hydroxyprogesterone mass spectrometry methods
- International normalized ratio (INR) testing in Europe: between-laboratory comparability of test results obtained by Quick and Owren reagents
- Detecting molecular forms of antithrombin by LC-MRM-MS: defining the measurands
- A design for external quality assessment for the analysis of thiopurine drugs: pitfalls and opportunities
- Harmonization of PCR-based detection of intestinal pathogens: experiences from the Dutch external quality assessment scheme on molecular diagnosis of protozoa in stool samples
- Harmonization of urine albumin/creatinine ratio (ACR) results: a study based on an external quality assessment program in Polish laboratories
- Standardization of autoimmune testing – is it feasible?
- Diagnostic laboratory tests for systemic autoimmune rheumatic diseases: unmet needs towards harmonization
- Clinically relevant discrepancies between different rheumatoid factor assays
- An international survey on anti-neutrophil cytoplasmic antibodies (ANCA) testing in daily clinical practice
- Predictive autoimmunity using autoantibodies: screening for anti-nuclear antibodies
- Harmonization in autoimmune thyroid disease diagnostics
- International consensus on antinuclear antibody patterns: definition of the AC-29 pattern associated with antibodies to DNA topoisomerase I
- Reference standards for the detection of anti-mitochondrial and anti-rods/rings autoantibodies
- International Consensus on Antinuclear Antibody Patterns: defining negative results and reporting unidentified patterns
Articles in the same Issue
- Frontmatter
- Editorials
- Harmonization in laboratory medicine: Blowin’ in the wind
- Standardization and harmonization of autoimmune diagnostics
- On the complexity of hemostasis and the need for harmonization of test practice
- Harmonization of laboratory hematology: a long and winding journey
- Section 1: Current Harmonization Activities at Global Level
- Harmonization in laboratory medicine: more than clinical chemistry?
- Harmonization of External Quality Assessment Schemes and their role – clinical chemistry and beyond
- An overview of EFLM harmonization activities in Europe
- Metrological traceability and harmonization of medical tests: a quantum leap forward is needed to keep pace with globalization and stringent IVD-regulations in the 21st century!
- Assessment of bone turnover in osteoporosis: harmonization of the total testing process
- Recent initiatives in harmonization of hemostasis practice
- EASI – European Autoimmunity Standardisation Initiative: facing the challenges of diagnostics in autoimmunity
- Harmonization of microbiology processes and standards: work in progress
- Harmonization initiatives in the generation, reporting and application of biological variation data
- Harmonization of accreditation to ISO15189
- External quality assessment programs in the context of ISO 15189 accreditation
- Section 2: Pre-Pre and Pre-Analytical Phase
- Laboratory testing in the emergency department: an Italian Society of Clinical Biochemistry and Clinical Molecular Biology (SIBioC) and Academy of Emergency Medicine and Care (AcEMC) consensus report
- The EFLM strategy for harmonization of the preanalytical phase
- Section 3: The Analytical Phase
- The roadmap for harmonization: status of the International Consortium for Harmonization of Clinical Laboratory Results
- The quest for equivalence of test results: the pilgrimage of the Dutch Calibration 2.000 program for metrological traceability
- Current state and recommendations for harmonization of serum/plasma 17-hydroxyprogesterone mass spectrometry methods
- International normalized ratio (INR) testing in Europe: between-laboratory comparability of test results obtained by Quick and Owren reagents
- Detecting molecular forms of antithrombin by LC-MRM-MS: defining the measurands
- A design for external quality assessment for the analysis of thiopurine drugs: pitfalls and opportunities
- Harmonization of PCR-based detection of intestinal pathogens: experiences from the Dutch external quality assessment scheme on molecular diagnosis of protozoa in stool samples
- Harmonization of urine albumin/creatinine ratio (ACR) results: a study based on an external quality assessment program in Polish laboratories
- Standardization of autoimmune testing – is it feasible?
- Diagnostic laboratory tests for systemic autoimmune rheumatic diseases: unmet needs towards harmonization
- Clinically relevant discrepancies between different rheumatoid factor assays
- An international survey on anti-neutrophil cytoplasmic antibodies (ANCA) testing in daily clinical practice
- Predictive autoimmunity using autoantibodies: screening for anti-nuclear antibodies
- Harmonization in autoimmune thyroid disease diagnostics
- International consensus on antinuclear antibody patterns: definition of the AC-29 pattern associated with antibodies to DNA topoisomerase I
- Reference standards for the detection of anti-mitochondrial and anti-rods/rings autoantibodies
- International Consensus on Antinuclear Antibody Patterns: defining negative results and reporting unidentified patterns

