Abstract
The goal of harmonizing laboratory information is to contribute to quality in patient care, ultimately improving upon patient outcomes and safety. The main focus of harmonization and standardization initiatives has been on analytical processes within the laboratory walls, clinical chemistry tests in particular. However, two major evidences obtained in recent years show that harmonization should be promoted not only in the analytical phase but also in all steps of the testing process, encompassing the entire field of laboratory medicine, including innovative areas (e.g. “omics”) rather than just conventional clinical chemistry tests. A large body of evidence demonstrates the vulnerability of the extra-analytical phases of the testing cycle. Because only “good biological samples” can assure good analytical quality, a closer interconnection between the different phases of the cycle is needed. In order to provide reliable and accurate laboratory information, harmonization activities should cover all steps of the cycle from the “pre-pre-analytical” phase (right choice of test at right time for right patient) through the analytical steps (right results with right report) to the “post-post-analytical” steps (right and timely acknowledgment of laboratory information, right interpretation and utilization with any necessary advice as to what to do next with the information provided). In addition, modern clinical laboratories are performing a broad menu of hundreds of tests, covering both traditional and innovative subspecialties of the discipline. In addition, according to a centered viewpoint, harmonization initiatives should not be addressed exclusively to clinical chemistry tests but should also include all areas of laboratory medicine.
Introduction
Harmonization is fundamental to quality in laboratory medicine, its end point being to improve patient outcomes by providing accurate and actionable information [1]. Although the importance of standardization and harmonization of laboratory results has been evident for more than four decades, only recently adequate attention has been paid to the increasing need to address this issue. Clinical laboratories can no longer work in isolation, and greater efforts are being made to promote standardization and harmonization in view of the challenges incurred by globalization. Harmonization in laboratory medicine is, in fact, crucial to ensuring that data from different laboratories are comparable and that improvement is made to the accuracy and consistency of results and their interpretation, thus facilitating clinical decision making and assuring valuable patient care [2], [3], [4]. Although patients, clinicians and other healthcare operators tend to assume that clinical laboratory tests performed by different laboratories at different times on the same sample and specimen can be compared and results can be reliably and consistently interpreted, this is not necessarily the case. Not only are many laboratory test results highly variable, poorly standardized and harmonized, but also harmonization efforts are often limited to the analytical phase. In addition, although the focus of initial standardization and harmonization activities has been on improving clinical chemistry assays, a broader approach involving the entire field of laboratory medicine is now urgently needed. As shown in Figure 1, in the last few years, the main developments in the field branch in two directions. Harmonization should, in fact, (a) be promoted not only in the analytical phase but in all steps of the testing process and (b) take into consideration not only conventional clinical chemistry tests but also the entire field of laboratory medicine, including innovative areas (e.g. “omics”).
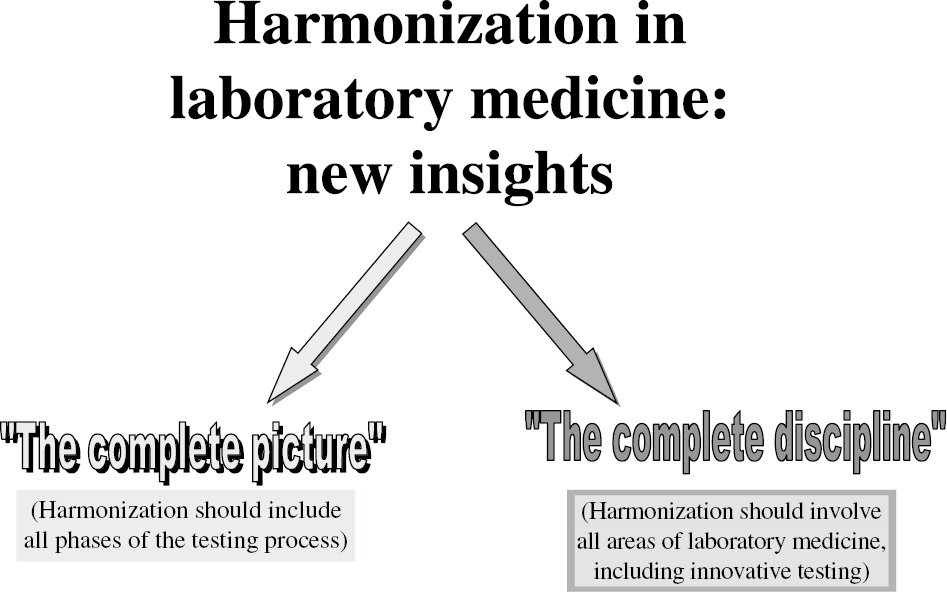
Harmonization in laboratory medicine as a complete picture for all disciplines.
Harmonization in laboratory medicine as a complete picture
The initial focus was on standardizing and harmonizing analytical processes and methods, but it has at last been acknowledged that harmonization must reach further, to include all steps of the total testing process (TTP) or, rather, the brain-to-brain loop [5], [6]. As recently stated by Miller [7], the history of the awareness of the importance of standardization can be traced back to the seminal paper by Belk and Sunderman [8] who, in 1947, demonstrated wide discrepancies between results of 59 US hospital laboratories. The findings made in their survey paved the way for the development of external quality assurance (EQA)/proficiency testing (PT) programs and prompted efforts to establish the hierarchy of certified reference materials (CRMs) and reference measurement procedures (RPMs) that could be accepted as high-order references for the standardization of measurand results [9], [10]. However, increasing consensus has been achieved on the need to focus on a global picture of harmonization, mainly in view of (a) the nature of errors in laboratory medicine [11]; (b) the evidence of large variations in terminology, units and reference intervals [12]; and (c) the risks for patient safety related to the above issues [1]. A body of evidence collected in recent years demonstrates the vulnerability of the extra-analytical phases, and the need to promote quality and harmonization in each and every step of the testing cycle [1], [2], [3], [4], [5], [6]. The importance is now well recognized of the right terminology, units, report formats, reference intervals and decision limits, as well as appropriate tests and test profiles, requests and criteria for interpretation. Harmonization activities should cover all steps of the cycle from the “pre-pre-analytical” phase (right test choice at right time on right patient) through the analytical steps (right results with right report) to the “post-post-analytical” steps (right and timely acknowledgment of laboratory information, right interpretation and utilization with any necessary right advice as to “what to do next with the information”) [1], [3]. The right environment for harmonization programs is the understanding of the interdependence and interconnection between the different phases of the cycle: preanalytical quality is needed for accurate results, and essential information should be introduced in the postanalytical phase to facilitate interpretation and clinical decision making [13]. Only “good samples make good assays” and only “good reports make good laboratory information”. This, in turn, calls for a global view of harmonization in laboratory medicine. In response to the new paradigm of quality and patient safety in laboratory medicine by the Clinical and Laboratory Standards Institute [14], I modified the definition of harmonization to include not only analytical results but also the ultimate laboratory information. According to this re-definition, harmonization is “the process of recognizing, understanding, and explaining differences in all phases of the TTP, while taking steps to achieve uniformity of the laboratory information, or at minimum, a means of identifying and spreading common policies and procedures, such as different groups can use the data obtained from different laboratories interchangeably” [5].
Harmonization in pre-pre- and preanalytical phases
To ensure the answer to a clinical question is reliable, and to obtain accurate laboratory results, “five rights” should be observed in the pre-pre-analytical phase: the right patient, the right time, the right test, the right sample collection/handling and the right sample transportation. Elsewhere, I reported on the evidence available in the literature on the problems of patient and sample identification, the issues of timing and re-testing intervals, the need for an appropriate management of test demand as well as variations in procedures for sample collection/handling and, finally, the overlooked question of adequate sample transportation [13]. Harmonization initiatives are needed to overcome the current state of the art and to promote better clinical practices [15]. Several programs are in progress for harmonizing the initial steps of the cycle, starting with the issue of demand management. The acceptance of the definition of “inappropriate test demand”, apparently “a request that is made outside some form of agreed guidance” [16], [17], is a seminal achievement. The type of guidance given should range from international guidelines, allowing a higher degree of harmonization, to locally agreed behaviors between laboratory professionals, clinicians and other care operators. An interesting proposal has been to use evidence-based re-testing intervals, defined as the “minimum time before a test should be repeated based on the properties of the measurand and the clinical context in which it is used” [18]. As underlined in several surveys, patient/sample identification errors, which are still frequent, can have dramatic consequences [19]. The Joint Commission for Accreditation of Healthcare Organizations and the College of American Pathologists have identified accurate patient identification as a cardinal patient safety goal, and the Working Group for the Preanalytical Phase (WG-PRE) of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) has issued recommendations for harmonizing patient identification and tube labeling [20]. The use of harmonized standard operating procedures (SOP), assuring multiple identifiers, as well as automated systems for patient/sample identification and information technology facilities, should obviate the risks of patient misidentification and “wrong blood in tube”, true nightmares for any clinical laboratory [21]. Major advancements have been achieved in standardizing patient preparation, sample collection and handling procedures, thanks also to the efforts of working groups, in particular, the WG-PRE of the EFLM [22], [23], [24], [25], [26], [27]. Finally, further work to optimize and standardize sample transportation procedures [28], [29], and to harmonize criteria for evaluating the quality of biological samples and accepting/rejecting them has been largely promoted and reported, also by means of automated workstations and serum indexes [30], [31]. The importance of pre-pre-analytical (and preanalytical) variables in affecting the integrity of biospecimens has recently been stressed as a major concern in the pipeline of biomarker discovery, development and translational steps [32], [33]. The harmonization of preanalytical processes, is therefore a fundamental pre-requisite for reducing and controlling preanalytical biological and analytical variability and assuring quality in all fields of laboratory medicine, including “omics” and innovative tests [34].
Harmonization in post- and post-post-analytical phases
The evidence of significant variations in the units used for reporting laboratory results, which can lead to misinterpretation of laboratory data and endanger patient safety, has prompted initiatives aimed at the adoption of a single standardized set of units [5], [12]. Major efforts have been made to promote the harmonization of reference intervals, with should be traceable to reference measurement procedures, and the use of common intervals in huge geographical areas such as Australasia, Asia and Canada [15], [35]. Several initiatives have been successfully promoted to harmonize the management and notification of critical results [36], [37], as well as the practice of providing interpretative comments [38]. As a part of the model of quality indicators, harmonized indicators of timeliness have been proposed to improve this fundamental category of quality of laboratory testing [39], [40]. Other quality indicators have been designed for recording and monitoring data transcription errors, erroneous and/or delayed reports [41].
Harmonization in laboratory medicine: not only clinical chemistry
In an editorial published in the journal some years ago, we stated that we “were clinical chemists once and young. Now we are getting older and specialists in laboratory medicine”, thus recognizing the terrific rate of recent advances and changes in laboratory testing, which has attained unprecedented levels [42]. In the last few decades, clinical needs and technological advancements have changed the landscape of laboratory medicine [43]. The consolidation of different specialties in clinical laboratories answers physicians’ need to receive a unique laboratory report with results from clinical chemistry, hematology, coagulation, molecular diagnostics and microbiological-virological tests. This, in turn, has been facilitated by technological improvements, automation and informatics. Now, a modern clinical laboratory is performing a broad menu of hundreds of tests, covering both traditional and innovative subspecialties of the discipline. A widely debated issue in the harmonization of measurements hinges on the evidence that standardization has been already achieved and seems to be promoted almost exclusively in the field of clinical chemistry and for a limited number of measurands. Efforts in standardizing laboratory tests began in the field of clinical chemistry, and specific requirements for calibration traceability to high order references (CRMs and RPMs) have been applied some clinical chemistry tests. However, in recent decades, awareness has been raised that the standardization principles, namely, those described in ISO 17511, have three major limitations. First, no pure-substance CRMs and RPMs are yet available, and they are unlikely to be forthcoming because of technical limitations for hundreds of important but complex measurands [7]. Moreover, not only is the number of measurands included in the list continuously updated, particularly for the development of new biomarkers, innovative techniques such as “omics”, but the list also includes measurands of significant clinical value. Second, many matrix-based CRMs have not been validated as commutable with patient samples, and in many cases it has been clearly demonstrated that they are not commutable and therefore not suitable for use in an ISO 17511 compliant calibration traceability hierarchy [44]. A body of evidence demonstrates that tracing calibration to a non-commutable CRM causes differences in results for clinical samples among different measurement procedures, which, in turn, may translate in errors in diagnostic and therapeutic decisions. Third, the uncertainty goals based on medical relevance of the measurand cannot be always achieved with RPMs [45], [46]. In view of the above considerations, and the pressing need to promote greater comparability in laboratory information, efforts are being made to achieve equivalent results even when it is not technically feasible to develop an RMP in a reasonable timeframe and the preparation of commutable matrix-based CRMs is challenging. Through harmonization, equivalent results can be achieved within medically meaningful limits, also when standardization is a “mission impossible”, because it is applicable to laboratory fields other than clinical chemistry. In coagulation, one of the most successful and effective examples of harmonization is prothrombin PT expression, for which there is an international normalized ratio (INR). PT results in INR are corrected mathematically by raising the PT ratio to a power equal to the international sensitivity index (ISI). Substantial improvement in interlaboratory harmonization of INR-reported values has been achieved by standardizing reagents/equipment, and by using a novel approach for the verification and harmonization of ISI and MNPT (mean normal PT) values [47]. The reduction of interlaboratory variation in INR reporting translates in the true clinical goal, which is to reduce INR value variability in patients receiving oral vitamin K antagonist anticoagulants, who might be tested at different sites and times, according to a truly patient-centered approach. Similar approaches have been used for harmonizing the results of other coagulation tests, including D-dimer [48]. In the field of autoimmunity, harmonization initiatives have been promoted as standardization still represents a challenging goal. In fact, autoantibodies are complex and structurally heterogeneous molecules due to posttranslational modification and are present in biological fluids in different types due to oligoclonality. This, in turn, precludes the definition of both pure substance CRMs and RPMs. However, the several initiatives underway in this diagnostic area include all the phases of the total testing process: in the preanalytical phase, appropriateness of test requests, harmonization of autoantibody terminology and adoption of uniform nomenclature for laboratory tests; in the analytical phase, harmonization of measurements and sharing of test profiles and diagnostic algorithms; and in the postanalytical phase, harmonization of data reporting and criteria for interpreting immunoserological results, especially harmonization of units, reference intervals, decision limits and definition and notification of critical values [49]. The diagnosis of celiac disease is a paradigmatic example of the importance of harmonization in this field. After the discovery of the clinical value of immunoglobulin A (IgA) anti-tissue transglutaminase (tTG) antibody assay, which is the test of choice for detecting the disease, it was suggested that a histological assessment might be omitted in symptomatic children who have anti-tTG IgA levels 10-fold the upper reference limit (URL), as shown in data from some primary studies [50], [51]. However, this is an arbitrary cutoff value, and a wide disparity of results and poor comparability, both between methods and between laboratories, has been reported [52]. Efforts to standardize and harmonize anti-tTG assays are therefore needed, particularly as the clinical goal, namely, the avoidance of an invasive examination, represents a major advantage for patients’ safety and healthcare costs. For other immunoassays of hormones and proteins with different circulating molecular forms (e.g. thyroid stimulating hormone, insulin, parathyroid hormone [PTH] and troponin), harmonization protocols using panels of authentic individual serum samples and commutable control materials used in EQA schemes have allowed between methods agreement to be achieved. In addition to the utilization of commutable sera, this approach is based on the development of robust statistical approaches for value assignment of the panel of individual samples and or EQA materials [53], [54], [55], [56], [57]. Serological testing and virological testing are other areas of interest in standardization and harmonization initiatives. For example, the standardization of antibody and DNA measurements and harmonization of laboratory procedures are crucial to the success of cancer prevention strategies through screening methods as well as for development and implementation of vaccination against human papilloma virus (HPV). The WHO supported the preparation and initial analysis of a panel of candidate serological and DNA reference reagents aimed at facilitating interlaboratory comparisons and the detection of HPV worldwide. HPV-DNA standards will contribute to the field of HPV prevention, diagnosis and treatment. If made available throughout the world, they will allow for the reference calibration of HPV-DNA tests, thereby enabling manufacturers to further validate and develop HPV detection reagents and kits, which will enable reliable disease and vaccination monitoring worldwide [58]. In tuberculosis (TB), human immunodeficiency virus (HIV) and malaria, molecular analytical tools are yielding the initial hints regarding the mechanisms underlying protection against, or susceptibility to, clinical disease. It is therefore high time that standardization and harmonization of sample collection, storage and molecular analysis are used to ensure highest quality data from these precious samples. Efforts have been made to achieve the standardization of immunological and global platforms that will allow their effective use in a clinical setting, their use for biomarker discovery and validation and their use in generating data sets that can be compared across different platforms and across different preclinical settings and/or different clinical trials [59]. Antimicrobial susceptibility testing with phenotypic methods requires breakpoints, namely, a minimum inhibitory concentration (MIC) categorizing microorganisms into susceptible, intermediately susceptible and resistant to the relevant antimicrobial agent [60]. Historically, there have been multiple guidelines for antimicrobial susceptibility testing, often with different MIC breakpoints dividing organisms into categories of susceptibility. Consequently, the same organism causing similar infections in different countries might be reported as susceptible or resistant, depending on the guidelines followed. The harmonization process calls for a comprehensive review of breakpoints, and includes the application of recent techniques, such as pharmacodynamic analysis, and current data, where available, on susceptibility distributions, resistance mechanisms and clinical outcome related to in vitro tests [61]. Molecular methods, used in a variety of ways, detect, quantify and sequence nucleic acid throughout many areas of laboratory medicine. These tests, in fact, are used to diagnose cancer, provide prognostic assessments, aid in treatment selection and monitor the effectiveness of therapy, also through the detection of minimal residual disease for a single patient. However, particularly in the field of nucleic acid testing for infectious diseases and clinical genetics, the proliferation of assay methods, the number of targets for molecular diagnostics and the absence of standard reference materials contribute to variability in test results among laboratories [62]. In particular, there is a lack of established and globally accepted reference materials to serve as primary standard for comparing performance characteristics and results performed in different laboratories and/or using different methods. In fact, it is increasingly important to harmonize quantitative assays assays, such as viral load measurements, and/or to establish the analytic sensitivity of qualitative assays. However, the availability of reference materials alone does not assure harmonization: reliable approaches for their implementation in test development and validation should be uniform according to guidelines, consensus documents, documentary standards and reference method publications [62]. In the last few years, the fast emergence and the great success of next-generation sequencing (NGS), allowing the fast generation of thousands to millions of base pairs of DNA sequence of an individual, herald a new era in molecular diagnostics. However, the new technologies bring challenges, both at the technical level and in terms of data management, as well as for the interpretation of results. Some guidelines issued appear valuable tools for the harmonization and quality assurance of NGS diagnostics, serving as yet another example of the need to consider the harmonization process a global picture, starting with the need to validate tests before introducing them into clinical practice, to include in the analysis only genes with a known relation between the aberrant genotype and the pathology and to report NGS results in clear and consistent manner [63]. In diagnostic areas based on the microscopic evaluation of tissue and cell morphology, interpretation is hampered by subjectivity, and its quality depends on the competence of professionals [64]. Repeated efforts should be made to effectively train laboratorians and pathologists, showing that interobserver variability can be decreased, but that reproducibility remains a challenge [65]. In turn, harmonization efforts should be reiterated, through both better accreditation and continuing education programs [66] and reference image databases developed with the input of experts in the field [67].
Conclusions
The landscape of laboratory medicine and clinical laboratories has dramatically changed in recent decades. The menu of laboratory tests has increased not only in terms of absolute numbers, but also, even more importantly, in terms of complexity and diagnostic areas. Now, most clinical laboratories perform not only traditional clinical chemistry tests, but also undertake coagulation, hematological, immunological, molecular and microbiological-virological examinations. It is widely recognized that effective patient care, patient safety and clinical research call for comparability of laboratory information independent of time, place and measurement procedure. Comparability is achieved by establishing metrological traceability, which assures that measurement procedures measure the same quantity and that the calibration of measurement procedure is traceable to a common reference system consisting of reference methods and materials [68]. Standardization ensures traceability to the International System of Units (SI), but it should be achieved only in the presence of both the following conditions: (a) the measurand is clearly defined and (b) the agreement of test results is attained by establishing traceability to a higher-order reference measurement procedure or pure substance reference material that can be defined by using the SI. Unfortunately, today the number of measurands that can be standardized is limited in relation to the hundreds of tests performed in laboratory medicine and mainly includes “clinical chemistry” tests. The other way of achieving result (and information) comparability is harmonization, which ensures traceability to a reference system agreed on by convention. For a multitude of measurands, SI and therefore standardization are not yet applicable, particularly when the components in the measurand are heterogeneous: complex molecules such as proteins, peptides, hormones, autoantibodies and many biomarkers create difficulties because they are often structurally heterogeneous due to posttranslational modifications (e.g. glycosylation, sialylation, sulfation, complexes and catabolic fragments). This complexity, in turn, can hinder our current understanding of whether one, several or all forms of a measurand are of clinical interest. Of the many examples, human chorionic gonadotropin (HCG) [5], troponin [56] and PTH [69] represent paradigmatic and commonly requested laboratory measurands. In the last few years, the increased awareness of the lack of clearly defined measurands, reference methods and/or reference materials for many measurands of absolute clinical value has prompted further efforts to achieve harmonization, particularly as the final goal is to assure the comparability not only of analytical results but also of the ultimate laboratory information and not only in clinical chemistry but also in all fields of laboratory medicine. The paradox of comparability in laboratory medicine is that standardization of assays does not always lead to harmonization of information, and harmonization of test results does not always call for standardized reference methods [70]. Many initiatives are in progress, promoted by scientific and professional organizations, including the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), EFLM, the American Association of Clinical Chemistry (AACC) and the International Consortium for Harmonization of Clinical Laboratory Results (ICHCLR). Of particular interest are the initiatives of the ICHCLR, designed to coordinate harmonization activities at an international level [71], but only a closer cooperation between these organizations, laboratory professionals, in vitro diagnostics (IVD) manufacturers, accreditation and regulatory bodies will allow us to achieve greater interchangeability and comparability of laboratory information. This – first and foremost – is a duty and an ethical mandate for laboratory professionals and their scientific organizations. These initiatives should have main drivers in common: (a) harmonization should be promoted in the total testing cycle (“global picture”), and (b) harmonization and standardization initiatives should involve not only the traditional area of clinical chemistry, but all fields of laboratory medicine.
Author contributions: The author has accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Plebani M. Harmonization in laboratory medicine: the complete picture. Clin Chem Lab Med 2013;51:741–51.10.1515/cclm-2013-0075Search in Google Scholar PubMed
2. Tate JR, Johnson R, Barth JH, Panteghini M. Harmonization of laboratory testing – a global activity. Clin Chim Acta 2014;432: 1–3.10.1016/j.cca.2014.02.006Search in Google Scholar PubMed
3. Tate JR, Johnson R, Barth J, Panteghini M. Harmonization of laboratory testing – current achievements and future strategies. Clin Chim Acta 2014;432:4–7.10.1016/j.cca.2013.08.021Search in Google Scholar PubMed
4. Miller WG, Tate JR, Barth JH, Jones GR. Harmonization: the sample, the measurement, and the report. Ann Lab Med 2014;34:187–97.10.3343/alm.2014.34.3.187Search in Google Scholar PubMed PubMed Central
5. Plebani M. Harmonization in laboratory medicine: requests, samples, measurements and reports. Crit Rev Clin Lab Sci 2016;53:184–96.10.3109/10408363.2015.1116851Search in Google Scholar PubMed
6. Aarsand AK, Sandberg S. How to achieve harmonisation of laboratory testing – the complete picture. Clin Chim Acta 2014;432:8–14.10.1016/j.cca.2013.12.005Search in Google Scholar PubMed
7. Miller WG. Harmonization: its time has come. [Editorial] Clin Chem 2017;63:1184–6.10.1373/clinchem.2017.274860Search in Google Scholar PubMed
8. Belk WP, Sunderman FW. A survey of the accuracy of chemical analyses in clinical laboratories. Am J Clin Pathol 1947;17:853–61.10.1093/ajcp/17.11.853Search in Google Scholar PubMed
9. White GH. Metrological traceability in clinical biochemistry. Ann Clin Biochem 2011;48:393–409.10.1258/acb.2011.011079Search in Google Scholar PubMed
10. Siekmann L. Metrological traceability – a concept for standardization in laboratory medicine. Clin Chem Lab Med 2013;51:953–7.10.1515/cclm-2012-0710Search in Google Scholar PubMed
11. Plebani M. The detection and prevention of errors in laboratory medicine. Ann Clin Biochem 2010;47:101–10.10.1258/acb.2009.009222Search in Google Scholar PubMed
12. Legg M, Swanepoel C. The australian pathology units and terminology standardization project – an overview. Clin Biochem Rev 2012;33:103–8.Search in Google Scholar
13. Plebani M. Towards a new paradigm in laboratory medicine: the five rights. Clin Chem Lab Med 2016;54:1881–91.10.1515/cclm-2016-0848Search in Google Scholar PubMed
14. Clinical and Laboratory Standards Institute (CLSI) Harmonized Terminology Database. Available at: http://htd.clsi.org/listallterms.asp. Accessed: 29 Jun 2017.Search in Google Scholar
15. Plebani M. Harmonization of clinical laboratory information – current and future strategies. E J Int Fed Clin Chem 2016;27:15–22.Search in Google Scholar
16. Fryer AA, Smellie WS. Managing demand for laboratory tests: a laboratory toolkit. J Clin Pathol 2013;66:62–72.10.1136/jclinpath-2011-200524Search in Google Scholar PubMed
17. Plebani M, Panteghini M. Promoting clinical and laboratory interaction by harmonization. Clin Chim Acta 2014;432:15–21.10.1016/j.cca.2013.09.051Search in Google Scholar PubMed
18. Lang T. The Association for Clinical Biochemistry & Laboratory Medicine. National minimum re-testing interval project. Prepared for the Clinical Practice Group of the ACB and supported by the Royal College of Pathologists 2013:1–36.Search in Google Scholar
19. College of American Pathologists, Valenstein PN, Raab SS, Walsh MK. Identification errors involving clinical laboratories: a College of American Pathologists Q-Probes study of patient and specimen identification errors at 120 institutions. Arch Pathol Lab Med 2006;130:1106–13.10.5858/2006-130-1106-IEICLSearch in Google Scholar PubMed
20. Van Dongen-Lases EC, Cornes MP, Grankvist K, Ibarz M, Kristensen GB, Lippi G, et al. Working Group for Preanalytical Phase (WG-PRE), European Federation of Clinical Chemistry and Laboratory Medicine (EFLM). Patient identification and tube labelling – a call for harmonisation. Clin Chem Lab Med 2016;54:1141–5.10.1515/cclm-2015-1089Search in Google Scholar PubMed
21. Bolton-Maggs PH, Wood EM, Wiersum-Osselton JC. Wrong blood in tube – potential for serious outcomes: can it be prevented? Br J Haematol 2015;168:3–13.10.1111/bjh.13137Search in Google Scholar PubMed
22. Simundic AM, Cornes M, Grankvist K, Lippi G, Nybo M, Kovalevskaya S, et al. Survey of national guidelines, education and training on phlebotomy in 28 European countries: an original report by the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) working group for the preanalytical phase (WG-PA). Clin Chem Lab Med 2013;51:1585–93.10.1515/cclm-2013-0283Search in Google Scholar PubMed
23. Simundic AM, Cornes M, Grankvist K, Lippi G, Nybo M. Standardization of collection requirements for fasting samples: for the working group on preanalytical phase (WG-PA) of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM). Clin Chim Acta 2014;432:33–7.10.1016/j.cca.2013.11.008Search in Google Scholar PubMed
24. Lippi G, Banfi G, Church S, Cornes M, De Carli G, Grankvist K, et al. Preanalytical quality improvement. In pursuit of harmony, on behalf of European Federation for Clinical Chemistry and Laboratory Medicine (EFLM) Working Group for Preanalytical Phase (WG-PRE). Clin Chem Lab Med 2015;53:357–70.10.1515/cclm-2014-1051Search in Google Scholar PubMed
25. Lippi G, Baird GS, Banfi G, Bölenius K, Cadamuro J, Church S, et al. Improving quality in the preanalytical phase through innovation, on behalf of the European Federation for Clinical Chemistry and Laboratory Medicine (EFLM) Working Group for Preanalytical Phase (WG-PRE). Clin Chem Lab Med 2017;55:489–500.10.1515/cclm-2017-0107Search in Google Scholar PubMed
26. Cornes M, van Dongen-Lases E, Grankvist K, Ibarz M, Kristensen G, Lippi G, et al. Order of blood draw: Opinion Paper by the European Federation for Clinical Chemistry and Laboratory Medicine (EFLM) working group for the preanalytical phase (WG-PRE).Clin Chem Lab Med 2017;55:27–31.10.1515/cclm-2016-0426Search in Google Scholar PubMed
27. Lippi G, Simundic A-M, on behalf of the European Federation for Clinical Chemistry and Laboratory Medicine (EFLM) Working Group for Preanalytical Phase (WG-PRE). The EFLM strategy for harmonization of the preanalytical phase. Clin Chem Lab Med 2018;56:1660–6.10.1515/cclm-2017-0277Search in Google Scholar PubMed
28. Zaninotto M, Tasinato A, Padoan A, Vecchiato G, Pinato A, Sciacovelli L, et al. Effects of sample transportation on commonly requested laboratory tests. Clin Chem Lab Med 2012;50:1755–60.10.1515/cclm-2012-0150Search in Google Scholar PubMed
29. Zaninotto M, Tasinato A, Vecchiato G, Legnaro A, Pinato A, Plebani M. Performance specifications in extra-analytical phase of laboratory testing: sample handling and transportation. Clin Biochem 2017;50:574–8.10.1016/j.clinbiochem.2017.04.008Search in Google Scholar PubMed
30. Lippi G, Avanzini P, Campioli D, Da Rin G, Dipalo M, Aloe R, et al. Systematical assessment of serum indices does not impair efficiency of clinical chemistry testing: a multicenter study. Clin Biochem 2013;46:1281–4.10.1016/j.clinbiochem.2013.06.007Search in Google Scholar PubMed
31. Dolci A, Panteghini M. Harmonization of automated hemolysis index assessment and use: is it possible? Clin Chim Acta 2014;432:38–43.10.1016/j.cca.2013.10.012Search in Google Scholar PubMed
32. Ellervik C, Vaught J. Preanalytical variables affecting the integrity of human biospecimens in biobanking. Clin Chem 2015;61:914–34.10.1373/clinchem.2014.228783Search in Google Scholar PubMed
33. Kellogg MD, Ellervik C, Morrow D, Hsing A, Stein E, Sethi AA. Preanalytical considerations in the design of clinical trials and epidemiological studies. Clin Chem 2015;61:797–803.10.1373/clinchem.2014.226118Search in Google Scholar PubMed
34. Basso D, Padoan A, Laufer T, Aneloni V, Moz S, Schroers H, et al. Relevance of pre-analytical blood management on the emerging cardiovascular protein biomarkers TWEAK and HMGB1 and on miRNA serum and plasma profiling. Clin Biochem 2017;50:186–93.10.1016/j.clinbiochem.2016.11.005Search in Google Scholar PubMed
35. Adeli K, Higgins V, Seccombe D, Collier CP, Balion CM, Cembrowski G, et al. National survey of adult and pediatric reference intervals in clinical laboratories across Canada: a report of the CSCC working group on reference interval harmonization. Clin Biochem 2017;50:925–35.10.1016/j.clinbiochem.2017.06.006Search in Google Scholar PubMed
36. Piva E, Plebani M. From “panic” to “critical” values: which path toward harmonization? Clin Chem Lab Med 2013;51:2069–71.10.1515/cclm-2013-0459Search in Google Scholar PubMed
37. Lam Q, Ajzner E, Campbell CA, Young A. Critical risk results – an update on international initiatives. Eur J Int Fed Clin Chem 2016;27:66–76.Search in Google Scholar
38. Vasikaran S, Sikaris K, Kilpatrick E, French J, Badrick T, Osypiw J, et al. Assuring the quality of interpretative comments in clinical chemistry. Clin Chem Lab Med 2016;54:1901–11.10.1515/cclm-2016-0709Search in Google Scholar PubMed
39. Plebani M, Astion ML, Barth JH, Chen W, de Oliveira Galoro CA, Escuer MI, et al. Harmonization of quality indicators in laboratory medicine. A preliminary consensus. Clin Chem Lab Med 2014;52:951–8.10.1515/cclm-2014-0142Search in Google Scholar PubMed
40. Sciacovelli L, Lippi G, Sumarac Z, West J, Garcia Del Pino Castro I, Furtado Vieira K, et al. Quality Indicators in Laboratory Medicine: the status of the progress of IFCC Working Group “Laboratory Errors and Patient Safety” project. Clin Chem Lab Med 2017;55:348–57.10.1515/cclm-2016-0929Search in Google Scholar PubMed
41. Plebani M. EFLM Task Force on Performance Specifications for the extra-analytical phases. Performance specifications for the extra-analytical phases of laboratory testing: why and how. Clin Biochem 2017;50:550–4.10.1016/j.clinbiochem.2017.02.002Search in Google Scholar PubMed
42. Siest G, Plebani M. CCLM: evolving to meet the needs of today’s laboratory professionals and scientists. [Editorial] Clin Chem Lab Med 2008;46:883–4.10.1515/CCLM.2008.200Search in Google Scholar PubMed
43. Plebani M. Quality in laboratory medicine: 50 years on. Clin Biochem 2017;50:101–4.10.1016/j.clinbiochem.2016.10.007Search in Google Scholar PubMed
44. Miller WG, Myers GL, Rej R. Why commutability matters. [Editorial] Clin Chem 2006;52:553–4.10.1373/clinchem.2005.063511Search in Google Scholar PubMed
45. Braga F, Infusino I, Panteghini M. Performance criteria for combined uncertainty budget in the implementation of metrological traceability. Clin Chem Lab Med 2015;53:905–12.10.1515/cclm-2014-1240Search in Google Scholar PubMed
46. Infusino I, Panteghini M. Serum albumin: accuracy and clinical use. Clin Chim Acta 2013;419:15–8.10.1016/j.cca.2013.01.005Search in Google Scholar PubMed
47. Favaloro EJ, McVicker W, Lay M, Ahuja M, Zhang Y, Hamdam S, et al. Harmonizing the International Normalized Ratio (INR): standardization of methods and use of novel strategies to reduce interlaboratory variation and bias. Am J Clin Pathol 2016;145:191–202.10.1093/ajcp/aqv022Search in Google Scholar PubMed
48. Tachil J, Lippi G, Favaloro E. D-Dimer testing: laboratory aspects and current issues. Methods Mol Biol 2017;1646:91–104.10.1007/978-1-4939-7196-1_7Search in Google Scholar PubMed
49. Tozzoli R, Villalta D, Bizzaro N. Challenges in the standardization of autoantibody testing: a comprehensive review. Clin Rev Allergy Immunol 2017;53:68–77.10.1007/s12016-016-8579-ySearch in Google Scholar PubMed
50. Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, et al. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of celiac disease. J Pediatr Gastroenterol Nutr 2012;54:136–60.10.1097/MPG.0b013e31821a23d0Search in Google Scholar PubMed
51. Hennes EM, Zeniya M, Czaja AJ, Pares A, Dalekos GN, Krawitt EL, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008;48:169–76.10.1002/hep.22322Search in Google Scholar PubMed
52. Beltran L, Koening M, Egner W, Howard M, Butt A, Austin MR, et al. High-titre circulating tissue transglutaminase-2 antibodies predict small bowel villous atrophy, but decision cut-off limits must be locally validated. Clin Exp Immunol 2014;176:190–8.10.1111/cei.12249Search in Google Scholar PubMed PubMed Central
53. Van Houcke SK, Van Aelst S, Van Uytfanghe K, Thienpont LM. Harmonization of immunoassays to the all-procedure trimmed mean – proof of concept by use of data from the insulin standardization project. Clin Chem Lab Med 2013;51:e103–5.10.1515/cclm-2012-0661Search in Google Scholar PubMed
54. Stöckl D, Van Uytfanghe K, Van Aelst S, Thienpont LM. A statistical basis for harmonization of thyroid stimulating hormone immunoassays using a robust factor analysis model. Clin Chem Lab Med 2014;52:965–72.10.1515/cclm-2013-1038Search in Google Scholar PubMed
55. Clerico A, Ripoli A, Zucchelli GC, Plebani M. Harmonization protocols for thyroid stimulating hormone (TSH) immunoassays: different approaches based on the consensus mean value. Clin Chem Lab Med 2015;53:377–82.10.1515/cclm-2014-0586Search in Google Scholar PubMed
56. Clerico A, Ripoli A, Masotti S, Prontera C, Storti S, Fortunato A, et al. Pilot study on harmonization of cardiac troponin I immunoassays using patients and quality control plasma samples. On behalf of the Italian Section of the European Ligand Assay Society (ELAS) and of the Study Group on Cardiovascular Biomarkers of the Società Italiana di Biochimica Clinica (SIBioC). Clin Chim Acta 2016;456:42–8.10.1016/j.cca.2016.02.017Search in Google Scholar PubMed
57. Thienpont LM, Van Uytfanghe K, De Grande LA, Reynders D, Das B, Faix JD, et al. IFCC Committee for Standardization of Thyroid Function Tests (C-STFT). Harmonization of serum thyroid-stimulating hormone measurements paves the way for the adoption of a more uniform reference interval. Clin Chem 2017;63:1248–60.10.1373/clinchem.2016.269456Search in Google Scholar PubMed
58. Pagliusi SR, Dillner J, Pawlita M, Quint WG, Wheeler CM, Ferguson M. Chapter 23: International Standard reagents for harmonization of HPV serology and DNA assays – an update. Vaccine 2006;24(Suppl 3):S3/193–200.10.1016/j.vaccine.2006.06.016Search in Google Scholar PubMed
59. Dutruel C, Thole J, Geels M, Mollenkopf HJ, Ottenhoff T, Guzman CA, et al. TRANSVAC workshop on standardisation and harmonisation of analytical platforms for HIV, TB and malaria vaccines: ‘how can big data help?’. Vaccine 2014;32:4365–8.10.1016/j.vaccine.2014.06.014Search in Google Scholar PubMed
60. Kahlmeter G. Defining antibiotic resistance-towards international harmonization. Ups J Med Sci 2014;119:78–86.10.3109/03009734.2014.901446Search in Google Scholar PubMed PubMed Central
61. Brown D, Macgowan A. Harmonization of antimicrobial susceptibility testing breakpoints in Europe: implications for reporting intermediate susceptibility. J Antimicrob Chemother 2010;65:183–5.10.1093/jac/dkp432Search in Google Scholar PubMed
62. Holden MJ, Madej RM, Minor P, Kalman LV. Molecular diagnostics: harmonization through reference materials, documentary standards and proficiency testing. Expert Rev Mol Diagn 2011;11:741–55.10.1586/erm.11.50Search in Google Scholar PubMed
63. Matthijs G, Souche E, Alders M, Corveleyn A, Eck S, Feenstra I, et al. Guidelines for diagnostic next-generation sequencing. Eur J Hum Genet 2016;24:1515.10.1038/ejhg.2015.226Search in Google Scholar PubMed PubMed Central
64. Comperat E, Egevad L, Lopez-Beltran A, Camparo P, Algaba F, Amin M, et al. An interobserver reproducibility study on invasiveness of bladder cancer using virtual microscopy and heatmaps. Histopathology 2013;63;756–66.10.1111/his.12214Search in Google Scholar PubMed
65. Van der Kwast T, Collette L, Van Poppel H, Van Cangh P, Vekemans K, DaPozzo L, et al. Impact of pathology review of stage and margin status of radical prostatectomy specimens (EORTC trial 22911). Virchows Arch 2006;449;428–34.10.1007/s00428-006-0254-xSearch in Google Scholar PubMed
66. Jones KN, Kreisle R, Geiss RW, Holliman JH, Lill PH, Anderson PG. Group for research in pathology education online resources to facilitate pathology instruction. Arch Pathol Lab Med 2002;126:346–50.10.5858/2002-126-0346-GFRIPESearch in Google Scholar PubMed
67. Egevad L, Cheville J, Evans AJ, Hombland J, Kench JG, Kristiansen G. Pathology imagebase – a reference image database for standardization of pathology. Histopathology 2017;71:677–85.10.1111/his.13313Search in Google Scholar PubMed
68. Vesper HW, Myers GL, Miller WG. Current practices and challenges in the standardization and harmonization of clinical laboratory tests. Am J Clin Nutr 2016;104:907S–12S.10.3945/ajcn.115.110387Search in Google Scholar PubMed PubMed Central
69. Sturgeon CM, Sprague S, Almond A, Cavalier E, Fraser WD, Algeciras-Schimnich A, et al. Perspective and priorities for improvement of parathyroid hormone (PTH) measurement – A view from the IFCC Working Group for PTH. Clin Chim Acta 2017;467:42–7.10.1016/j.cca.2016.10.016Search in Google Scholar PubMed PubMed Central
70. Klee GG. Harmonization and standardization of thyroid function tests. [Editorial] Clin Chem 2010;56:879–80.10.1373/clinchem.2010.145540Search in Google Scholar PubMed
71. Myers GL, Miller WG. Challenge to coordinate harmonization activities on an international level. [Editorial] Clin Chem 2017;63:1429–30.10.1373/clinchem.2017.275669Search in Google Scholar PubMed
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorials
- Harmonization in laboratory medicine: Blowin’ in the wind
- Standardization and harmonization of autoimmune diagnostics
- On the complexity of hemostasis and the need for harmonization of test practice
- Harmonization of laboratory hematology: a long and winding journey
- Section 1: Current Harmonization Activities at Global Level
- Harmonization in laboratory medicine: more than clinical chemistry?
- Harmonization of External Quality Assessment Schemes and their role – clinical chemistry and beyond
- An overview of EFLM harmonization activities in Europe
- Metrological traceability and harmonization of medical tests: a quantum leap forward is needed to keep pace with globalization and stringent IVD-regulations in the 21st century!
- Assessment of bone turnover in osteoporosis: harmonization of the total testing process
- Recent initiatives in harmonization of hemostasis practice
- EASI – European Autoimmunity Standardisation Initiative: facing the challenges of diagnostics in autoimmunity
- Harmonization of microbiology processes and standards: work in progress
- Harmonization initiatives in the generation, reporting and application of biological variation data
- Harmonization of accreditation to ISO15189
- External quality assessment programs in the context of ISO 15189 accreditation
- Section 2: Pre-Pre and Pre-Analytical Phase
- Laboratory testing in the emergency department: an Italian Society of Clinical Biochemistry and Clinical Molecular Biology (SIBioC) and Academy of Emergency Medicine and Care (AcEMC) consensus report
- The EFLM strategy for harmonization of the preanalytical phase
- Section 3: The Analytical Phase
- The roadmap for harmonization: status of the International Consortium for Harmonization of Clinical Laboratory Results
- The quest for equivalence of test results: the pilgrimage of the Dutch Calibration 2.000 program for metrological traceability
- Current state and recommendations for harmonization of serum/plasma 17-hydroxyprogesterone mass spectrometry methods
- International normalized ratio (INR) testing in Europe: between-laboratory comparability of test results obtained by Quick and Owren reagents
- Detecting molecular forms of antithrombin by LC-MRM-MS: defining the measurands
- A design for external quality assessment for the analysis of thiopurine drugs: pitfalls and opportunities
- Harmonization of PCR-based detection of intestinal pathogens: experiences from the Dutch external quality assessment scheme on molecular diagnosis of protozoa in stool samples
- Harmonization of urine albumin/creatinine ratio (ACR) results: a study based on an external quality assessment program in Polish laboratories
- Standardization of autoimmune testing – is it feasible?
- Diagnostic laboratory tests for systemic autoimmune rheumatic diseases: unmet needs towards harmonization
- Clinically relevant discrepancies between different rheumatoid factor assays
- An international survey on anti-neutrophil cytoplasmic antibodies (ANCA) testing in daily clinical practice
- Predictive autoimmunity using autoantibodies: screening for anti-nuclear antibodies
- Harmonization in autoimmune thyroid disease diagnostics
- International consensus on antinuclear antibody patterns: definition of the AC-29 pattern associated with antibodies to DNA topoisomerase I
- Reference standards for the detection of anti-mitochondrial and anti-rods/rings autoantibodies
- International Consensus on Antinuclear Antibody Patterns: defining negative results and reporting unidentified patterns
Articles in the same Issue
- Frontmatter
- Editorials
- Harmonization in laboratory medicine: Blowin’ in the wind
- Standardization and harmonization of autoimmune diagnostics
- On the complexity of hemostasis and the need for harmonization of test practice
- Harmonization of laboratory hematology: a long and winding journey
- Section 1: Current Harmonization Activities at Global Level
- Harmonization in laboratory medicine: more than clinical chemistry?
- Harmonization of External Quality Assessment Schemes and their role – clinical chemistry and beyond
- An overview of EFLM harmonization activities in Europe
- Metrological traceability and harmonization of medical tests: a quantum leap forward is needed to keep pace with globalization and stringent IVD-regulations in the 21st century!
- Assessment of bone turnover in osteoporosis: harmonization of the total testing process
- Recent initiatives in harmonization of hemostasis practice
- EASI – European Autoimmunity Standardisation Initiative: facing the challenges of diagnostics in autoimmunity
- Harmonization of microbiology processes and standards: work in progress
- Harmonization initiatives in the generation, reporting and application of biological variation data
- Harmonization of accreditation to ISO15189
- External quality assessment programs in the context of ISO 15189 accreditation
- Section 2: Pre-Pre and Pre-Analytical Phase
- Laboratory testing in the emergency department: an Italian Society of Clinical Biochemistry and Clinical Molecular Biology (SIBioC) and Academy of Emergency Medicine and Care (AcEMC) consensus report
- The EFLM strategy for harmonization of the preanalytical phase
- Section 3: The Analytical Phase
- The roadmap for harmonization: status of the International Consortium for Harmonization of Clinical Laboratory Results
- The quest for equivalence of test results: the pilgrimage of the Dutch Calibration 2.000 program for metrological traceability
- Current state and recommendations for harmonization of serum/plasma 17-hydroxyprogesterone mass spectrometry methods
- International normalized ratio (INR) testing in Europe: between-laboratory comparability of test results obtained by Quick and Owren reagents
- Detecting molecular forms of antithrombin by LC-MRM-MS: defining the measurands
- A design for external quality assessment for the analysis of thiopurine drugs: pitfalls and opportunities
- Harmonization of PCR-based detection of intestinal pathogens: experiences from the Dutch external quality assessment scheme on molecular diagnosis of protozoa in stool samples
- Harmonization of urine albumin/creatinine ratio (ACR) results: a study based on an external quality assessment program in Polish laboratories
- Standardization of autoimmune testing – is it feasible?
- Diagnostic laboratory tests for systemic autoimmune rheumatic diseases: unmet needs towards harmonization
- Clinically relevant discrepancies between different rheumatoid factor assays
- An international survey on anti-neutrophil cytoplasmic antibodies (ANCA) testing in daily clinical practice
- Predictive autoimmunity using autoantibodies: screening for anti-nuclear antibodies
- Harmonization in autoimmune thyroid disease diagnostics
- International consensus on antinuclear antibody patterns: definition of the AC-29 pattern associated with antibodies to DNA topoisomerase I
- Reference standards for the detection of anti-mitochondrial and anti-rods/rings autoantibodies
- International Consensus on Antinuclear Antibody Patterns: defining negative results and reporting unidentified patterns

