Serological diagnosis and prognosis of severe acute pancreatitis by analysis of serum glycoprotein 2
-
Dirk Roggenbuck
, Alexander Goihl
Abstract
Background:
Glycoprotein 2 (GP2), the pancreatic major zymogen granule membrane glycoprotein, was reported to be elevated in acute pancreatitis in animal models.
Methods:
Enzyme-linked immunosorbent assays (ELISAs) were developed to evaluate human glycoprotein 2 isoform alpha (GP2a) and total GP2 (GP2t) as specific markers for acute pancreatitis in sera of 153 patients with acute pancreatitis, 26 with chronic pancreatitis, 125 with pancreatic neoplasms, 324 with non-pancreatic neoplasms, 109 patients with liver/biliary disease, 67 with gastrointestinal disease, and 101 healthy subjects. GP2a and GP2t levels were correlated with procalcitonin and C-reactive protein in 152 and 146 follow-up samples of acute pancreatitis patients, respectively.
Results:
The GP2a ELISA revealed a significantly higher assay accuracy in contrast to the GP2t assay (sensitivity ≤3 disease days: 91.7%, specificity: 96.7%, positive likelihood ratio [LR+]: 24.6, LR–: 0.09). GP2a and GP2t levels as well as prevalences were significantly elevated in early acute pancreatitis (≤3 disease days) compared to all control cohorts (p<0.05, respectively). GP2a and GP2t levels were significantly higher in patients with severe acute pancreatitis at admission compared with mild cases (p<0.05, respectively). Odds ratio for GP2a regarding mild vs. severe acute pancreatitis with lethal outcome was 7.8 on admission (p=0.0222). GP2a and GP2t levels were significantly correlated with procalcitonin [Spearman’s rank coefficient of correlation (ρ)=0.21, 0.26; p=0.0110, 0.0012; respectively] and C-reactive protein (ρ=0.37, 0.40; p<0.0001; respectively).
Conclusions:
Serum GP2a is a specific marker of acute pancreatitis and analysis of GP2a can aid in the differential diagnosis of acute upper abdominal pain and prognosis of severe acute pancreatitis.
Introduction
The serological diagnosis of acute pancreatitis, the main cause for hospitalization in case of acute abdominal pain in developed countries [1], is still a laboratory challenge [2]. The incidence of acute pancreatitis, a life-threatening disease, ranges from 17.5 to 73.4 cases per 100,000 individuals globally [2]. Although the pathophysiology of acute pancreatitis is not understood entirely yet, it is now widely acknowledged that premature intra-pancreatic activation of proenzymes in particular trypsinogen stored in zymogen granules (ZGs) plays a pivotal role [3], [4], [5], [6], [7]. Thus, acute pancreatitis onset is characterized by acinar cell injury resulting in an impaired polarity of proenzyme secretion and basolateral release of ZG contents [4]. The ensuing inflammation can lead to a systemic response syndrome and even shock [8]. Thus, the leakage of ZG-related molecules and release of inflammatory cytokines generates a plethora of potential serological acute pancreatitis-specific markers [9]. Despite the continuous identification of novel biomarkers, serum lipase analysis is still the only serological tool with high strength of evidence according to the revised 2012 Atlanta Classification and preferred to amylase testing [10], [11]. False positive lipase testing has been reported recently [12] and lipase analysis is not recommended for severity stratification [9]. Instead, C-reactive protein (CRP) and recently procalcitonin (PCT) are used for severity assessment of acute pancreatitis [10], [13], [14].
Interestingly, a well-characterized animal model of acute pancreatitis revealed elevated major ZG membrane glycoprotein 2 (GP2) levels as a potential serum marker [15]. Alike digestive proenzymes, two isoforms of GP2, alpha (GP2a) and beta (GP2b), both expressed at equal levels and referred to as total GP2 (GP2t) together are released into the pancreatic duct upon exocrine pancreatic stimulation [16], [17]. Significantly higher levels of GP2 could be detected by a research enzyme-linked immunosorbent assay (ELISA) in sera of acute pancreatitis patients compared to controls [18]. Assessment of GP2 showed a better assay accuracy and at least 1 day longer increased levels in patients with acute pancreatitis compared to established lipase/amylase testing. However, elevated GP2 levels were also observed in patients with chronic pancreatitis and pancreatic cancer which questioned the clinical usefulness of GP2 as a specific marker.
Thus, we developed ELISAs for the detection of acute pancreatitis-specific GP2 and investigated the diagnostic and prognostic value of serum GP2 levels in a large cohort of patients with acute pancreatitis and an extensive disease-control group.
Materials and methods
Subjects
Characteristics of the 153 patients with acute pancreatitis and 651 controls collected at the intensive care unit of the department of surgery (Otto-von-Guericke-University Magdeburg, 05/1995–03/2013) and 101 healthy subjects (in.vent Diagnostica, Hennigsdorf, Germany) are given in Table 1. Apart from acute pancreatitis, the following recruited disease controls are relevant for the differential diagnosis of acute upper abdominal in particular epigastric pain: chronic pancreatitis, pancreatic neoplasms, gastric and esophageal cancer, as well as peptic ulcer.
Patients’ and healthy subjects’ (BD) characteristics.
| Disorder | Number (%) | Age (IQR) | Gender (F/M) |
|---|---|---|---|
| Acute pancreatitis | 153 | 50.0 (28.0) | 58/95 |
| Etiology | |||
| Alcohol abuse | 59 (38.6) | 40.0 (12.0) | 11/48 |
| Biliary disease | 54 (35.3) | 65.0 (24.0) | 30/24 |
| Drug induced | 1 (0.7) | 50.0 | 0/1 |
| Idiopathic | 21 (13.7) | 61.0 (24.8) | 11/10 |
| Post-trauma | 11 (7.2) | 57.0 (19.0) | 2/9 |
| Post-ERCP | 7 (4.6) | 59.0 (39.5) | 4/3 |
| Severity | |||
| Mild | 22 (14.4) | 57.5 (29.0) | 14/8 |
| Local complications | 74 (48.4) | 45.5 (30.0) | 27/47 |
| Systemic complications | 41 (26.8) | 54.0 (28.5) | 11/30 |
| Lethal outcome | 16 (10.5) | 65.6 (29.0) | 6/10 |
| Chronic pancreatitis | 26 | 48.0 (19.0)a | 13/13b |
| Pancreatic neoplasm | 125 | 60.0 (16.0) | 68/57 |
| Pancreatic adenocarcinoma | 25 (20.0) | 60.5 (15.2)a | 8/17b |
| Periampullary carcinoma | 36 (28.8) | 63.0 (17.5) | 16/20b |
| Benign pancreatic cystic neoplasm | 34 (27.2) | 61.0 (16.0) | 27/7 |
| IPMN | 18 (14.4) | 64.0 (12.0) | 10/8b |
| Insulinoma | 12 (9.6) | 49.0 (14.5)a | 7/5b |
| Liver or biliary cancer | 118 | 64.0 (11.0) | 59/59 |
| Hepatocellular carcinoma | 40 (33.9) | 64.0 (12.2) | 20/20b |
| Cholangiocellular carcinoma | 38 (32.2) | 63.0 (8.2) | 19/19b |
| Gall bladder cancer | 40 (33.9) | 66.0 (9.0) | 20/20b |
| Gastrointestinal cancer | 126 | 63.3 (16.8) | 54/72b |
| Esophageal cancer | 26 (20.6) | 63.5 (9.5) | 6/20b |
| Gastric cancer | 40 (31.7) | 69.0 (14.2) | 20/20b |
| GIST | 26 (20.6) | 62.5 (16.5) | 11/15b |
| Colon carcinoma | 34 (27.0) | 58.0 (24.2)a | 17/17b |
| Neuroendocrine tumors | 40 | 62.5 (19.5) | 20/20b |
| Sarcoma | 40 | 61.0 (16.2) | 20/20b |
| Benign liver or biliary disease | 109 | 56.0 (24.0) | 78/31 |
| Benign liver tumors | 46 (42.2) | 43.5 (23.8) | 38/8 |
| Hepatic cyst | 23 (21.1) | 59.0 (9.0)a | 20/3 |
| Gall stone disease | 40 (36.7) | 61.0 (19.8) | 20/20b |
| Peptic ulcer (bleeding or perforated) | 27 | 63.0 (12.0) | 7/20b |
| Peritonitis | 40 | 66.0 (24.8) | 20/20b |
| Healthy subjects | 101 | 27.5 (15.0) | 39/62b |
ERCP, endoscopic retrograde cholangiopancreatography; F, female; GIST, gastrointestinal stromal tumor; IPMN, intraductal papillary mucinous neoplasm; IQR, interquartile range; M, male. aAge not significantly different to the age of patients with acute pancreatitis (p>0.05). bGender distribution not significantly different to the one of patients with acute pancreatitis (p>0.05).
Patients with acute pancreatitis were stratified retrospectively according to severity using clinical and imaging data [10], [19]. Furthermore, the etiology of acute pancreatitis was determined in accordance with international guidelines [10]. For 30/152 patients, 3 or more consecutive samples could be obtained which resulted in 128 follow-up samples [median observation period: 28 days, interquartile range (IQR): 28 days]. Disease duration from clinical onset until admission was determined for all patients. For 152 and 146 acute pancreatitis patients, CRP and PCT levels were obtained, respectively.
All clinical diagnoses were based upon standard clinical, imaging, endoscopic and histological criteria. The diagnosis of chronic pancreatitis was established using a scoring system whereby patients with acute exacerbations were excluded [20].
The study was approved by the Local Ethics Committee of Magdeburg Medical Faculty and complies with the World Medical Association Declaration of Helsinki. Aliquots of the sera were stored at −80°C.
Anti-GP2 antibody production
Human GP2a and GP2b were expressed recombinantly and purified by Ni-chelate chromatography [21]. Polyclonal antibodies to total GP2 (GP2t) were produced by immunizing rabbits with an equal mixture of GP2a and GP2b (Pineda Antikörperservice, Berlin, Germany).
Monoclonal antibodies recognizing GP2a (K9) and GP2b (P102) were generated by immunizing mice with immunoconjugates [22] and selected by ELISA [23]. The animal experiments were approved by the local government (V3-2347-A16-4-2012).
ELISAs for the detection of GP2a/GP2t
The monoclonal anti-GP2a antibody K9 or polyclonal rabbit anti-GP2 antibodies at 1 μg/mL were coated onto Maxisorb plates (Nunc, Roskilde, Denmark) at 4°C overnight. After blocking with 1% bovine serum albumin in 50 mM Tris-buffered saline (Sigma Co, Taufkirchen, Germany) at room temperature for 1h, serum samples diluted 1 in 100 in 50 mM Tris-buffered saline with 0.2% bovine serum albumin were incubated for 1h and washed with Tris-buffered saline containing 0.1% Tween20 (Sigma) to detect GP2a and GP2t. Horseradish-peroxidase labeled polyclonal anti-human GP2 antibodies were added for 1h and developed with tetramethylbenzidine for 15 min (Seramun, Heidesee, Germany). The optical density was read using a microplate reader (SLT, Crailsheim, Germany) at 450 nm/620 nm. Purified recombinant GP2a or an equal mixture of GP2a and GP2b were used as standard material ranging from 0.1 to 10.0 ng/mL for the GP2a and GP2t ELISAs, respectively. The only obtained monoclonal antibody to the shorter GP2b interacted readily with both immobilized isoforms but as solid-phase antibody with the soluble isoforms at a low binding strength only. This did not allow developing a sensitive GP2b ELISA.
For interference experiments, GP2 containing sera were spiked with human hemoglobin (0.0–2.5 g/L), triglycerides as 20% emulsion of soybean oil (5.7–25.0 g/L), unconjugated bilirubin (30.0–100.0 mg/L), and GP2’s urinary homolog Tamm-Horsfall protein (uromodulin, 0.0–1.0 µg/L) (Sigma Co, respectively).
Statistical analysis
Data were checked for normality by the Kolmogorov–Smirnov test. The two-tailed, Mann-Whitney and Kruskal–Wallis tests were used to test for statistically significant differences of independent samples in 2 and more groups, respectively. Spearman’s rank correlation test was applied for within group comparison. Prevalence comparison between groups was performed by two-tailed Fisher’s exact test. p-Values of less than 0.05 were considered significant. Assay performance was analyzed by receiver-operating characteristics (ROC) curve analysis (Medcalc, Mariakerke, Belgium).
Results
Regression of GP2a/GP2t levels
Serum GP2a and GP2t levels were linearly related to each other [coefficient of determination (R2)=0.49] when all patients with acute pancreatitis and controls were analyzed (Figure S1). Regression analysis in acute pancreatitis patients alone revealed a higher R2 of 0.72 compared to controls (R2=0.29) and a significantly different slope and intercept (p<0.0001, =0.0001, respectively).
Correlation of GP2a/GP2t levels with disease duration
Both GP2a and GP2t demonstrated a significantly negative correlation with disease duration in acute pancreatitis (Figure S2) [Spearman’s coefficient of rank correlation (ρ): –0.22, –0.41, p=0.0053, <0.0001; respectively]. For further evaluation, patients with acute pancreatitis were stratified into three groups covering patients with disease duration of ≤3 days, with disease duration from 4 to 10 days and >10 days (Table 2). Onset of abdominal pain was defined as day 1 of disease.
Prevalences of glycoprotein 2 isoform alpha (GP2a) and total GP2 (GP2t) positives detected by enzyme-linked immunosorbent assay (ELISA) in 153 patients with acute pancreatitis and 752 controls employing 0.7 and 2.3 ng/mL as optimized cut-offs, respectively.
| Disorder | n | GP2a (%) | GP2t (%) |
|---|---|---|---|
| Acute pancreatitis | 153 | 40 (26.1) | 39 (25.5) |
| Disease duration | |||
| ≤third disease day | 12 | 11 (91.7)c | 11 (91.7)c |
| ≤fourth disease day | 30 | 16 (53.3)b,d | 14 (46.7)a,d |
| ≤tenth disease day | 59 | 26 (44.1)a,e | 25 (42.4)a,e |
| Etiology | |||
| Alcohol abuse | 59 | 19 (32.2) | 17 (28.8) |
| Biliary disease | 54 | 9 (16.7) | 8 (14.8) |
| Drug induced | 1 | 1 (100.0) | 1 (100.0) |
| Idiopathic | 21 | 8 (38.1) | 9 (42.9) |
| Post-trauma | 11 | 3 (27.3) | 4 (36.4) |
| Post-ERCP | 7 | 0 (0.0) | 0 (0.0) |
| Severity | |||
| Mild | 22 | 2 (9.1) | 2 (9.1) |
| Local complications | 74 | 24 (32.4) | 25 (33.8) |
| Systemic complications | 41 | 7 (17.1) | 6 (14.6) |
| Lethal outcome | 16 | 7 (43.8) | 6 (37.5) |
| Disease controls and blood donors | 752 | 28 (3.7)c,f | 56 (7.4)c,f |
| Chronic pancreatitis | 24 | 3 (12.5)f | 3 (12.5)f |
| Pancreatic neoplasm | 125 | 11 (8.8)c,f | 14 (11.2)b,f |
| Pancreatic adenocarcinoma | 25 | 2 (8.0)a,f | 4 (16.0)f |
| Periampullary carcinoma | 36 | 4 (11.1)f | 5 (13.9)f |
| Benign pancreatic cystic neoplasm | 34 | 1 (2.9)b,f | 3 (8.8)a,f |
| IPMN | 18 | 3 (16.7)f | 2 (11.1)f |
| Insulinoma | 12 | 1 (8.3)e | 0 (0.0)f |
| Liver or biliary cancer | 118 | 5 (4.2)c,f | 18 (15.2)f |
| Hepatocellular carcinoma | 40 | 1 (2.5)c,f | 9 (22.5)e |
| Cholangiocellular carcinoma | 38 | 2 (5.3)b,f | 5 (13.2)f |
| Gall bladder cancer | 40 | 2 (5.0)b,f | 4 (10.0)f |
| Gastrointestinal cancer | 126 | 2 (1.6)c,f | 6 (4.8)c,f |
| Esophageal cancer | 26 | 0 (0.0)b,f | 0 (0.0)a,f |
| Gastric cancer | 40 | 1 (2.5)b,f | 3 (7.5)a,f |
| GIST | 26 | 0 (0.0)b,f | 1 (3.8)a,f |
| Colon carcinoma | 34 | 1 (2.9)b,f | 2 (5.9)a,f |
| Neuroendocrine tumors | 40 | 1 (2.5)b,f | 3 (7.5)a,f |
| Sarcoma | 40 | 1 (2.5)b,f | 1 (2.5)b,f |
| Benign liver or biliary disease | 109 | 2 (1.8)c,f | 2 (1.8)c,f |
| Benign liver tumors | 46 | 0 (0.0)c,f | 0 (0.0)c,f |
| Hepatic cyst | 23 | 1 (4.3)a,f | 1 (4.3)a,f |
| Gall stone disease | 40 | 1 (2.5)b,f | 1 (2.5)c,f |
| Peptic ulcer (bleeding or perforated) | 27 | 1 (3.7)b,f | 2 (7.4)a,f |
| Peritonitis | 40 | 2 (5.0)b,f | 5 (12.5)f |
| Healthy subjects | 101 | 0 (0.0)c,f | 2 (2.0)c,f |
GIST, gastrointestinal stromal tumor; IPMN, intraductal papillary mucinous neoplasm. ap<0.05; bp<0.01; cp<0.0001; comparison of GP2 prevalence with the one in all acute pancreatitis cases by Fisher’s test. dp<0.05; ep<0.01; fp<0.0001; comparison of GP2 prevalence with the one in acute pancreatitis ≤third disease day by Fisher’s test.
Comparison of GP2a/GP2t levels in acute pancreatitis and control groups
Acute pancreatitis patients were age and gender matched with patients suffering from chronic pancreatitis, pancreatic neoplasms, insulinoma, and colon carcinoma (p>0.05).
Serum GP2a and GP2t by ELISA demonstrated significantly different levels in 153 acute pancreatitis patients and 752 controls (p<0.0001) (Figure 1A,B). Thus, median levels of GP2a and GP2t in acute pancreatitis patients demonstrated a significant decline from time period to time period (≤3 days, 4–10 days, >10 days) (p>0.05, respectively). Of note, several sera demonstrated GP2a and GP2t levels above the highest standard of 10 ng/mL and dilutions thereof could not be retested due to lack of material.

Serum glycoprotein 2 isoform alpha (GP2a) (A) and total GP2 (GP2t) (B) levels in patients with acute pancreatitis (AP) and controls.
GP2a and GP2t were analyzed in 153 patients with AP, 651 disease controls, and 101 healthy subjects (HS) by enzyme-linked immunosorbent assay. Patients with AP were stratified into patients with up to 3 days of disease duration (n=12), between 4 and 10 days (n=47), and more than 10 days (n=94). As disease controls, 26 patients with chronic pancreatitis (CP), 125 with pancreatic neoplasms (PNpl), 118 with liver or biliary cancer (LBCa), 126 with gastrointestinal cancer (GICa), 40 with neuroendocrine tumor (NET), 40 with sarcoma (SA), 109 with benign liver or biliary diseases (bL/BD), 27 with bleeding or perforated peptic ulcer (PU), and 40 with peritonitis (PT) were investigated. (Optimized cut-offs for GP2a and GP2t obtained by receiver-operating characteristic curve analysis were illustrated by dashed horizontal lines. Data are displayed in Box-and-Whisker plots with far out values, defined as values that are smaller than the lower quartile minus 3 times the interquartile range, or larger than the upper quartile plus 3 times the interquartile range, displayed as solid triangles). p, Kruskal Wallis test, Conover post-hoc analysis.
Whereas the median GP2a and GP2t levels in acute pancreatitis patients until the 3rd day were significantly elevated in comparison with all control groups, the median GP2a level of patients until the 10th day was not significantly different from those in patients with pancreatic neoplasms, peptic ulcer, and peritonitis (p<0.05, respectively). In contrast, the corresponding GP2t level was not significantly different in patients with gastrointestinal cancer, neuroendocrine tumor, sarcoma and chronic pancreatitis (p<0.05, respectively). Furthermore, the median GP2t level in patients with liver/biliary cancer was even significantly higher.
Comparison of GP2a/GP2t assay performance
To consider the decline in GP2 levels over time, respective optimized GP2 cut-offs were obtained by ROC curve analysis employing acute pancreatitis patients till the 3rd day as positive criterion and all 752 controls as negative criterion (Figure S3). The comparison of ROC curves revealed a significantly higher area under the curve (AUC) for GP2a in contrast to GP2t (0.93 vs. 0.90, p=0.0427).
Optimal cut-off levels for GP2a and GP2t were determined at 0.7 and 2.3 ng/mL, respectively. Thus, GP2a possessed a significantly better accuracy with a sensitivity of 91.7% and a specificity of 96.7%. GP2t demonstrated the same sensitivity but a poorer specificity (92.6%). Altogether, these data resulted in positive/negative likelihood ratios of 24.6 and 0.09 for GP2a and 12.3 and 0.09 for GP2t, respectively.
Comparison of GP2a/GP2t positivity in acute pancreatitis and controls
The obtained cut-offs of 0.7 ng/mL (GP2a) and 2.3 ng/mL (GP2t) were used to define the prevalence of positives in patients and controls (Table 2). There was a decrease in the prevalence of GP2a and GP2t positives after the 3rd disease day which dropped to 44.1% and 42.4% till the 10th day, respectively.
The GP2a ELISA revealed significantly less false positives compared with the GP2t assay (28/752 vs. 56/752, p=0.0022). This was basically due to the significantly higher prevalence of positive GP2t findings in patients with liver/biliary cancer (18/118 vs. 5/118, p=0.0073) and particularly with hepatocellular carcinoma (9/40 vs. 1/40, p=0.0143).
GP2a/GP2t levels and positivity in etiological acute pancreatitis variants
GP2a and GP2t levels were significantly different in patients with varying etiology (p=0.0144, 0.0199, respectively). Patients with idiopathic and alcoholic illness demonstrated significantly elevated GP2a and GP2t levels in contrast to patients with biliary and post-trauma acute pancreatitis (p<0.05, respectively).
In contrast to GP2a, there was a significantly higher prevalence of GP2t in patients with idiopathic disease compared with biliary acute pancreatitis patients (9/21 vs. 8/54, p=0.0143).
Correlation of GP2a/GP2t with CRP and PCT
GP2a and GP2t levels were significantly correlated with PCT (ρ=0.21, 0.26, p=0.0110, 0.0012, respectively) and CRP values (ρ=0.37, 0.40, p<0.0001; respectively) in 152 and 146 follow-up samples of acute pancreatitis patients, respectively (Figure S4).
In general, GP2a and GP2t levels declined rapidly after the 3rd disease day. However, patients with severe illness demonstrated elevated GP2a levels until the 21st day with a steady decline over time (Figure 2A). CRP levels demonstrated a similar modulation corresponding with therapy success (Figure 2A–D). In two lethal cases, there was a strikingly similar behavior of GP2a and PCT levels at time points beyond the 10th day (Figure 2E,F). One patient showed a simultaneous increase and subsequent decrease of GP2a and PCT levels in response to pancreatic necrosectomy on day 34 (Figure 2E). Of note, increases in GP2a levels could be determined even later than the 50th day (Figure 2G,H). GP2t levels showed similar alterations like GP2a (data not shown).
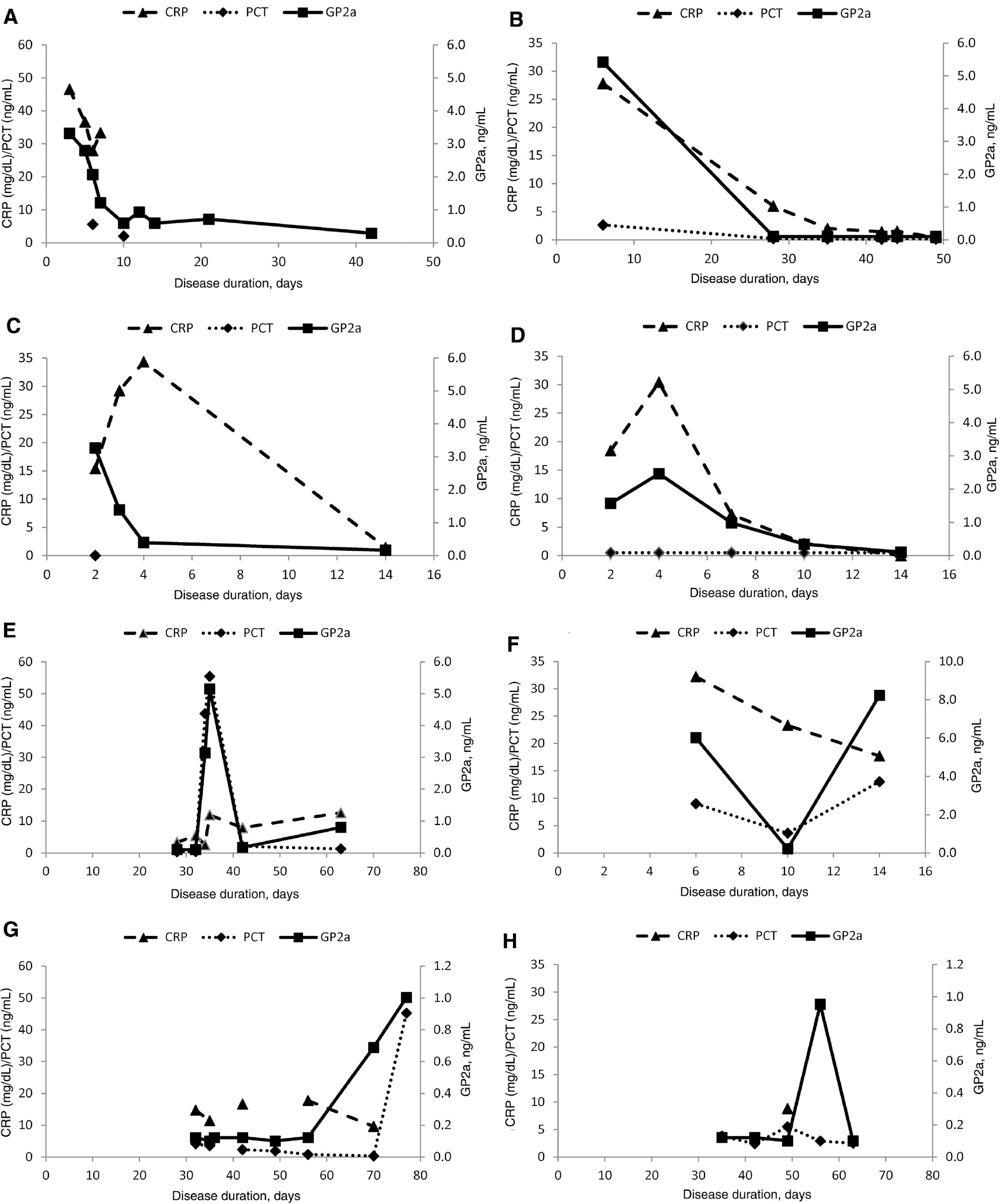
Serum glycoprotein 2 isoform alpha (GP2a), procalcitonin (PCT), and C-reactive protein (CRP) levels in follow-up samples of patients with acute pancreatitis between the 2nd and 49th disease day.
(A) Male, 47 years, alcohol abuse, severe acute pancreatitis with local complications. (B) Male, 31 years, alcohol abuse, severe acute pancreatitis with systemic complications. (C) Male, 48 years, alcohol abuse, severe acute pancreatitis with local complications. (D) Male, 31 years, alcohol abuse, severe acute pancreatitis with systemic complications. Acute pancreatitis patients with late increase of GP2a (>10th day). (E) Female, 65 years, biliary, severe acute pancreatitis with lethal outcome. (F) Female, 83 years, biliary, severe acute pancreatitis with lethal outcome. (G) Male, 59 years, alcohol abuse, severe acute pancreatitis with systemic complications. (H) Male, 26 years, alcohol abuse, severe acute pancreatitis with systemic complications.
Prediction of acute pancreatitis severity by GP2a/GP2t levels
Given the association of GP2a and GP2t levels with PCT and CRP, acute pancreatitis patients were stratified in 4 groups according to disease severity retrospectively (Figure 3).

Association of serum glycoprotein 2 isoform alpha (GP2a) and total GP2 (GP2t) with severity of disease.
GP2a (A,C) and GP2t (B,D) levels were detected by enzyme-linked immunosorbent assays in acute pancreatitis (AP) patients with mild disease (I), severe disease with local complications (II), severe disease with systemic complications (III), and severe disease with lethal outcome (IV). Of note, disease duration was significantly different in the AP severity stages (p=0.0330) whereby severe cases with local complications had a significantly shorter disease duration at first presentation than severe acute pancreatitis patients with systemic disease (p<0.05). In contrast, the differences in etiology in the four severity stages of AP patients did not reach significance (p=0.0586). Optimized cut-offs for GP2a and GP2t obtained by receiver-operating characteristic curve analysis were illustrated by dashed horizontal lines. Data are displayed in Box-and-Whisker plots with far out values, defined as values that are smaller than the lower quartile minus 3 times the interquartile range, or larger than the upper quartile plus 3 times the interquartile range, displayed as solid triangles.
Remarkably, patients with lethal outcome did demonstrate a significantly higher prevalence of GP2a and GP2t compared with mild cases at admission (7/16 vs. 2/22, 6/16 vs. 2/22, p=0.0211, 0.0497; respectively). This resulted in an odds ratio of 7.8 [95% confidence interval (CI): 1.3–45.1, p=0.0222] for GP2a and 6.0 (95% CI: 1.0–35.3, p=0.0474) for GP2t positivity regarding the prognosis for a lethal outcome. When acute pancreatitis patients admitted until the 10th disease day were considered only, there was also a significantly higher GP2a positivity in lethal cases compared to mild disease (5/6 vs. 1/7, p=0.0291).
There were no significantly different GP2a levels in the four disease severity stages of the 153 patients with acute pancreatitis (p=0.1167) (Figure 3A). In contrast, GP2t levels differed significantly (p=0.0220) (Figure 3B). Thus, mild acute pancreatitis cases had significantly lower GP2t levels compared to severe illness with local complications and lethal outcome (p<0.05, respectively).
When all severe cases (n=131) were compared to patients with mild disease, GP2a and GP2t levels were significantly increased in the severe acute pancreatitis cohort (p=0.0329, 0.0042, respectively) (Figure 3C, D).
Discussion
The search for serological parameters for the diagnosis of acute pancreatitis continues unabatedly [2], [9], [24], [25]. Thus, the recent report of GP2 being a superior marker for acute pancreatitis serology deserves closer attention [18]. The self-aggregating, non-digestive autoantigenic GP2 is secreted along with digestive proenzymes and represents a major pancreatic plug component [4], [16], [26], [27], [28]. GP2 does not appear to play a decisive intracellular role during secretion but rather an antimicrobial and immunomodulating one after its release into the intestine despite its reported regulation of acinar endocytosis or ZG assembly [29], [30], [31], [32], [33], [34], [35], [36].
Pancreatic autodigestion as the putative leading pathogenic mechanism in acute pancreatitis results in acinar polarity impairment with basolateral release of active enzymes and other ZG contents such as GP2 [4], [37]. Consequently, a significant elevation of GP2t and GP2a in acute pancreatitis was demonstrated in our study. Hao et al. reported even a significantly higher accuracy for GP2 testing compared to lipase analysis (AUC: 0.96 vs. 0.81) which underscores GP2’s potential as a novel acute pancreatitis marker [18]. Likewise, we found a similar AUC of 0.93 for GP2a investigating acute pancreatitis patients until the 3rd disease day. In contrast to GP2t, GP2a revealed a higher specificity (96.7% vs. 92.6%) after having established optimized cut-offs for both ELISA. Of note, the poorer specificity of the GP2t analysis was not due to cross-reactivity with the urinary GP2-homolog uromodulin (Supplemental Data, Assay performance of the GP2a ELISA), a chronic kidney-disease marker [38]. Thus, the very good specificity of GP2a obtained with an extended control group is in stark contrast to GP2’s assay specificity reported recently [18]. Elevated GP2 levels were detected in patients with chronic pancreatitis and pancreatic cancer by these authors. In this context, the novel GP2a ELISA appears to be a better diagnostic tool for the differential diagnosis of patients with acute upper abdominal pain.
The reported equal secretion of two GP2 isoforms was supported by our regression analysis indicating that approximately 50% of serum GP2 detected in acute pancreatitis patients belonged to GP2a [17].
Consistent with the assumption, that GP2 is not involved in chronic pancreatitis pathophysiology, GP2a testing seems to be closer associated with the acute pancreatic inflammation. The negative correlation of GP2a with disease duration and the significantly higher levels in alcoholic in contrast to biliary illness support this notion. Chronic ethanol consumption decreased the GP2 content of rodent ZGs due to the impairment of ZG stability [39]. Nonetheless, pathogenic processes in biliary acute pancreatitis also appear to interfere with the stability of ZG [4].
Thus, the elevated GP2 levels found in patients with pancreatic cancer and chronic pancreatitis by Hao et al. were either a result of false-positive findings or brought about by the detection of a special variant of GP2 recognized by their assay design. Since GP2t testing by affinity-purified polyclonal anti-GP2 antibodies in our study confirmed the lower serum GP2 content in these controls generally, the former assumption seems to hold true. Indeed, Hao et al. did not report assay performance characteristics of their research ELISA [18]. The GP2a ELISA of our study demonstrated little interference by hemolysis and icteric samples as well as by its renal homolog uromodulin present in serum (Supplemental Data, Assay performance of the GP2a ELISA). Furthermore, its established inter-assay variation of 10% near the cut-off level enabled an excellent positive/negative differentiation (Supplemental Data, Table S1).
Consistent with the low serum GP2a levels in pancreatic cancer in our study, diminished levels of serum lipase have been reported as an independent marker of pancreatic cancer recently [40]. In contrast, elevated GP2 levels were found in pancreatic ductal fluid of pancreatic cancer but not of pancreatitis patients recently [41].
Another reason for the poor differentiation of GP2 levels by Hao et al. might be GP2’s negative correlation with disease duration which was not reported for the 31 acute pancreatitis patients recruited [18]. In our study, GP2a and GP2t levels of acute pancreatitis patients with >10 disease days did not differ significantly from those in patients with pancreatic neoplasms and other controls. Significantly increased serum GP2 levels could be detected even beyond the 50th day which indicates the possible development of new acinar damage at later time points. This fact can be beneficial particularly for cases of delayed presentation as discussed for other putative acute pancreatitis markers with longer half-lives [42]. We detected continuously elevated GP2a in severe cases up to the 21st disease day. One explanation could be that GP2a is linked to the ZG membrane via a glycosyl phosphatidylinositol anchor cleaved by a phospholipase which can delay its release [43], [44], [45].
The significant association of GP2a with PCT as well as CRP and its elevation in severe acute pancreatitis with lethal outcome were intriguing findings of this study. Although CRP is used to predict severity of disease, it did not identify patients with a high risk of dying in a recent study [13]. This may add new impetus to the search for prognostic biomarkers of acute pancreatitis resulting in simpler severity assessment strategies since current scoring systems are rather cumbersome [9], [46], [47], [48], [49].
Our study has certain limitations. Despite the large number of acute pancreatitis patients recruited, only 12 samples of patients up to the 3rd disease day could be analyzed because of delayed admission. All these 12 patients demonstrated a severe disease course. Thus, the high sensitivity of GP2a for the diagnosis of acute pancreatitis needs to be corroborated by future studies and samples with levels higher than 10 ng/mL need to be diluted further for the determination of the final GP2 levels.
Another limitation is the significantly different disease duration of the four severity stages. The pre-existing longer disease duration on admission of patients with systemic complications could have resulted in an underestimation of GP2 levels. Additionally, mild cases were not age matched with severe acute pancreatitis patients.
Furthermore, PCT and CRP levels were obtained retrospectively and were not available for all patient samples. The respective correlation data should be interpreted cautiously due to the high number of GP2 levels below the cut-offs. A major limitation of this study is obviously the lack of pancreatic lipase data. Thus, the value of GP2a testing for the diagnosis of acute pancreatitis could only be discussed in the context of previous data. Indeed, in daily clinical praxis, acute pancreatitis is diagnosed as a synopsis of clinical symptoms, medical imaging and laboratory tests such as CRP, PCT, pancreatic amylase and lipase. If GP2a could improve the diagnosis of acute pancreatitis should be the topic of additional studies.
Conclusions
GP2a is a specific marker for acute pancreatitis and enables the differentiation to chronic pancreatitis and pancreatic neoplasms. Furthermore, GP2a can be used to predict the mortality of the disease which renders GP2a a promising diagnostic and prognostic acute pancreatitis marker.
Acknowledgments
We thank Juliane Cuccato for expert technical assistance.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: Bundesministerium für Wirtschaft und Technologie, Zentrales Innovationsprogramm Mittelstand KF2379005 CS2.
Employment or leadership: Dirk Roggenbuck is a shareholder of GA Generic Assays GmbH and Medipan GmbH. Pamela Holzlöhner and Katja Hanack are co-founders of new/era/mabs GmbH – a company that offers customized generation of monoclonal antibodies. The remaining authors declare that they have no competing financial interests.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143:1179–87.10.1053/j.gastro.2012.08.002Search in Google Scholar PubMed PubMed Central
2. Lippi G, Valentino M, Cervellin G. Laboratory diagnosis of acute pancreatitis: in search of the Holy Grail. Crit Rev Clin Lab Sci 2012;49:18–31.10.3109/10408363.2012.658354Search in Google Scholar PubMed
3. Muller CA, Appelros S, Uhl W, Buchler MW, Borgstrom A. Serum levels of procarboxypeptidase B and its activation peptide in patients with acute pancreatitis and non-pancreatic diseases. Gut 2002;51:229–35.10.1136/gut.51.2.229Search in Google Scholar PubMed PubMed Central
4. Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet 2015;386:85–96.10.1007/978-3-642-60870-4_4Search in Google Scholar
5. Afghani E, Pandol SJ, Shimosegawa T, Sutton R, Wu BU, Vege SS, et al. Acute pancreatitis-progress and challenges: a report on an international symposium. Pancreas 2015;44:1195–210.10.1097/MPA.0000000000000500Search in Google Scholar PubMed PubMed Central
6. Lerch MM, Halangk W. Human pancreatitis and the role of cathepsin B. Gut 2006;55:1228–30.10.1136/gut.2006.092114Search in Google Scholar PubMed PubMed Central
7. Sendler M, Maertin S, John D, Persike M, Weiss FU, Krueger B, et al. Cathepsin-B activity initiates apoptosis via digestive protease activation in pancreatic acinar cells and experimental pancreatitis. J Biol Chem 2016;291:14717–31.10.1074/jbc.M116.718999Search in Google Scholar PubMed PubMed Central
8. Leppkes M, Maueroder C, Hirth S, Nowecki S, Gunther C, Billmeier U, et al. Externalized decondensed neutrophil chromatin occludes pancreatic ducts and drives pancreatitis. Nat Commun 2016;7:10973.10.1038/ncomms10973Search in Google Scholar PubMed PubMed Central
9. Meher S, Mishra TS, Sasmal PK, Rath S, Sharma R, Rout B, et al. Role of Biomarkers in Diagnosis and Prognostic Evaluation of Acute Pancreatitis. J Biomark 2015;2015:519–34.10.1155/2015/519534Search in Google Scholar PubMed PubMed Central
10. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–11.10.1136/gutjnl-2012-302779Search in Google Scholar PubMed
11. Keim V, Teich N, Fiedler F, Hartig W, Thiele G, Mossner J. A comparison of lipase and amylase in the diagnosis of acute pancreatitis in patients with abdominal pain. Pancreas 1998;16:45–9.10.1097/00006676-199801000-00008Search in Google Scholar
12. Da BL, Shulman IA, Joy LC, Buxbaum J. Origin, presentation, and clinical course of nonpancreatic hyperlipasemia. Pancreas 2016;45:846–9.10.1097/MPA.0000000000000561Search in Google Scholar
13. Mantke R, Pross M, Kunz D, Ebert M, Kahl S, Peters B, et al. Soluble thrombomodulin plasma levels are an early indication of a lethal course in human acute pancreatitis. Surgery 2002;131:424–32.10.1067/msy.2002.122379Search in Google Scholar
14. Mofidi R, Suttie SA, Patil PV, Ogston S, Parks RW. The value of procalcitonin at predicting the severity of acute pancreatitis and development of infected pancreatic necrosis: systematic review. Surgery 2009;146:72–81.10.1016/j.surg.2009.02.013Search in Google Scholar
15. Lowe AW, Luthen RE, Wong S, Grendell JH. The level of the zymogen granule protein GP2 is elevated in a rat model for acute pancreatitis. Gastroenterology 1994;107:1819–27.10.1016/0016-5085(94)90826-5Search in Google Scholar
16. Rindler MJ, Hoops TC. The pancreatic membrane protein GP-2 localizes specifically to secretory granules and is shed into the pancreatic juice as a protein aggregate. Eur J Cell Biol 1990;53:154–63.Search in Google Scholar
17. Fukuoka S. Molecular cloning and sequences of cDNAs encoding alpha (large) and beta (small) isoforms of human pancreatic zymogen granule membrane-associated protein GP2. Biochim Biophys Acta 2000;1491:376–80.10.1016/S0167-4781(00)00057-9Search in Google Scholar
18. Hao Y, Wang J, Feng N, Lowe AW. Determination of plasma glycoprotein 2 levels in patients with pancreatic disease. Arch Pathol Lab Med 2004;128:668–74.10.5858/2004-128-668-DOPGLISearch in Google Scholar PubMed
19. Lerch MM. Classifying an unpredictable disease: the revised Atlanta classification of acute pancreatitis. Gut 2013;62:2–3.10.1136/gutjnl-2012-303724Search in Google Scholar PubMed
20. Hoffmeister A, Mayerle J, Beglinger C, Buchler MW, Bufler P, Dathe K, et al. English language version of the S3-consensus guidelines on chronic pancreatitis: definition, aetiology, diagnostic examinations, medical, endoscopic and surgical management of chronic pancreatitis. Z Gastroenterol 2015;53:1447–95.10.1055/s-0041-107379Search in Google Scholar PubMed
21. Roggenbuck D, Reinhold D, Wex T, Goihl A, von Arnim U, Malfertheiner P, et al. Autoantibodies to GP2, the major zymogen granule membrane glycoprotein, are new markers in Crohn’s disease. Clin Chim Acta 2011;412:718–24.10.1016/j.cca.2010.12.029Search in Google Scholar PubMed
22. Messerschmidt K, Hempel S, Holzlohner P, Ulrich RG, Wagner D, Heilmann K. IgA antibody production by intrarectal immunization of mice using recombinant major capsid protein of hamster polyomavirus. Eur J Microbiol Immunol (Bp) 2012;2:231–8.10.1556/EuJMI.2.2012.3.9Search in Google Scholar PubMed PubMed Central
23. Roggenbuck D, Rober N, Bogdanos DP, Goihl A, Reinhold D, Conrad K, et al. Autoreactivity to isoforms of glycoprotein 2 in inflammatory bowel disease. Clin Chim Acta 2015;442:82–3.10.1016/j.cca.2015.01.018Search in Google Scholar PubMed
24. Viljoen A, Patrick JT. In search for a better marker of acute pancreatitis: third time lucky? Clin Chem 2011;57:1471–3.10.1373/clinchem.2011.173385Search in Google Scholar PubMed
25. Maksimow M, Kyhala L, Nieminen A, Kylanpaa L, Aalto K, Elima K, et al. Early prediction of persistent organ failure by soluble CD73 in patients with acute pancreatitis. Crit Care Med 2014;42:2556–64.10.1097/CCM.0000000000000550Search in Google Scholar PubMed
26. Fukuoka S, Freedman SD, Scheele GA. A single gene encodes membrane-bound and free forms of GP-2, the major glycoprotein in pancreatic secretory (zymogen) granule membranes. Proc Natl Acad Sci USA 1991;88:2898–902.10.1073/pnas.88.7.2898Search in Google Scholar PubMed PubMed Central
27. Roggenbuck D, Hausdorf G, Martinez-Gamboa L, Reinhold D, Buttner T, Jungblut PR, et al. Identification of GP2, the major zymogen granule membrane glycoprotein, as the autoantigen of pancreatic antibodies in Crohn’s disease. Gut 2009;58:1620–8.10.1136/gut.2008.162495Search in Google Scholar PubMed
28. Roggenbuck D, Reinhold D, Schierack P, Bogdanos DP, Conrad K, Laass MW. Crohn’s disease specific pancreatic antibodies: clinical and pathophysiological challenges. Clin Chem Lab Med 2014;52:483–94.10.1515/cclm-2013-0801Search in Google Scholar PubMed
29. Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature 2009;462:226–30.10.1038/nature08529Search in Google Scholar PubMed
30. Schierack P, Rodiger S, Kolenda R, Hiemann R, Berger E, Grzymajlo K, et al. Species-specific and pathotype-specific binding of bacteria to zymogen granule membrane glycoprotein 2 (GP2). Gut 2015;64:517–9.10.1136/gutjnl-2014-307854Search in Google Scholar PubMed
31. Werner L, Paclik D, Fritz C, Reinhold D, Roggenbuck D, Sturm A. Identification of pancreatic Glycoprotein 2 as an endogenous immunomodulator of innate and adaptive immune responses. J Immunol 2012;189:2774–83.10.4049/jimmunol.1103190Search in Google Scholar PubMed
32. Roggenbuck D, Reinhold D, Werner L, Schierack P, Bogdanos DP, Conrad K. Glycoprotein 2 in Crohn’s disease. Adv Clin Chem 2013;60:187–208.10.1016/B978-0-12-407681-5.00006-4Search in Google Scholar
33. Yu S, Michie SA, Lowe AW. Absence of the major zymogen granule membrane protein, GP2, does not affect pancreatic morphology or secretion. J Biol Chem 2004;279:50274–9.10.1074/jbc.M410599200Search in Google Scholar
34. Parker EM, Zaman MM, Freedman SD. GP2, a GPI-anchored protein in the apical plasma membrane of the pancreatic acinar cell, co-immunoprecipitates with src kinases and caveolin. Pancreas 2000;21:219–25.10.1097/00006676-200010000-00001Search in Google Scholar
35. Scheele GA, Fukuoka S, Freedman SD. Role of the GP2/THP family of GPI-anchored proteins in membrane trafficking during regulated exocrine secretion. Pancreas 1994;9:139–49.10.1097/00006676-199403000-00001Search in Google Scholar
36. Yu S, Lowe AW. The pancreatic zymogen granule membrane protein, GP2, binds Escherichia coli Type 1 fimbriae. BMC Gastroenterol 2009;9:58.10.1186/1471-230X-9-58Search in Google Scholar
37. Chiari H. Über die Selbstverdauung des menschlichen Pankreas. Z Heilkunde 1896;17:69–96.Search in Google Scholar
38. Steubl D, Block M, Herbst V, Nockher WA, Schlumberger W, Satanovskij R, et al. Plasma Uromodulin correlates with kidney function and identifies early stages in chronic kidney disease patients. Medicine (Baltimore) 2016;95:e3011.10.1097/MD.0000000000003011Search in Google Scholar
39. Apte MV, Norton ID, Haber PS, Korsten MA, McCaughan GW, Pirola RC, et al. Chronic ethanol administration decreases rat pancreatic GP2 content. Biochim Biophys Acta 1997;1336:89–98.10.1016/S0304-4165(97)00015-9Search in Google Scholar
40. Gultepe I, Basaranoglu M, Zorlu M, Senyigit A, Taskale EZ, Zarali S, et al. Low lipase levels as an independent marker of pancreatic cancer: a frequently neglected condition in clinical setting. Turk J Gastroenterol 2016;27:197–200.10.5152/tjg.2016.16056Search in Google Scholar
41. Porterfield M, Zhao P, Han H, Cunningham J, Aoki K, Von Hoff DD, et al. Discrimination between adenocarcinoma and normal pancreatic ductal fluid by proteomic and glycomic analysis. J Proteome Res 2014;13:395–407.10.1021/pr400422gSearch in Google Scholar
42. Chase CW, Barker DE, Russell WL, Burns RP. Serum amylase and lipase in the evaluation of acute abdominal pain. Am Surg 1996;62:1028–33.Search in Google Scholar
43. LeBel D, Beattie M. The major protein of pancreatic zymogen granule membranes (GP-2) is anchored via covalent bonds to phosphatidylinositol. Biochem Biophys Res Commun 1988;154:818–23.10.1016/0006-291X(88)90213-6Search in Google Scholar
44. Paul E, Leblond FA, LeBel D. In resting conditions, the pancreatic granule membrane protein GP-2 is secreted by cleavage of its glycosylphosphatidylinositol anchor. Biochem J 1991;277(Pt 3):879–81.10.1042/bj2770879Search in Google Scholar PubMed PubMed Central
45. Havinga JR, Slot JW, Strous GJ. Membrane detachment and release of the major membrane glycoprotein of secretory granules in rat pancreatic exocrine cells. Eur J Cell Biol 1985;39:70–6.Search in Google Scholar
46. Zubia-Olaskoaga F, Maravi-Poma E, Urreta-Barallobre I, Ramirez-Puerta MR, Mourelo-Farina M, Marcos-Neira MP. Comparison between revised Atlanta classification and determinant-based classification for acute pancreatitis in intensive care medicine. Why do not use a modified determinant-based classification? Crit Care Med 2016;44:910–7.10.1097/CCM.0000000000001565Search in Google Scholar PubMed
47. Segre E, Pigozzi L, Lison D, Pivetta E, Bosco O, Vizio B, et al. May thrombopoietin be a useful marker of sepsis severity assessment in patients with SIRS entering the emergency department? Clin Chem Lab Med 2014;52:1479–83.10.1515/cclm-2014-0219Search in Google Scholar PubMed
48. Hu ZD, Wei TT, Zhong RQ. Red blood cell distribution: an index without additional cost in estimating the prognosis of acute pancreatitis. Clin Chem Lab Med 2016;54:e389–90.10.1515/cclm-2016-0351Search in Google Scholar PubMed
49. Hu ZD, Wei TT, Tang QQ, Fu HT, Yang M, Ma N, et al. Prognostic value of red blood cell distribution width in acute pancreatitis patients admitted to intensive care units: an analysis of a publicly accessible clinical database MIMIC II. Clin Chem Lab Med 2016;54:e195–7.10.1515/cclm-2015-1021Search in Google Scholar PubMed
Supplemental Material:
The online version of this article (DOI: 10.1515/cclm-2016-0797) offers supplementary material, available to authorized users.
©2016, Dirk Roggenbuck et al., published by De Gruyter.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Frontmatter
- Editorial
- Biotin interference on immunoassay methods: sporadic cases or hidden epidemic?
- Reviews
- False biochemical diagnosis of hyperthyroidism in streptavidin-biotin-based immunoassays: the problem of biotin intake and related interferences
- Vitamin K plasma levels determination in human health
- Mini Review
- Point-of-care testing INR: an overview
- Opinion Paper
- Could accreditation bodies facilitate the implementation of medical guidelines in laboratories?
- Genetics and Molecular Diagnostics
- QMPSF is sensitive and specific in the detection of NPHP1 heterozygous deletions
- General Clinical Chemistry and Laboratory Medicine
- High-dose biotin therapy leading to false biochemical endocrine profiles: validation of a simple method to overcome biotin interference
- Multicenter performance evaluation of a second generation cortisol assay
- A rapid UPLC-MS/MS assay for the simultaneous measurement of fluconazole, voriconazole, posaconazole, itraconazole, and hydroxyitraconazole concentrations in serum
- A microplate assay to measure classical and alternative complement activity
- Serological diagnosis and prognosis of severe acute pancreatitis by analysis of serum glycoprotein 2
- Retrospective evaluation of the clinical utility of serological biomarkers in Chinese patients with inflammatory bowel disease: 2-year clinical experience
- Infrared analysis of lipoproteins in the detection of alcohol biomarkers
- Coexistence of anti-β2-glycoprotein I domain I and anti-phosphatidylserine/prothrombin antibodies suggests strong thrombotic risk
- Antiphosphatidylserine/prothrombin antibodies as biomarkers to identify severe primary antiphospholipid syndrome
- Cardiovascular Diseases
- The impact of admission neutrophil-to-platelet ratio on in-hospital and long-term mortality in patients with infective endocarditis
- Letters to the Editor
- Biotin interference in immunoassays mimicking subclinical Graves’ disease and hyperestrogenism: a case series
- A simple method to detect biotin interference on immunoassays
- Proposed classification for a variant of Kounis syndrome
- Lyophilized hemoglobin E control material for the dichlorophenol-indophenol (DCIP) test
- Procalcitonin variation before and after 100-km ultramarathon
- Preliminary study in specific activity of molecular components in allergy: implications for diagnostics and relationship with disease severity
- A 2-min at 4500 g rather than a 15-min at 2200 g centrifugation does not impact the reliability of 10 critical coagulation assays
- Immunoassay interference caused by heterophilic antibodies interacting with biotin
- Red blood cell distribution width and mean platelet volume are potential prognostic indices for patients with primary biliary cirrhosis
- Neutrophil CD64 molecule expression can predict bloodstream infection in septic shock patients
Articles in the same Issue
- Frontmatter
- Editorial
- Biotin interference on immunoassay methods: sporadic cases or hidden epidemic?
- Reviews
- False biochemical diagnosis of hyperthyroidism in streptavidin-biotin-based immunoassays: the problem of biotin intake and related interferences
- Vitamin K plasma levels determination in human health
- Mini Review
- Point-of-care testing INR: an overview
- Opinion Paper
- Could accreditation bodies facilitate the implementation of medical guidelines in laboratories?
- Genetics and Molecular Diagnostics
- QMPSF is sensitive and specific in the detection of NPHP1 heterozygous deletions
- General Clinical Chemistry and Laboratory Medicine
- High-dose biotin therapy leading to false biochemical endocrine profiles: validation of a simple method to overcome biotin interference
- Multicenter performance evaluation of a second generation cortisol assay
- A rapid UPLC-MS/MS assay for the simultaneous measurement of fluconazole, voriconazole, posaconazole, itraconazole, and hydroxyitraconazole concentrations in serum
- A microplate assay to measure classical and alternative complement activity
- Serological diagnosis and prognosis of severe acute pancreatitis by analysis of serum glycoprotein 2
- Retrospective evaluation of the clinical utility of serological biomarkers in Chinese patients with inflammatory bowel disease: 2-year clinical experience
- Infrared analysis of lipoproteins in the detection of alcohol biomarkers
- Coexistence of anti-β2-glycoprotein I domain I and anti-phosphatidylserine/prothrombin antibodies suggests strong thrombotic risk
- Antiphosphatidylserine/prothrombin antibodies as biomarkers to identify severe primary antiphospholipid syndrome
- Cardiovascular Diseases
- The impact of admission neutrophil-to-platelet ratio on in-hospital and long-term mortality in patients with infective endocarditis
- Letters to the Editor
- Biotin interference in immunoassays mimicking subclinical Graves’ disease and hyperestrogenism: a case series
- A simple method to detect biotin interference on immunoassays
- Proposed classification for a variant of Kounis syndrome
- Lyophilized hemoglobin E control material for the dichlorophenol-indophenol (DCIP) test
- Procalcitonin variation before and after 100-km ultramarathon
- Preliminary study in specific activity of molecular components in allergy: implications for diagnostics and relationship with disease severity
- A 2-min at 4500 g rather than a 15-min at 2200 g centrifugation does not impact the reliability of 10 critical coagulation assays
- Immunoassay interference caused by heterophilic antibodies interacting with biotin
- Red blood cell distribution width and mean platelet volume are potential prognostic indices for patients with primary biliary cirrhosis
- Neutrophil CD64 molecule expression can predict bloodstream infection in septic shock patients

