The attenuation of pain behaviour and serum interleukin-6 concentration by nimesulide in a rat model of neuropathic pain
-
Taraneh Moini Zanjani
, Masoumeh Sabetkasaei
Abstract
Background
Evidence for a role of immune system in hyperalgesic pain states is increasing. Recent work in neuroimmunology suggests that the immune system does more than simply perform its well known functions of recognizing and removing invading pathogens and tumors. Interest in neuroinflammation and neuroimmune activation has grown rapidly in recent years with the recognition of the role of central nervous system inflammatiom and immune responses in the aetiology of pain states. Among various theories, the role of inflammatory responses of the injured nerve has recently received attention. Cytokines are heterogenous group of polypeptides that activate the immune system and mediate inflammatory responses, acting on a variety of tissue, including the peripheral and central nervous system. Interleukin-6 (IL-6) a pro-inflammatory cytokine, is potentially important in pain aetiology, have pronociceptive actions. Neuropathic pain may be due to a primary insult to the peripheral or central nervous system. Substances released during inflammation from immune cells play an important role in the development and maintenance of chronic pain. Nimesulide, a highly selective cox-2 inhibitor, effectively reduces hyperalgesia due to peripherally administration of inflammatory agents like formalin. The safety of nimesulide was reported for some conditions in which other NSAIDs are contraindicated. Here we have determined the effect of nimesulide on pain behaviour and serum IL-6 level in chronic constriction injury (CCI) model of neuropathic pain.
Methods
Experiments were carried out on male Wistar rats, (weight 150–200 g, n = 8). Rats were divided into 3 different groups: 1-CCI + saline 0.9% 2Sham + saline 0.9% (control) 3CCI + drug. Nimesulide (1.25, 2.5, 5 mg/kg, i.p.) was injected 1h before surgery and continued daily to day 14 post-ligation. 42 °C water for thermal hyperalgesia, von Frey filaments for mechanical allodynia, acetone test for cool allodynia and 10 °C water for cold hyperalgesia were respectively used as pain behavioural tests. Behavioural tests were recorded before surgery and on postoperative days 1, 3, 5, 7, 10, 14 and the serum concentration of IL-6 was determined at the day 14.
Results
The results of this study showed a decrease in hyperalgesia and allodynia following nimesulide administration.
Conclusions
It appears that nimesulide was able to reduce pain behaviour due to nerve inflammation and a parallel decrease in the serum IL-6 concentration was observed.
Implications
The immune system is an important mediator in the cascade of events that ultimately results in hyperalgesia. Cytokines contribute to the patheogenesis of neuropathic pain, therefore drugs that inhibit cytokine release from immune cells may reduce inflammatory pain states.
1 Introduction
There is increasing evidence for a role of the immune system in hyperalgesic pain states. The immune system is an important mediator in the cascade of events that ultimately results in hyperalgesia. Among several theories, the role of inflammatory responses in the injured nerve has recently received attention [1,2,3]. Recent work in neuroimmunology suggests that the immune system does more than simply perform its well-known functions of recognizing and removing invading pathogens and tumours [4,5]. The current literature strongly suggests a role for pro-inflammatory cytokines in the genesis or maintenance of pain states [5,6]. Cytokines are a heterogeneous group of polypeptides that activate the immune system and mediate inflammatory responses, acting on a variety of tissues, including the peripheral and central nervous system [7,8]. Interleukin-6 (IL-6), a pro-inflammatory cytokine which is potentially important in pain aetiology, has been implicated in neuropathic pain manifested as spontaneous pain, hyperalgesia and allodynia, with multiple aetiologies [6,9,10]. The chronic constriction injury (CCI) model, in which four loose ligatures are applied to the rat sciatic nerve, produces hyperalgesia that is similar to that seen in painful human peripheral neuropathies [11]. Greater hyperalgesia is produced by chromic than by silk sutures [12]. These observations suggest that inflammation in and around the nerve appears to be a critical mechanism of hyperalgesia in the CCI model and cytokines may play an important role [13]. Intrathecal anti-IL-6 antibody attenuated this reaction suggesting a role for IL-6 in the development of pain [14]. Plasma levels of IL-6 also increase following peripheral nerve injury [15,16]. It has been reported that central or peripheral injection of IL-6 increases the concentration of prostaglandin E2 (PG E2) in the cerebrospinal fluid (CSF), and this increase is prevented by inhibition of cyclooxygenase [17,18]. Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most commonly used drugs world-wide to treat pain and inflammation. However, just when and where NSAIDs exert their anti-hyperalgesic actions has not been completely defined [19]. Nimesulide, a non-steroidal anti-inflammatory drug, possesses the ability to reduce chronic inflammatory pain via multiple mechanisms including preferential inhibition of prostaglandin (PG) synthesis via cyclooxygenase-2 (COX-2), reduction in cytokine action or release, and other effects [20,21]. Nimesulide reduced inflammatory pain produced by formalin injection in rats [22].
The present study was designed to evaluate the inflammatory response associated with sciatic nerve injury under the influence of nimesulide. Here, we have determined the effect of nimesulide on hyperalgesia, allodynia and the serum level of IL-6 in the CCI model of neuropathic pain.
2 Materials and methods
2.1 Animals
Experiments were carried out on male Wistar rats (weight 150–200 g) that were housed one rat per cage and placed under a 12 h light/dark cycle in a temperature-controlled room (22±1 °C). Animals had free access to food and water. Rats were divided randomly into several experimental groups, each made-up of eight animals. All experiments followed the IASP guidelines on ethical standards for investigation of experimental pain in animals [23]. The animals were allowed to habituate to the housing facilities for 1 week before the experiments began. Behavioural studies were performed in a quiet room between the hours of 9:00 and 11:00 am. Efforts were made to limit distress and use the minimum number of animals necessary to achieve statistical significance.
2.2 Surgery
We used the CCI model of neuropathic pain [11]. The surgical procedure was performed under ketamine (60 mg/kg) and xylazine (10 mg/kg) anaesthesia. The left sciatic nerve was exposed and four loose chromic gut ligatures were placed around the nerve proximal to the trifurcation. The distance between the two adjacent ligatures was 1 mm. The wound was irrigated with saline (0.9%) and closed in two layers with 4-0 silk (fascial plane) and surgical skin staples. In the saline-treated sham group, rats underwent the same surgical procedure except for the ligation.
2.3 Drug preparation
Nimesulide (Sigma, U.S.A.) was dissolved in saline 0.9% and prepared as suspension. Ketamine hydrochloride (Sigma, U.S.A.) and xylazine hydrochloride (Sigma, U.S.A.) were used for anaesthesia. All drugs were injected by the intra-peritoneal (i.p.) route.
2.4 Drug administration
Animals were randomly divided into three experimental groups: (1) saline-treated CCI group, (2) saline-treated Sham group, and (3) drug-treated CCI group. Taking into account the pharmacokinetics of nimesulide [24], nimesulide 1.25, 2.5 and 5 mg/kg [22] were injected 1 h before surgery and continued daily to day 14 post-ligation. All behavioural tests were recorded on day 0 (control day) before the surgery and on days 1, 3, 5, 7, 10, and 14 post-nerve injury. The order of pain testing was thermal hyperalgesia, mechanical allodynia, cool allodynia and cold hyperalgesia (the interval between each test was 30 min). On day 14, rats were euthanized by CO2 asphyxiation, then they were rapidly guillotined and the blood was collected for serum evaluation of IL-6 [25].
2.5 Behavioral tests and experimental design
The sciatic nerve territory (mid-plantar hind paw) was tested for sensitivity to noxious and innocuous stimuli using standard behavioural assays done sequentially at several intervals up to 14 days following surgery. Animals were acclimated to the testing chambers for 30 min prior to testing. Hyperalgesia (a decreased threshold for noxious stimuli) and allodynia (a heightened response to normally non-noxious stimuli), were evaluated in the animals.
2.6 Thermal hyperalgesia
We used the paw immersion test (hot bath) to assess the sensitivity to a heat stimulus, which consisted of the immersion of the paw in a 42 °C water bath [26]. We recorded the latency of withdrawal for each paw. Three measurements with an interval of 5 min between them were made for each hind paw, and the mean was calculated. The paw withdrawal latency (PWL) was obtained by subtracting the latency of the contralateral unaffected hind paw from the ipsilateral experimental paw. A cut-off time of 15 s was used to avoid tissue damage. Negative difference scores are indicative of thermal hyperalgesia [11,26,27,28].
2.7 Mechanical allodynia
Mechanical sensitivity to non-noxious stimuli was measured by applying a set of calibrated nylon monofilaments (Stoelting, U.S.A.). The von Frey methodology was used to assess the sensitivity of the skin to tactile stimulation. von Frey filaments are calibrated to have a characteristic bending force when pressure is applied. Each rat was placed under a transparent Plexiglas cage on an elevated metal screen surface with 1 cm mesh openings. Increasing strengths of von Frey filaments were applied sequentially to the plantar surface of the left hind paw of each animal. The minimum paw withdrawal threshold (PWT), defined as the minimum gram strength eliciting two sequential responses with 3 min intervals between them (withdrawal from pressure), was recorded for the left paw. The intensity of mechanical stimulation was increased from 2 to 60 g in a graded manner using successively greater diameter filaments until the hind paw was withdrawn. For successive tests, the placement of these stimuli was varied slightly from one trial to the next to avoid sensitization of the hind paw [29].
2.8 Cool allodynia
The acetone test, a method slightly modified from Choi et al. [30], was used to determine the reactivity to an acetone stimulus. Rats were placed under a transparent Plexiglas cage, as described previously, and an acetone bubble was formed at the end of a piece of small polyethylene tubing that was connected to a syringe; then, the bubble was lightly touched to the heel. The acetone was applied 5 times with an interval of 1 min between application, and the number of paw lifts from surface was the response measured. The response was calculated as the percent of paw withdrawal frequency (% PWF) using the following equation: (number of paw withdrawals/5 trials) × 100 [29].
2.9 Cold hyperalgesia
The paw immersion test (cold bath) was used to test cold hyperalgesia. Using a method similar to the hot bath (see above), the paw was immersed in a 10 °C water bath, and the latency of withdrawal was recorded. A cut-off time of 15 s was used to avoid tissue damage [27].
2.10 IL-6 protein analysis by ELISA
Cytokine levels in the serum of rats were measured using a commercially available ELISA specific for IL-6 (Biosource International, England) with a lower detection limit of <8 pg/ml. Blood samples from the different groups were centrifuged at 2500 rpm for 20 min, and the serum was collected and frozen at −70 °C. The analysis of cytokine protein expression was done according to the manufacturer’s instructions. The rat IL-6 kit is a solid phase Sandwich Enzyme-linked-Immuno-Sorbent-Assay (ELISA). An antibody specific for rat IL-6 has been coated onto the wells of the microtiter strips provided. Samples, including standards of known rat IL-6 concentration, control specimens, and unknowns, were pipetted into these wells. During the first incubation, the rat interleukin-6 antigen binds to the immobilized (capture) antibody on one site. After washing, a biotinylated antibody specific for rat IL-6 was added. During the second incubation, this antibody binds to the immobilized rat IL-6 captured during the first incubation. After removal of excess secondary antibody, Streptavidin-Peroxidase (enzyme) was added. This enzyme binds to the biotinylated antibody and completes the four-member sandwich. After a third incubation and washing to remove all of the unbound enzyme, a substrate solution that the bound enzyme acts upon to produce colour was added. The intensity of this coloured product is directly proportional to the concentration of rat IL-6 present in the original specimen. Then, the plates were read using a microplate reader at 450 nm. The cytokine protein concentration was obtained from a standard curve (Fig. 2A).
2.11 Statistical analysis
Parametric data were analysed for significance using an analysis of variance (ANOVA) followed by a post-hoc Tukey’s test. Non-parametric data were analysed using two related samples followed by the Wilcoxon test. In all cases, P < 0.05 was considered significant.
3 Results
All the animals experienced normal weight gain over the course of the study. Different stimuli were tested over a 14-day time frame and included the measurement of thermal, mechanical, cool allodynia and cold stimuli.
3.1 Response to thermal hyperalgesia
In the paw immersion test (hot bath), while the saline-treated sham group did not exhibit pain behaviour during the 14 days of the study compared to the pre-ligation day, all saline-treated CCI groups exhibited confirmed heat hyperalgesia at the third day post-ligation (P < 0.05) compared to the pre-surgery control day. This hyperalgesia was sustained throughout the rest of the experimental period. In the drug-treated CCI group, nimesulide produced an anti-hyperalgesic effect at the 2.5 and 5 mg/kg doses, but not at the 1.25 g/kg dose (P < 0.05) (Fig. 1A).
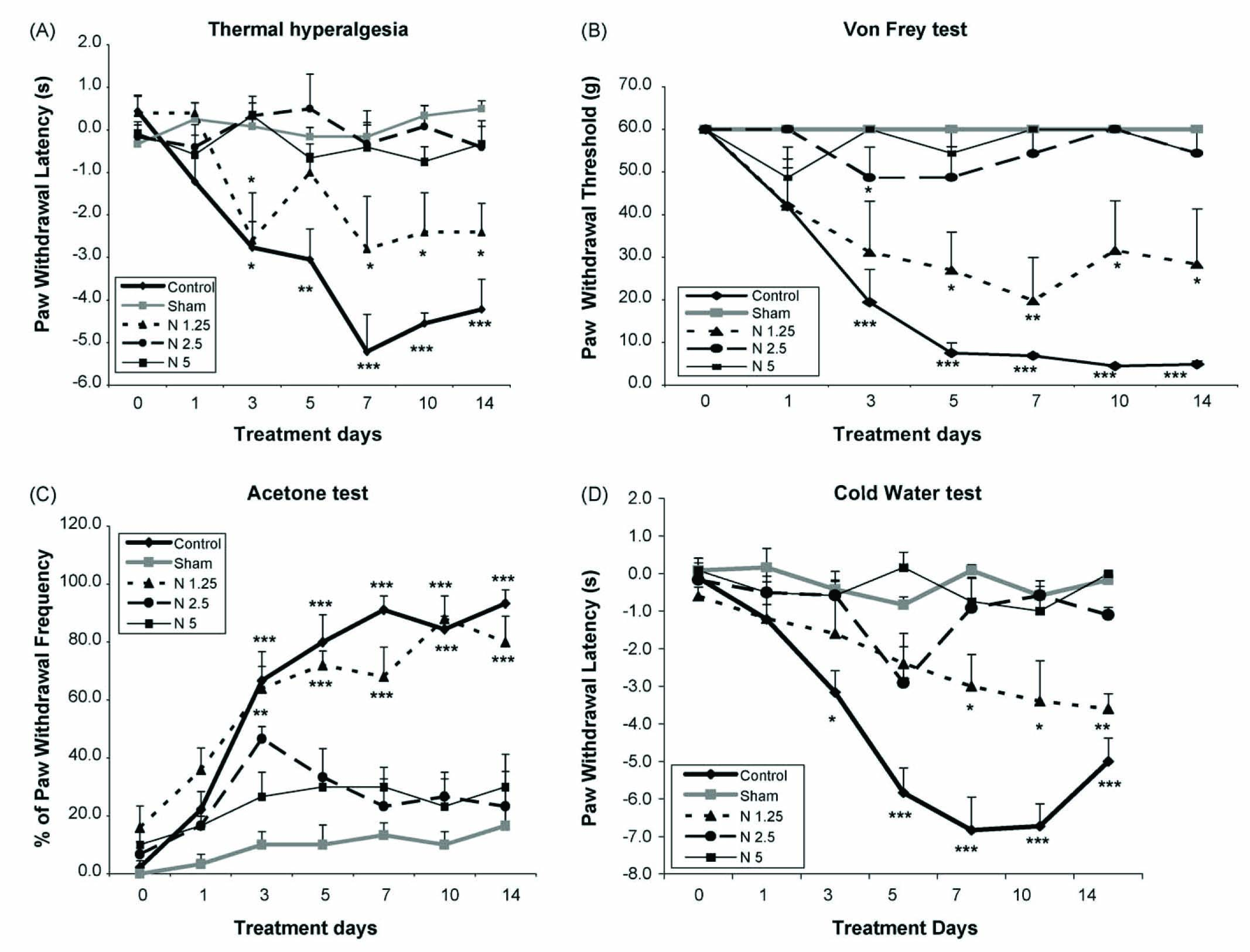
(A) The latency of paw withdrawal (in seconds) in response to 42 °C hot water bath before and at several time points after surgery in saline-treated CCI group, saline-treated sham group and drug-treated CCI group. Nimesulide (1.25, 2.5 and 5 mg/kg) was injected i.p. Data are presented as mean ± S.E.M. of 6–8 rats in each group. Asterisks (*P < 0.05; **P < 0.01; ***P < 0.001) indicate a statistically significant difference when compared to day 0 before surgery. (B) Paw withdrawal threshold in response to von Frey filaments before and at several time points after surgery in saline-treated CCI group, saline-treated sham group and drug-treated CCI group. Nimesulide (1.25, 2.5 and 5 mg/kg) was injected i.p. Data are presented as means ± S.E.M. of 6–8 rats in each group. Asterisks (***P < 0.001) indicate a statistically significant difference when compared to day 0 before surgery. (C) The frequency of paw withdrawal in response to acetone before and at several time points after surgery in saline-treated CCI group, saline-treated sham group and drug-treated CCI group. Nimesulide (1.25, 2.5 and 5 mg/kg) was injected i.p. Data are presented as means ± S.E.M. of 6–8 rats in each group. Asterisks (*P < 0.05; ***P < 0.001) indicate a statistically significant difference when compared to day 0 before surgery. (D) The latency of paw withdrawal (in seconds) in response to 10 °C cold water bath before and at several time points after surgery in saline-treated CCI group, saline-treated sham group and drug-treated CCI group. Nimesulide (1.25, 2.5 and 5 mg/kg) was injected i.p. Data are presented as means ± S.E.M. of 6–8 rats in each group. Asterisks (**P < 0.01; ***P < 0.001) indicate a statistically significant difference when compared to day 0 before surgery.
3.2 Response to mechanical allodynia
In the von Frey test, all saline-treated CCI groups were strongly allodynic at the third day post-ligation (P < 0.001) compared to the control day; this effect was sustained until the end of the study. In contrast, the saline-treated sham group did not show any mechanical allodynia for the duration of the experimental period as compared to the pre-surgery day. During the period of the study, in the drug-treated CCI group, nimesulide produced a tactile antihypersensitivity effect at the 2.5 and 5 mg/kg doses, but not at the 1.25 mg/kg dose (P < 0.05) (Fig. 1B).
3.3 Response to cool allodynia
Acetone test: In the saline-treated CCI group, a significant difference in pain behaviour (P < 0.001) was seen at the third day post-injury compared to day 0; this effect continued until the end of the study. However, cool allodynia was not observed in the saline-treated sham group. In the drug-treated CCI group, nimesulide produced an anti-allodynic effect at the 2.5 and 5 mg/kg doses but not at the of 1.25 mg/kg dose (P < 0.01) (Fig. 1C).
3.4 Response to cold hyperalgesia
In the paw immersion test, cold hyperalgesia developed at 5 days post-ligation in the saline-treated CCI group (P < 0.001) compared to the control day, an effect which persisted for the duration of the study. However, the saline-treated sham group did not exhibit pain behaviour at any point in the study. In the drug-treated CCI group, nimesulide produced an anti-hyperalgesic effect at the 2.5 and 5 mg/kg doses but not at the 1.25 mg/kg dose (P < 0.05) (Fig. 1D).
3.5 Cytokine protein analysis
Protein analysis (by ELISA) revealed that the IL-6 serum level was increased in the saline-treated CCI group compared to the saline-treated sham group (P < 0.001). Pre-treatment with nimesulide 1.25, 2.5 and 5 mg/kg attenuated the production of IL-6 in the drug-treated CCI group (P < 0.001) compared to the saline-treated CCI group (Fig. 2B).
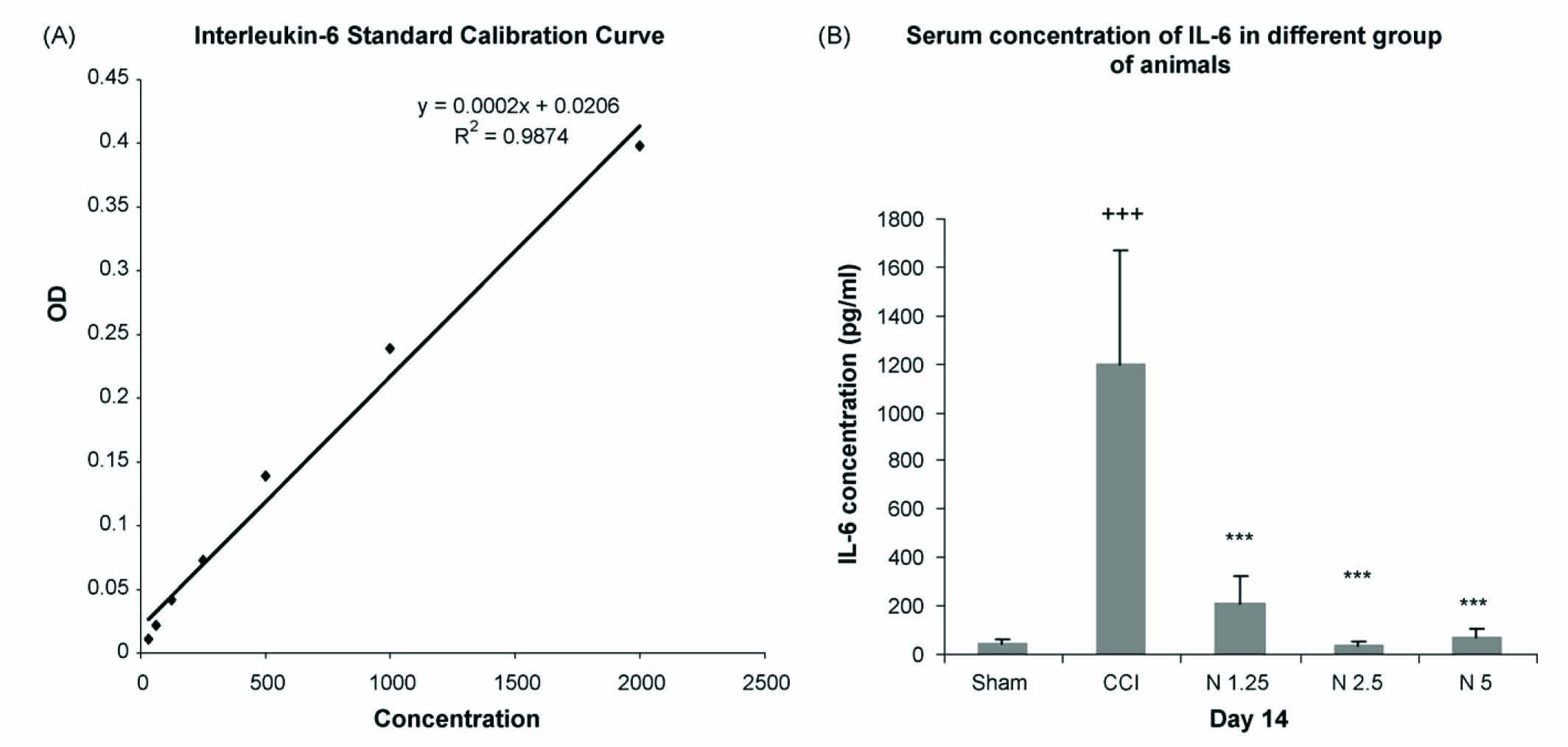
(A) Interleukin-6 standard calibration curve for serum evaluation of IL-6 in different groups of animals. (B) Serum concentration of IL-6 in saline-treated CCI group, saline-treated sham group and drug-treated CCI group on day 14 post-ligation. Data are presented as means ± S.E.M. of 6–8 rats in each group. Asterisks (+++P < 0.001) indicate a statistically significant difference between saline-treated CCI group compared to saline-treated sham group and (***P < 0.001) indicate a statistically significant difference between drug-treated CCI group compared to saline-treated CCI group. N1.25 = nimesulide 1.25 mg/kg, N2.5 = nimesulide 2.5 mg/kg, N5= nimesulide 5 mg/kg.
4 Discussion
The aim of the present study was to determine the antiinflammatory and analgesic effects of nimesulide in neuropathic pain in the rat. We evaluated the serum levels of IL-6 because it is an important pro-inflammatory cytokine released during inflammatory pain. The mechanisms of neuropathic pain are not well understood, and there is no satisfactory treatment for this pain. Prostanoids have been regarded as the main mediator in the maintenance of inflammation and pain [31]. Evidence for a spinal site of prostanoid action in some pain states has been demonstrated by the fact that spinal administration of PG E2 to mice produces hyperalgesia and that intrathecal administration of NSAIDs is antinociceptive [32,33]. Prostanoids synthesized in the spinal cord in response to noxious stimuli, are known to facilitate nociceptive transmission, and disrupting their production prevents allodynia and hyperalgesia, raising questions about their role in neuropathic pain [34]. The analgesic action of NSAIDs has been explained on the basis of the peripheral inhibition of prostaglandin synthesis via the blockage of cyclooxygenase and the prevention of bradykinin and cytokineinduced hyperalgesia [35,36]. It appears that NSAIDs exert their analgesic effect through a variety of other peripheral and central mechanisms. The localization of COX-2 in the brain provides an additional central mechanisms of action for several NSAIDs, in particular for those that have a preferential inhibitory effect upon COX-2 such as nimesulide [36,37]. Nimesulide has been reported to be safe for some conditions in which other NSAIDs are contraindicated [21,38]. Nimesulide completely inhibited the development of thermal hind paw hyperalgesia induced by peripheral injection of formalin and showed a significant analgesic effect in the tail flick test [22,36]. In another study, nimesulide and diclofenac were significantly more effective than celecoxib and rofecoxib in reducing the mechanical hind paw hyperalgesia induced by the intraplantar injection of Freund’s Complete Adjuvant (FCA) [22]. In rats undergoing partial transection of the sciatic nerve, subcutaneous injection of indomethacin, meloxicam, and an EP1 prostaglandin receptor blocker reduced mechanical hypersensitivity [39]. Taking these data into account, in the present study, we have determined the effect of nimesulide on nerve injury-induced neuropathic pain with an inflammatory component. Our data showed that nimesulide prevented hyperalgesia and allodynia due to sciatic nerve ligation. These data are consistent with the above-mentioned reports. In neuropathic pain models and in model of inflammatory pain, pro-inflammatory cytokines (IL-1, IL-6 and TNFα) and other endogenous hyperalgesic mediators, including nerve growth factor, bradykinin and PGs, are produced and are thought to influence nociception or pain [4,40,41]. Thermal hyperalgesia has been reported when these cytokines are injected into the rat paw [10]. IL-6, a mediator in the cascade of pain, is secreted by a wide range of cells including immune cells, fibroblasts, endothelial cells, and neurons [10,1]. In addition, it has been shown that neurons of the spinal cord produce IL-6 mRNA in response to peripheral nerve injury resulting in neuropathic pain behaviours, and mechanical allodynia has been correlated with levels of IL-6 in the sciatic nerve. The importance of IL-6 in nociception has been further demonstrated with IL-6 knock-out mice which do not develop heat and pressurehypersensitivity in response to a chronic constriction injury [18]. It is possible that cytokines trigger the release of directly acting algogenic compounds such as PGs and bradykinin [35,40]. Clinically, IL-6 may have a role in nerve injury and pain, as patients with persistent sciatic nerve pain display elevated blood levels of IL-6 [15]. In chronic inflammatory pain, nimesulide and celecoxib treatment have produced reductions in IL-6 in synovial fluid of osteoarthritis patients [42]. In our study, we found that in the presence of nimesulide, the serum concentration of IL-6 declined in the drug-treated CCI group compared to saline-treated CCI animals. Although all doses of nimesulide were able to reduce the IL-6 serum concentration, analgesia was not observed for the low dose. It seems that immune cells producing IL-6 are sensitive even to the low dose of nimesulide, while a suitable analgesic dose of nimesulide is required to reduce allodynia.
In conclusion, our results showed that nimesulide could attenuate pain behaviours in the CCI model of neuropathic pain. It appears that the anti-hyperalgesic and anti-allodynic effects of nimesulide may be due, in part, to the prevention of IL-6 production following sciatic nerve injury. This reduction in cytokine action by nimesulide seems to be unique among non-steroidal anti-inflammatory drugs (NSAIDs) [38,21]. To understand the role of IL-6, and considering the fact that pro-inflammatory cytokines are mediators involved in the early stage of inflammation after nerve injury, further studies are needed to determine the level of IL-6 immunoreactivity or mRNA in the sciatic nerve and in the immune cells of the peripheral and central nervous system under the influence of nimesulide during neuropathic pain.
DOI of refers to article: 10.1016/j.sjpain.2010.09.002.
Acknowledgements
This research was supported by a grant provided by the Neuroscience Research Centre of Shahid Beheshti University of Medical Sciences. The authors thank the staff of the Department of Pharmacology for their kind collaboration.
References
[1] Marchand MP, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci 2005;6:521–32.Search in Google Scholar
[2] Strouse TB. The relationship between cytokines and pain/depression: a review and current status. Curr Pain Headache Rep 2007;11:98–103.Search in Google Scholar
[3] Hansson E. Long-term pain, neuroinflammation and glial activation. Scan J Pain 2010;1:67–72.Search in Google Scholar
[4] Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev 2006;51(2):240–64.Search in Google Scholar
[5] Watkins LR, Hutchinson MR, Milligan ED, Maier SF. & ldquo;Listening” and “talking” to neurons: implication of immune activation for pain control and increasing the efficacy of opioids. Brain Res Rev 2007;56(1):148–69.Search in Google Scholar
[6] Sweitzer SM, Martin D, Deleo JA. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience 2001;103:529–39.Search in Google Scholar
[7] Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci 2008;28:5189–94.Search in Google Scholar
[8] Uceyler N, Sommer C. Cytokine regulation in animal models of neuropathic pain and in human diseases. Neurosci Lett 2008;6;437(3):194–8.Search in Google Scholar
[9] Deleo JA, Yezierski RP. The role of neuroimmune activation in persistent pain. Pain 2001;90:1–6.Search in Google Scholar
[10] De Jongh RF, Vissers KC, Meert TF, Booij Leo HDJ, De Deyne CS, Heylen RJ. The role of interleukin-6 in nociception and pain. Anesth Analg 2003;96:1099–103.Search in Google Scholar
[11] Bennett GJ, Xie YKA. Peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in men. Pain 1988;33:87–107.Search in Google Scholar
[12] Maves TJ, Pechman PS, Gebhart GF, Meller ST. Possible chemical contribution from chromic gut sutures produces disorders of pain sensation like those seem in man. Pain 1993;54:57–69.Search in Google Scholar
[13] Wieseler-Frank J, Maier SF, Watkins LR. Central proinflammatory cutokines and pain enhancement. Neurosignals 2005;14(4):166–74.Search in Google Scholar
[14] Zhang J-H, Hang Y-G. The immune system: a new look at pain. Chin Med 2006;11(11):930–8.Search in Google Scholar
[15] Flatters SJL, Alyson JF, Dickenson AH. Nerve injury alters the effect of interleukin-6 on nociceptive transmission in peripheral afferents. Eur J Pharmacol 2004;484:183–91.Search in Google Scholar
[16] Moini Zanjani T, Sabetkasaei M, Mosaffa N, Manaheji H, Labibi F, Farokhi B. Suppression of interleukin-6 by minocycline in a rat model of neuropathic pain. Eur J Pharmacol 2006;538(1–3):66–72.Search in Google Scholar
[17] Ma W, Quirion R. Targeting invading macrophage-derived PGE2, IL-6 and calcitonin gene-related peptide in injured nerve to treat neuropathic pain. Expert Opin Ther Targets 2006;10(4):533–46.Search in Google Scholar
[18] Thacker MA, Clark AK, Marchand F, McMahon SB. Pathophisiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg 2007;105:838–47.Search in Google Scholar
[19] Kalgutkar AS, Zhao Z. Discovery and design of selectivecyclooxygenase-2 inhibitors as non-ulcerogenic anti-inflammatory drug with potential utility as anti-cancer agents. Curr Drug Targets 2001;2:79–106.Search in Google Scholar
[20] Bennett A, Villa G. Nimesulide: an NSAID that preferentially inhibits Cox-2 and has various unique pharmacological activities. Expert Opin Pharmacother 2000;1(2):277–8.Search in Google Scholar
[21] Leong Wai F, Vishvanatha M, Suchitra AD, Manoj K, Vikram S, Asif P, Garima S, Asma P, Shoukath A. Nimesulide vs other NSAIDs: efficacy and safety. CMP Medica India Pvt. Ltd.; 2009.Search in Google Scholar
[22] Bianchi M, Broggini M. Anti-hyperalgesic effects of nimesulide: study in rats and humans. Int J Clin Pract 2002;128:11–9.Search in Google Scholar
[23] Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983;16:109–10.Search in Google Scholar
[24] Rainsford KD. Nimesulide actions and uses. Switzelland: Birkhauser Verlag; 2005. p. 66–70.Search in Google Scholar
[25] Raghavendra V, Tanga F, Deleo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther 2003;306:624–30.Search in Google Scholar
[26] Seltzer Z, Dubner R, Shir YA. Novel behavioral model of neuropathic pain disorders in rats by partial sciatic nerve injury. Pain 1990;43:205–18.Search in Google Scholar
[27] Attal N, Jazal F, Kayser V, Guilbaud G. Further evidence for “pain related” behaviors in a model of unilateral peripheral mononeuropathy. Pain 1990;41:235–51.Search in Google Scholar
[28] Boivie J, Hansson P, Lindblom U, Touch. Temperature and assessment, progress in pain research and management. Seatle: IASP Press; 1994. p. 325–9.Search in Google Scholar
[29] Stuesse SL, Grisp T, McBurney DL, Schechter JB, Lovell JA, Cruce WL. Neuropathic pain in aged rats: behavioral responses and astrocytic activation. Exp Brain Res 2001;137:219–27.Search in Google Scholar
[30] Choi Y, Yoon YW, Na HS, Kim SH, Chung SM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 1994;59:369–76.Search in Google Scholar
[31] Weiya M, Eisenach JC. Morphological and pharmacological evidence for the role of peripheral prostaglandins in the pathogenesis of neuropathic pain. Eur J Neurosci 2002;15:1037–47.Search in Google Scholar
[32] Ma W, Quirion R. Does COX2-dependent PGE2 play a role in neuropathic pain? Neurosci Lett 2008;437(3(6)):165–9.Search in Google Scholar
[33] Thungo V, Rice Andrew SC, Robert DH. Non steroidal anti-inflammatory drugs for neuropathic pain: how do we explain continued widespread use? Pain 2009;143:169–71.Search in Google Scholar
[34] Hefferan MP, O’Rielly DD, Loomis CW. Inhibition of prostaglandins synthesis early after L5/L6 nerve ligation prevents the development of prostaglandin-dependent and prostaglandin-independent allodynia in the rat. Anesthesiology 2003;99(5):1180–5.Search in Google Scholar
[35] Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, poole S, Bonventre JV, Woolf CJ. Interleukin-1β-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 2001;410:471–5.Search in Google Scholar
[36] Tassorelli C, Rosaria G, Sandrini G, Nappi G. Central components of the analgesic/antihyperalgesic effect of nimesulide: studies in animal models of pain and hyperalgesia. Drugs 2003;63(1):9–22.Search in Google Scholar
[37] Ghilardi JR, Svensson CL, Roger SD, Yaksh TL, Manthy PW. Constitutive spinal cyclooxygenase-2 participates in the initiation of tissue injury-induced hyperalgesia. J Neurosci 2004;24(11):2727–32.Search in Google Scholar
[38] Bennett A. Overview of nimesulide. Rheumatology 1999;38:11–9.Search in Google Scholar
[39] Vo T, Rice AS, Dworkin RH. Non-steroidal anti-inflammatory drugs for neuropathic pain: how do we explain continued wide spread use? Pain 2009;143:169–71.Search in Google Scholar
[40] Cui JG, Holmni S, Mathiesen T, Meyesson BA, Linderoth B. Possible role of inflammatory mediators in tactile hypersensivity in rat models of mononeuropathy. Pain 2000;88:239–48.Search in Google Scholar
[41] Rittner HL, Brack A, Stein C. Pain and the immune system: friend or foe? Anesthesist 2002;51:351–8.Search in Google Scholar
[42] Bianchi M, Broggeni M, Balzarini P, Franchi S, Sacerdote P. Effects of nimesulide on pain and on synovial fluid concentrations of substance P, interleukin-6 and interleukin-8 in patients with knee osteoarthritis: comparison with celecoxib. Int J Clin Pract 2007;61(8):1270–7.Search in Google Scholar
© 2010 Scandinavian Association for the Study of Pain
Articles in the same Issue
- Editorial comment
- Importance of clinical neurophysiological tests in the evaluation of pain: Indispensable in complex pain conditions
- Educational case report
- Difficult diagnosis of facial pain: A case report and mini-review
- Editorial comment
- Nerve block—A reliable diagnostic tool?
- Review
- Diagnostic blocks for chronic pain
- Editorial comment and review
- What do maltreatment and schemas have to do with the treatment of chronic pain?
- Clinical pain research
- Early maladaptive schemas in Finnish adult chronic male and female pain patients
- Editorial comment
- Electrically induced pain models: The benefit of “electric feel”
- Original experimental
- Cross-over evaluation of electrically induced pain and hyperalgesia
- Editorial comment
- Social work in a pain clinic
- Observational studies
- Patients referred from a multidisciplinary pain clinic to the social worker, their socio-demographic profile and the contribution of the social worker to the management of the patients
- Clinicial pain research
- Patients referred from a multidisciplinary pain clinic to the social worker, their general health, pain condition, treatment and outcome
- Editorial comment
- Suppression of pain behavior in nerve-injured rats by an anti-inflammatory drug: Promises and caveats for translation to clinical applications in man
- Original experimental
- The attenuation of pain behaviour and serum interleukin-6 concentration by nimesulide in a rat model of neuropathic pain
- Corrigendum
- Corrigendum to “A 6-months, randomised, placebo-controlled evaluation of efficacy and tolerability of 7 day buprenorphine transdermal patch in osteoarthritis patients naïve to potent-opioids” [Scandinavian Journal of Pain 1 (2010) 122-141]
Articles in the same Issue
- Editorial comment
- Importance of clinical neurophysiological tests in the evaluation of pain: Indispensable in complex pain conditions
- Educational case report
- Difficult diagnosis of facial pain: A case report and mini-review
- Editorial comment
- Nerve block—A reliable diagnostic tool?
- Review
- Diagnostic blocks for chronic pain
- Editorial comment and review
- What do maltreatment and schemas have to do with the treatment of chronic pain?
- Clinical pain research
- Early maladaptive schemas in Finnish adult chronic male and female pain patients
- Editorial comment
- Electrically induced pain models: The benefit of “electric feel”
- Original experimental
- Cross-over evaluation of electrically induced pain and hyperalgesia
- Editorial comment
- Social work in a pain clinic
- Observational studies
- Patients referred from a multidisciplinary pain clinic to the social worker, their socio-demographic profile and the contribution of the social worker to the management of the patients
- Clinicial pain research
- Patients referred from a multidisciplinary pain clinic to the social worker, their general health, pain condition, treatment and outcome
- Editorial comment
- Suppression of pain behavior in nerve-injured rats by an anti-inflammatory drug: Promises and caveats for translation to clinical applications in man
- Original experimental
- The attenuation of pain behaviour and serum interleukin-6 concentration by nimesulide in a rat model of neuropathic pain
- Corrigendum
- Corrigendum to “A 6-months, randomised, placebo-controlled evaluation of efficacy and tolerability of 7 day buprenorphine transdermal patch in osteoarthritis patients naïve to potent-opioids” [Scandinavian Journal of Pain 1 (2010) 122-141]

