Abstract
Background:
The need to harmonize laboratory information is particularly intense in the field of plasma proteins, considering their clinical impact and relevance in monitoring diseases.
Methods:
We evaluated units and reference intervals (RIs) utilized by participants of the External Quality Assessment Scheme (EQAS) for plasma proteins of the Centre of Biomedical Research. Moreover, we evaluated inter-laboratory analytical variability from 2001 to 2017.
Results:
The census of participants’ units employed in 2017 showed that for albumin (ALB), ~66% of laboratories still used dL instead of L, and for most other proteins, ~70% still expressed the results in mg/dL. Laboratories primarily used the RIs reported in the packaging inserts of their analytical systems, but for each protein, there was a wide variability of RIs, also among laboratories using the same analytical method. Mean CVs% of the 13 certified proteins in the last five EQA cycles ranged from 3.8% of haptoglobin (HPT) to 12.4% of α1-antitrypsin (AAT) and decreased from 2001 to 2017 for most of them, in particular for C3, ALB, α2-macroglobulin (A2M), HPT and transferrin (TRF).
Conclusions:
In the face of a reduction in inter-laboratory variability for a lot of proteins, there has not been a substantial change in the units and in the RIs used by the participants. To change old habits is difficult and requires coordination and collaboration. The EQAS plays an important role in the assessment and monitoring of all elements that contribute to the formulation of laboratory information and may be useful to contribute to their harmonization.
Introduction
Harmonization represents a fundamental aspect of quality in laboratory medicine, as its ultimate goal is to improve patient outcomes through the provision of an accurate and actionable laboratory information. Therefore, the scope of harmonization goes beyond the analytical phase to include all other aspects of the total testing process, such as terminology and units, report formats, reference intervals (RIs) and decision limits, as well as test profiles request and criteria for interpretation [1], [2], [3], [4], [5], [6].
To fulfil this need, the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) has created a working group on harmonization of the total testing process (WG-H) to promote the use of harmonized nomenclature for measurands, of amounts of substance units and the implementation of common RIs for the measurands where this approach is feasible at the European level [7].
The need for harmonizing laboratory results is particularly intense in the field of quantitative protein assays considering the clinical impact of plasma protein measurements and their relevance in monitoring diseases.
The main step toward achieving the standardization of plasma protein measurements has been the production, by the Committee on Plasma Proteins of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), of the Certified Reference Materials, CRM470 for 15 human serum proteins [8], [9], [10], [11]. Subsequently, the value has been transferred to a new replacement material; thus, from 2008, the ERM-DA470k/IFCC for 12 proteins is available: α2-macroglobulin (A2M), α1-acid glycoprotein (AAG), α1-antitrypsin (AAT), albumin (ALB), C3, C4, haptoglobin (HPT), immunoglobulin (Ig) A, IgG, IgM, transferrin (TRF), and prealbumin or transthyretin (TTR) and the ERM-DA472/IFCC for C-reactive protein (CRP) [12], [13], [14]. The new material has demonstrated the full traceability for all certified proteins, except for ceruloplasmin (CER) [15], [16], and its utility to continue the process of harmonization of patient proteins results [17].
Other initiatives toward the harmonization process are undertaken by IFCC regarding the post-analytical phase of the process, in particular, reporting units [18], RIs and decision levels.
Units
Harmonized reporting of units remains an important challenge to be undertaken by the clinical laboratory community. The EFLM WG-H has begun a campaign, articulated in various steps, to promote the harmonization of the units of measure in the report (step 1: changing from mL to L as unit of volume; step 2: changing to the liter for reporting protein concentrations; step 3: promotion of the use of mmol/L for reporting electrolytes and minerals).
Particularly, step 2 foresees that all laboratories still reporting plasma proteins in mg/dL or g/dL must change to mg/L or g/L. As indicated by Dybkaer and Jorgensen 50 years ago [19], in fact, the recommended unit of volume is liter. EFLM WG-H proposed 31st October 2016 as the deadline for the implementation of this step. At a national level, the Scientific Societies of Clinical Chemistry and Laboratory Medicine play an important role in supporting this campaign [20].
Reference intervals (RIs)
The harmonization process of RIs seems to have an uphill road [21]: despite their role in providing clinical information to guide clinicians in their decision-making, there appears to be little conformity in laboratory practice, and differences in RIs persist between laboratories that use the same platforms and reagents [22], [23].
The same RI across different assays for a specific analyte could be optimally shared when there is sound calibration traceability, and evidence from a between-method comparison shows that bias would not prevent the use of a common RI [24], [25].
In the field of plasma proteins, after the use of the CRM470, which has resulted in significant changes in reference values for some proteins, studies on plasma proteins RIs began to be performed in different geographical areas [26], [27], [28], and in 1995, several professional societies and diagnostic companies agreed to use interim consensus reference ranges for all immunochemical methods, independently of the system or instrument used, until further studies documented more accurate values [29], [30]. As a part of the latter, the IFCC Committee for RIs and Decision Limits (C-RIDL) began a study to evaluate protein concentrations in serum for several racial and ethnic groups in different geographical locations [31] and recently arranged a global multicenter study to explore rational and harmonizable procedures for derivation of RIs and investigate the feasibility of sharing RIs, which includes also some plasma proteins such as ALB, IgA, IgG, IgM, C3, C4 and CRP [32], [33], [34], [35].
In addition, EFLM recently began a campaign to improve the harmonization of RIs used by European laboratories, whose first step is a survey to understand which are the origin of the RIs presently in use and if partitioning criteria for the use of RIs are the same all over Europe.
Aim
The aim of this work was to analyze the units and the RIs used by laboratories participating in the EQA program of the Centre of Biomedical Research (CRB) in reporting plasma proteins. Moreover, the inter-laboratory variability of the 13 certified proteins was evaluated to report the state of the art and changes in time of their measurement.
Materials and methods
The units and RIs of 85 laboratories participating in the EQA Program of CRB for the 23 plasma proteins included in the scheme were analyzed. In particular IgA, IgG, IgM, C3, C4, AAT, TTR, ALB, AAG, A2M, β2-microglobulin (B2M), HPT, TRF, CRP, CER, rheumatoid factor (RF), anti-streptolysin O (ASO), total IgE, total κ and λ chains (TLC), free κ and λ-chains (FLC), retinol binding protein (RBP).
The collection of units and RIs was done through the dedicated CRB website that allows laboratories to input results and RIs expressed with the units used in their medical report; units are then converted with predefined factors in units of the International System (SI). RIs are referred to an adult male.
To verify the degree of harmonization of units of measure, we analyzed before (September 2016) and after (September 2017) step 2 of EFLM WG-H campaign, the units employed by the same participants in the last two cycles of EQA program.
To verify the degree of harmonization of RIs utilized by participants, we analyzed those reported in the 3rd EQA survey 2017. Moreover, we analyzed the RIs reported in the manufacturers’ packaging inserts of the mostly used commercial kits.
To evaluate the overall inter-laboratory analytical variability of the 13 certified proteins, we reviewed all data from 2001 to 2017. The number of results varied from a protein to another and in the years, but it was sufficiently high to allow a robust statistical elaboration because the CRB exchanges results with another European EQA provider, having in common the same control materials.
The mean inter-laboratory variability observed in the last five EQA cycles (consisting of five surveys of two liquid control samples each) was obtained by the CVs% survey for overall participants’ results, independently from the method they used, calculated on data from 48 control samples. Moreover, to evaluate the change in time, we reported the mean inter-laboratory variability (CV%) observed in 2001, 2004, 2007, 2009, 2013 and 2017.
The CVs% were calculated on the basis of a non-parametric approach: median and standard deviation robust (DSrob), after exclusion of outliers (values exceeded±3DSrob).
Results
Units
Table 1 shows the units utilized by participants in 2017.
Units of measure (%) utilized by participants to CRB External Quality Assessment program for 23 plasma proteins.
| Proteins | Units, % | |||||||
|---|---|---|---|---|---|---|---|---|
| g/dL | mg/dL | g/L | mg/L | ng/mL | μg/mL | kU/L | U/mL | |
| Ig A-G-M | 71.0 | 29.0 | ||||||
| C3-C4 | 68.2 | 31.8 | ||||||
| AAT | 63.9 | 36.1 | ||||||
| TTR | 65.0 | 30.0 | 5.0 | |||||
| ALB | 58.4 | 7.8 | 33.8 | |||||
| AAG | 73.2 | 26.8 | ||||||
| A2M | 66.7 | 33.3 | ||||||
| B2M | 8.0 | 82.0 | 2.0 | 8.0 | ||||
| HPT | 72.3 | 27.7 | ||||||
| TRF | 76.6 | 23.4 | ||||||
| CRP | 48.3 | 1.1 | 50.6 | |||||
| CER | 66.7 | 30.3 | 3.0 | |||||
| Ig E | 38.5 | 61.5 | ||||||
| RF | 5.4 | 94.6 | ||||||
| ASO | 4.3 | 95.7 | ||||||
| TLC (κ – λ) | 37.1 | 61.4 | 1.5 | |||||
| FLC (κ – λ) | 100 | |||||||
| RBP | 9.1 | 90.9 | ||||||
AAT, α1-antitrypsin; TTR, prealbumin or transthyretin; ALB, albumin; AAG, α1-acid glycoprotein; A2M, α2-macroglobulin; B2M, β2-microglobulin; HPT, haptoglobin; TRF, transferrin; CRP, C-reactive protein; CER, ceruloplasmin; RF, rheumatoid factor; ASO, anti-streptolysin O; TLC, total light chains; FLC, free light chains; RBP, retinol binding protein.
For ALB, ~66% of laboratories still use dL instead of L. Only for FLC the totality of laboratories report the result with the recommended units (mg/L), while for TLC and RBP, 37.1% and 9.1%, respectively, still utilize mg/dL. For CRP, mg/L and mg/dL are used equally; for B2M, four different units are used: mg/L (82.0%), mg/dL (8.0%), μg/mL (8.0%) and ng/mL (2.0%); for all the other proteins, most laboratories (~70%, range 63.9%–76.6%) still express the result in mg/dL.
In 2016, before the deadline, the state of the art was nearly identical: only two laboratories shifted from mg/dL to g/L.
Reference intervals
Table 2 reports the RIs utilized by the participants, related to an adult male (45 years old), for all the plasma proteins included in the EQA scheme.
Reference intervals utilized by Italian laboratories participating in the CRB EQA program for plasma proteins.
| n, % | Min | Max | n, % | Min | Max | n, % | Min | Max | n, % | Min | Max | n, % | Min | Max |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgA, g/L | C3, g/L | AAG, g/L | TTR, g/L | IgE, kU/L | ||||||||||
| 2 | 0.8 | 3.1 | 12 | 0.8 | 1.5 | 3 | 0.50 | 1.10 | 7 | 0.18 | 0.38 | 9 | 0 | 87 |
| 2 | 0.4 | 3.5 | 2 | 0.7 | 1.6 | 10 | 0.51 | 1.17 | 7 | 0.10 | 0.40 | 61 | 0 | 100 |
| 63 | 0.7 | 4.0 | 4 | 0.8 | 1.6 | 63 | 0.50 | 1.20 | 13 | 0.15 | 0.40 | 4 | 0 | 114 |
| 4 | 0.8 | 4.0 | 71 | 0.9 | 1.8 | 3 | 0.47 | 1.25 | 73 | 0.20 | 0.40 | 4 | 0 | 150 |
| 2 | 1.4 | 4.0 | 4 | 0.8 | 1.9 | 3 | 0.40 | 1.30 | TRF, g/L | 4 | 3 | 150 | ||
| 2 | 0.8 | 4.1 | 2 | 0.9 | 1.9 | 7 | 0.43 | 1.30 | 2 | 1.9 | 2.8 | 9 | 0 | 165 |
| 2 | 0.7 | 4.4 | 2 | 0.9 | 2.1 | 7 | 0.50 | 1.30 | 2 | 1.8 | 3.3 | 4 | 1 | 165 |
| 2 | 0.7 | 4.5 | 4 | 0.9 | 2.2 | 3 | 0.55 | 1.40 | 3 | 2.0 | 3.3 | 4 | 0 | 200 |
| 6 | 0.8 | 4.5 | C4, g/L | A2M, g/L | 3 | 2.0 | 3.4 | κTLC, g/L | ||||||
| 4 | 0.9 | 4.5 | 2 | 0.10 | 0.30 | 17 | 1.0 | 2.6 | 2 | 1.8 | 3.5 | 38 | 1.7 | 3.7 |
| 4 | 0.9 | 4.7 | 6 | 0.16 | 0.38 | 50 | 1.3 | 3.0 | 3 | 2.1 | 3.5 | 25 | 1.4 | 3.8 |
| 4 | 0.6 | 4.8 | 80 | 0.10 | 0.40 | 17 | 1.5 | 3.5 | 3 | 1.7 | 3.6 | 6 | 1.2 | 4.4 |
| 2 | 1.0 | 4.9 | 2 | 0.15 | 0.40 | 17 | 1.2 | 3.9 | 66 | 2.0 | 3.6 | 31 | 6.3 | 13.5 |
| IgG, g/L | 2 | 0.20 | 0.40 | B2M, mg/L | 2 | 2.1 | 3.6 | λTLC, g/L | ||||||
| 2 | 7.4 | 14.4 | 2 | 0.15 | 0.48 | 3 | 0.7 | 1.3 | 5 | 2.2 | 3.7 | 38 | 0.9 | 2.1 |
| 4 | 6.5 | 15.0 | 2 | 0.15 | 0.53 | 3 | 1.0 | 1.7 | 2 | 1.7 | 3.8 | 6 | 0.6 | 2.3 |
| 2 | 7.0 | 15.6 | 2 | 0.17 | 0.53 | 8 | 0.7 | 1.8 | 2 | 1.8 | 3.8 | 13 | 0.9 | 2.4 |
| 4 | 7.5 | 15.6 | 2 | 0.18 | 0.55 | 3 | 0.0 | 2.0 | 3 | 1.9 | 3.8 | 6 | 1.1 | 2.4 |
| 4 | 6.0 | 16.0 | AAT, g/L | 3 | 0.8 | 2.0 | 2 | 2.1 | 3.8 | 6 | 1.0 | 2.5 | ||
| 2 | 6.5 | 16.0 | 19 | 0.9 | 1.7 | 5 | 0.9 | 2.0 | 2 | 2.0 | 4.0 | 31 | 3.1 | 7.2 |
| 59 | 7.0 | 16.0 | 4 | 0.8 | 1.9 | 5 | 0.0 | 2.2 | CRP, mg/L | κFLC, mg/L | ||||
| 2 | 7.1 | 16.0 | 4 | 0.8 | 2.0 | 10 | 0.8 | 2.2 | 1 | 0.0 | 3.0 | 78 | 3.3 | 19.4 |
| 2 | 8.0 | 16.0 | 4 | 0.8 | 2.0 | 3 | 1.0 | 2.2 | 1 | 0.0 | 3.3 | 22 | 6.7 | 22.4 |
| 2 | 7.9 | 16.4 | 4 | 0.9 | 2.0 | 10 | 0.8 | 2.4 | 66 | 0.0 | 5.0 | λFLC, mg/L | ||
| 2 | 6.8 | 16.5 | 56 | 0.9 | 2.0 | 3 | 0.0 | 2.5 | 1 | 1.0 | 5.0 | 78 | 5.7 | 26.3 |
| 2 | 6.2 | 16.6 | 7 | 0.9 | 2.0 | 3 | 0.7 | 2.5 | 12 | 0.0 | 6.0 | 22 | 8.3 | 27.0 |
| 4 | 7.0 | 17.0 | 4 | 1.1 | 2.5 | 3 | 0.9 | 2.5 | 4 | 0.0 | 7.0 | RF, kU/L | ||
| 2 | 8.0 | 17.0 | ALB, g/L | 10 | 1.1 | 2.5 | 1 | 1.0 | 7.0 | 2 | 0 | 10 | ||
| 2 | 8.0 | 17.6 | 2 | 38 | 47 | 13 | 1.2 | 2.5 | 3 | 0.0 | 7.5 | 2 | 0 | 12 |
| 2 | 8.0 | 18.0 | 3 | 41 | 47 | 3 | 1.5 | 2.5 | 3 | 0.0 | 8.0 | 39 | 0 | 14 |
| 4 | 5.4 | 18.2 | 2 | 33 | 48 | 3 | 1.0 | 2.6 | 1 | 0.1 | 8.0 | 4 | 1 | 14 |
| IgM, g/L | 18 | 34 | 48 | 3 | 0.8 | 3.0 | 4 | 0.0 | 10.0 | 21 | 0 | 15 | ||
| 2 | 0.7 | 2.1 | 2 | 38.4 | 48 | 3 | 0.7 | 3.2 | CER, g/L | 18 | 0 | 20 | ||
| 2 | 0.4 | 2.2 | 2 | 32 | 50 | 5 | 1.2 | 3.2 | 11 | 0.20 | 0.50 | 2 | 1 | 20 |
| 58 | 0.4 | 2.3 | 6 | 34 | 50 | 3 | 0.0 | 3.5 | 4 | 0.23 | 0.55 | 9 | 0 | 30 |
| 2 | 0.5 | 2.3 | 26 | 35 | 50 | HPT, g/L | 15 | 0.22 | 0.58 | 4 | 0 | 40 | ||
| 4 | 0.2 | 2.4 | 3 | 36.6 | 51 | 3 | 0.3 | 1.9 | 4 | 0.15 | 0.60 | ASO, kU/L | ||
| 6 | 0.4 | 2.4 | 3 | 33 | 52 | 59 | 0.3 | 2.0 | 59 | 0.20 | 0.60 | 4 | 0 | 116 |
| 2 | 0.4 | 2.8 | 2 | 34 | 52 | 16 | 0.4 | 2.0 | 4 | 0.20 | 0.63 | 4 | 0 | 145 |
| 4 | 0.6 | 2.8 | 23 | 35 | 52 | 5 | 0.5 | 2.0 | 4 | 0.20 | 0.70 | 2 | 0 | 160 |
| 2 | 0.4 | 3.0 | 2 | 37 | 53 | 5 | 0.3 | 2.1 | RBP, mg/L | 75 | 0 | 200 | ||
| 10 | 0.5 | 3.0 | 5 | 35 | 55 | 3 | 0.3 | 2.3 | 100 | 30 | 60 | 7 | 1 | 200 |
| 4 | 0.6 | 3.0 | 2 | 38 | 55 | 3 | 0.4 | 2.4 | 2 | 2 | 200 | |||
| 2 | 0.5 | 3.1 | 2 | 10 | 60 | 3 | 0.1 | 2.6 | 2 | 25 | 200 | |||
| 4 | 0.5 | 3.2 | 3 | 0.5 | 3.2 | 2 | 0 | 240 | ||||||
| 4 | 0 | 408 | ||||||||||||
AAT, α1-antitrypsin; TTR, prealbumin or transthyretin; ALB, albumin; AAG, α1-acid glycoprotein; A2M, α2-macroglobulin; B2M, β2-microglobulin; HPT, haptoglobin; TRF, transferrin; CRP, C-reactive protein; CER, ceruloplasmin; RF, rheumatoid factor; ASO, anti-streptolysin O; TLC, total light chains; FLC, free light chains; RBP, retinol binding protein.
For Igs, ~60% of laboratories utilized the same RI: 0.7–4.0 g/L for IgA, 7–16 g/L for IgG and 0.4–2.3 g/L for IgM.
The more commonly used RIs for the others certified proteins were: C3=0.9–1.8 g/L (71%), C4=0.1–0.4 g/L (80%), AAT=0.9–2.0 g/L (56%), TTR=0.2–0.4 g/L (73%), AAG=0.5–1.2 g/L (63%), A2M=1.3–3.0 g/L (50%), HPT=0.3–2.0 g/L (59%), TRF=2.0–3.6 g/L (66%) and CRP=<5 g/L (66%).
For ALB, it was not observed a RI employed with a large prevalence by participants; the RIs mostly used were: 35–50 g/L (26%), 35–52 g/L (23%) and 34–48 g/L (18%).
Only 19.4% of participants employed nephelometric or turbidimetric methods while 80.6% utilized a colorimetric method, precisely 59.6% the bromocresol green method (BCG) and 21.0% the bromocresol purple method (BCP). The more commonly used RI among the participants utilizing immunometric methods was 35–52 g/L (42%), while 35–50 g/L was the most used RI both for BCG (27%) and for BCP (31%) methods (Table 3).
Reference intervals for albumin utilized by Italian laboratories participating to CRB EQA program for plasma proteins, grouped for method.
| n, % | Min, g/L | Max, g/L |
|---|---|---|
| Colorimetric, BCG | ||
| 3 | 38 | 47 |
| 3 | 41 | 47 |
| 3 | 33 | 48 |
| 24 | 34 | 48 |
| 3 | 38.4 | 48 |
| 3 | 32 | 50 |
| 27 | 35 | 50 |
| 3 | 33 | 52 |
| 3 | 34 | 52 |
| 19 | 35 | 52 |
| 5 | 35 | 55 |
| 3 | 38 | 55 |
| 3 | 10 | 60 |
| Colorimetric, BCP | ||
| 15 | 34 | 48 |
| 31 | 34 | 50 |
| 31 | 35 | 50 |
| 15 | 35 | 52 |
| 8 | 35 | 55 |
| Immunometric | ||
| 8 | 41 | 47 |
| 17 | 35 | 50 |
| 17 | 36.6 | 51 |
| 8 | 33 | 52 |
| 42 | 35 | 52 |
| 8 | 37 | 53 |
Laboratories primarily used the RI reported in the manufacturers’ packaging inserts of the commercial kits (Table 4), but for each protein, we observed a wide variability of others RIs, also among the laboratories using the same analytical method (Figure 1).
Reference intervals reported in the package inserts of the most commercial kits utilized in Italy.
| Proteins | Unit | Proposed reference range | Turbidimetric methods | Nephelometric methods | ||||
|---|---|---|---|---|---|---|---|---|
| Roche, cobas | Abbott | Beckman, AU | Beckman, immage | Siemens, BN | Siemens, vista | |||
| IgA | g/L | 0.7–4.0 | 0.7–4.0 | 0.63–4.84 | 0.7–4.0 | 0.82–4.53 | 0.7–4.0 | 0.7–4.0 |
| IgG | g/L | 7–16 | 7–16 | 5.4–18.22 | 7–16 | 7.51–15.6 | 7–16 | 7–16 |
| IgM | g/L | 0.4–2.3 | 0.4–2.3 | 0.22–2.40 | 0.4–2.3 | 0.46–3.04 | 0.4–2.3 | 0.4–2.3 |
| C3 | g/L | 0.9–1.8 | 0.9–1.8 | 0.82–1.85 | 0.9–1.8 | 0.79–1.52 | 0.9–1.8 | 0.9–1.8 |
| C4 | g/L | 0.1–0.4 | 0.1–0.4 | 0.15–0.53 | 0.1–0.4 | 0.16–0.38 | 0.1–0.4 | 0.1–0.4 |
| AAT | g/L | 0.9–2.0 | 0.9–2.0 | / | 0.9–2.0 | 0.88–1.74 | 0.9–2.0 | 0.9–2.0 |
| TTR | g/L | 0.2–0.4 | 0.2–0.4 | / | 0.2–0.4 | 0.18–0.38 | 0.2–0.4 | 0.2–0.4 |
| ALB | g/L | 35–52 | 35–52 | / | / | 36.6–51 | 35–52 | 35–52 |
| AAG | g/L | 0.5–1.2 | 0.5–1.2 | / | 0.5–1.2 | 0.51–1.17 | 0.5–1.2 | 0.5–1.2 |
| A2M | g/L | 1.3–3.0 | / | / | / | 1.02–2.59 | 1.3–3.0 | 1.3–3.0 |
| B2M | mg/L | – | 0.8–2.2 | / | 0.8–2.4 | /(Dako) | 1.09–2.53 | 1.09–2.53 |
| HPT | g/L | 0.3–2.0 | 0.3–2.0 | 0.14–2.58 | 0.3–2.0 | 0.36–1.95 | 0.3–2.0 | 0.3–2.0 |
| TRF | g/L | 2.0–3.6 | 2.0–3.6 | 1.74–3.64 | 2.0–3.6 | 2.02–3.36 | 2.0–3.6 | 2.0–3.6 |
| CRP | mg/L | <5 | < 5 | <5 (Sentinel) | <5 | <8 | <3 | <3 |
| CER | g/L | 0.2–0.6 | M 0.15–0.30 | 0.2–0.6 (Sentinel) | 0.2–0.6 | 0.22–0.58 | 0.2–0.6 | 0.2–0.6 |
| F 0.16–0.45 | ||||||||
| IgE | kU/L | – | <100 | <100 (Biokit) | / | <165 | <100 | <100 |
| RF | kU/L | – | <14 | <30 (Biokit) | <14 | <20 | <10 | <15 |
| ASO | kU/L | – | <200 | <200 (Biokit) | <200 | <116 | <408 | <408 |
| κ TLC | g/L | – | 1.38–3.75 | / | 1.4–3.8 | 6.29–13.5 | 1.7–3.7 | 1.7–3.7 |
| λ TLC | g/L | 0.93–2.42 | / | 0.95–2.45 | 3.13–7.23 | 0.9–2.1 | 0.9–2.1 | |
| κ FLC | mg/L | – | / | / | / | / | 6.7–22.4 | / |
| λ FLC | mg/L | / | / | / | / | 8.3–27.0 | / | |
| RBP | mg/L | – | / | / | / | / | 30–60 | 30–60 |

Graph reporting the RIs (blue rectangles) of participants to CRB External Quality Assessment program for plasma proteins and results (black circles) obtained on one control sample.
Inter-laboratory variability
For the 13 certified proteins, the results of the last five cycles of EQA showed the mean CV% reported in Table 5. Mean CV% was: <5% for IgG, C3, ALB, HPT and TRF; <10% for IgA, IgM, AAG, TTR, A2M and CPR; <13% for C4 and AAT.
Interlaboratory variability of the 13 certified proteins observed in the last five EQA cycles (2013–2017) calculated on data from 48 control samples.
| Proteins | n | Concentration range | Mean CV%±SD | CRB allowable limits of performancea, % |
|---|---|---|---|---|
| IgA | 407 | 1.23–4.33 g/L | 8.10±0.72 | 10.2 |
| IgG | 393 | 7.00–22.17 g/L | 4.82±0.69 | 8.0 |
| IgM | 390 | 0.59–2.90 g/L | 6.18±1.17 | 12.6 |
| C3 | 291 | 0.72–2.32 g/L | 4.89±0.23 | 8.4 |
| C4 | 306 | 0.16–0.56 g/L | 11.10±1.48 | 12.0 |
| AAT | 126 | 0.62–2.83 g/L | 12.35±2.59 | 13.8 |
| TTR | 102 | 0.15–0.53 g/L | 9.53±2.51 | 14.5 |
| ALB | 193 | 18.0–59.0 g/L | 3.95±0.88 | 6.1 |
| AAG | 84 | 0.39–1.68 g/L | 6.03±0.69 | 12.1 |
| A2M | 29 | 0.87–3.58 g/L | 5.27±0.84 | 11.3 |
| HPT | 187 | 0.50–2.25 g/L | 3.82±0.38 | 12.5 |
| TRF | 364 | 1.25–4.47 g/L | 4.47±0.35 | 8.5 |
| CRP | 787 | 23.7–82.0 mg/L | 8.72±0.66 | 12.0 |
aMinimum grade of quality.
HPT and ALB presented the lowest variability with a mean CV% of 3.82%±0.38% and 3.95%±0.88%, respectively.
The mean CV% of TTR resulted in 9.53% due to 2014 results (CV%=13.10%) because in the other years, it remains <10%. Instead, the inter-laboratory variability of AAT, despite the drop during 2013 to 2017 (CV%=16.41%, 13.17%, 11.33%, 11.22%, 9.62%, respectively), it remains the highest, with a mean CV%=12.35%. The inter-laboratory variability of C4, constantly over 11.5% until 2016, decreased to 8.5% in 2017.
The inter-laboratory variability over the time is reported in Figure 2. We observed a substantial and progressive reduction for C3, ALB, A2M, HPT and TRF.
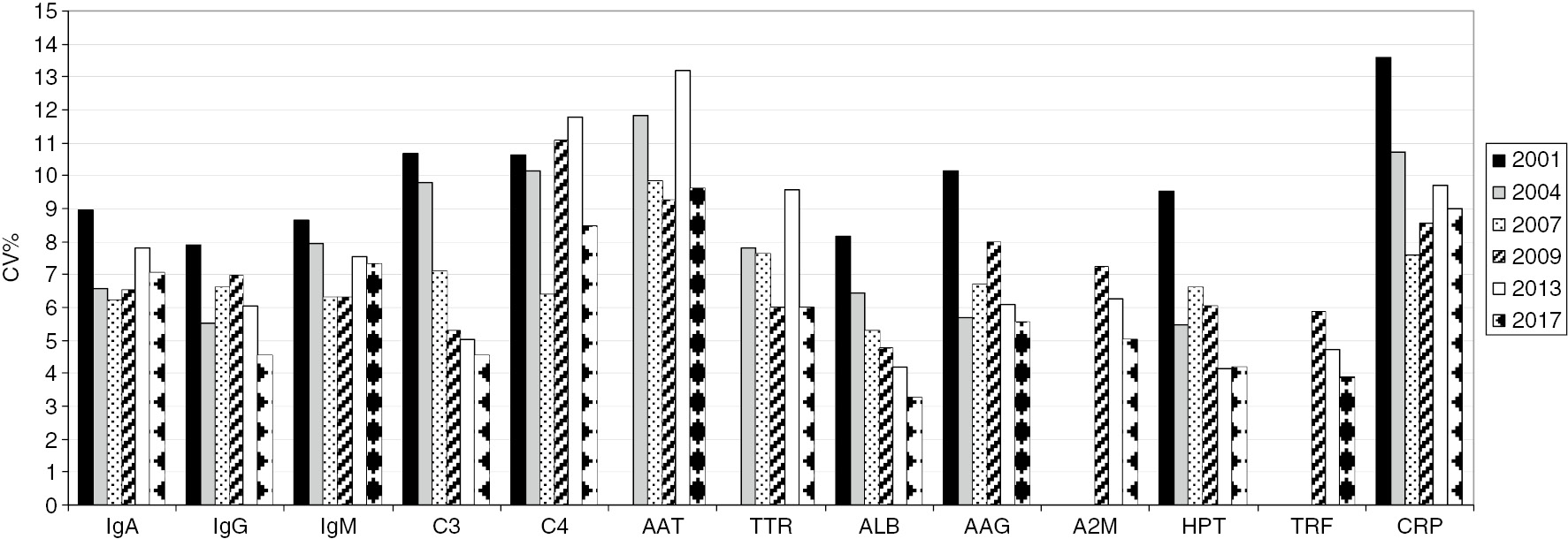
CRB External Quality Assessment program for plasma proteins: mean coefficients of variation (CVs).
AAT, α1-antitrypsin; TTR, prealbumin or transthyretin; ALB, albumin; AAG, α1-acid glycoprotein; A2M, α2-macroglobulin; HPT, haptoglobin; TRF, transferrin, CRP, C-reactive protein.
Discussion
Units
The harmonization in reporting measurands with the same units is a universal problem. On a local basis, a pragmatic solution should be sought, at least by different laboratories in the same geographical area, in order to obviate the reporting of confusing results.
At a European level, the EFLM WG-H campaign foresaw that all laboratories still reporting plasma proteins in mg/dL or g/dL would have to change to mg/L or g/L. In particular, it suggested to change from mg/dL to mg/L for B2M, κ and λ FLC and CRP; from g/dL to g/L for ALB and from mg/dL to g/L for AAT, AAG, A2M, C3, C4, CER, HPT, Ig A, G, M, TTR, RBP and TFR.
At a national level, the Società Italiana di Biochimica Clinica e Biologia Molecolare Clinica (SIBioC) supported this campaign [20] and invited all members to make these changes within 31st October 2016. At the same time, all EQAS providers were asked to change the units in their periodical reports. The CRB, which already used L as the unit of volume for all proteins, following the indications of the EFLM, changed the unit for C4, TTR and CER from mg/L to g/L, except for RBP that remained in mg/L. Moreover, the CRB communicated the reason of the changes to participants with a letter, encouraging them, at the same time, to make the change from mg/dL to g/L or mg/L.
Despite these suggestions, the census of participants’ units used in 2017 demonstrated that their harmonization is far from coming. In Italy, only ~30% of laboratories used the recommended units of measure, and different units were used to express the same concentration of protein, for example, mg/L, g/L and mg/dL for TTR. This may lead clinicians to wrong interpretations, risking patients’ safety.
Why are the laboratories so reticent to change? There are many aspects to be considered: first of all, changing old habits is difficult both for the laboratorist and for the clinician; second, a series of actions have to be undertaken before and during the change.
A particularly troublesome aspect is when the units cause a change in numeric values, which may lead physicians to misinterpretation. The change from dL to L introduces a 10- or 100-fold modification of the numbers and results will increase 10 times for B2M, CRP (from mg/dL to mg/L) and for ALB (from g/dL to g/L) while results will decrease by 100-fold for AAT, AAG, A2M, CER, HPT, Ig A, G, M, TTR and TFR.
Moreover, going from mg/dL to g/L, the values reported in whole numbers must be reported in decimal places and it could be an issue that hinders change, in particular for some of the lower concentration proteins, for example, C4, TTR, CER and RBP must be reported with three decimal places rather than in whole numbers.
The following planning and actions should be undertaken by laboratories when changing units from mg/dL to mg/L causing a 10-fold increase or when changing units from mg/dL to g/L causing a 100-time reduction: (1) synchronized adjustment of analyzer and computer systems; (2) communication and liaison with all service users; (3) updating of all documentation.
Communication to hospital users and general practitioners is fundamental; they should be informed of the intention to change units of measurement. EFLM suggests to insert a message with every report for a period of time prior to the change to provide advance notification: “Please, note: from XX.XX.XX, protein xyz, results will be reported in g/L (or mg/L) instead of mg/dL in line with the national and international guidelines. This means, for example, that a transferrin currently reported as 300 mg/dL will be reported as 3.0 g/L”. Moreover, from the moment of the change, a standard comment to every report sent out for a period of 12 months is suggested: “Please note new units and the change of reference intervals”.
Thus far, there has not been the desired improvement toward harmonization of units, but this should not let the scientific community and the EQAS providers give up encouraging the laboratories to implement all the activities necessary for the change.
Reference intervals
Harmonization of RIs is a continuing project that aims to create uniform interpretation of results and prevent misdiagnosis caused by a greater variation in RI than in the measurement result. However, the situation is complex: regulations, e.g. the European Directive on in vitro diagnostics device [36], lay down that manufacturers should mention RIs in the package inserts, and clinical laboratories, seeking accreditation for compliance with ISO 15189:2012, need to demonstrate that RIs communicated to all users of the laboratory service are appropriate for the patient population served and for the measurement systems used [37]. Laboratories should also verify if they use RIs from literature or manufacturers: in fact, although the International Organization for Standardization (ISO) and Clinical and Laboratory Standard Institute (CLSI) encourage every laboratory to establish its own RIs [38], producing RIs is actually too expensive and a heavy task for most laboratories. The use of the same RI for a specific analyte, for homogeneous populations shared by all laboratories within a country or a region is possible if there are some prerequisites to determine common RIs, one of them is the use of analytical methods traceable to a reference system [39]. If a reference system exists for calibration traceability and well-characterized RIs obtained with a well-standardized measurement procedure are published, the manufacturer can provide those RIs without the need of a new RI study, on condition that its measurements procedure has the same level of standardization [25].
The results of our study show that laboratories primarily used the RIs reported in the packaging inserts of the commercial kits, which, for the 13 certified proteins, should be the same since the manufacturers declared to be traceable to ERM-DA470k/IFCC, but the evidence demonstrates otherwise: Roche cobas, Beckman AU, Siemens BN and Siemens Vista all use IFCC-recommended RIs, but Abbott Architect and Beckman Immage RIs are different because they suggest RIs deriving from a manufaturer’s study on a small groups of subjects. These last RIs should be revised on the basis of larger studies.
Few multicenter RI studies on proteins, measured with a specific reagent system, are available and results are slightly different from the expected value in the manufacturer’s package [40], [41], but it can be seen, for example, that the results of IgA, IgG, IgM and C3 from Beckman AU system aligned well with the results of the Beckman Immage system; hence, for these proteins, common RIs could be given on the two platforms [41].
For ALB, a wide variability of RIs was observed, even among the laboratories using the same analytical method. In particular, the BCP method reads 2 g/L lower than the BCG method; thus, RIs should be different [42], whereas the most used RI for both colorimetric methods was the same: 35–50 g/L.
In addition, for each other protein, a large variability of other RIs exists, even among laboratories using the same analytical system.
To promote harmonization, CRB EQAS provides information on the RIs used by participating laboratories, thus enabling them to verify the appropriateness of their RIs. The laboratory can compare its own RI with those of laboratories using the same or different analytical systems.
We do not recommend using a specific RI, but when a laboratory highlights a lack of agreement between its RI and those of other laboratories using the same analytical system, CRB advises it to verify the correctness of RI employed. For example, a laboratory may change its analytical system, but fails to review the RIs applied, resulting in an incorrect clinical significance.
This extremely important aspect cannot be ignored by EQAS, and organizers must strongly encourage laboratories to carefully verify the reliability of their values [43].
Inter-laboratory variability
The overall inter-laboratory analytical variability, which included both within- and between-manufacturer variances, was very different among the studied proteins, ranging from 3.8% to 12.4%, and decreased in the years for most of them. Results of the present study and those previously found [44], [45], [46] are essentially in agreement with total between-laboratory variability observed in the UK National External Quality Assessment Scheme (UK NEQAS) until 2009 [17].
Certainly, the introduction of ERM-DA470 has resulted in a sudden reduction of the between-assay variation for the majority of the certified proteins [17]; however, persisting bias among manufacturers were reported shortly thereafter for some proteins [47].
Method discrepancy remains a significant gap between different analytical systems and laboratories: for example, results of a recent study highlight that harmonization of the results of three immunoassays (Beckman Immage, Siemens BNI and Roche cobas) for serum Ig measurement has not been completely achieved, even if their traceability to ERM-DA470 [48]. The causes of the differences observed among systems in the measurements of EQA materials and individual patient serum samples, excluding commutability problems, may be related to the nature of antibodies used in the assays with various differences in epitope recognition. In addition, the process of value transfer from the ERM-DA470k/IFCC to the manufacturer’s master calibrators, and subsequently to product calibrants, may somehow deviate from the IFCC protocol [49].
Additionally, for ALB, despite the low inter-laboratory analytical variability observed (CV%=3.95), studies on measurement accuracy and clinical use of ALB demonstrated that the dominating error component was bias for most measurement procedures and that significant differences among immunochemical, BCG and BCP methods compromise interpretations of serum ALB results [42], [50].
Conclusions
Result comparability for some of the certified proteins is not as good as required for their clinical application, suggesting that further investments of diagnostic manufacturers are still necessary, just to reduce the differences existing today above all for IgA, IgM, C4, AAT, TTR and CRP.
A lot of laboratories do not use recommended units and use different RIs for certified proteins where there is evidence, proven by a between-method comparison, that bias does not prevent the use of a common RI. Changing old habits is difficult and requires coordination and collaboration. EQAS reports, providing information on these aspects, are useful to highlight the need to change, stimulate participating laboratories to act and facilitate harmonization of units and RIs.
The ISO15189 [41] requires a redefinition of duties and accountability as a prerequisite to develop and achieve an overall improvement in clinical care through a culture of assessment and monitoring of quality. Currently, the mission of laboratory information is to provide an answer to the clinical question based on the whole result, involving reference range/decisional levels, interpretative comments and diagnostic algorithms, and can indicate possible actions to be taken by clinicians on the patient that produces an outcome. In this context, EQAS, provide a powerful mechanism to survey units, RIs and between-laboratory comparability, so playing a primary role in the assessment and monitoring of all elements that contribute to the formulation of laboratory information.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Plebani M. Harmonization in laboratory medicine: the complete picture. Clin Chim Acta 2013;51.741–51.10.1515/cclm-2013-0075Suche in Google Scholar PubMed
2. Myers GL, Miller WG. The International Consortium for Harmonization of Clinical Laboratory Results (ICHCLR) – a pathway for harmonization. EJIFCC 2016;27:30–6.Suche in Google Scholar
3. Tate JR, Johnson R, Barth J, Panteghini M. Harmonization of laboratory testing – current achievements and future strategies. Clin Chim Acta 2014;432:4–7.10.1016/j.cca.2013.08.021Suche in Google Scholar PubMed
4. Miller WG, Tate JR, Barth JH, Jones GR. Harmonization: the sample, the measurement and the report. Ann Lab Med 2014;34:187–97.10.3343/alm.2014.34.3.187Suche in Google Scholar PubMed PubMed Central
5. Plebani M. Harmonization in laboratory medicine: requests, samples, measurements and reports. Crit Rev Clin Lab Sci 2016;53:184–96.10.3109/10408363.2015.1116851Suche in Google Scholar PubMed
6. Plebani M, Panteghini M. Promoting clinical and laboratory interaction by harmonization. Clin Chim Acta 2014;432:15–21.10.1016/j.cca.2013.09.051Suche in Google Scholar PubMed
7. Ceriotti F. Harmonization initiatives in Europe. eJIFCC 2016;27: 23–9.Suche in Google Scholar
8. Whicher JT, Ritchie RF, Johnson AM, Baudner S, Bienvenu J, Blirup-Jensen S, et al. New international reference preparation for proteins in human serum (RPPHS). Clin Chem 1994;40:934–8.10.1093/clinchem/40.6.934Suche in Google Scholar
9. Johnson AM, Whicher JT, Ledue TB, Carlström A, Itoh Y, Petersen PH. Effect of a new international reference preparation for proteins in human serum (certified reference material 470) on results of the College of American Pathologists survey for plasma proteins. Arch Pathol Lab Med 2000;124:1496–501.10.5858/2000-124-1496-EOANIRSuche in Google Scholar PubMed
10. Johnson AM, Whicher JT. Effect of certified reference material 470 (CRM 470) on national quality assurance programs for serum proteins in Europe. Clin Chem Lab Med 2001;39:1123–8.10.1515/CCLM.2001.177Suche in Google Scholar PubMed
11. Franzini C, Dalla Dea E, Merlini G, Moratti R, Plebani M, Prencipe L, et al. IFCC International plasma protein survey: preliminary analysis of data from Italy. Clin Chem Lab Med 2002;40:A22.Suche in Google Scholar
12. Zegers I, Schreiber W, Munoz A, Sheldon J, Merlini G, Itoh Y, et al. Certification of proteins in the human serum reference material ERM-DA470k/IFCC EUR 23431 EN, European Communities, Luxembourg, 2008, ISBN 978-92-79-094903. In: European Communities, ed., Vol. Luxembourg 2008:1–54.Suche in Google Scholar
13. Zegers I, Keller T, Schreiber W, Sheldon J, Albertini R, Merlini G, et al. Characterization of the new serum protein reference material ERM-DA470k/IFCC: value assignment by immunoassay. Clin Chem 2010;56:1880–8.10.1373/clinchem.2010.148809Suche in Google Scholar PubMed
14. Zegers I, Charout-Got J, Rzychon M, Trapmann S, Emons H, Schimmel H, et al. Certification of C-reactive protein in reference material ERM-DA472/IFCC EUR 23756 EN, European Communities, Luxembourg, 2009, ISBN 978-92-79-11326-0. In: European Communities, ed., Vol. Luxembourg 2009:1–28.Suche in Google Scholar
15. Infusino I, Valente C, Dolci A, Panteghini M. Standardization of ceruloplasmin measurements is still an issue despite the availability of a common reference material. Anal Bioanal Chem 2010;397:521–5.10.1007/s00216-009-3248-0Suche in Google Scholar PubMed
16. Zegers I, Beetham R, Keller T, Sheldon J, Bullok D, MacKenzie F, et al. The importance of commutability of reference materials used as calibrators: the example of ceruloplasmin. Clin Chem 2013;59.1322–9.10.1373/clinchem.2012.201954Suche in Google Scholar
17. Merlini G, Blirup-Jensen S, Johnson AM, Sheldon J, Zegers I. Standardizing plasma protein measurements worldwide: a challenging enterprise. Clin Chem Lab Med 2010;48:1567–75.10.1515/CCLM.2010.314Suche in Google Scholar
18. Flatman R, Ferard G, Dybkaer R, IFCC IUPAC Joint Committee on Nomenclature, Properties and Units (C-NPU). Understanding the ‘Silver Book’ – An important reference for standardized nomenclature in clinical laboratory sciences. Chim Clin Acta 2016;467:4–7.10.1016/j.cca.2016.06.035Suche in Google Scholar
19. Dybkaer K, Jorgensen R. Quantities and units in clinical chemistry. Including recommendation 1966 of commission on clinical chemistry of IUPAC and IFCC. Kǿberhavn: Munksgaard, 1967.Suche in Google Scholar
20. Ceriotti F. Standardizzazione e armonizzazione: SIBioC in prima linea. Biochim Clin 2015;39:48–55.Suche in Google Scholar
21. Siest G, Henny J, Gräsbeck R, Wilding P, Peticlerc C, Queraltó JM, et al. The theory of reference values: an unfinished symphony. Clin Chem Lab Med 2013;51:47–64.10.1515/cclm-2012-0682Suche in Google Scholar
22. Zardo L, Secchiero S, Sciacovelli L, Bonvicini P, Plebani M. Reference intervals: are interlaboratory differences appropriate? Clin Chem Lab Med 1999;37:1131–3.10.1515/CCLM.1999.165Suche in Google Scholar
23. Tate JR, Sikaris KA, Jones GR, Yen T, Koerbin G, Ryan J, et al. on behalf of the AACB Committee for common reference intervals. Harmonizing adult and paediatric reference intervals in Australia and New Zealand: an evidence-based approach for establishing a first panel of chemistry analytes. Clin Biochem Rev 2014;35:213–35.Suche in Google Scholar
24. JR Tate, Koerbin G, Adeli K. Opinion paper: deriving harmonised reference intervals – global activities. EJIFCC 2016;27:48–65.Suche in Google Scholar
25. Miller WG, Horowitz GL, Ceriotti F, Fleming JK, Greenberg N, Katayev A, et al. Reference Intervals: strengths, weaknesses, and challenges. Clin Chem 2016;62.916–23.10.1373/clinchem.2016.256511Suche in Google Scholar
26. Blaabjerg O, Petersen PH, Blom M, Irjala K, Uldall A, Gry H, et al. Common reference intervals for plasma protein in the Nordic Countries. Upsala J Med Sci 1994;99.357–62.10.3109/03009739409179379Suche in Google Scholar
27. Aguzzi F, Gasparro C, Somenzini M, Calatroni S. I nuovi valori di riferimento per le 14 sieroproteine del CRM 470 (RPPHS). Dati preliminary. Biochim Clin 1994;18:612–6.Suche in Google Scholar
28. Ichihara K, Kawai T. Determination of reference intervals for 13 plasma proteins based on IFCC international reference preparation (CRM470) and NCCLS proposed guideline (C28-P, 1992): trial to select reference individuals by results of screening tests and application of maximal likelihood method. J Clin Lab Anal 1997;11:117–24.10.1002/(SICI)1098-2825(1996)10:2<110::AID-JCLA9>3.0.CO;2-GSuche in Google Scholar
29. Dati F, Schumann G, Thomas L, Aguzzi F, Baudner S, Bienvenu J, et al. Consensus of a group of professional societies and diagnostic companies on guidelines for interim reference ranges for 14 proteins in serum based on the standardization against the IFCC/BCR/CAP referece material (CRM 470). Eur J Clin Chem Clin Biochem 1996;34:517–20.Suche in Google Scholar
30. Dati F, Johnson AM, Whicher JT. The existing interim consensus reference ranges and the future approach. Clin Chem Lab Med 2001;39:1134–6.10.1515/CCLM.2001.179Suche in Google Scholar
31. Johnson AM, Petersen PH, Wicher JT, Carlstöm A, MacLennan S, on behalf of the International Federation of Clinical Chemistry and Laboratory Medicine, Committee on Plasma Proteins. Reference intervals for serum proteins: similarities and differences between adult Caucasian and Asian Indian males in Yorkshire, UK. Clin Chem Lab Med 2004;42:792–9.10.1515/CCLM.2004.132Suche in Google Scholar
32. Henny J. Multicenter reference intervals studies: a promising perspective for the future? Clin Chem Lab Med 2013;51:1335–8.10.1515/cclm-2013-0410Suche in Google Scholar PubMed
33. Ichihara K, Ceriotti F, Tam TH, Sueyoshi S, Poon PM, Thong ML, et al. The Asian project for collaborative derivation of reference intervals: (1) strategy and major results of standardized analytes. Clin Chem Lab Med 2013;51:1429–42.10.1515/cclm-2012-0421Suche in Google Scholar PubMed
34. Ichihara K, Ozarda Y, Barth JH, Klee G, Qiu L, Erasmus R, et al. on behalf of Committee on Reference Intervals and Decision Limits, IFCC. A global multicenter study on reference values: 1. Assessment of methods for derivation and comparison of reference intervals. Clin Chim Acta 2017;467:70–82.10.1016/j.cca.2016.09.016Suche in Google Scholar PubMed
35. Ichihara K, Ozarda Y, Barth JH, Klee G, Shimizu Y, Xia L, et al. on behalf of Committee on Reference Intervals and Decision Limits, IFCC. A global multicenter study on reference values: 2. Exploration of sources of variation across the countries. Clin Chim Acta 2017;467:83–97.10.1016/j.cca.2016.09.015Suche in Google Scholar PubMed
36. The new in Vitro Diagnostic Device Regulation (EU) 2017/746. Official Journal of the European Union, Brussels, 2017.Suche in Google Scholar
37. International Organization for Standardization. ISO 15189:2012: Medical laboratories: particular requirements for quality and competence. Geneva, Switzerland: International Organization for Standardization, 2012.Suche in Google Scholar
38. Clinical and Laboratory Standard Institute. Defining, establishing and verifying reference intervals in the clinical laboratory; approved guideline, 3rd ed. CLSI document C28-A3. Wayne, PA: Clinical and Laboratory Standards Institute, 2008:28–61.Suche in Google Scholar
39. Ceriottti F. Common reference intervals: the IFCC position. Clin Biochem 2009;42:297.10.1016/j.clinbiochem.2008.09.017Suche in Google Scholar PubMed
40. Fuentes-Arderiu X, Alonso-Gregorio E, Alvarez-Funes V, Ambrós-Marigómez C, Coca-Fabregás L, Cruz-Placer M, et al. Multicentre physiological reference intervals for serum concentrations of immunoglobulins A, G and M, complement C3c and C4 measured with Tina-Quant reagents systems. Clin Chem Lab Med 2007;45:387–90.10.1515/CCLM.2007.069Suche in Google Scholar PubMed
41. Qin X, Tang G, Qui L, Chang Li P, Xia L, Chen M, et al. A multicenter reference study for specific proteins in China. Medicine 2015;94:1–12.10.1097/MD.0000000000002211Suche in Google Scholar PubMed PubMed Central
42. Bachmann LM, Yu M, Boyd JC, Bruns DE, Miller WG. State of harmonization of 24 serum albumin measurement procedures and implications for medical decisions. Clin Chem 2017;63:770–9.10.1373/clinchem.2016.262899Suche in Google Scholar PubMed
43. Plebani M, Secchiero S, Sciacovelli L, Zardo L, Fietta MB. Utility of External Quality Assessment Programs in checking reference intervals used by clinical laboratories. Clin Chem Lab Med 2002;40:Special Supplement:S140.Suche in Google Scholar
44. Zardo L, Secchiero S, Sciacovelli L, Plebani M. The standardization of plasma protein checked by an External Quality Assessment Scheme (EQAS). Chim Clin Acta 2005;355:S415–6.Suche in Google Scholar
45. Graziani MS, Righetti G, Marini M, Zardo L, Rizzotti P. Il consolidamento della diagnostica proteica. Biochim Clin 2008;32:94–101.Suche in Google Scholar
46. Secchiero S, Sciacovelli L, Plebani M. Armonizzazione della misura dell’albumina sierica: quali informazioni dal programma di Valutazione Esterna di Qualità (VEQ) del Centro di Ricerca Biomedica. Biochim Clin 2015;39:431.Suche in Google Scholar
47. Ledue TB, Johnson AM. Commutability of serum proteins values: persisting bias among manufacturers using values assigned from the certified reference material 470 (CRM 470) in the United States. Clin Chem Lab Med 2001;39:1129–33.10.1515/CCLM.2001.178Suche in Google Scholar PubMed
48. Zhang K, Lin G, Wang L, Sun Y, Zhang R, Jiehong X, et al. Harmonization of results has not been fully achieved for serum immunoglobulin measurements. Clin Chem Lab Med 2015;53:e309–12.10.1515/cclm-2015-0145Suche in Google Scholar PubMed
49. Blirup-Jensen S, Johnson AM, Larsen M. IFCC Committee on plasma proteins. Protein standardization V: value transfer procedure for the assignment of serum protein values from a reference preparation to a target material. Clin Chem lab Med 2008;46:1470–9.10.1515/CCLM.2008.289Suche in Google Scholar PubMed
50. Infusino L, Panteghini M. Accuratezza nella misura e impiego clinico dell’esame di laboratorio: l’esempio dell’albumina sierica. Biochim Clin 2013;37:48–52.Suche in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- The Post-Analytical Phase
- Terminology, units and reporting – how harmonized do we need to be?
- A pragmatic bottom-up approach to harmonize the units of clinical chemistry tests among Belgian clinical laboratories, focusing on immunoassays
- Indirect methods for reference interval determination – review and recommendations
- Verification of reference intervals in routine clinical laboratories: practical challenges and recommendations
- An update report on the harmonization of adult reference intervals in Australasia
- NUMBER: standardized reference intervals in the Netherlands using a ‘big data’ approach
- Pediatric and adult reference interval harmonization in Canada: an update
- Report formatting in laboratory medicine – a call for harmony
- Harmonization of interpretative comments in laboratory hematology reporting: the recommendations of the Working Group on Diagnostic Hematology of the Italian Society of Clinical Chemistry and Clinical Molecular Biology (WGDH-SIBioC)
- Toward harmonization of clinical molecular diagnostic reports: findings of an international survey
- An evidence- and risk-based approach to a harmonized laboratory alert list in Australia and New Zealand
- Harmonization of units and reference intervals of plasma proteins: state of the art from an External Quality Assessment Scheme
- Harmonization activities of Noklus – a quality improvement organization for point-of-care laboratory examinations
- Towards harmonization of external quality assessment/proficiency testing in hemostasis
- The Post-Post-Analytical Phase
- Extra-analytical quality indicators – where to now?
- Role of laboratory medicine in collaborative healthcare
- Acknowledgment
Artikel in diesem Heft
- Frontmatter
- The Post-Analytical Phase
- Terminology, units and reporting – how harmonized do we need to be?
- A pragmatic bottom-up approach to harmonize the units of clinical chemistry tests among Belgian clinical laboratories, focusing on immunoassays
- Indirect methods for reference interval determination – review and recommendations
- Verification of reference intervals in routine clinical laboratories: practical challenges and recommendations
- An update report on the harmonization of adult reference intervals in Australasia
- NUMBER: standardized reference intervals in the Netherlands using a ‘big data’ approach
- Pediatric and adult reference interval harmonization in Canada: an update
- Report formatting in laboratory medicine – a call for harmony
- Harmonization of interpretative comments in laboratory hematology reporting: the recommendations of the Working Group on Diagnostic Hematology of the Italian Society of Clinical Chemistry and Clinical Molecular Biology (WGDH-SIBioC)
- Toward harmonization of clinical molecular diagnostic reports: findings of an international survey
- An evidence- and risk-based approach to a harmonized laboratory alert list in Australia and New Zealand
- Harmonization of units and reference intervals of plasma proteins: state of the art from an External Quality Assessment Scheme
- Harmonization activities of Noklus – a quality improvement organization for point-of-care laboratory examinations
- Towards harmonization of external quality assessment/proficiency testing in hemostasis
- The Post-Post-Analytical Phase
- Extra-analytical quality indicators – where to now?
- Role of laboratory medicine in collaborative healthcare
- Acknowledgment

