Abstract
Telomeres are protective caps at the end of eukaryotic chromosomes, whose length is correlated with health and lifespan. Telomere attrition is a common feature of the aging process and can be accelerated by oxidative stress and chronic inflammation. Various nutrients influence the telomere length, partially due to their antioxidant and anti-inflammatory properties. The aim of this review was to meta-analytically assess the effect of omega-3 fatty acids on the telomere length. We searched four databases (PubMed, Web of Sciences, Scopus, and the Cochrane Library) from inception until November 2021. Of 573 records, a total of 5 clinical trials were included for the quantitative meta-analysis, comprising a total of 337 participants. The results revealed an overall beneficial effect of omega-3 fatty acids on the telomere length (mean difference = 0.16; 95% CI, 0.02, 0.30; p = 0.02). Despite a limited number of studies, the available evidence suggests that omega-3 fatty acids may positively affect the telomere length. However, larger clinical trials are needed to confirm our findings, along with studies aimed to clarify the underlying molecular mechanisms.
Introduction
Phenotypically, aging is a biological process characterized by a wide variety of hallmarks at the molecular and cellular levels [1]. However, short telomeres are sufficient to trigger age-related pathologies and decrease lifespan in mice and humans [2,3]. Telomeres are dynamic structures formed by proteins and repeated sequences of DNA (5′-TAGGG-3′) at the end of eukaryotic chromosomes. Together, telomeric DNA and telomeric proteins maintain the structural integrity of chromosomes, thus keeping genomic stability [4].
Telomeres are subject to shortening at each cycle of cell division, losing approximately 50-to 100 base pairs per mitotic division in human cells [5]. However, the rate of telomere loss is affected by numerous factors other than the mitotic replication rate. Oxidative stress and chronic low-grade inflammation (also known as inflammaging) are thought to be the major contributors to telomere shortening. Due to the high guanine-cytosine content, telomeres are extremely prone to oxidative damage compared to nontelomeric sequences. Likewise, the proinflammatory phenotype that accompanies aging in mammals is also linked to the onset of age-associated diseases and telomere shortening [6,7]. Additionally, extensive evidence supports that the telomere length (TL) is a dynamic trait sensitive to environmental factors. An accelerated telomere shortening has been associated with smoking, air pollution, excessive food intake, and psychological stress. Exposure to these factors may promote telomere attrition by increasing oxidative stress and inflammation [8,9,10].
Although the association between diet and telomere maintenance is currently under investigation, recent human studies indicate that specific dietary components may be considered a potential nutritional tool for preserving TL throughout the lifespan [11,12]. Given that the TL is affected by an inflammatory/oxidative status, it follows that a higher intake of antioxidant-rich foods and/or greater adherence to an anti-inflammatory diet may play a role in telomere maintenance and influence the overall health and longevity [13,14]. Indeed, current epidemiological and clinical data showed that higher consumption of vegetables, fruits, nuts, legumes, and seaweed is associated with longer TL [15,16,17]. These foods provide a range of bioactive compounds affecting endogenous antioxidant response, anti-inflammatory pathways and, at least in part, telomere maintenance [18,19,20].
During the last three decades, omega-3 (ω-3) polyunsaturated fatty acids (PUFAs) have attracted increasing interest because of their various roles in disease risk reduction. They are essential dietary nutrients, existing in many different forms primarily from marine sources, fish oil supplements, and certain plant sources. However, eicosapentaenoic acid (EPA; 20:5ω-3) and docosahexaenoic acid (DHA; 22:6ω-3) have been most widely investigated with regard to their health benefits [21]. The influence of ω-3 PUFAs on the inflammatory responses has been widely reported. These fatty acids can attenuate many mechanisms associated with inflammation, including inhibition of pro-inflammatory transcription factors, leucocyte chemotaxis, and eicosanoid production [22]. Likewise, the impact of ω-3 PUFAs on oxidative stress parameters has been evaluated in several studies. Supplementation with omega-3 PUFAs improved malondialdehyde (MDA), total antioxidant capacity (TAC), and glutathione peroxidase (GPx) activity in different clinical conditions [23].
To our knowledge, no study has reviewed meta-analytically the evidence concerning the clinical effect of ω-3 PUFAs on the TL. Therefore, this meta-analysis assesses whether ω-3 PUFAs administration can modulate the TL in clinical trials.
Methods
Search strategy
The search strategy, screening, and selection criteria were developed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statement [24]. A literature search was conducted in the following databases: PubMed, Web of Sciences, Scopus, and the Cochrane Library. Clinical studies assessing the effect of ω-3 PUFAs on TL, published up to 15 November 2021, were included in the review. Articles were excluded from the review for the following reasons: studies not published in English; articles that used secondary data; studies on animal models or in vitro experiments; observational studies; and studies in individuals younger than 18 years of age.
A comprehensive systematic literature search was conducted using both controlled vocabulary and free text terms. Using Boolean operators, we combined the following terms: “polyunsaturated fatty acids” OR “pufa” OR “unsaturated fatty acid” OR omega-3” OR “n-3” OR “n3” OR “ω-3” “docosahexaenoic acid” OR “DHA” OR “eicosapentaenoic acid” OR “EPA” OR “alpha linolenic acid” OR “ALA” AND “telomere” OR “telomeric” OR “telomere shortening” OR “telomere homeostasis” OR “telomere length” OR “telomere length maintenance” OR “telomere maintenance”. Similar queries were used for controlled vocabulary search.
Data extraction and study quality assessment
Titles and abstracts obtained from all the databases were independently reviewed by two authors (S.D. and S.A.). The removal of duplicate records was conducted with reference management software (EndNote X8; Clarivate Analytics, Philadelphia, PA, USA). The full texts were screened by S.D. and S.A., excluding all articles that did not meet the inclusion criteria. In the case of disagreement, the assistance of a third author (G.S.) was sought. The following data were extracted and tabulated: author’s name, publication year, study country, study design, study characteristics (sample size, age, gender, duration of intervention, and health status), intervention (type of ω-3 fatty acids and dose), outcome assessment for TL, fluid analyzed, and results.
To assess the methodological quality and risk of bias of the included randomized clinical trials (RCTs), we used the Cochrane risk of bias tool [25]. The tool evaluates seven components: (1) sequence generation, (2) allocation sequence concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective outcome reporting, and (7) other bias. For nonrandomized and single-arm clinical trials, we used Risk Of Bias In Non-randomised Studies – of Interventions (ROBINS-I) [26]. This tool assesses seven components: (1) bias due to confounding, (2) bias in the selection of participants into the study, (3) bias in classification of interventions, (4) bias due to deviations from intended interventions, (5) bias due to missing data, (6) bias in the measurement of outcomes, and (7) bias in the selection of the reported result.
Statistical analysis
Continuous data were expressed as the mean difference with a 95% confidence interval. The summary statistics were the number of participants, the mean change from baseline, and the standard deviation of the mean change. If change from-baseline scores were not provided post-test means and standard deviations were used. The mean difference was used to express the results across studies. We used the I 2 test to describe the proportion of the total variation in the study estimates that is due to heterogeneity. The following grades were applied: <25% (very low), from 25 to <50% (low), from 50 to <75% (moderate) and ≥75% (large) [27]. A random effect model was chosen for the meta-analyses because this method of analysis is favored when there is evidence of heterogeneity among studies. To assess whether the pooled estimate was biased by the effect of any particular study, we also carried out a sensitivity analysis, recalculating the pooled estimate. The meta-analysis was conducted using R Software, version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria), and the interface R-Studio version 1.4.1717 (R studio, PBC, Boston, MA, USA). A p value <0.05 was considered to be statistically significant.
Results
Study selection
As shown in Figure 1, the combined search resulted in 573 published studies from the four databases, among which 313 were duplicates. After evaluation of the title and abstract, 241 records were discarded because they did not meet the inclusion criteria. The remaining 19 articles were examined for eligibility assessment through full-text reading. Of these, 14 records did not meet the eligibility criteria. Therefore, a total of five studies were included in the final analysis.

PRISMA flow diagram.
Study characteristics
The five included clinical studies were conducted between 2013 and 2019 [28,29,30,31,32]. Three out of the 5 studies selected were RCTs. The sample size ranged from 33 to 106 subjects per study with an average number of 67.4 participants. The clinical trials varied in time duration from 2 to 12 months. The age of all participants varied from 33 to >65 years. Three studies assessed both men and women, 1 study assessed only women, and 1 study did not provide information about the sex of participants. The quantitative polymerase chain reaction was the preferred method for measuring telomeres and 1 study used quantitative-fluorescence in situ hybridization. The characteristics of all the included studies are presented in Table 1.
Characteristics of the included clinical trials
| Study (Author, year, Ref.) | Country | Design | Study characteristics | Intervention | Telomere length assessment method | Fluid analyzed | Results |
|---|---|---|---|---|---|---|---|
| Balcerczyk et al., 2014 [28] | Poland | Intervention trial | 66 women (age range: 35–55 years) | ω-3 PUFAs (1,350 mg/day) | qPCR (T:S ratio) | Blood | No effect on telomere length |
| Duration: 3 months | |||||||
| Condition: healthy | |||||||
| Barden et al., 2016 [29] | Australia | Randomized double-blind placebo-controlled trial | 85 subjects (mean age: 56.5 ± 1.4 years) (men and women) | ω-3 PUFAs (4 g/day ω-3) | qPCR (kb/genome) | Blood | Significant increase of neutrophil telomere length after correction for neutrophil count (p = 0.015) |
| Duration: 2 months | |||||||
| Condition: kidney disease | |||||||
| Kiecolt-Glasera et al., 2013 [30] | USA | Randomized double-blind placebo-controlled trial | 106 subjects (mean age: 50.7 years) (37 men and 69 women) | ω-3 PUFAs (2.5 g/day or l.25 g/day) | qPCR (bp) | Blood | Telomere length increased but not significantly |
| Duration: 4 months | |||||||
| Condition: overweight | |||||||
| O’Callaghan et al., 2014 [31] | Australia | Randomized double-blind controlled pilot study | 33 subjects (age > 65 years) | EPA-rich fish oil (1.67 g EPA + 0.16 g DHA/day) or DHA-rich fish oil (1.55 g DHA + 0.40 g EPA/day) | qPCR (kb/genome) | Blood | Significant reduction of telomere shortening (p < 0.02) |
| Duration: 6 months | |||||||
| Condition: mild cognitive impairment | |||||||
| Tsoukalast et al., 2019 [32] | Greece | Intervention trial | 47 subjects (mean age 47.1 years) (24 men and 23 women) | ALA (370 mg/day); EPA (312,6 mg/day); DHA (154,2 mg/day) | Q-FISH (bp) | Blood | Significant increase in telomere length (p < 0.05) |
| Duration: 6–12 months | |||||||
| Condition: healthy |
Abbreviations: ω-3, omega-3; PUFAs, polyunsaturated fatty acids; qPCR, quantitative real-time polymerase chain reaction; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; ALA, alpha-linolenic acid; Q-FISH, quantitative-fluorescent in situ hybridization.
Meta-analysis
Overall, we meta-analyzed 5 clinical trials involving a total of 337 participants. Using a random effect model, the meta-analysis showed a statistically significant effect of ω-3 fatty acids supplementation on TL (mean difference = 0.16; 95% CI, 0.02, 0.30; p = 0.02) (Figure 2a). However, there was significant evidence of high heterogeneity (I 2 85%) in the overall analysis. Thus, we conducted a sensitivity analysis by omitting 1 study with no control from the primary analysis and recalculating the effect size. After the sensitivity analysis, the positive effects of ω-3 fatty acids on TL remained significant (mean difference = 0.21; 95% CI, 0.07, 0.36; p < 0.01) (Figure 2b). Although the heterogeneity was reduced to I 2 = 69%, the grade remained moderate.
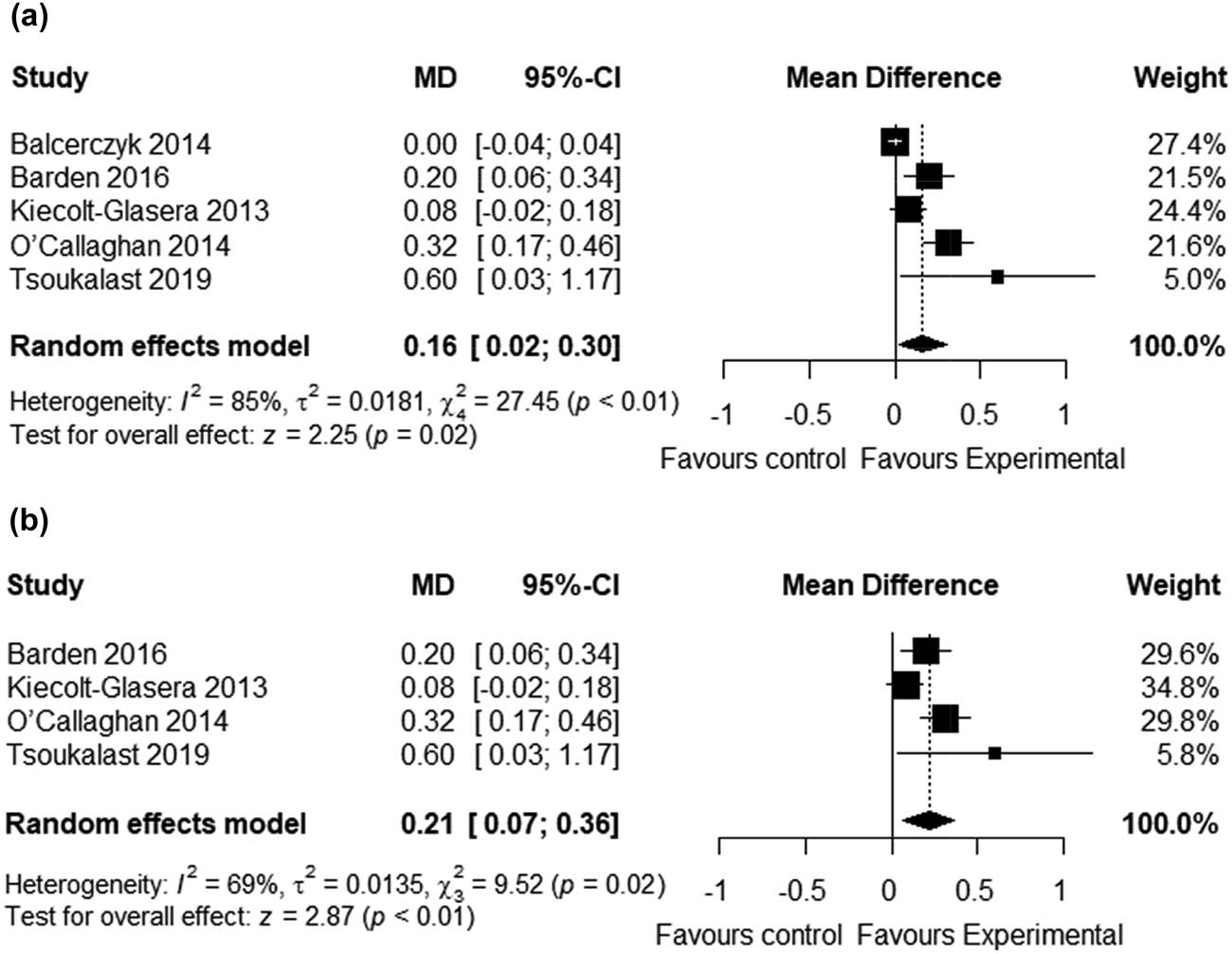
Forest plots showing the effect of omega-3 fatty acids supplementation on the telomere length. (a) Forest plot of the overall analysis. (b) Forest plot of the sensitivity analysis.
The publication bias was assessed using funnel plots. As shown in Figure 3, the funnel plot analysis revealed that the risk of publication bias was low. Results for the risk of bias are shown in Table 2 and Figure 4. The critical appraisal tools of the intervention trials show good quality in the methodology.

Funnel plots of risk of publication bias: (a) overall analysis and (b) sensitivity analysis.
Quality assessment of the included interventional trials
| Articles | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data (attrition bias) | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Barden et al., 2016 [29] | Low | Low | Low | Low | Unclear | Low | Low |
| Kiecolt-Glasera et al., 2013 [30] | Low | Low | Low | Low | Low | Low | Low |
| O’Callaghan et al., 2014 [31] | Low | Low | Low | Low | Low | Low | Low |
| Bias due to confounding | Bias in the selection of participants into the study | Bias in the classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | |
| Balcerczyk et al., 2014 [28] | — | — | — | Low | Low | Low | Low |
| Tsoukalast et al., 2019 [32] | Moderate | Low | Low | Low | Low | Low | Low |

Risk of bias graph.
Discussion
To the best of our knowledge, the present study is the first meta-analysis conducted to date showing a beneficial association between supplementation of ω-3 fatty acids and TL. Despite the limited number of clinical studies included in this meta-analysis, there is evidence that ω-3 fatty acids may have a role in telomere maintenance. Moreover, these results are consistent with previous studies showing that key foods rich in antioxidants and anti-inflammatory components may positively influence telomere attrition [33,34]. However, most of the trials examined here used different sample sizes, treatment durations, and involved a large variety of dosages of ω-3 fatty acids.
In the RCT conducted by O’ Callaghan et al., six months of daily supplementation with two different doses of EPA and DHA (1.67 g EPA + 0.16 g DHA; 1.55 g DHA + 0.40 g EPA) reduced telomere shortening in older adults with mild cognitive impairment when compared to the group supplemented with linoleic fatty acid [31]. Although this study has a longer duration than others, the main limitation was the limited sample size (n = 33). In contrast, Kiecolt-Glasera et al. conducted an RCT with a larger sample size (n = 106) involving overweight subjects. After four months of supplementation with two different doses of ω-3 PUFAs (2.5 or l.25 g/day), TL increased in the active group while it tended to decrease in the control group. Likewise, a reduction of F2-isoprostanes, an oxidative stress biomarker, was observed in the supplemented group compared to the placebo [30]. Despite a small number of participants (n = 47), the beneficial effects of ω-3 PUFAs on the length of short telomeres were also demonstrated by Tsoukalast et al. in a cohort of healthy volunteers. They used a nutraceutical supplement with 466.8 mg of EPA + DHA and the supplementation period lasted for 6–12 months [32]. In another two studies included in this meta-analysis, no statistically significant effect on TL was observed after supplementation with ω-3 PUFAs [28,29]. However, Barden et al. obtained a significant result when TL was corrected for neutrophil counts. This finding was associated with reduced levels of F2-isoprostanes and it may relate to increased clearance of neutrophils with shorter telomeres from the circulation.
Currently, oxidative stress appears to play a primary role in telomere shortening [35]. The molecular mechanisms underlying the effect of ω-3 fatty acids against oxidative stress have been investigated in experimental models, and several findings support the involvement of NF-E2-related factor-2 (NRF2) [36,37]. This transcription factor is known as the master regulator of the antioxidant response and it is responsible for both constitutive and inducible expression of cytoprotective proteins and detoxification enzymes [38]. A recent clinical study in patients with type 2 diabetes established a translational link between DHA and EPA and their antioxidant properties through modulation of NRF2 [39]. Oxidative stress also represents the most frequent cause of DNA damage at the telomeric level and it is related to telomere shortening/dysfunction [40]. It was reported that ω-3 fatty acids may attenuate DNA oxidative damage and protect chromosomal integrity through upregulation of NRF2 [41]. As mentioned, the ω-3 fatty acids may also exert beneficial effects on TL through their anti-inflammatory properties. Prospective cohort studies found that a higher plasma concentration of ω-3 fatty acids was associated with lower levels of proinflammatory markers and reduced attrition of TL [42,43]. Therefore, this anti-inflammatory potential could be a possible mechanism by which ω-3 fatty acids exert their effect on telomere maintenance.
The present meta-analysis has some limitations. The number of studies included is small due to limited data. For this reason, we could not perform a subgroup analysis and, therefore, the effects of dosage, duration, age, and gender on findings remained unclear. Then, the clinical trials assessed different samples sizes, dosages, and clinical conditions, which may have been responsible for the observed heterogeneity.
However, although our findings should be interpreted cautiously, this meta-analysis provides preliminary evidence that treatments with ω-3 fatty acids may potentially have some clinical efficacy on telomere maintenance.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated and analyzed during the current review are available from the corresponding author on reasonable request.
References
[1] López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217.10.1016/j.cell.2013.05.039Search in Google Scholar
[2] Kong CM, Lee XW, Wang X. Telomere shortening in human diseases. FEBS J. 2013;280:3180–93.10.1111/febs.12326Search in Google Scholar
[3] Whittemore K, Vera E, Martínez-Nevado E, Sanpera C, Blasco MA. Telomere shortening rate predicts species life span. Proc Natl Acad Sci U S A. 2019;116:15122–27.10.1073/pnas.1902452116Search in Google Scholar
[4] Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350:1193–8.10.1126/science.aab3389Search in Google Scholar
[5] Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992;89:10114–18.10.1073/pnas.89.21.10114Search in Google Scholar
[6] Zhang J, Rane G, Dai X, Shanmugam MK, Arfuso F, Samy RP, et al. Ageing and the telomere connection: an intimate relationship with inflammation. Ageing Res Rev. 2016;25:55–69.10.1016/j.arr.2015.11.006Search in Google Scholar
[7] Lin J, Epel E. Stress and telomere shortening: insights from cellular mechanisms. Ageing Res Rev. 2022;73:101507.10.1016/j.arr.2021.101507Search in Google Scholar
[8] Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–4.10.1016/S0140-6736(05)66630-5Search in Google Scholar
[9] Zhao B, Vo HQ, Johnston FH, Negishi K. Air pollution and telomere length: a systematic review of 12,058 subjects. Cardiovasc Diagn Ther. 2018;8:480–92.10.21037/cdt.2018.06.05Search in Google Scholar PubMed PubMed Central
[10] Davinelli S, De Vivo I. Lifestyle choices, psychological stress and their impact on ageing: the role of telomeres. Centenarians. Cham: Springer; p. 135–48.10.1007/978-3-030-20762-5_8Search in Google Scholar
[11] Davinelli S, Trichopoulou A, Corbi G, De Vivo I, Scapagnini G. The potential nutrigeroprotective role of Mediterranean diet and its functional components on telomere length dynamics. Ageing Res Rev. 2019;49:1–10.10.1016/j.arr.2018.11.001Search in Google Scholar PubMed
[12] Ruiz-Narváez EA, Baylin A, Azofeifa J, Leal A, Rosero-Bixby L. Diet and leukocyte telomere length in a population with extended longevity: the costa rican longevity and healthy aging study (creles). Nutrients. Epub ahead of print 1 August 2021;13:13. 10.3390/nu13082585.Search in Google Scholar PubMed PubMed Central
[13] Prasad KN, Wu M, Bondy SC. Telomere shortening during aging: attenuation by antioxidants and anti-inflammatory agents. Mechanisms Ageing Dev. 2017;164:61–6.10.1016/j.mad.2017.04.004Search in Google Scholar PubMed
[14] García-Calzón S, Zalba G, Ruiz-Canela M, Shivappa N, Hébert JR, Martínez JA, et al. Dietary inflammatory index and telomere length in subjects with a high cardiovascular disease risk from the PREDIMED-NAVARRA study: cross-sectional and longitudinal analyses over 5 y. Am J Clin Nutr. 2015;102:897–904.10.3945/ajcn.115.116863Search in Google Scholar PubMed PubMed Central
[15] Lee JY, Jun NR, Yoon D, Shin C, Baik I. Association between dietary patterns in the remote past and telomere length. Eur J Clin Nutr. 2015;69:1048–52.10.1038/ejcn.2015.58Search in Google Scholar PubMed
[16] Tucker LA. Fruit and Vegetable Intake and Telomere Length in a Random Sample of 5448 U.S. Adults. Nutrients. Epub ahead of print 1 May 2021;13:13. 10.3390/NU13051415.Search in Google Scholar
[17] Rafie N, Golpour Hamedani S, Barak F, Safavi SM, Miraghajani M. Dietary patterns, food groups and telomere length: a systematic review of current studies. Eur J Clin Nutr. 2017;71:151–8.10.1038/ejcn.2016.149Search in Google Scholar PubMed
[18] Maleki M, Khelghati N, Alemi F, Bazdar M, Asemi Z, Majidinia M, et al. Stabilization of telomere by the antioxidant property of polyphenols: anti-aging potential. Life Sci. Epub ahead of print 15 October 2020;259:259. 10.1016/j.lfs.2020.118341.Search in Google Scholar PubMed
[19] Alibakhshi A, Ranjbari J, Pilehvar-Soltanahmadi Y, Nasiri M, Mollazade M, Zarghami N. An update on phytochemicals in molecular target therapy of cancer: potential inhibitory effect on telomerase activity. Curr Med Chem. 2016;23:2380–93.10.2174/0929867323666160425113705Search in Google Scholar PubMed
[20] Davinelli S, Scapagnini G. Lifespan and healthspan extension by nutraceuticals: an overview. Centenarians. Cham: Springer; p. 169–79.10.1007/978-3-030-20762-5_11Search in Google Scholar
[21] Shahidi F, Ambigaipalan P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu Rev Food Sci Technol. 2018;9:345–81.10.1146/annurev-food-111317-095850Search in Google Scholar PubMed
[22] Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75:645–62.10.1111/j.1365-2125.2012.04374.xSearch in Google Scholar PubMed PubMed Central
[23] Heshmati J, Morvaridzadeh M, Maroufizadeh S, Akbari A, Yavari M, Amirinejad A, et al. Omega-3 fatty acids supplementation and oxidative stress parameters: a systematic review and meta-analysis of clinical trials. Pharmacol Res. Epub ahead of print 1 November 2019;149:149. 10.1016/j.phrs.2019.104462.Search in Google Scholar PubMed
[24] Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.10.1186/2046-4053-4-1Search in Google Scholar PubMed PubMed Central
[25] Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.10.1136/bmj.d5928Search in Google Scholar PubMed PubMed Central
[26] Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. Epub ahead of print 2016;355:355. 10.1136/bmj.i4919.Search in Google Scholar PubMed PubMed Central
[27] Higgins J, Green S Cochrane Handbook for Systematic Reviews of Interventions|The Cochrane Collaboration, http://www.cochrane.org/training/cochrane-handbook (2011).Search in Google Scholar
[28] Balcerczyk A, Gajewska A, Macierzyńska-Piotrowska E, Pawelczyk T, Bartosz G, Szemraj J. Enhanced antioxidant capacity and anti-ageing biomarkers after diet micronutrient supplementation. Molecules. 2014;19:14794–14808.10.3390/molecules190914794Search in Google Scholar PubMed PubMed Central
[29] Barden A, O'callaghan N, Burke V, Mas E, Beilin LJ, Fenech M, et al. N-3 fatty acid supplementation and leukocyte telomere length in patients with chronic kidney disease. Nutrients. Epub ahead of print 19 March 2016;8:8. 10.3390/nu8030175.Search in Google Scholar PubMed PubMed Central
[30] Kiecolt-Glaser JK, Epel ES, Belury MA, Andridge R, Lin J, Glaser R, et al. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: a randomized controlled trial. Brain Behav Immun. 2013;28:16–24.10.1016/j.bbi.2012.09.004Search in Google Scholar PubMed PubMed Central
[31] O'callaghan N, Parletta N, Milte CM, Benassi-Evans B, Fenech M, Howe PR. Telomere shortening in elderly individuals with mild cognitive impairment may be attenuated with ω-3 fatty acid supplementation: a randomized controlled pilot study. Nutrition. 2014;30:489–91.10.1016/j.nut.2013.09.013Search in Google Scholar PubMed
[32] Tsoukalas D, Fragkiadaki P, Docea AO, Alegakis AK, Sarandi E, Vakonaki E, et al. Association of nutraceutical supplements with longer telomere length. Int J Mol Med. 2019;44:218–26.10.3892/ijmm.2019.4191Search in Google Scholar
[33] Freitas-Simoes TM, Ros E, Sala-Vila A. Nutrients, foods, dietary patterns and telomere length: update of epidemiological studies and randomized trials. Metabolism: Clin Exp. 2016;65:406–15.10.1016/j.metabol.2015.11.004Search in Google Scholar
[34] Karimi B, Nabizadeh R, Yunesian M, Mehdipour P, Rastkari N, Aghaie A. Foods, dietary patterns and occupational class and leukocyte telomere length in the male population. Am J Mens Health. 2018;12:479–92.10.1177/1557988317743385Search in Google Scholar
[35] Coluzzi E, Leone S, Sgura A. Oxidative stress induces telomere dysfunction and senescence by replication fork arrest. Cells. 2019;8:19.10.3390/cells8010019Search in Google Scholar
[36] Zgórzyńska E, Dziedzic B, Gorzkiewicz A, Stulczewski D, Bielawska K, Su KP, et al. Omega-3 polyunsaturated fatty acids improve the antioxidative defense in rat astrocytes via an Nrf2-dependent mechanism. Pharmacol Rep. 2017;69:935–42.10.1016/j.pharep.2017.04.009Search in Google Scholar
[37] Tatsumi Y, Kato A, Sango K, Himeno T, Kondo M, Kato Y, et al. Omega-3 polyunsaturated fatty acids exert anti-oxidant effects through the nuclear factor (erythroid-derived 2)-related factor 2 pathway in immortalized mouse Schwann cells. J Diabetes Investig. 2019;10:602–12.10.1111/jdi.12931Search in Google Scholar
[38] Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–95.10.1074/jbc.R900010200Search in Google Scholar
[39] Golpour P, Nourbakhsh M, Mazaherioun M, Janani L, Nourbakhsh M, Yaghmaei P. Improvement of NRF2 gene expression and antioxidant status in patients with type 2 diabetes mellitus after supplementation with omega-3 polyunsaturated fatty acids: a double-blind randomised placebo-controlled clinical trial. Diabetes Res Clin Pract. Epub ahead of print 1 April 2020;162:162. 10.1016/j.diabres.2020.108120.Search in Google Scholar
[40] Oikawa S, Kawanishi S. Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. FEBS Lett. 1999;453:365–8.10.1016/S0014-5793(99)00748-6Search in Google Scholar
[41] Sakai C, Ishida M, Ohba H, Yamashita H, Uchida H, Yoshizumi M, et al. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS One. Epub ahead of print 1 November 2017;12:12. 10.1371/journal.pone.0187934.Search in Google Scholar PubMed PubMed Central
[42] Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–46.10.1210/jc.2005-1303Search in Google Scholar PubMed
[43] Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA - J Am Med Assoc. 2010;303:250–7.10.1001/jama.2009.2008Search in Google Scholar PubMed PubMed Central
© 2022 Sawan Ali et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Diagnostic accuracy of genetic markers for identification of the Lr46/Yr29 “slow rusting” locus in wheat (Triticum aestivum L.)
- NADPH-derived ROS generation drives fibrosis and endothelial-to-mesenchymal transition in systemic sclerosis: Potential cross talk with circulating miRNAs
- Effect of omega-3 fatty acids on the telomere length: A mini meta-analysis of clinical trials
- Analysis of differentially expressed genes and signaling pathways involved in atherosclerosis and chronic obstructive pulmonary disease
- The epigenetic dimension of protein structure
- Gamma-induced mutants of Bacillus and Streptomyces display enhanced antagonistic activities and suppression of the root rot and wilt diseases in pulses
- Corticosterone potentiates ochratoxin A-induced microglial activation
- Supercomplex supercomplexes: Raison d’etre and functional significance of supramolecular organization in oxidative phosphorylation
- Insights into functional connectivity in mammalian signal transduction pathways by pairwise comparison of protein interaction partners of critical signaling hubs
- The effects of supplementation of Nannochloropsis oculata microalgae on biochemical, inflammatory and antioxidant responses in diabetic rats
- Molecular epidemiology of human papillomavirus in pregnant women in Burkina Faso
- Review Articles
- Interaction of cervical microbiome with epigenome of epithelial cells: Significance of inflammation to primary healthcare
- Seaweeds’ pigments and phenolic compounds with antimicrobial potential
- The capture of host cell’s resources: The role of heat shock proteins and polyamines in SARS-COV-2 (COVID-19) pathway to viral infection
- Erratum
- Erratum to “Plant growth-promoting properties of bacterial endophytes isolated from roots of Thymus vulgaris L. and investigate their role as biofertilizers to enhance the essential oil contents”
- Special Issue on XXV Congress of the Italian Society for Pure and Applied Biophysics
- Low-temperature librations and dynamical transition in proteins at differing hydration levels
- The phosphoinositide PI(3,5)P2 inhibits the activity of plant NHX proton/potassium antiporters: Advantages of a novel electrophysiological approach
- Targeted photoimmunotherapy for cancer
- Calorimetry of extracellular vesicles fusion to single phospholipid membrane
- Calcium signaling in prostate cancer cells of increasing malignancy
- Oxygen diffusion pathways in mutated forms of a LOV photoreceptor from Methylobacterium radiotolerans: A molecular dynamics study
- A photosensitizing fusion protein with targeting capabilities
- Ion channels and neuronal excitability in polyglutamine neurodegenerative diseases
- Is styrene competitive for dopamine receptor binding?
- Diffusion of molecules through nanopores under confinement: Time-scale bridging and crowding effects via Markov state model
- Quantitative active super-resolution thermal imaging: The melanoma case study
- Innovative light sources for phototherapy
- Electrophysiological study of the effects of side products of RuBi-GABA uncaging on GABAA receptors in cerebellar granule cells
- Subcellular elements responsive to the biomechanical activity of triple-negative breast cancer-derived small extracellular vesicles
Articles in the same Issue
- Research Articles
- Diagnostic accuracy of genetic markers for identification of the Lr46/Yr29 “slow rusting” locus in wheat (Triticum aestivum L.)
- NADPH-derived ROS generation drives fibrosis and endothelial-to-mesenchymal transition in systemic sclerosis: Potential cross talk with circulating miRNAs
- Effect of omega-3 fatty acids on the telomere length: A mini meta-analysis of clinical trials
- Analysis of differentially expressed genes and signaling pathways involved in atherosclerosis and chronic obstructive pulmonary disease
- The epigenetic dimension of protein structure
- Gamma-induced mutants of Bacillus and Streptomyces display enhanced antagonistic activities and suppression of the root rot and wilt diseases in pulses
- Corticosterone potentiates ochratoxin A-induced microglial activation
- Supercomplex supercomplexes: Raison d’etre and functional significance of supramolecular organization in oxidative phosphorylation
- Insights into functional connectivity in mammalian signal transduction pathways by pairwise comparison of protein interaction partners of critical signaling hubs
- The effects of supplementation of Nannochloropsis oculata microalgae on biochemical, inflammatory and antioxidant responses in diabetic rats
- Molecular epidemiology of human papillomavirus in pregnant women in Burkina Faso
- Review Articles
- Interaction of cervical microbiome with epigenome of epithelial cells: Significance of inflammation to primary healthcare
- Seaweeds’ pigments and phenolic compounds with antimicrobial potential
- The capture of host cell’s resources: The role of heat shock proteins and polyamines in SARS-COV-2 (COVID-19) pathway to viral infection
- Erratum
- Erratum to “Plant growth-promoting properties of bacterial endophytes isolated from roots of Thymus vulgaris L. and investigate their role as biofertilizers to enhance the essential oil contents”
- Special Issue on XXV Congress of the Italian Society for Pure and Applied Biophysics
- Low-temperature librations and dynamical transition in proteins at differing hydration levels
- The phosphoinositide PI(3,5)P2 inhibits the activity of plant NHX proton/potassium antiporters: Advantages of a novel electrophysiological approach
- Targeted photoimmunotherapy for cancer
- Calorimetry of extracellular vesicles fusion to single phospholipid membrane
- Calcium signaling in prostate cancer cells of increasing malignancy
- Oxygen diffusion pathways in mutated forms of a LOV photoreceptor from Methylobacterium radiotolerans: A molecular dynamics study
- A photosensitizing fusion protein with targeting capabilities
- Ion channels and neuronal excitability in polyglutamine neurodegenerative diseases
- Is styrene competitive for dopamine receptor binding?
- Diffusion of molecules through nanopores under confinement: Time-scale bridging and crowding effects via Markov state model
- Quantitative active super-resolution thermal imaging: The melanoma case study
- Innovative light sources for phototherapy
- Electrophysiological study of the effects of side products of RuBi-GABA uncaging on GABAA receptors in cerebellar granule cells
- Subcellular elements responsive to the biomechanical activity of triple-negative breast cancer-derived small extracellular vesicles

