Reactions of strontium anorthite with H2O+CaCl2 fluids at 500 °C and high pressure: Kinetic information from in situ synchrotron-radiation XRF analyses of the fluid
Abstract
The assemblage strontium anorthite, quartz, and kyanite was reacted with H2O+CaCl2 solutions at 500 °C and pressures between 460 and ~1300 MPa using a hydrothermal diamond-anvil cell. Information on the kinetics was obtained in situ based on time-resolved synchrotron-radiation X-ray fluorescence analyses of the Sr concentration in the fluid. The reaction products (anorthite or zoisite) were studied using transmission electron microscopy to obtain information on the reaction mechanism and mineral-fluid partitioning of strontium. The time required for equilibration was primarily controlled by the reaction mechanism, but not discernibly affected by pressure or chloride concentration. Nucleation and growth of zoisite at the expense of strontium anorthite was much faster than the Sr-Ca exchange reaction of strontium anorthite to anorthite, and resulted in chemically homogeneous crystals. The anorthite had developed a high nanoporosity during the reaction, which is indicative of coupled dissolution-precipitation. A zoisite-fluid exchange coefficient
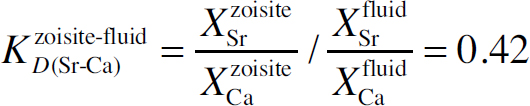
was obtained for the Sr-Ca fractionation at 500 °C and ~1300 MPa. At low bulk Sr/Ca, this value is in very good agreement with literature data, which are based on zoisite syntheses from oxide and hydroxide mixtures in chloridic fluids at 600 °C, 2 GPa and analyses after quench. This suggests that the Ca-Sr ratios in fluid and zoisite were not affected by back reactions during quenching. The constrained anorthite-fluid Sr partition coefficient for 500 °C, 460 MPa is, likewise, consistent with literature data, but determination of mineral-fluid partition and exchange coefficients can be hampered by quench phases in nanopores if coupled dissolution-precipitation acted as reaction mechanism.
© 2015 by Walter de Gruyter Berlin/Boston
Articles in the same Issue
- MSA Roebling medal lecture. Mineralogy, petrology, U-Pb geochronology, and geologic evolution of the Dabie-Sulu classic ultrahigh-pressure metamorphic terrane, East-Central China
- Brittle-ductile microfabrics in naturally deformed zircon: Deformation mechanisms and consequences for U-Pb dating
- In situ hot-stage AFM study of the dissolution of the barite (001) surface in water at 30–55 °C
- A multi-domain gem-grade Brazilian apatite
- High-temperature structural behaviors of anhydrous wadsleyite and forsterite
- Rates and mechanism of Y, REE, and Cr diffusion in garnet
- EPR discrimination of microcrystalline calcite geomaterials
- Crystal chemistry of Bi- and Mn-bearing vesuvianite from Långban, Sweden
- Cation ordering in Pb2+-bearing, Mn3+-rich pargasite from Långban, Sweden
- Long-term solid-phase fate of co-precipitated U(VI)-Fe(III) following biological iron reduction by Thermoanaerobacter
- The sulfur speciation in S-bearing minerals: New constraints by a combination of electron microprobe analysis and DFT calculations with special reference to sodalite-group minerals
- Simultaneous sound velocity and density measurements of NaCl at high temperatures and pressures: Application as a primary pressure standard
- The temperature and compositional dependence of disordering in Fe-bearing dolomites
- Quantifying crystallization and devitrification of rhyolites by means of X-ray diffraction and electron microprobe analysis
- Reactions of strontium anorthite with H2O+CaCl2 fluids at 500 °C and high pressure: Kinetic information from in situ synchrotron-radiation XRF analyses of the fluid
- Solubility of xenotime in a 2 M HCl aqueous fluid from 1.2 to 2.6 GPa and 300 to 500 °C
- Elastic and anelastic anomalies due to spin-state transitions in orthorhombic perovskite from isoelectronic behavior of Co3+ and Fe2+
- Accurate determination of ferric iron in garnets by bulk Mössbauer spectroscopy and synchrotron micro-XANES
- Re-investigation of the crystal structure of enstatite under high-pressure conditions
- Second-order P6̄c2-P31c transition and structural crystallography of the cyclosilicate benitoite, BaTiSi3O9, at high pressure
- High-pressure structural studies of eskolaite by means of single-crystal X-ray diffraction
- Almandine: Lattice and non-lattice heat capacity behavior and standard thermodynamic properties
- Witzkeite: A new rare nitrate-sulphate mineral from a guano deposit at Punta de Lobos, Chile
- Krasheninnikovite, KNa2CaMg(SO4)3F, a new mineral from the Tolbachik volcano, Kamchatka, Russia
- Crystal structure of pseudojohannite, with a revised formula, Cu3(OH)2[(UO2)4O4(SO4)2](H2O)12
- Letter. A natural photoelectrochemical cell for water splitting: Implications for early Earth and Mars
- Letter. In situ observation of the breakdown of magnetite (Fe3O4) to Fe4O5 and hematite at high pressures and temperatures
- Letter. The crystal structure of bartelkeite, with a revised chemical formula, PbFeGeVI(Ge2IVO7) (OH)2·H2O, isotypic with high-pressure P21/m lawsonite
Articles in the same Issue
- MSA Roebling medal lecture. Mineralogy, petrology, U-Pb geochronology, and geologic evolution of the Dabie-Sulu classic ultrahigh-pressure metamorphic terrane, East-Central China
- Brittle-ductile microfabrics in naturally deformed zircon: Deformation mechanisms and consequences for U-Pb dating
- In situ hot-stage AFM study of the dissolution of the barite (001) surface in water at 30–55 °C
- A multi-domain gem-grade Brazilian apatite
- High-temperature structural behaviors of anhydrous wadsleyite and forsterite
- Rates and mechanism of Y, REE, and Cr diffusion in garnet
- EPR discrimination of microcrystalline calcite geomaterials
- Crystal chemistry of Bi- and Mn-bearing vesuvianite from Långban, Sweden
- Cation ordering in Pb2+-bearing, Mn3+-rich pargasite from Långban, Sweden
- Long-term solid-phase fate of co-precipitated U(VI)-Fe(III) following biological iron reduction by Thermoanaerobacter
- The sulfur speciation in S-bearing minerals: New constraints by a combination of electron microprobe analysis and DFT calculations with special reference to sodalite-group minerals
- Simultaneous sound velocity and density measurements of NaCl at high temperatures and pressures: Application as a primary pressure standard
- The temperature and compositional dependence of disordering in Fe-bearing dolomites
- Quantifying crystallization and devitrification of rhyolites by means of X-ray diffraction and electron microprobe analysis
- Reactions of strontium anorthite with H2O+CaCl2 fluids at 500 °C and high pressure: Kinetic information from in situ synchrotron-radiation XRF analyses of the fluid
- Solubility of xenotime in a 2 M HCl aqueous fluid from 1.2 to 2.6 GPa and 300 to 500 °C
- Elastic and anelastic anomalies due to spin-state transitions in orthorhombic perovskite from isoelectronic behavior of Co3+ and Fe2+
- Accurate determination of ferric iron in garnets by bulk Mössbauer spectroscopy and synchrotron micro-XANES
- Re-investigation of the crystal structure of enstatite under high-pressure conditions
- Second-order P6̄c2-P31c transition and structural crystallography of the cyclosilicate benitoite, BaTiSi3O9, at high pressure
- High-pressure structural studies of eskolaite by means of single-crystal X-ray diffraction
- Almandine: Lattice and non-lattice heat capacity behavior and standard thermodynamic properties
- Witzkeite: A new rare nitrate-sulphate mineral from a guano deposit at Punta de Lobos, Chile
- Krasheninnikovite, KNa2CaMg(SO4)3F, a new mineral from the Tolbachik volcano, Kamchatka, Russia
- Crystal structure of pseudojohannite, with a revised formula, Cu3(OH)2[(UO2)4O4(SO4)2](H2O)12
- Letter. A natural photoelectrochemical cell for water splitting: Implications for early Earth and Mars
- Letter. In situ observation of the breakdown of magnetite (Fe3O4) to Fe4O5 and hematite at high pressures and temperatures
- Letter. The crystal structure of bartelkeite, with a revised chemical formula, PbFeGeVI(Ge2IVO7) (OH)2·H2O, isotypic with high-pressure P21/m lawsonite

