Abstract
This case report elucidates pitfalls of clinical and radiologic investigations of neuropathic pain due to trigeminal pathology, and utility of neurophysiologic examination when diagnosing facial pain. Our patient was a 63-year-old woman who developed acute, severe facial pain, first located behind the left eye. Neuralgic exacerbations, paresthesia within lower face on the left and restricted mouth opening occurred during the course of the disease with gradual progression. Brain MRI and CT scans were interpreted as normal at 4 and 10 months after symptom onset. At 9 months, detailed neurophysiologic examination showed severe chronic mandibular neuropathy at the left oval foramen with more prominent disturbance of the thick myelinated nerve fibers than the small fibers suggesting compressive etiology. Guided by the neurophysiologic findings, 11 months after the onset of the symptoms, a new brain MRI with contrast enhancement revealed metastatic adenocarcinoma of the left temporal bone along the mandibular nerve, exactly matching the site indicated by the neurophysiologic examination. Neurophysiologic tests offer cost-effective, sensitive tools for screening and accurate level diagnostics of neuropathy and neuropathic pain, which can be utilized also in the diagnosis of facial pain. In addition, whenever there are progressing neurologic deficits or neurophysiologic signs indicating expansive lesion, despite initially normal findings in the brain imaging studies, repeated MRI examinations are warranted, preferably focusing to the ‘neurophysiologic region of interest’ to avoid radiologic sampling errors. As no isolated technique achieves 100% diagnostic accuracy, only rational combinations of different methods will result in correct diagnosis of facial pain without unnecessary delays. Treatment of neuropathic pain is often delayed because of difficulties in reaching the correct diagnosis. During the work-up, many differential diagnostic alternatives have to be considered, also in patients with chronic orofacial pain. Table 1 shows the most important differential diagnoses of orofacial pain.
1 Case presentation
A 63-year-old woman in good general health developed acute, severe facial pain, first located behind the left eye. She had a history of ductal breast cancer (T1N0M0G2) successfully treated 8 years earlier. Her deep aching pain was continuous spreading over time to the entire left side of the head. There were atypical neuralgic exacerbations lasting for up to 30 min with continuous smarting pain, numbness and paresthesia on the lower left side of the face and the tongue. The patient reported slowly progressive chewing difficulties, restricted mouth opening, and fluctuating eyelid edema. She was treated by a private neurologist during the first 8 months, with diagnoses of acute idiopathic trigeminal neuropathy, atypical facial pain, and trigeminal neuralgia. An ophthalmologic examination and brain MRI (Fig. 1A) had been normal at 4 months after symptom onset. Gabapentin 600 mg/day partially relieved the pain. Because of progressive chewing difficulties and suspected ptosis, she was referred to the University Hospital of Turku for further investigations to exclude myasthenic syndrome.
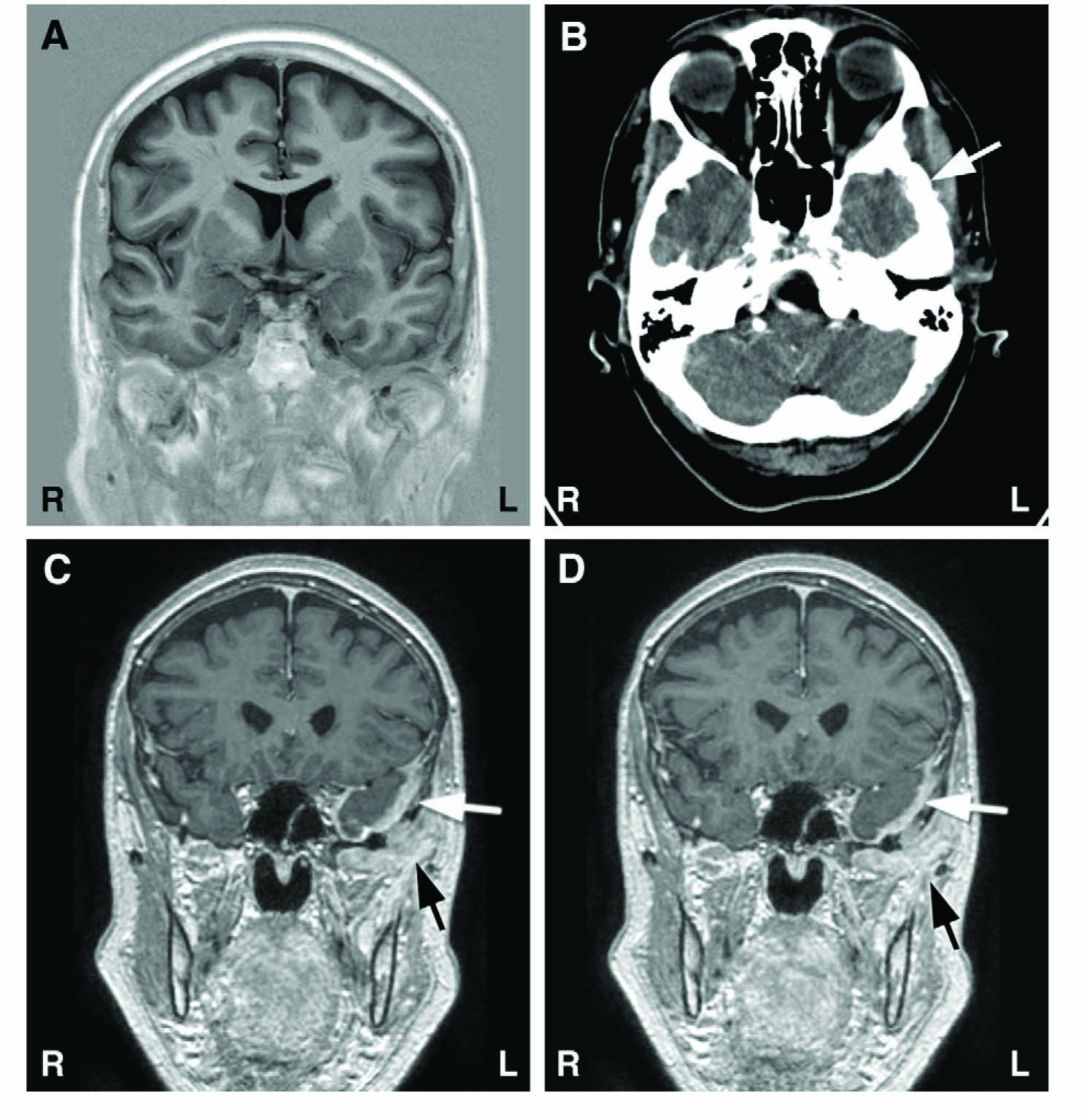
Results of structural brain imaging during the course of the disease. (A) Normal native MRI of the brain taken 4 months after onset of facial pain symptoms. (B) CT image taken 10 months after symptom onset with brain window setting; incipient lytic process of low temporal bone on left side marked with an arrow (hardly visible at this magnification). (C and D) Eleven months after symptom onset, gadolinium enhanced T1-weighted MR images show clearly visible pathologic enhancement of dura (white arrows) and enhancing soft tissue metastatic mass below temporal bone (black arrows).
Ten months after the onset, a neurologic examination revealed diminished sensibility to light touch and sharp objects within the left infraorbital (ION) and mental nerve (MN) distributions. Edema of the left eyelid was noted, but there were no clinical signs of myasthenia. Mouth opening was restricted to 20mm. The neurologic examination was otherwise normal. All blood tests were within normal limits, except for a slight elevation of C-reactive protein (15; normal <10). The patient received a diagnosis of idiopathic trigeminal neuropathy with pain.
Because of progressive chewing difficulties, a clinical oral examination was performed at 11 months to exclude temporomandibular disorders. There was slight edema of the left cheek, and tenderness on palpation of the left temporomandibular joint. Mouth opening was restricted to 16mm. Tactile detection and sharp-blunt discrimination were impaired within the left MN distribution.
Neurophysiologic examination was done 9 months after the symptom onset with previously described methods and equipment [1,2]. Neurography of the extremities were normal. Except for the muscles of mastication on the left, needle EMG of the limb and bulbar muscles, including quantitative motor unit potential (MUP) analysis, and neuromuscular transmission tests were normal. Masticatory muscles on the left were severely atrophied and showed abundant spontaneous activity indicating active denervation process. There were no voluntarily activated MUPs in the masseter and temporal muscles, and no muscle action potential could be evoked from the masseter muscle with needle stimulation of the masseter nerve, compatible with total loss of functioning motor axons. In the lateral pterygoid muscle, there were a few large, polyphasic MUPs of long duration signifying collateral reinnervation and a chronic state. Masseter reflex response and sensory action potential of the inferior alveolar nerve were absent on the left, normal on the right side (Fig. 2A and C). Blink reflex responses were bilaterally missing with left MN stimulation, but normal with stimulation of the right MN (Fig. 2B), the infraorbital and supraorbital nerves. Thermal quantitative sensory testing (QST) revealed slightly elevated warm detection threshold only in the left MN distribution (Fig. 2D). The neurophysiologic findings indicated a severe, chronic axonal injury of the left mandibular nerve below the ganglion Gasseri but above the branching point of the motor nerves to the masticatory muscles, i.e. at the region of the oval foramen. Because of more severe damage to large myelinated nerve fibers than to small fibers, a compressive lesion was suspected and urgent radiologic re-imaging was recommended.
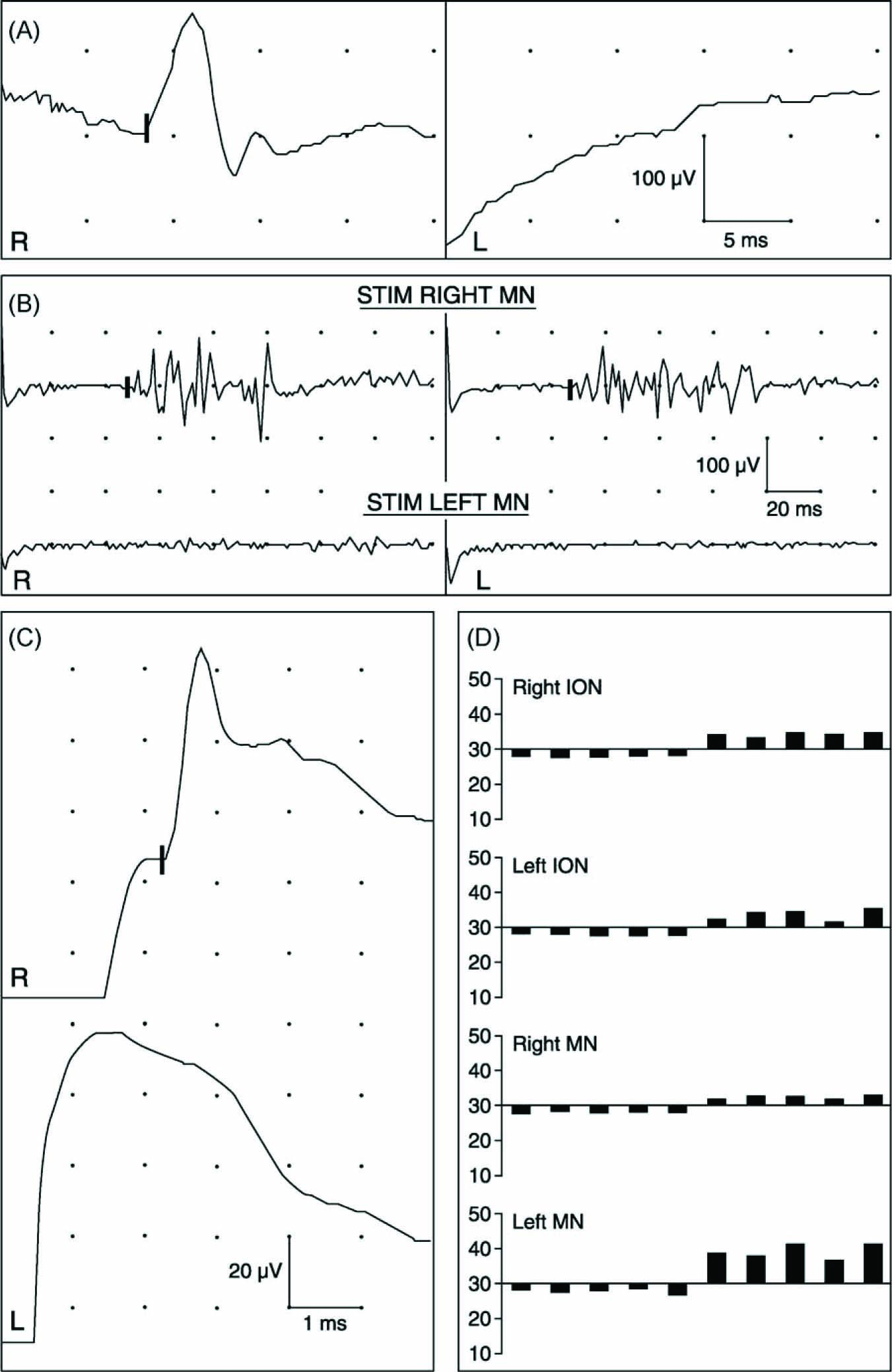
Results of neurophysiologic examination 9 months after the onset of facial pain. (A) Masseter reflex recording with normal response on right (R) side, and absent response on left (L). (B) Blink reflex recording showing normal responses bilaterally with stimulation of right mental nerve (MN) and absent responses with stimulation of left MN. (C) Neurography of inferior alveolar nerve with normal sensory action potential on right side (onset of negative peak is marked on upper trace) and absent response on left side. (D) Cold (first five bars below 30 °C) and warm (next five bars above 30 °C) detection threshold measurements with stimulation at infraorbital (ION) and MN distributions on both sides. Five repetitions of each test are shown. Only warm detection threshold within left MN distribution is elevated, indicating hypoesthesia to warming.
The first MR imaging performed at the private clinic had been normal (Fig. 1A), excluding also temporal arteritis. After neurophysiologic examination, native and enhanced CT (Fig. 1B) scans of the brain were done with routine brain protocol including a bone window, and they were interpreted as normal. Because of the neurophysiologic findings and progressing clinical symptoms, a new MRI scan covering the brain and the basal cranial bones was done with gadolinium enhancement 11 months after symptom onset. MRI showed increased meningeal enhancement in the region of the left temporal pole and base (Fig. 1C and 1D); a metastatic tumor mass invaded the basal parts of the temporal bone extending to the left orbit. A biopsy confirmed the metastasis of an adenocarcinoma. Residual tumor growth was also found in the operated left breast area.
2 Discussion
This patient case illustrates some pitfalls in the diagnosis of neuropathic facial pain and brain neoplasms that may contribute to delayed diagnosis and treatment of a malignancy. Radiologic imaging even with MRI can be normal at the first investigations of patients with brain tumors [3,4]. As in our patient, more peripheral trigeminal pathology can easily be overlooked [5,6] in standard radiologic brain imaging [7,8] although this is regarded as the ‘gold standard’ for structural pathology behind neurologic symptoms. When only CT scans are done, up to 20% of the initial imaging studies may result in false normal findings in patients with a brain neoplasm [3,4]. Tumors of the temporal lobe region are especially prone to lead to false interpretation of CT scans [4], as happened in our patient.
The neurophysiologic tests currently available enable detailed analysis of trigeminal system pathology, providing good topographic-level diagnosis [1,2,9,10], and aiding in the difficult clinical assessment of neuropathic facial pain [2,5,7,11,12]. This good diagnostic accuracy was evident in our patient, but the neurophysiologic findings were probably not fully utilized in the initial analysis of the CT scans, interpreted as normal. At retrospective inspection of the same scans with high resolution and magnification, bony lytic invasion of the temporal bone could be distinguished by a specialist in neuroradiology (Fig. 1B).
Some difficulties inherent in the clinical examination of trigeminal neuropathy were evident. Severe atrophy of the muscles of mastication was not mentioned in the clinical notes. Masticatory muscle atrophy and unilateral diminishment or absence of the masseter reflex are difficult to verify clinically, yet easily diagnosed with neurophysiologic techniques [7,8,10], as shown here. It has been indicated that without neurophysiologic recordings [13] and quantitative small fiber function tests [14], the diagnosis of peripheral neuropathy cannot be reliably made; these tests significantly improve both the sensitivity and negative predictive value when peripheral neuropathy is suspected [2,9,12,14]. In our patient, the sensory deficits were clinically clear, and neurophysiology was not necessary for the diagnosis of neuropathy as such. However, neurophysiologic examination accurately profiled the fiber-type damage, the preferential involvement of the large myelinated nerve fibers, correctly suggesting a compressive lesion. In addition, the neurophysiologic findings precisely located the neuropathy to the mandibular nerve at the oval foramen providing the region of interest for further imaging studies. The clinical signs were not restricted to the neuroanatomical distribution of the injured nerve, suggesting more widespread trigeminal involvement at a higher level of the neuraxis. This, with falsely normal brain MRI and CT scans, resulted in several incorrect diagnoses during the course of the disease and a significant delay before appropriate treatment of the malignancy could be initiated.
Table 1 shows differential diagnostic etiologies to be considered in facial pain. According to the present definitions, neuropathic pain requires demonstration of a causative lesion or dysfunction of the nervous system [9,15] (cf. www.IASP.org). Trigeminal nerve lesions constitute the main etiology of orofacial neuropathic pain that is most frequent after iatrogenic nerve injuries and post herpetic neuralgia [16], but may also occur in systemic and central nervous system diseases [17]. In our patient, some of the symptoms and signs were related to trigeminal neuropathy, while others were due to direct nociceptive mechanisms associated with tumor invasion and reactive inflammation. The continuous aching and smarting pain with atypical neuralgic exacerbations at the lower jaw and tongue were apparently related to the mandibular neuropathy, and responded to gabapentin medication, whereas the more widespread background pain did not. The sensory symptoms and signs involved also the infraorbital and periorbital regions, although only the lowest trigeminal division showed neurophysiologic abnormalities, and the MRI did not show involvement of the trigeminal main root, the ganglion Gasseri, or the maxillary nerve. These widespread and subtle sensory alterations might represent non-specific sensory aberrations that may occur in chronic musculoskeletal pain conditions [18]. However, in neuropathic pain, symptoms and signs may also spread outside the originally involved nerve territory [2], which may complicate the clinical diagnostics.
Differential diagnosis of facial pain.
| Neuropathic pain |
| Facial pain associated with cranial neuropathies (most common: trigeminal neuropathy) |
| Peripheral nerve lesions (facial trauma, iatrogenic, infections, tumors) |
| Systemic diseases (diabetes, borreliosis, sarcoidosis, connective tissue diseases and otherautoimmune disorders, direct neoplastic infiltration or paraneoplasia) |
| Central nervous system diseases (brain infarct or haemorrhagic lesions, tumors, infections, multiple sclerosis, Parkinson’s disease) |
| Acute idiopathic neuropathy |
| Cranial nerve involvement in generalized peripheral neuropathy: advanced sensorimotor or pure sensory polyneuropathy, small fiber neuropathy |
| Cranial neuralgias (trigeminal, glossopharyngeal, nervus intermedius) |
| Idiopathic pain conditions with potential neuropathic involvement |
| Persistent idiopathic facial pain/atypical facial pain |
| Burning mouth syndrome |
| Disorders of the craniocervical junction and upper cervical spine (traumatic or non-traumatic) |
| Nociceptive pain and musculoskeletal pain |
| Dental pathology |
| Sinus, ear, eye, and salivary gland infections |
| Malignant neoplasms of head and neck |
| Other bone or soft tissue lesions (traumatic, inflammatory, vascular, cysts, polyps) |
| Temporal arteritis |
| Temporomandibular dysfunction (muscular, joint, both) |
| Ophthalmologic causes (refraction errors, increased intraophthalmic pressure) |
| Facial dyskinesia (blepharospasm, oromandibular dyskinesia, hemifacial spasm) |
| Trigeminal autonomic cephalalgias |
| Cluster headache, paroxysmal hemicranias, short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing(SUNCT) |
Trigeminal neuralgia was one of the diagnostic alternatives considered in our patient but with clear neurologic deficits, long-lasting neuralgic attacks, and ongoing background pain from the beginning she obviously did not fulfill its diagnostic criteria [19]. She also received the diagnosis of acute idiopathic trigeminal nerve neuropathy. The acute, painful onset of her symptoms with subsequent neurologic deficits following within the next weeks could have fitted this entity [20]. Yet, persistent pain, gradual progression of symptoms, and focal edema were not typical for idiopathic neuropathy.
Atypical facial pain, chronic facial pain without demonstrable neurologic deficits [19], can be difficult to differentiate from neuropathic facial pain [21], in which the subjective symptoms may be subtle, and unambiguous clinical signs may be absent [2,5]. The clear clinical signs of trigeminal neuropathy excluded this diagnosis in our patient. Temporomandibular dysfunction, musculoskeletal chronic pain condition around the temporomandibular joint [22], may induce restriction of mouth opening, but is not associated with neurologic deficits that were evident in our case.
Under-diagnostics, inappropriate treatment, and severely delayed adequate treatment of neuropathic pain are a challenge to the medical profession world wide [23]. In this patient group, both direct and indirect costs of healthcare are manifold compared to age- and sex-matched controls [23,24]. Neurophysiologic tests offer cost-effective, sensitive tools for screening and accurate level diagnostics of neuropathy and neuropathic pain, which should be utilized also in the diagnosis of facial pain. In addition, whenever there are progressing neurologic deficits or neurophysiologic signs indicating expansive lesion, despite initially normal findings in the brain imaging studies, repeated, detailed MRI examinations are warranted, preferably focusing to the ‘neurophysiologic region of interest’ to avoid radiologic sampling errors. As no isolated technique achieves 100% diagnostic accuracy, only rational combinations of different methods will result in successful diagnosis of facial pain without unnecessary delays.
DOI of refers to article: 10.1016/j.sjpain.2010.09.001.
Acknowledgements
This work was financially supported by research grants from Finnish Medical Foundation and Sigrid Jusélius Foundation.
References
[1] Jääskeläinen SK. A new technique for recording sensory conduction velocity of the inferior alveolar nerve. Muscle Nerve 1999;22:455–9.Search in Google Scholar
[2] Jääskeläinen SK, Teerijoki-Oksa T, Forssell H. Neurophysiologic and quantitative sensory testing in the diagnosis of trigeminal neuropathy and neuropathic pain. Pain 2005;117:349–57.Search in Google Scholar
[3] Omuro AMP, Leite CC, Mokhtari K, Delattre J-Y. Pitfalls in the diagnosis of brain tumours. Lancet Neurol 2006;5:937–48.Search in Google Scholar
[4] Grant R. Overview: brain tumour diagnosis and management/Royal College of Physicians guidelines. J Neurol Neurosurg Psychiatry 2004;75(Suppl. II):ii18–23.Search in Google Scholar
[5] Jääskeläinen S, Forssell H, Tenovuo O. Electrophysiological testing of the trigeminofacial system: aid in the diagnosis of atypical facial pain. Pain 1999;80:191–200.Search in Google Scholar
[6] Forssell H, Jääskeläinen SK, Tenovuo O, Hinkka S. Sensory dysfunction in burning mouth syndrome. Pain 2002;99:41–7.Search in Google Scholar
[7] Majoie CBLM, Aramideh M, Hulsmans FJH, Castelijns JA, van Beek EJR, Ongerboer de Visser BW. Correlation between electromyographic reflex and MR imaging examinations of the trigeminal nerve. Am J Neuroradiol 1999;20:1119–25.Search in Google Scholar
[8] Wedekind C, Hesselmann V, Klug N. Comparison of MRI and electrophysiological studies for detecting brainstem lesions in traumatic brain injury. Muscle Nerve 2002;26:270–3.Search in Google Scholar
[9] Cruccu G, Anand P, Attal N, Garcia-Larrea L, Haanpää M, Jorum E, Serra J. EFNS guidelines on neuropathic pain assessment. Eur J Neurol 2004;11:153–62.Search in Google Scholar
[10] Hopf HC. Topodiagnostic value of brainstem reflexes. Muscle Nerve 1994;17:475–84.Search in Google Scholar
[11] Zuniga JR, Meyer RA, Gregg JM, Miloro M, Davis LF. The accuracy of clinical neurosensory testing for nerve injury diagnosis. J Oral Maxillofac Surg 1998;56:2–8.Search in Google Scholar
[12] Teerijoki-Oksa T, Jääskeläinen SK, Forssell K, Forssell H. Recovery of nerve injury after mandibular sagittal split osteotomy. Diagnostic value of clinical and electrophysiologic tests in the follow-up. Int J Oral Maxillofac Surg 2004;33:134–40.Search in Google Scholar
[13] England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, Cohen JA, Fisher MA, Howard JF, Kinsella LJ, Latov N, Lewis RA, Low PA, Sumner AJ. Distal symmetric polyneuropathy: a definition for clinical research. Report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005;64:199–207.Search in Google Scholar
[14] Løseth S, Lindal S, Stålberg E, Mellgren SI. Intraepidermal nerve fibre density, quantitative sensory testing and nerve conduction studies in a patient material with symptoms and signs of sensory polyneuropathy. EFNS task force/CME article. Eur J Neurol 2006;13:105–11.Search in Google Scholar
[15] Treede R-D, Jensen TS, Campbell JN. Neuropathic pain. Redefinition and a grading system for clinical and research purposes. Neurology 2008;70: 1630–5.Search in Google Scholar
[16] Bennett GJ. Neuropathic pain in the orofacial region: clinical and research challenges. J Orofac Pain 2004;18:281–5.Search in Google Scholar
[17] Chadwick D. The cranial nerves and special senses. The fifth or trigeminal nerve. In: Walton J, editor. Brain’s diseases of the nervous system. 10th ed. Oxford: Oxford University Press; 1993. p. 102–6.Search in Google Scholar
[18] Leffier A-S. Pain influences somatosensory perception—an experimental and clinical study. Doctoral thesis, Karolinska Institute. Stockholm: Repro Print AB; 2002.Search in Google Scholar
[19] Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders, 2nd ed. Cephalalgia 2004;24(Suppl. 1):9–160.Search in Google Scholar
[20] Weaver K, Kraft GH. Idiopathic shoulder girdle neuropathy. Phys Med Rehabil Clin N Am 2001;12:353–64.Search in Google Scholar
[21] Forssell H, Tenovuo O, Silvoniemi P, Jääskeläinen SK. Differences and similarities between atypical facial pain and trigeminal neuropathic pain. Neurology 2007;69:1451–9.Search in Google Scholar
[22] Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders. Review, criteria, examination and specifications, critique. J Craniomandib Disord 1992;6:301–55.Search in Google Scholar
[23] Freynhagen R, Bennet MI. Diagnosis and management of neuropathic pain. BMJ 2009;339:391–5.Search in Google Scholar
[24] Berger A, Dukes EM, Oster G. Clinical characteristics and economic costs of patients with painful neuropathic disorders. J Pain 2004;5:143–9.Search in Google Scholar
Abbreviations
- CT
-
computerized tomography
- ION
-
infraorbital nerve
- MN
-
mental nerve
- MRI
-
magnet resonance imaging
- MUP
-
motor unit potential
- QST
-
quantitative sensory testing.
© 2010 Scandinavian Association for the Study of Pain
Articles in the same Issue
- Editorial comment
- Importance of clinical neurophysiological tests in the evaluation of pain: Indispensable in complex pain conditions
- Educational case report
- Difficult diagnosis of facial pain: A case report and mini-review
- Editorial comment
- Nerve block—A reliable diagnostic tool?
- Review
- Diagnostic blocks for chronic pain
- Editorial comment and review
- What do maltreatment and schemas have to do with the treatment of chronic pain?
- Clinical pain research
- Early maladaptive schemas in Finnish adult chronic male and female pain patients
- Editorial comment
- Electrically induced pain models: The benefit of “electric feel”
- Original experimental
- Cross-over evaluation of electrically induced pain and hyperalgesia
- Editorial comment
- Social work in a pain clinic
- Observational studies
- Patients referred from a multidisciplinary pain clinic to the social worker, their socio-demographic profile and the contribution of the social worker to the management of the patients
- Clinicial pain research
- Patients referred from a multidisciplinary pain clinic to the social worker, their general health, pain condition, treatment and outcome
- Editorial comment
- Suppression of pain behavior in nerve-injured rats by an anti-inflammatory drug: Promises and caveats for translation to clinical applications in man
- Original experimental
- The attenuation of pain behaviour and serum interleukin-6 concentration by nimesulide in a rat model of neuropathic pain
- Corrigendum
- Corrigendum to “A 6-months, randomised, placebo-controlled evaluation of efficacy and tolerability of 7 day buprenorphine transdermal patch in osteoarthritis patients naïve to potent-opioids” [Scandinavian Journal of Pain 1 (2010) 122-141]
Articles in the same Issue
- Editorial comment
- Importance of clinical neurophysiological tests in the evaluation of pain: Indispensable in complex pain conditions
- Educational case report
- Difficult diagnosis of facial pain: A case report and mini-review
- Editorial comment
- Nerve block—A reliable diagnostic tool?
- Review
- Diagnostic blocks for chronic pain
- Editorial comment and review
- What do maltreatment and schemas have to do with the treatment of chronic pain?
- Clinical pain research
- Early maladaptive schemas in Finnish adult chronic male and female pain patients
- Editorial comment
- Electrically induced pain models: The benefit of “electric feel”
- Original experimental
- Cross-over evaluation of electrically induced pain and hyperalgesia
- Editorial comment
- Social work in a pain clinic
- Observational studies
- Patients referred from a multidisciplinary pain clinic to the social worker, their socio-demographic profile and the contribution of the social worker to the management of the patients
- Clinicial pain research
- Patients referred from a multidisciplinary pain clinic to the social worker, their general health, pain condition, treatment and outcome
- Editorial comment
- Suppression of pain behavior in nerve-injured rats by an anti-inflammatory drug: Promises and caveats for translation to clinical applications in man
- Original experimental
- The attenuation of pain behaviour and serum interleukin-6 concentration by nimesulide in a rat model of neuropathic pain
- Corrigendum
- Corrigendum to “A 6-months, randomised, placebo-controlled evaluation of efficacy and tolerability of 7 day buprenorphine transdermal patch in osteoarthritis patients naïve to potent-opioids” [Scandinavian Journal of Pain 1 (2010) 122-141]

