Abstract
Matrix metalloproteinases (MMPs) are implicated in many physiological and pathological processes, including contraction, migration, differentiation, and proliferation. These processes all involve cell phenotype changes, known to be accompanied by reorganization of actin cytoskeleton. Growing evidence indicates a correlation between MMP activity and the dynamics of actin system, suggesting their mutual regulation. Here, data on the influence of MMPs on the actin microfilament system, on the one hand, and the dependence of MMP expression and activation on the organization of actin structures, on the other hand, are reviewed. The different mechanisms of putative actin-MMP regulation are discussed.
Introduction
Matrix metalloproteinases (MMPs) are zinc- and calcium-dependent endopeptidases that can degrade all the extracellular matrix (ECM) structural elements. They play important roles in many physiological processes, such as embryonic development, morphogenesis, reproduction, and tissue remodeling (1), (2), (3), (4), as well as different pathologies (5), (6), (7), (8). These processes all involve changes in cell phenotype, which are typically accompanied by actin cytoskeleton dynamics. This review is aimed to highlight interdependence between actin microfilament system and MMPs in different cell types and speculate on their mutual regulation.
General structure of MMPs
MMPs all share a number of common features and include several domains. The N-terminal signal domain enhances protein export across the cell membrane into extracellular space. Propeptide domain binding with Zn2+ in active site maintains MMP molecule in latent form. The majority of MMPs release as inactive proenzymes. Hydrolytic removal of propeptide domain and release of the Zn2+-binding center is followed by the activation of MMP. Endopeptidase activity of MMPs is provided by the catalytic domain that includes two Zn2+ ions and three Ca2+ ions (9).
Digestion of ECM by MMPs
All MMPs are able to degrade at least one component of the ECM. The different members of this family are specialized in degrading different sets of matrix proteins, although their substrate specificities may overlap. To date about 30 types of MMPs are described and classified into several groups based on their matrix substrate specificities and amino acid sequence similarity, i.e. collagenases (MMP-1, -8, and -13), gelatinases (MMP-2 and -9), stromelysins (MMP-3, -10, and -11), matrilysins (MMP-7 and -26), membrane-type MMPs (MMP-14, -15, -16, -17, -24, and -25), enamelysin (MMP-20), and others (MMP-19, -21, -23, -27, and -28) (10), (11). Collagenases cleave interstitial collagens I, II, and III and other ECM molecules and soluble proteins. Gelatinases (MMP-2 and MMP-9) readily digest gelatin (11). They also digest a number of other ECM molecules including type IV, V, and XI collagens, laminin, aggrecan core protein, etc. MMP-2 further digests collagens I, II, and III. The membrane-bound MT-MMPs may digest collagens on or near the cell surface. All MT-MMPs, except MT4-MMP (MMP-17), can activate proMMP-2. For a detailed description of ECM-dependent activities of different MMPs, see the review by Nagase et al. (11).
Non-matrix substrates for MMPs
Although MMPs were initially described as ECM-remodeling proteases, data indicate that their role is not limited to matrix digestion. MMPs can also cleave a variety of non-matrix proteins including cytokines, growth factors, lipoproteins, and clotting factors, leading to their shedding, activation or inactivation (12). Moreover, it was shown that some MMPs may be involved in direct N-cadherin shedding, thereby inducing proliferative phenotype in cells. For example, MMP9 and MMP12, but not MMP2 or MMP14, were shown to cause N-cadherin shedding and activating the β-catenin signaling pathway on vascular smooth muscle cells (VSMCs) and promote their proliferation (13). Moreover, Goldsmith et al. (14) proposed a role for MMPs in integrin shedding. The authors have shown that, in neonatal rat cardiac fibroblasts, but not in myocytes, inhibition of MMPs leads to a clear dose-dependent inhibition of shedding of the extracellular β1-integrin domain into the extracellular environment. However, the function of the shed β1-integrin fragment was not defined. The authors suggest that it may provide a feedback signal to regulate receptor distribution on the cell surface or bind to specific ECM components in turn enhancing or inhibiting some cellular functions (14).
Besides a lot of studies relating the extracellular role of MMPs, their intracellular action is also contemplated. In cardiomyocytes, the activity of MMPs in mitochondria was shown (15), where the mitochondrial accumulation of MMP-9 was related to arrhythmia and impaired contractility (16), (17). MMP-2 was further detected in the nuclei of heart and liver cells (18). Moreover, a truncated yet active fragment of MMP-3 was localized to the nuclei of several human cancer cell lines (19). However, the biological role for MMPs within the nucleus remains unclear. Some authors credit it with nuclear matrix degradation (20).
A further role for MMPs in myofibril degradation is proposed. In the hearts exposed to oxygen stress-induced ischemic reperfusion injury MMP-2 was shown to be involved in digestion of sarcomeric proteins, including α-actinin (21), troponin I (15), myosin light chain-1 (22), myosin light chain-2 (23), and titin (24). Sarcomeric protein degradation led to contractile dysfunction, which was protected by the inhibition of MMP-2 action (25). In normal skeletal muscle, MMP-2 was detected in Z-lines of the sarcomeres, in the nuclear membrane, and in mitochondria (26).
Therefore, MMPs are likely to act as intracellular proteases, modulating different cell functions. However, mechanisms targeting active MMPs to intracellular compartments and the mechanisms of their intracellular activation are not fully understood. It is believed that intracellular MMPs may be splice variants lacking a signal sequence or MMPs with an ineffective signal sequence to target them to the endoplasmic reticulum (25), (27).
The ECM-independent functions of MMPs are discussed in greater detail in Ref. (28).
Regulation of MMPs
MMPs are tightly regulated at several levels including transcriptional, post-transcriptional, and post-translational. The expression of most MMPs is primarily regulated at transcriptional level, with the different MMPs having varying temporal and spatial expression patterns (29). The expression of MMPs can be up-regulated in different cells in response to many factors including proinflammatory cytokines and growth factors (10). Various exogenous and endogenous stimuli may also regulate MMPs at post-transcriptional level by stabilizing and/or destabilizing mRNA transcripts (30). The stabilization of MMP-1 and -3 transcripts by phorbol esters and epidermal growth factor in fibroblasts and stabilization of MMP-13 transcripts in osteoblasts by platelet-derived growth factor and glucocorticoids as well as destabilization by transforming growth factor beta (TGF-β) were shown (31), (32). Several studies indicate that MMP-2 activity is also regulated via non-proteolytic post-translational modifications of the full-length zymogen, by S-glutathiolation, S-nitrosylation, and phosphorylation (33), (34), (35), (36). In addition, MMP activity may be regulated directly by their endogenous inhibitors named tissue inhibitors of metalloproteinases (TIMMPs), which can reversibly bind to activated MMPs (32), (37), (38).
Correlation between MMPs and actin cytoskeleton organization
Many cellular functions such as migration, contraction, proliferation, and differentiation are associated with MMP dynamics. Significantly, these functions are characterized by distinct cellular phenotypes, dependent considerably on the organization of actin microfilament system. Increasing evidence describes a correlation between MMP activity and the dynamics of actin structures in different cell types. It was shown, e.g. that MMPs are influenced by actin polymerization. Induced production of MMP-9 in SNB19 glioma cells and constitutive expression of MMP-9 in HT1080 fibrosarcoma cells were lost following treatment of these cells with an inhibitor of actin polymerization cytochalasin-D. However, treatment with cytochalasin-D did not affect MMP-2 expression in HT1080 cells (39). According to another study, disruption of actin cytoskeleton organization in human trabecular meshwork cells, induced by direct inhibitors of actin polymerization cytochalasin D, and latrunculin A, was followed by the activation of MMP-2 and the reduction in pro-MMP-2, suggesting that MMP-2 is regulated via proteolytic activation of its latent form. This increase in active MMP-2 correlated with the increased expression of MT1-MMP, the activator of MMP-2, and with decreases in protein levels of the MMP inhibitors TIMP-1 and TIMP-2 (40). MMP-2 activation was regulated by actin cytoskeleton organization in human palmar fascial fibroblasts as well (41). The additional study demonstrates that MMP-9 is suppressed by an alteration in cell shape and actin disruption in melanoma cells (42). Moreover, actin cytoskeleton remodeling is shown to be a critical regulator of MT1-MMP trafficking, and disruption of the actin cytoskeleton potently affects the MT1-MMP cell surface accumulation (43), (44).
Taken together, these data indicate that expression and/or activation of different MMPs may be regulated by actin polymerization/depolymerization process, where, in most cases, polymerized actin is likely to down-regulate MMP-2 and up-regulate MMP-9.
MMPs and contractility
In VSMCs, changes in MMP-2 mRNA expression were shown to inversely relate to changes in mRNA levels for smooth muscle actin (α-SM actin) (45). In these cells, α-SM actin is the predominating actin isoform (46) and is a key marker of contractile phenotype, which is often referred to as differentiated. Induced VSMC de-differentiation, involving changes in the actin cytoskeleton with decreased level of α-SM actin expression resulted in up-regulation of MMP-2 expression (45).
In fibroblasts, transition to myofibroblast phenotype, characterized by the expression of high levels of contractile proteins, particularly α-SM actin was shown to suppress the expression of MMP-2. Factors that promote cell tension and favor the expression of contractile proteins, including serum factors, TGF-β, pro-contractile GPCR ligands, or culturing fibroblasts in collagen lattices under tension, down-regulated MMP-2. Jasplakinolide, that promotes F-actin assembly, also suppressed MMP-2 gene expression (47). Suppression described could be overcome by forcing the disassembly of F-actin, either through the addition of F-actin depolymerizing agents or by culturing cells in non-attached 3-D lattices that prevent force generation. However, the loss of contractility alone was not sufficient to promote MMP-2 up-regulation. Stimulating cells with the actin depolymerizing agent, latrunculin B, in the absence of serum factors, caused contractile protein down-regulation but did not up-regulate MMP-2 expression, suggesting several mechanisms involved in MMP-2 regulation in these cells.
α-SM actin is not always considered to be a marker of a differentiated state. For example, the visceral smooth muscle cells express higher levels of α-SM actin when de-differentiated, which is contrary to what occurs in VSMCs (48). Notably, in these cells, γ-SM actin is the predominating variant of actin in contrast to VSMCs, where α-SM actin is the main actin isoform (46).
In cardiomyocytes, differentiated contractile status is associated with α-cardiac actin expression, while α-SM actin up-regulation corresponds to a less contractile phenotype and is considered to be a de-differentiation marker of these cells (49). Recently we have shown that reorganization of myofibrillar apparatus in neonatal rat cardiomyocytes in culture is characterized by the loss of contractility and α-SM actin expression (50) and is accompanied by MMP-2 production (51).
It appears therefore that, in different cell types, more contractile phenotype, associated either with α-SM actin up-regulation or with α-SM actin down-regulation, generally corresponds to MMP-2 depletion.
MMPs in migration
Some authors suggest contractile vs. migratory phenotype to be mutually exclusive (47). Transition to migratory phenotype in different cell types includes actin cytoskeleton reorganizations (Figure 1), such as lamellipodia formation (52). For smooth muscle cells, the ability to alter their phenotype from a contractile to a migratory state is shown to be accompanied by proliferation (53). During this phenotypic modulation, VSMCs down-regulate a set of contractile proteins, including α-SM actin, and up-regulate genes involved in cell migration, ECM deposition, and cell growth (54), (55).
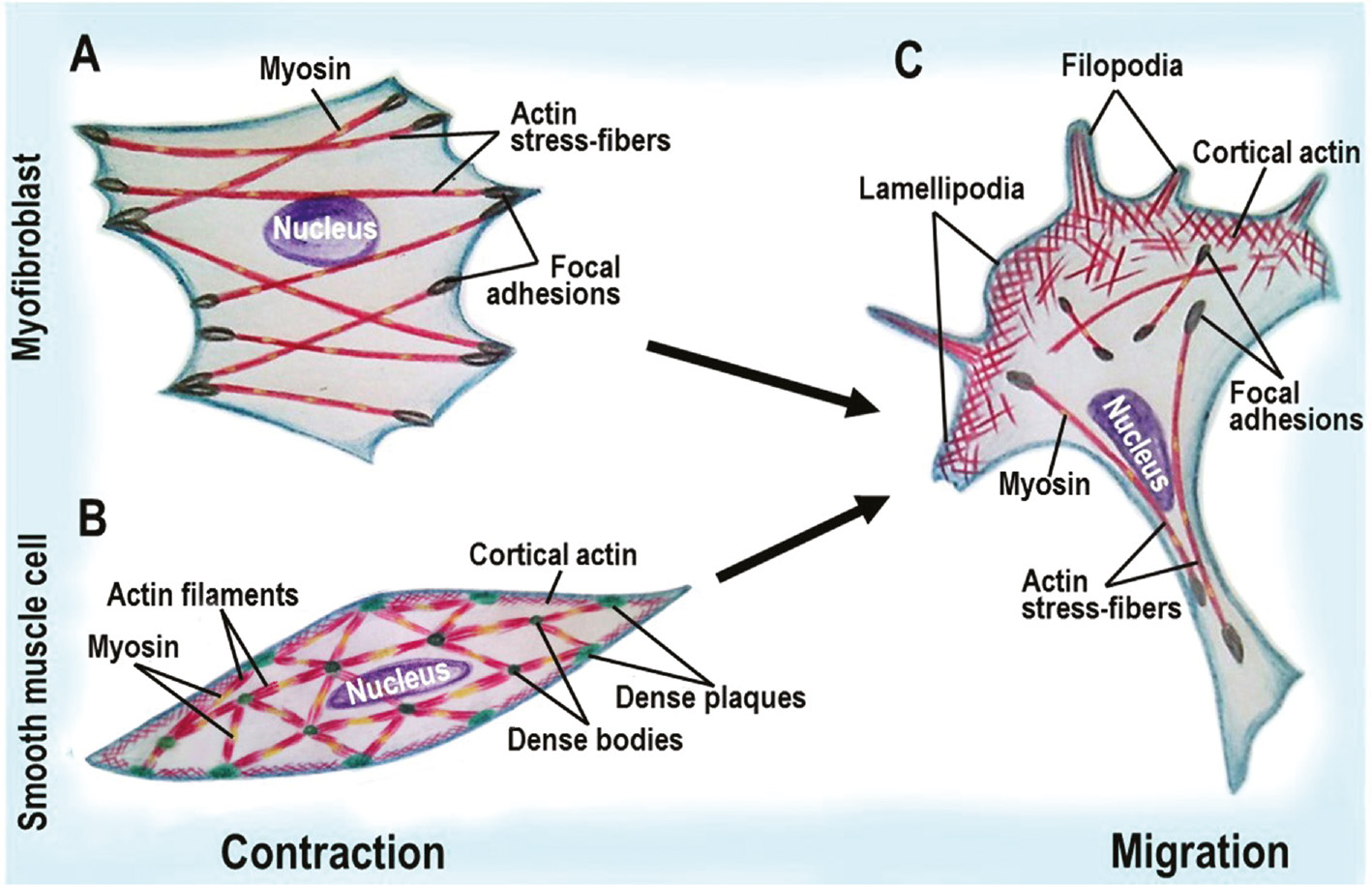
Schematic diagram illustrating contraction vs. migration phenotype in fibroblasts and smooth muscle cells.
(A) contractile fibroblasts, myofibroblasts, are characterized by the formation of myosin-containing actin contractile stress fibers. (B) in contracting smooth muscle cells, actin assembles into myosin-containing actin contractile apparatus and cortical actin network. (C) migration of both fibroblasts and smooth muscle cells is accompanied by a similar reorganization of actin microfilament system, including lamellipodia and filopodia formation.
Migration is accompanied by MMP-2 expression in the majority of cells. MMP-2 is therefore typically referred to as pro-migratory protease. Up-regulation of MMP-2 was linked to migration and proliferation of different smooth muscle cells (56), (57), (58), (59), as well as myoblast migration (60). Increased MMP-2 activity stimulated melanoma cell migration (61) as well as migration and invasion of endometriotic cells (62). In contrast, inhibited MMP-2 activity and expression blocked migration, and invasion of human fibrosarcoma cells (63).
In general, MMP-2 activation accompanies migration and inversely correlates with contractile activity.
MMPs in differentiation
Interdependent dynamics of actin and MMPs may be further observed during the differentiation process. For example, muscle cell differentiation is known to be accompanied by significant actin rearrangements with actin isoform switching as cells progress from their non-muscle precursors (64). Cardiogenic differentiation of the human heart L9TB progenitor cells was shown to include the decreased expression of MMP-1 and increased expression of MMP-2 and MMP-9 compared to non-differentiating cells, with MMP-9 up-regulation observed at the early stage of differentiation only. MMP-2 expression at the later differentiation stages might be surprising in view of data described above, as MMP-2 is expected to be incompatible with the differentiated contractile phenotype. However, its expression through the whole differentiation period is likely to be accountable for the fact that fully differentiated cells were not obtained in this study (65). The role of MMPs was shown in the cardiac differentiation of embryonic stem cells as well (66). Moreover, MMP changes were shown in the differentiation of chondroblasts, osteoblasts, and adipoblasts, derived from adult mesenchymal stem cells (67).
Actin-MMP mutual regulation
Data described demonstrate a strong relation between actin system dynamics and MMP activity in different cell types and various processes, which may suggest actin-MMP mutual regulation. Although the regulation mechanisms have not been determined, relevant knowledge allows their contemplation.
As cytoskeleton changes often precede MMP modulation, actin microfilament dynamics might be linked to the expression of MMP genes. A role for actin in gene expression is well known [for a review see ref. (68)]. The best known actin-regulated transcription factor is serum response factor (SRF), which controls the expression of many cytoskeleton-associated genes in response to changes in actin dynamics. The signal from the actin cytoskeleton to SRF is mediated by the megakaryoblastic leukemia 1 (MKL1) transcription coactivator (also known as MAL or MRTF-A) (69). There is also evidence that polymerized actin could regulate the expression of specific genes (70), (71). Dynamics of cytoplasmic actin can further regulate the activity of transcription factors, and thus indirectly influence nuclear events. For example, presequence protease 2 (PREP2) (72) and Yin-Yang1 (YY1) (73) are transcription factors, bound to actin filaments in the cytoplasm, and depolymerization of actin filaments releases them to the nucleus to activate transcription. It is in addition well accepted for the actin cytoskeleton to affect gene expression via NF-κB. Regulation of NF-κB by actin polymerization is supported by NF-κB activation following disruption of the actin cytoskeleton in human intestinal epithelial cells (74). Altogether, actin can control gene expression by regulation of the localization and activity of transcription factors, in both the cytoplasm and the nucleus.
This allows us to assume a possible involvement of actin microfilament system in the regulation of MMP expression and thereby ECM remodeling, as MMPs are key modulators of ECM.
It is well accepted that deformation of the ECM, in turn, can affect cell actin cytoskeleton and thereby alter cell shape, motility, and function. ECM is shown to influence the actin cytoskeleton organization in non-muscle cells (75), (76), (77) and smooth muscle cells (78), as well as myofibrillar apparatus in striated muscle cells (79), (80). The interaction between ECM and actin cytoskeleton is provided by integrin receptors (Figure 2), which are transmembrane heterodimers capable of transmitting mechanical signals from ECM to anchored cells and transfer them into the intracellular stimuli, leading to the alteration of the cell phenotype (81), (82). This process is known as mechanotransduction, which differs from those involved in adhesion (83). So, it would be reasonable to suggest that the actin system dynamics are regulated by MMPs via ECM remodeling followed by the integrin-mediated mechanotransduction.
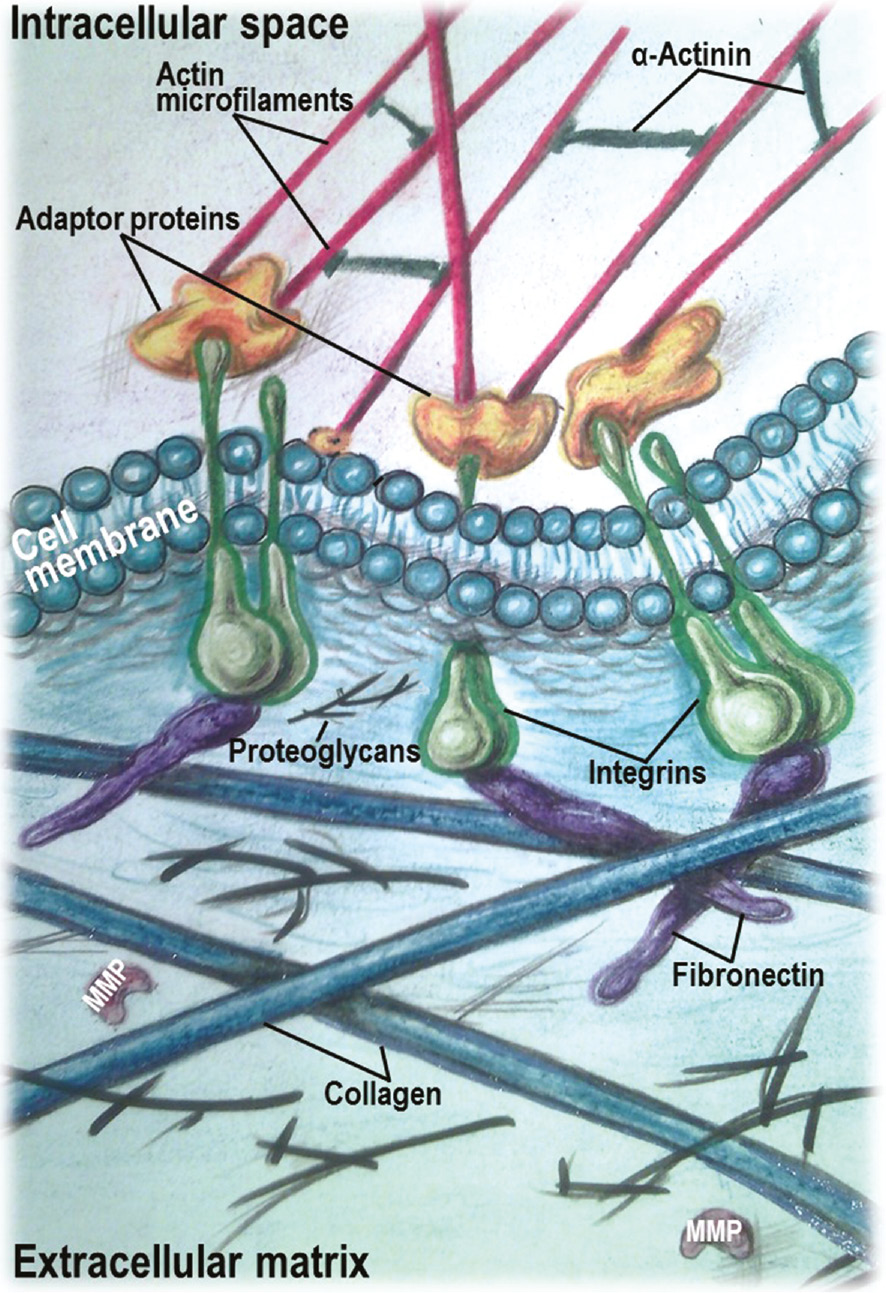
Schematic diagram illustrating a linkage between the extracellular matrix and the actin cytoskeleton by integrins.
The extracellular domain of integrins interacts with the extracellular matrix components, while the cytoplasmic tail binds to actin microfilaments through adaptor cytoskeletal proteins.
On the other hand, some data suggest a direct interaction between MMPs and integrins. For example, integrin shedding by MMPs was proposed in the cardiovascular system (14). Moreover, MMP-2 in its active form was able to directly bind with integrin αvβ3 on the surface of invasive cells (84), suggesting regulatory relationships between these two molecules.
Another regulation mechanism may involve the MMP-induced release of different stimulating factors from ECM. MMP-3, e.g. was shown to release TGF-β from the ECM by proteolytic cleavage of the latent TGF-β binding protein (85), where TGF-β is known to stimulate α-SM actin expression (86).
Interestingly, it is α-SM actin that was shown to provide a mechanical linkage between mechanosensory elements and appropriate targets required for mechanotransduction (87). In myofibroblasts, cell-generated traction forces, associated with α-SM actin, were shown to contribute to matrix remodeling (87). Our recent study suggests that α-SM actin can modulate the synthesis of ECM by cardiomyocytes, which in turn suppresses α-SM actin expression (50). This evidence implies a feedback regulation between ECM and actin system dynamics. The involvement of MMPs in this process is supported by the recent data (88), suggesting that mesenchymal stem cell function is controlled by MMP activity, which in turn is regulated by mechanical stimulation of cells. In this regard, MMP regulation by mechanical stimuli, applied to the cells, is well described (89), (90), (91), (92), (93), (94). Moreover, it was shown that type VIII collagen signals through β1-integrin receptors to allow optimal configuration of the cytoskeleton by stress-fiber formation and thereby facilitates MMP-2-dependent cell migration (95).
Thus, an increasing body of data suggests that actin system dynamics is involved in regulation of MMP expression and activation, which in turn may regulate actin structure organization and isoform expression.
Conclusions
Processes, strongly regulated by actin system dynamics, such as migration, contraction, proliferation, and differentiation, involve MMP changes in both muscle and non-muscle cells, normal or transformed. The obvious correlation between actin organization and expression, on the one hand, and MMP expression and activity, on the other hand, suggests their mutual regulation. The mechanisms underlying are not clear but are likely to be mediated by integrin mechanotransduction. Investigation of these mechanisms may shed light on cell adaptation to the extracellular environment during key physiological and pathological processes via actin-ECM feedback regulation, which is becoming apparent.
Acknowledgments
Supported by the Russian Science Foundation and the Federal Agency for Scientific Organizations (grant 14-50-00068).
- List of abbreviations
- ECM
extracellular matrix
- MMP
matrix metalloproteinase
- SM actin
smooth muscle actin
- TGF-β
transforming growth factor beta
- VSMC
vascular smooth muscle cell
References
1. Brenner CA, Adler RR, Rappolee DA, Pedersen RA, Werb Z. Genes for extracellular matrix degrading metalloproteinases and their inhibitor, TIMP, are expressed during early mammalian development. Genes Dev 1989; 3: 848–59.10.1101/gad.3.6.848Search in Google Scholar
2. Matrisian LM, Hogan BL. Growth factor-regulated proteases and extracellular matrix remodeling during mammalian development. Curr Top Dev Biol 1990; 24: 219–59.10.1016/S0070-2153(08)60089-7Search in Google Scholar
3. Woessner J. Matrix metalloproteinases and their inhibitors in connective tissue remodeling, FASEB J 1991; 5: 2145–54.10.1096/fasebj.5.8.1850705Search in Google Scholar
4. Tyagi S, Kumar S, Glover G. Induction of tissue inhibitor and matrix metalloproteinases by serum in human heart-derived fibroblast and endomyocardial endothelial cells. J Cell Biochem 1995; 58: 360–71.10.1002/jcb.240580309Search in Google Scholar PubMed
5. Chakrabarti S, Patel KD. Matrix metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp Lung Res 2005; 31: 599–621.10.1080/019021490944232Search in Google Scholar PubMed
6. Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation 2006; 113: 2089–96.10.1161/CIRCULATIONAHA.105.573865Search in Google Scholar PubMed
7. Benjamin MM, Khalil RA. Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. EXS 2012; 103: 209–79.10.1007/978-3-0348-0364-9_7Search in Google Scholar PubMed PubMed Central
8. Gialeli C, Theocharis AD, Karamanos NK. Metalloproteinases in health and disease: roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 2010; 278: 16–27.10.1111/j.1742-4658.2010.07919.xSearch in Google Scholar PubMed
9. Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem 1997; 378: 151–60.Search in Google Scholar
10. Nagase H, Woessne JF Jr. Matrix metalloproteinases. J Biol Chem 1999; 274: 21491–4.10.1074/jbc.274.31.21491Search in Google Scholar PubMed
11. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 2006; 69: 562–73.10.1016/j.cardiores.2005.12.002Search in Google Scholar PubMed
12. Rodriguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim Biophys Acta 2010; 1803: 39–54.10.1016/j.bbamcr.2009.09.015Search in Google Scholar
13. Dwivedi A, Slater SC, George SJ. MMP-9 and -12 cause N-cadherin shedding and thereby beta-catenin signalling and vascular smooth muscle cell proliferation. Cardiovasc Res 2009; 81: 178–86.10.1093/cvr/cvn278Search in Google Scholar
14. Goldsmith EC, Carver W, McFadden A, Goldsmith JG, Price RL, Sussman M, Lorell BH, Cooper G, Borg TK. Integrin shedding as a mechanism of cellular adaptation during cardiac growth. Am J Physiol Heart Circ Physiol 2003; 284: H2227–34.10.1152/ajpheart.00920.2002Search in Google Scholar
15. Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JRB, Sawicki G, Schulz R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation 2002; 106: 1543–9.10.1161/01.CIR.0000028818.33488.7BSearch in Google Scholar
16. Moshal KS, Metreveli N, Frank I, Tyagi SC. Mitochondrial MMP activation, dysfunction and arrhythmogenesis in hyperhomocysteinemia. Curr Vasc Pharmacol 2008; 6: 84–92.10.2174/157016108783955301Search in Google Scholar
17. Moshal KS, Tipparaju SM, Vacek TP, Kumar M, Singh M, Frank IE, Patibandla PK, Tyagi N, Rai J, Metreveli N, Rodriguez WE, Tseng MT, Tyagi SC. Mitochondrial matrix metalloproteinase activation decreases myocyte contractility in hyperhomocysteinemia. Am J Physiol Heart Circ Physiol. 2008; 295: H890–7.10.1152/ajpheart.00099.2008Search in Google Scholar
18. Kwan JA, Schulze CJ, Wang W, Leon H, Sariahmetoglu M, Sung M, Sawicka J, Sims DE, Sawicki G, Schulz R. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J 2004; 18: 690–2.10.1096/fj.02-1202fjeSearch in Google Scholar
19. Si-Yayeb K, Monvoisin A, Mazzocco C, Lepreux S, Rosenbaum J. Unexpected localization of the matrix metalloproteinase-3 (MMP-3) within the cell nucleus in liver cancer cells. J Hepatology 2003; 38: 353.10.1016/S0168-8278(03)80622-0Search in Google Scholar
20. Si-Tayeb K, Monvoisin A, Mazzocco C, Lepreux S, Decossas M, Cubel G, Taras D, Blanc JF, Robinson DR, Rosenbaum J. Matrix metalloproteinase 3 is present in the cell nucleus and is involved in apoptosis. Am J Pathol 2006; 169: 1390–401.10.2353/ajpath.2006.060005Search in Google Scholar PubMed PubMed Central
21. Sung MM, Schulz CG, Wang W, Sawicki G, Bautista-López NL, Schulz R. Matrix metalloproteinase-2 degrades the cytoskeletal protein α-actinin in peroxynitrite mediated myocardial injury. J Mol Cell Cardiol 2007;43: 429–36.10.1016/j.yjmcc.2007.07.055Search in Google Scholar PubMed
22. Sawicki G, Leon H, Sawicka J, Sariahmetoglu M, Schulze CJ, Scott PG, Szczesna-Cordary D, Schulz R. Degradation of myosin light chain in isolated rat hearts subjected to ischemia-reperfusion injury: a new intracellular target for matrix metalloproteinase-2. Circulation 2005; 112: 544–52.10.1161/CIRCULATIONAHA.104.531616Search in Google Scholar PubMed
23. Doroszko A, Polewicz D, Cadete VJ, Sawicka J, Jones M, Szczesna-Cordary D, Cheung PY, Sawicki G. Neonatal asphyxia induces the nitration of cardiac myosin light chain 2 that is associated with cardiac systolic dysfunction. Shock 2010; 34: 592–600.10.1097/SHK.0b013e3181e14f1dSearch in Google Scholar PubMed PubMed Central
24. Ali MA, Cho WJ, Hudson B, Kassiri Z, Granzier H, Schulz R. Titin is a target of matrix metalloproteinase-2: implications in myocardial ischemia/reperfusion injury. Circulation 2010; 122: 2039–47.10.1161/CIRCULATIONAHA.109.930222Search in Google Scholar PubMed PubMed Central
25. Ali MA, Fan X, Schulz R.Cardiac sarcomeric proteins: novel intracellular targets of matrix metalloproteinase-2 in heart disease.Trends Cardiovasc Med 2011; 21: 112–8.10.1016/j.tcm.2012.03.008Search in Google Scholar PubMed
26. Hadler-Olsen E, Solli AI, Hafstad A, Winberg JO, Uhlin-Hansen L. Intracellular MMP-2 activity in skeletal muscle is associated with type II fibers. J Cell Physiol. 2015; 230: 160–9.10.1002/jcp.24694Search in Google Scholar PubMed
27. Ali MA, Chow AK, Kandasamy AD, Fan X, West LJ, Crawford BD, Simmen T, Schulz R. Mechanisms of cytosolic targeting of matrix metalloproteinase-2. J Cell Physiol 2012; 227: 3397–404.10.1002/jcp.24040Search in Google Scholar PubMed
28. Butler GS, Overall CM. Updated biological roles for matrix metalloproteinases and new “intracellular” substrates revealed by degradomics. Biochemistry 2009; 48: 10830–45.10.1021/bi901656fSearch in Google Scholar PubMed
29. Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 1993; 4: 197–250.10.1177/10454411930040020401Search in Google Scholar PubMed
30. Limaye AM, Desai KV, Chavalmane AK, Kondaiah P. Regulation of mRNAs encoding MMP-9 and MMP-2, and their inhibitors TIMP-1 and TIMP-2 by androgens in the rat ventral prostate. Mol Cell Endocrinol 2008; 294: 10–8.10.1016/j.mce.2008.07.003Search in Google Scholar PubMed
31. Delany AM, Jeffrey JJ, Rydziel S, Canalis E. Cortisol increases interstitial collagenase expression in osteoblasts by post-transcriptional mechanisms. J Biol Chem 1995; 270: 26607–12.10.1074/jbc.270.44.26607Search in Google Scholar PubMed
32. Vincenti MP. The matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) genes. Transcriptional and posttranscriptional regulation, signal transduction and cell-type-specific expression. Methods Mol Biol 2001; 151: 121–48.10.1385/1-59259-046-2:121Search in Google Scholar
33. Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem 2001; 276: 29596–602.10.1074/jbc.M102417200Search in Google Scholar
34. Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science 2002; 297: 1186–90.10.1126/science.1073634Search in Google Scholar
35. Sariahmetoglu M, Crawford BD, Leon H, Sawicka JLi L, Ballermann BJ, Holmes C, Berthiaume LG, Holt A, Sawicki G, Schulz R. Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J 2007; 21: 2486–95.10.1096/fj.06-7938comSearch in Google Scholar
36. Viappiani S, Nicolescu AC, Holt A, Sawicki G, Crawford BD, Leon H, van Mulligen T, Schulz R. Activation and modulation of 72 kDa matrix metalloproteinase-2 by peroxynitrite and glutathione. Biochem Pharmacol 2009; 77: 826–34.10.1016/j.bcp.2008.11.004Search in Google Scholar
37. Li YY, McTiernan CF, Feldman AM. Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res 2000; 46: 214–24.10.1016/S0008-6363(00)00003-1Search in Google Scholar
38. Chou J, Chan MF, Werb Z. Metalloproteinases: A functional pathway for myeloid cells. Microbiol Spectr 2016; 4: 10.10.1128/9781555819194.ch36Search in Google Scholar
39. Chintala SK, Sawaya R, Aggarwal BB, Majumder S, Giri DK, Kyritsis AP, Gokaslan ZL, Rao JS. Induction of matrix metalloproteinase-9 requires a polymerized actin cytoskeleton in human malignant gliomacells. J Biol Chem 1998; 273: 13545–51.10.1074/jbc.273.22.13545Search in Google Scholar PubMed
40. Sanka K, Maddala R, Epstein DL, Rao PV. Influence of actin cytoskeletal integrity on matrix metalloproteinase-2 activation in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci 2007; 48: 2105–14.10.1167/iovs.06-1089Search in Google Scholar PubMed
41. Tomasek JJ, Halliday NL, Updike DL, Ahern-Moore JS, Vu TK, Liu RW, Howard EW. Gelatinase A activation is regulated by the organization of the polymerized actin cytoskeleton. J Biol Chem 1997; 272: 7482–7.10.1074/jbc.272.11.7482Search in Google Scholar PubMed
42. MacDougall JR, Kerbel RS. Constitutive production of 92-kDa gelatinase B can be suppressed by alterations in cell shape. Exp Cell Res 1995; 218: 508–15.10.1006/excr.1995.1185Search in Google Scholar PubMed
43. Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion, and extracellular matrix degradation in invadopodia. Cancer Res 2007; 67: 4227–35.10.1158/0008-5472.CAN-06-3928Search in Google Scholar PubMed
44. Ispanovic E, Serio D, Haas TL. Cdc42 and RhoA have opposing roles in regulating membrane type 1-matrix metalloproteinase localization and matrix metalloproteinase-2 activation. Am J Physiol Cell Physiol 2008; 295: C600–10.10.1152/ajpcell.00460.2007Search in Google Scholar PubMed
45. Risinger GM, Jr., Hunt TS, Updike DL, Bullen EC, Howard EW. Matrix metalloproteinase-2 expression by vascular smooth muscle cells is mediated by both stimulatory and inhibitory signals in response to growth factors. J Biol Chem 2006; 281: 25915–25.10.1074/jbc.M513513200Search in Google Scholar PubMed
46. Arnoldi R, Hiltbrunner A, Dugina V, Tille JC, Chaponnier C. Smooth muscle actin isoforms: a tug of war between contraction and compliance. Eur J Cell Biol 2013; 92: 187–200.10.1016/j.ejcb.2013.06.002Search in Google Scholar PubMed
47. Howard EW, Crider BJ, Updike DL, Bullen EC, Parks EE, Haaksma CJ, Sherry DM, Tomasek JJ. MMP-2 expression by fibroblasts is suppressed by the myofibroblast phenotype. Exp Cell Res 2012; 318: 1542–53.10.1016/j.yexcr.2012.03.007Search in Google Scholar PubMed PubMed Central
48. Saga H, Kimura K, Hayashi K, Gotow T, Uchiyama Y, Momiyama T, Tadokoro S, Kawashima N, Jimbou A, Sobue K. Phenotype-dependent expression of alpha-smooth muscle actin in visceral smooth muscle cells. Exp Cell Res 1999;. 247: 279–92.10.1006/excr.1998.4339Search in Google Scholar PubMed
49. Driesen RB, Verheyen FK, Debie W, Blaauw E, Babiker FA, Cornelussen RN, Ausma J, Lenders MH, Borgers M, Chaponnier C, Ramaekers FC. Re-expression of alpha skeletal actin as a marker for dedifferentiation in cardiac pathologies. J Cell Mol Med 2009; 13: 896–908.10.1111/j.1582-4934.2008.00523.xSearch in Google Scholar PubMed PubMed Central
50. Bildyug N, Bozhokina E, Khaitlina S. Contribution of α-smooth muscle actin and extracellular matrix to the in vitro reorganization of cardiomyocyte contractile system. Cell Biol Int 2016; 40: 472–7.10.1002/cbin.10577Search in Google Scholar PubMed
51. Bildyug NB, Voronkina IV, Smagina LV, Yudintseva NM, Pinaev GP. Matrix Metalloproteinases in Primary Culture of Cardiomyocytes. Biochemistry (Mosc) 2015; 80: 1318–26.10.1134/S0006297915100132Search in Google Scholar PubMed
52. Krause M, Gautreau A. Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat Rev Mol Cell Biol 2014; 15: 577–90.10.1038/nrm3861Search in Google Scholar
53. Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004; 84: 767–801.10.1152/physrev.00041.2003Search in Google Scholar
54. Thyberg J. Differentiated properties and proliferation of arterial smooth muscle cells in culture. Int Rev Cytol 1996; 169: 183–265.10.1016/S0074-7696(08)61987-7Search in Google Scholar
55. Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 1995; 75: 487–517.10.1152/physrev.1995.75.3.487Search in Google Scholar
56. Kenagy RD, Hart CE, Stetler-Stevenson WG, Clowes AW. Primate smooth muscle cell migration from aortic explants is mediated by endogenous platelet-derived growth factor and basic fibroblast growth factor acting through matrix metalloproteinases 2 and 9. Circulation. 1997; 96: 3555–60.10.1161/01.CIR.96.10.3555Search in Google Scholar
57. Bendeck MP, Zempo N, Clowes AW, Galardy RE, Reidy MA. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res 1994; 75: 539–45.10.1161/01.RES.75.3.539Search in Google Scholar
58. Pauly RR, Passaniti A, Bilato C, Monticone R, Cheng L, Papadopoulos N, Gluzband YA, Smith L, Weinstein C, Lakatta EG, et al Migration of cultured vascular smooth muscle cells through a basement membrane barrier requires type IV collagenase activity and is inhibited by cellular differentiation. Circ Res. 1994; 75: 41–54.10.1161/01.RES.75.1.41Search in Google Scholar
59. Uzui H, Lee JD, Shimizu H, Tsutani H, Ueda T. The role of protein-tyrosine phosphorylation and gelatinase production in the migration and proliferation of smooth muscle cells. Atherosclerosis 2000; 149: 51–9.10.1016/S0021-9150(99)00295-6Search in Google Scholar
60. El Fahime E, Torrente Y, Caron NJ, Bresolin MD, Tremblay JP. In vivo migration of transplanted myoblasts requires matrix metalloproteinase activity. Exp Cell Res 2000; 258: 279–87.10.1006/excr.2000.4962Search in Google Scholar PubMed
61. Zhang Y, Li X, Li J, Hu H, Miao X, Song X, Yang W, Zeng Q, Mou L, Wang R. Human hemokinin-1 promotes migration of melanoma cells and increases MMP-2 and MT1-MMP expression by activating tumor cell NK1 receptors. Peptides 2016; 83: 8–15.10.1016/j.peptides.2016.07.004Search in Google Scholar PubMed
62. Ahn JH, Choi YS, Choi JH. Leptin promotes human endometriotic cell migration and invasion by up-regulating MMP-2 through the JAK2/STAT3 signaling pathway. Mol Hum Reprod 2015; 21: 792–802.10.1093/molehr/gav039Search in Google Scholar
63. Lee SK, Chun HK, Yang JY, Han DC, Son KH, Kwon BM. Inhibitory effect of obovatal on the migration and invasion of HT1080 cells via the inhibition of MMP-2. Bioorg Med Chem 2007; 15: 4085–90.10.1016/j.bmc.2007.03.081Search in Google Scholar
64. Ruzicka DL, Schwartz RJ Sequential activation of alpha-actin genes during avian cardiogenesis: vascular smooth muscle alpha-actin gene transcripts mark the onset of cardiomyocyte differentiation. J Cell Biol 1988; 107: 2575–86.10.1083/jcb.107.6.2575Search in Google Scholar
65. Bax NA, van Marion MH, Shah B, Goumans MJ, Bouten CV, van der Schaft DW. Matrix production and remodeling capacity of cardiomyocyte progenitor cells during in vitro differentiation. J Mol Cell Cardiol 2012; 53: 497–508.10.1016/j.yjmcc.2012.07.003Search in Google Scholar
66. Hong S, Kang JK, Park JJ, Ryu ES, Choi SS, Lee SH, Lee JH, Seo JS. Association of matrix metalloproteinase-3 with cardiogenic activity during Noggin-induced differentiation of mouse embryonic stem cells. Int J Cardiol 2010; 141: 49–60.10.1016/j.ijcard.2008.11.156Search in Google Scholar
67. Mannello F, Tonti GA, Bagnara GP, Papa S. Role and function of matrix metalloproteinases in the differentiation and biologicalcharacterization of mesenchymal stem cells. Stem Cells 2006; 24: 475–81.10.1634/stemcells.2005-0333Search in Google Scholar
68. Viita T, Vartiainen MK. From Cytoskeleton to Gene Expression: Actin in the Nucleus. Handb Exp Pharmacol 2016. [Epub ahead of print].10.1007/164_2016_27Search in Google Scholar
69. Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 2003; 113: 329–42.10.1016/S0092-8674(03)00278-2Search in Google Scholar
70. Ferrai C, Naum-Ongania G, Longobardi E, Palazzolo M, Disanza A, Diaz VM, Crippa MP, Scita G, Blasi F. Induction of HoxB transcription by retinoic acid requires actin polymerization. Mol Biol Cell 2009; 20: 3543–51.10.1091/mbc.e09-02-0114Search in Google Scholar PubMed PubMed Central
71. Huang W, Ghisletti S, Saijo K, Gandhi M, Aouadi M, Tesz GJ, Zhang DX, Yao J, Czech MP, Goode BL, Rosenfeld MG, Glass CK. Coronin 2A mediates actin-dependent de-repression of inflammatory response genes. Nature 2011; 470: 414–8.10.1038/nature09703Search in Google Scholar PubMed PubMed Central
72. Haller K, Rambaldi I, Daniels E, Featherstone M. Subcellular localization of multiple PREP2 isoforms is regulated by actin, tubulin, and nuclear export. J Biol Chem 2004; 279: 49384–94.10.1074/jbc.M406046200Search in Google Scholar
73. Favot L, Hall SM, Haworth SG, Kemp PR. Cytoplasmic YY1 is associated with increased smooth muscle-specific gene expression: implications for neonatal pulmonary hypertension. Am J Path 2005; 167: 1497–509.10.1016/S0002-9440(10)61236-9Search in Google Scholar
74. Németh ZH, Deitch EA, Davidson MT, Szabó C, Vizi ES, Haskó G. Disruption of the actin cytoskeleton results in nuclear factor-kappaB activation and inflammatory mediator production in cultured human intestinal epithelial cells. J Cell Physiol. 2004; 200: 71–81.10.1002/jcp.10477Search in Google Scholar
75. Petukhova OA, Turoverova LV, Kropacheva IV, Pinaev GP. Morphology of epidermoid carcinoma A431 cells spread on immobilized ligands. Tsitologiia 2004; 46: 5–15.Search in Google Scholar
76. Azorín E, Romero-Pérez B, Solano-Agama C, de la Vega MT, Toriz CG, Reyes-Márquez B, González-Pozos S, Rosales-García VH, Del Pliego MG,Sabanero M, Mendoza-Garrido ME. GH3 tumor pituitary cell cytoskeleton and plasma membrane arrangement are determined by extracellularmatrix proteins: implications on motility, proliferation and hormone secretion. Int J Physiol Pathophysiol Pharmacol 2014; 6: 66–83.Search in Google Scholar
77. Starke J, Wehrle-Haller B, Friedl P. Plasticity of the actin cytoskeleton in response to extracellular matrix nanostructure and dimensionality. Biochem Soc Trans 2014; 42: 1356–66.10.1042/BST20140139Search in Google Scholar
78. Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar Collagen Inhibits Arterial Smooth Muscle Proliferation Through Regulation of Cdk2 Inhibitors. Cell 1996; 87: 1069–78.10.1016/S0092-8674(00)81801-2Search in Google Scholar
79. Hilenski LL1, Terracio L, Sawyer R, Borg TK. Effects of extracellular matrix on cytoskeletal and myofibrillar organization in vitro. Scanning Microsc 1989; 3: 535–48.Search in Google Scholar
80. Bil’diug NB, Pinaev GP. Extracellular matrix dependence of the cardiomyocyte contractile apparatus organization. Tsitologiia 2013; 55: 713–24.Search in Google Scholar
81. Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol 1995; 11: 549–99.10.1146/annurev.cb.11.110195.003001Search in Google Scholar
82. Jalali S, del Pozo MA, Chen K, Miao H, Li Y, Schwartz MA, Shyy JY, Chien S. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci USA 2001; 98: 1042–6.10.1073/pnas.98.3.1042Search in Google Scholar
83. Wilson E, Sudhir K, Ives HE. Mechanical Strain of Rat Vascular Smooth Muscle Cells is Sensed by Specific Extracellular Matrix/ Integrin Interactions. J Clin Invest 1995; 96: 2364–72.10.1172/JCI118293Search in Google Scholar
84. Brooks PC, Strömblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell 1996; 85: 683–93.10.1016/S0092-8674(00)81235-0Search in Google Scholar
85. Maeda S, Dean DD, Gomez R, Schwartz Z, Boyan BD. The first stage of transforming growth factor beta1 activation is release of the large latent complex from the extracellular matrix of growth plate chondrocytes by matrix vesicle stromelysin-1 (MMP-3). Calcif Tissue Int 2002; 70: 54–65.10.1007/s002230010032Search in Google Scholar PubMed
86. Shannon DB, McKeown STW, Lundy FT, Irwin CR. Phenotypic differences between oral and skin fibroblasts in wound contraction and growth factor expression. Wound Rep Regen 2006; 14: 172–8.10.1111/j.1743-6109.2006.00107.xSearch in Google Scholar
87. Wang J, Zohar R, McCulloch CA. Multiple roles of alpha-smooth muscle actin in mechanotransduction. Exp Cell Res 2006; 312: 205–14.10.1016/j.yexcr.2005.11.004Search in Google Scholar
88. Kasper G, Glaeser JD, Geissler S, Ode A, Tuischer J, Matziolis G, Perka C, Duda GN. Matrix Metalloprotease activity is an essential link between mechanical stimulus and mesenchymal stem cell behavior. Stem Cells 2007; 25: 1985–94.10.1634/stemcells.2006-0676Search in Google Scholar
89. Fujisawa T, Hattori T, Takahashi K, Kuboki T, Yamashita A, Takigawa M. Cyclic mechanical stress induces extracellular matrix degradation in cultured chondrocytes via gene expression of matrix metalloproteinases and interleukin-1. J Biochem 1999; 125: 966–75.10.1093/oxfordjournals.jbchem.a022376Search in Google Scholar
90. Sun HB, Yokota H. Altered mRNA level of metalloproteinase-13 in MH7A synovial cells under mechanical loading and unloading. Bone 2001; 28: 399–403.10.1016/S8756-3282(00)00459-2Search in Google Scholar
91. Sun HB, Yokota H. Reduction of cytokine-induced expression and activity of MMP-1 and MMP-13 by mechanical strain in MH7A rheumatoid synovial cells. Matrix biol 2002; 21: 263–7.10.1016/S0945-053X(02)00003-3Search in Google Scholar
92. Asanuma K, Magid R, Johnson C, Nerem RM, Galis ZS. Uniaxial strain upregulates matrix-degrading enzymes produced by human vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol 2003; 284: H1778–84.10.1152/ajpheart.00494.2002Search in Google Scholar PubMed
93. Yang G, Im HJ, Wang JH. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene 2005; 363: 166–72.10.1016/j.gene.2005.08.006Search in Google Scholar PubMed PubMed Central
94. Sasaki K, Takagi M, Konttinen YT, Sasaki A, Tamaki Y, Ogino T, Santavirta S, Salo J. Upregulation of matrix metalloproteinase (MMP)-1 and its activator MMP-3 of human osteoblast by uniaxial cyclic stimulation. J Biomed Mater Res B Appl Biomater 2007; 80: 491–8.10.1002/jbm.b.30622Search in Google Scholar PubMed
95. Adiguzel E, Hou G, Sabatini PJ, Bendeck MP. Type VIII collagen signals via β1-integrin and RhoA to regulate MMP-2 expression and smooth muscle cell migration. Matrix Biol 2013; 32: 332–41.10.1016/j.matbio.2013.03.004Search in Google Scholar PubMed
©2016 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Reviews
- Coupling de novo protein folding with subunit exchange into pre-formed oligomeric protein complexes: the ‘heritable template’ hypothesis
- Role of non-motile microtubule-associated proteins in virus trafficking
- Advanced glycation end products mediated cellular and molecular events in the pathology of diabetic nephropathy
- Short Conceptual Overviews
- The microRNA-200 family: still much to discover
- Matrix metalloproteinases: an emerging role in regulation of actin microfilament system
- Unusual structures of CCTG repeats and their participation in repeat expansion
Articles in the same Issue
- Frontmatter
- Reviews
- Coupling de novo protein folding with subunit exchange into pre-formed oligomeric protein complexes: the ‘heritable template’ hypothesis
- Role of non-motile microtubule-associated proteins in virus trafficking
- Advanced glycation end products mediated cellular and molecular events in the pathology of diabetic nephropathy
- Short Conceptual Overviews
- The microRNA-200 family: still much to discover
- Matrix metalloproteinases: an emerging role in regulation of actin microfilament system
- Unusual structures of CCTG repeats and their participation in repeat expansion

