Abstract
During the systematic search of active compounds from endophytic fungi, two new butyrolactones, namely aspernolides L (2) and M (4), together with four known compounds: 1-O-acetylglycerol (1), butyrolactone I (3), butyrolactone VI (5), and (+) alantrypinone (6) were characterized from the EtOAc extract of the endophytic fungus Aspergillus versicolor isolated from the roots of Pulicaria crispa (Asteraceae). Extensive spectroscopic analysis, including 1D, 2D NMR, and HRESIMS, was used to elucidate their structures. Compounds 1, 5, and 6 are reported for the first time from this fungus.
1 Introduction
Bioactive secondary metabolites from endophytic fungi, isolated from higher plants, are a major focus of natural product research [1], [2], [3], [4]. They have been utilized as drugs and/or lead compounds in the pharmaceutical industry [1], [2], [3], [4], [5]. The specific habitats, metabolic pathways, and bioactivities of the endophytic fungi make them a good source of structurally novel and/or biologically active secondary metabolites [5], [6]. Fungi of the genus Aspergillus (Moniliaceae) have been reported as prolific producers of bioactive compounds as xanthones, butyrolactones, anthraquinones, polyketides, and alkaloids [1], [2], [5], [6], [7], [8], [9], [10], some of them possess various biological activities [11], [12], [13], [14]. In the course of our search for secondary metabolites from endophytic fungi, we investigated the fungal strain Aspergillus versicolor isolated from the roots of Pulicaria crispa (Asteraceae), which was collected from Gabal Al-aquiq, Al Madinah Al Munawwarah, Saudi Arabia. As a result, two new butyrolactones, namely aspernolides L (2) and M (4), and four known compounds (1, 3, 5, and 6) were isolated from this strain (Figure 1). The structures of these compounds were elucidated on the basis of a comprehensive analysis of 1D and 2D NMR spectra.
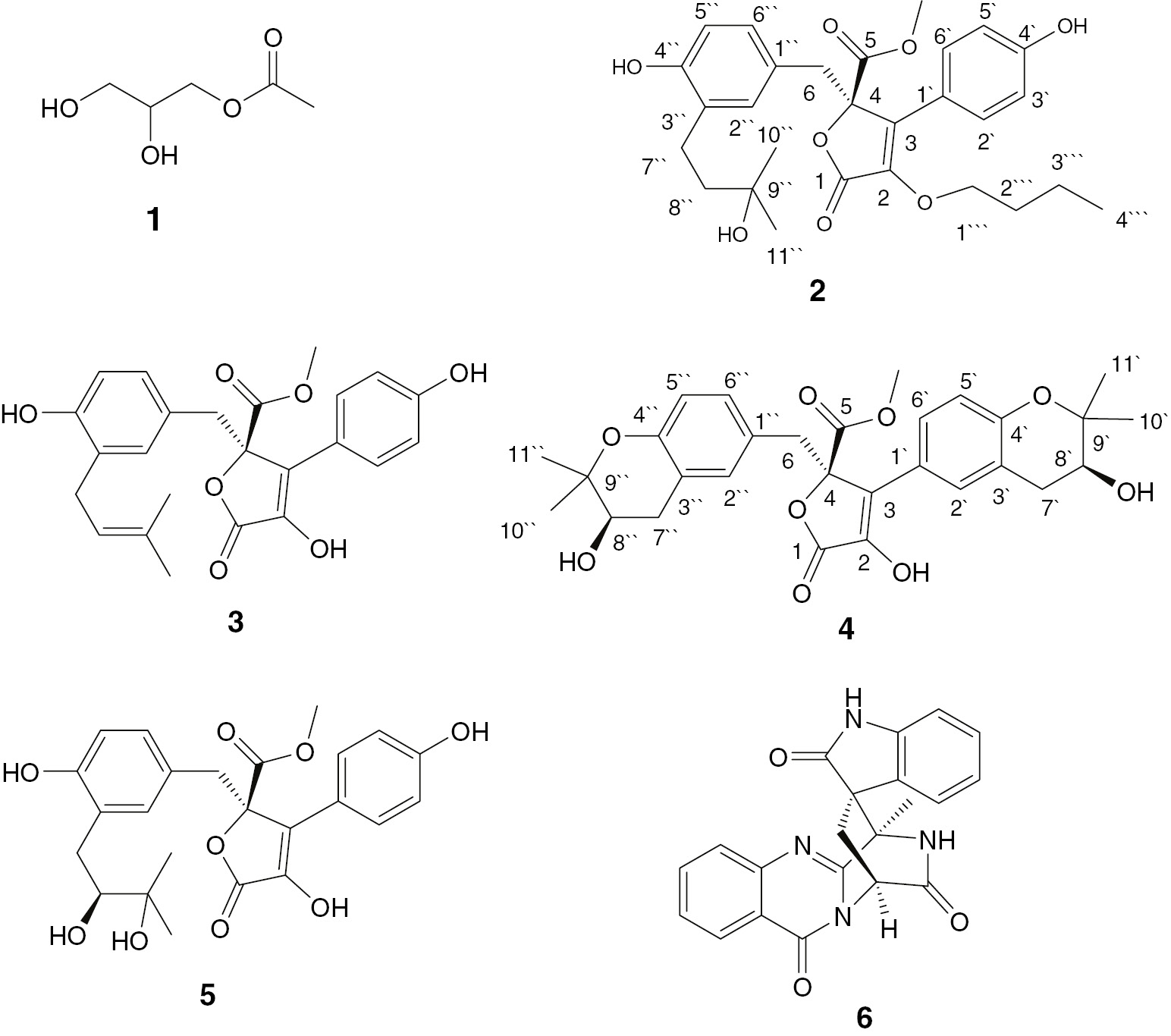
Structures of isolated compounds 1–6.
2 Materials and methods
2.1 General
Optical rotations were measured with a Perkin-Elmer 241 automatic polarimeter (Perkin-Elmer Inc., Waltham, MA, USA). UV spectra were recorded in MeOH on a Shimadzu 1601 UV/VIS spectrophotometer (Shimadzu, Kyoto, Japan). The IR spectra were measured on a Shimadzu Infrared-400 spectrophotometer (Shimadzu, Kyoto, Japan). ESIMS spectra were obtained with an LCQ DECA mass spectrometer (ThermoFinnigan, Bremen, Germany) coupled to an Agilent 1100 HPLC system equipped with a photodiode array detector. HRESIMS was recorded on LTQ Orbitrap mass spectrometer (ThermoFinnigan, Bremen, Germany). 1D and 2D NMR spectra (chemical shifts in ppm, coupling constants in Hz) were recorded on Bruker Avance DRX 500 MHz spectrometers (Bruker BioSpin, Billerica, MA, USA) using DMSO-d6 as solvent. For column chromatography, silica gel (0.063–0.200 mm, Merck, Darmstadt, Germany), RP18 (0.04–0.063 mm, Merck, Darmstadt, Germany), and Sephadex LH-20 (0.25–0.1 mm, Sigma-Aldrich) were used. Pre-coated silica gel 60 F254 plates (0.2 mm, Merck, Darmstadt, Germany) were used for thin-layer chromatography (TLC).
2.2 Isolation and cultivation of the fungal material
Pulicaria crispa was collected from Gabal Al-aquiq, Al Madinah Al Munawwarah, Saudi Arabia in March 2014. The plant was authenticated by Dr. Emad Alsherif, Associate Professor of Plant Ecology, Department of Biology, Faculty of Science and Arts, Khulais, King Abdulaziz University, Saudi Arabia. A specimen (PC-3-2014) was preserved at the herbarium of the Department of Natural Products and Alternative Medicine, Faculty of Pharmacy, King Abdulaziz University. Aspergillus versicolor was isolated from the internal roots tissue of P. crispa. The inner root tissues were carefully dissected under sterile conditions and placed on potato dextrose agar plates (PDA, Difco), containing chloramphenicol and gentamicin as antibacterial agents to prevent bacterial growth. The dishes were incubated at 27 °C for 4–6 weeks. Then, hyphal tips of the fungi were periodically removed and transferred to fresh PDA plates. The fungi were identified on the basis of their colonial morphological trait and microscopic observation, using light microscopy (CX31RBSF, Olympus) [15], which was genetically reinforced by the analysis of ITS sequence (Genbank Accession number AV191832.1). The fungus was deposited at the Department of Microbiology, Faculty of Pharmacy, Taibah University, Al Madinah Al Munawwarah, Saudi Arabia (PC No. MAR32014). The fresh fungal culture was transferred into 10 Erlenmeyer flasks (1 L each) for isolation and identification of secondary metabolites, containing rice solid cultures (100 mL of distilled water were added to 100 g commercially available rice and kept overnight prior to autoclaving). The cultures were then incubated at room temperature for 30 days under septic conditions.
2.3 Extraction and isolation
The rice cultures were extracted with EtOAc and concentrated under vacuum. The concentrated extract was mixed with 100 mL distilled H2O and partitioned between n-hexane and 90% MeOH. The total 90% MeOH extract (5.9 g) was subjected to SiO2 vacuum liquid chromatography (VLC) using n-hexane, EtOAc, and MeOH, which were separately concentrated to give FV1 (1.4 g), FV2 (1.9 g), and FV3 (2.3 g), respectively. Fraction FV-2 (1.9 g) was subjected to normal phase VLC using CHCl3:MeOH gradients (100% CHCl3 to 50:50 CHCl3:MeOH), 100 mL fractions were collected and monitored by TLC to obtain nine sub-fractions: FVE2-1 to FVE2-9. Sub-fraction FVE2-2 (95 mg) was chromatographed over SiO2 CC (20 g, 50×2 cm) using CHCl3:MeOH (99.5:0.5 to 97:3) as an eluent to give impure 1, which was further purified by repeated chromatography on a SiO2 CC using n-hexane:EtOAc gradient to afford 1(12.7 mg, white amorphous powder). SiO2 CC (40 g, 50×2 cm) of sub-fraction FVE2-3 (153 mg) using CHCl3:MeOH in order of increasing polarity afforded impure 2, which was purified on RP18 column (0.04–0.063 mm; 40 g, 50×2 cm) using H2O:MeOH gradient to give 2 (4.7 mg, yellow gum). Sub-fraction FVE2-4 (391 mg) was chromatographed over sephadex LH-20 (30 g, 50×2 cm) using MeOH as an eluent to yield impure 3 and 4. Separately, each one was purified on RP-18 column (0.04–0.063 mm; 40 g, 50×2 cm) using H2O:MeOH gradient to obtain 3 (9.9 mg, yellow gum) and 4 (3.8 mg, yellow gum). Separately, sub-fractions FVE2-5 (186 mg) and FVE2-6 (142 mg), each one was subjected to repeated SiO2 CC (50 g, 50×2 cm) using CHCl3: MeOH (95 to 85:15) as an eluent to give 5(15.1 mg, yellow gum, sub-fraction FVE2-5) and 6(21.0 mg, white powder, sub-fraction FVE2-6).
Aspernolide L (2): Yellow gum. - [α]D25 +48 (c 0.05, MeOH). - UV (MeOH): λmax (log ε)=209 (4.31), 226 (4.03), 316 (3.86) nm. - IR (KBr): 3436, 2978, 1729, 1667, 1056 cm−1. - NMR data (DMSO-d6, 500 and 125 MHz) see Table 1. - HRESIMS m/z 499.2329 (calcd for 499.2332 [M+H]+, C28H35O8).
NMR spectroscopic data of compounds 2and 4(DMSO-d6, 500 and 125 MHz).
| Position | 2 | Position | 4 | ||
|---|---|---|---|---|---|
| δH (mult., J [Hz]) | δC (mult.) | δH (mult., J [Hz]) | δC (mult.) | ||
| 1 | – | 168.1 C | 1 | – | 168.5 C |
| 2 | – | 138.2 C | 2 | – | 138.2 C |
| 3 | – | 128.1 C | 3 | – | 128.7 C |
| 4 | – | 84.7 C | 4 | – | 84.7 C |
| 5 | – | 169.8 C | 5 | – | 169.9 C |
| 6 | 3.42 d (14.9) | 38.1 CH2 | 6 | 3.41 d (16.0) | 38.1 CH2 |
| 3.38 d (14.9) | 3.36 d (16.0) | ||||
| 1′ | – | 119.9 C | 1′ | – | 121.4 C |
| 2′ | 7.50 d (8.2) | 128.8 CH | 2′ | 6.87 d (2.5) | 128.8 CH |
| 3′ | 6.87 d (8.2) | 115.7 CH | 3′ | – | 119.6 C |
| 4′ | – | 157.9 C | 4′ | – | 157.5 C |
| 5′ | 6.87 d (8.2) | 115.7 CH | 5′ | 6.51 d (7.5) | 115.8 CH |
| 6′ | 7.50 d (8.2) | 128.8 CH | 6′ | 7.49 dd (7.5, 2.5) | 131.6 CH |
| 1″ | – | 123.9 C | 7′ | 2.64 dd (16.1, 4.8) | 31.0 CH2 |
| 2.40 dd (16.1, 7.3) | |||||
| 2″ | 6.43 d (2.2) | 131.4 CH | 8′ | 3.71 dd (7.3, 4.8) | 68.5 CH |
| 3″ | – | 121.1 C | 9′ | – | 77.2 C |
| 4″ | – | 152.5 C | 10′ | 1.18 s | 25.7 CH3 |
| 5″ | 6.49 d (8.0) | 116.0 CH | 11′ | 1.18 s | 25.7 CH3 |
| 6″ | 6.47 dd (8.0, 2.2) | 128.7 CH | 1″ | – | 124.4 C |
| 7″ | 2.31 t (6.8) | 28.1 CH2 | 2″ | 6.42 d (2.0) | 131.7 CH |
| 8″ | 1.59 t (6.8) | 39.6 CH2 | 3″ | – | 119.6 C |
| 9″ | – | 73.9 C | 4″ | – | 151.7 C |
| 10″ | 1.23 s | 26.6 CH3 | 5″ | 6.52 d (8.0) | 115.6 CH |
| 11″ | 1.24 s | 26.4 CH3 | 6″ | 6.47 dd (8.0, 2.0) | 129.0 CH |
| 1″′ | 4.14 dd (14.5, 6.8) | 65.5 CH2 | 7″ | 2.64 dd (16.1, 5.5) | 31.0 CH2 |
| 3.90 dd (14.5, 5.3) | 2.40 dd (16.1, 8.0) | ||||
| 2″′ | 1.62 m | 31.9 CH2 | 8″ | 3.74 dd (8.0, 5.5) | 68.0 CH |
| 3″′ | 1.25 m | 21.7 CH2 | 9″ | – | 77.0 C |
| 4″′ | 0.84 t (6.6) | 14.0 CH3 | 10″ | 1.21 s | 25.8 CH3 |
| -OCH3 | 3.76 s | 53.5 CH3 | 11″ | 1.21 s | 25.8 CH3 |
| 4′-OH | 10.71 s | – | -OCH3 | 3.73 s | 53.5 CH3 |
| 4″-OH | 9.85 s | – | OH | 5.13 brs | – |
The bold values are the atoms positions.
Aspernolide M (4): Yellow. - [α]D25 +85 (c 0.03, MeOH). - UV (MeOH): λmax (log ε)=216 (4.12), 236 (3.97), 331 (3.41) nm. - IR (KBr): 3423, 2945, 1733, 1669, 1440, 1117, 1025 cm−1. - NMR data (DMSO-d6, 500 and 125 MHz) see Table 1. - HRESIMS m/z 525.2121 [M+H]+ (calcd C29H33O9, 525.2125).
3 Results and discussion
The culture of A. versicolor was extracted with EtOAc. The extract was subjected repeatedly to SiO2, sephadex, and RP18 CC to afford two new and four known compounds. Compounds 1, 3, 5, and 6 were identified by combined spectroscopic analyses as 1-O-acetylglycerol [16], butyrolactone I [17], butyrolactone VI [1], [2], and (+) alantrypinone [18], [19], respectively. The spectroscopic data for these compounds were in good agreement with those reported in the literature.
Compound 2 was obtained as a yellow gum. Its molecular formula C28H34O8was determined by HRESIMS pseudo-molecular ion peak atm/z499.2329 (calculated for 499.2332 [M+H]+, C28H35O8), indicating 12 degrees of unsaturation. The 1H and 13C NMR spectral data of 2 revealed that 8 of the 12 units of unsaturation were attributed to two phenyl moieties (Figures S3 and S4). In addition, two carbonyls and two olefinic carbons account for three degrees of unsaturation. The IR spectrum showed absorption bands of hydroxyl, carbonyl group, and C–H aromatic at 3436, 1729, and 1667 cm−1, respectively. The 13C, DEPT, and HSQC spectra of 2 revealed the presence of 28 carbon resonances: three methyls, one methoxy (δC 53.5, 5-OCH3), five methylenes, an oxymethylene δC 65.5 (C-1″′), seven methines, and 11 quaternary carbons, including two carbonyls at δC 169.8 (C-5) and 168.1 (C-1), two oxygen-bonded aromatic carbons at δC 157.9 (C-4′) and 152.5 (C-4″), and an oxygenated aliphatic carbon (δC 84.7, C-4), which suggested that 2 had a butyrolactone skeleton. The 1H NMR and 1H-1H COSY spectra displayed four ortho-coupled aromatic protons of a 1,4-disubstituted benzene moiety at δH 7.50 (d, 2H,J=8.2 Hz, H-2′,6′) and 6.87 (d, 2H,J=8.2 Hz, H-3′,5′) (Figure 2). They correlated to the carbons at δC 128.8 and 115.7, respectively, in the HSQC spectrum (Figure S5, Table1).Three aromatic signals at δH 6.43 (d, J=2.2 Hz, H-2″)/δC 131.4, 6.49 (d, J=8.0 Hz, H-5″)/116.0, and 6.47 (dd, J=8.0, 2.2 Hz, H-6″)/128.7 characteristic for a 1,3,4-tri-substituted benzene ring were observed. The observed HMBC cross peaks of H-2′ and H-6′/C-1′ and C-4′, H-3′ and H-5′/C-1′, C-4′, and C-6′, H-2″/C-1″, C-3″, C-4″, and C-6″, H-5″/C-1″ and C-3″, and H-6″/C-1″ and C-2″ confirmed these moieties (Figure 3). Furthermore, a side chain consisting of an oxygenated quaternary carbon (δC 73.9 C-9″), two methylenes at δH 1.59 (t, J=6.8 Hz, H-8″)/δC 39.6 (C-8″) and 2.31 (t, J=6.8 Hz, H-7″)/δC 28.1 (C-7″), and two singlet methyls at δH 1.23 (H-10″)/26.6 (C-10″), and 1.24 (H-11″)/26.4 (C-11″) was observed characteristic for 3-hydroxy-3-methylbutyl moiety. This was confirmed by the observed 1H-1H COSY and HMBC correlations (Figure 3). The HMBC correlations of H-7″/C-2″ and C-4″, H-8″/C-3″, and H-2″/C-7″ indicated its attachment at C-3″ (Figure S6). A methylene group at δH 3.42 and 3.38 (2H, each d, J=14.9 Hz, H-6)/δC 38.1 (C-6) was observed. The HMBC cross peaks of H-6/C-4, C-1″, C-2″, and C-6″ indicated that this methylene was connected to C-4 and C-1″ of the tri-substituted phenyl residue. The singlet signal at δH 3.76 showed HSQC cross peak to the carbon at δC 53.5, attributable to a methoxy group. It had HMBC cross peak to C-5 (δC 169.8). Furthermore, the oxygen-bearing methylene group at δH 4.14 (dd, J=14.5, 6.8 Hz, H-1″′A) and 3.90 (dd, J=14.5, 5.3 Hz, H-1″′B)/δC 65.5 (C-1″′), two multiplet methylenes at δH 1.62 (H-2″′)/31.9 (C-2″′) and 1.25 (H-3″′)/21.7 (C-3″′), and a triplet methyl at δH 0.84 (J=6.6 Hz, H-4″′)/14.0 (C-4″′), indicated the presence of a butyloxy group in 2. The HMBC cross peaks of H-1″′ to C-2 confirmed the connectivity of this group at C-2. The configuration at C-4 of 2 was deduced to be 4R based on biosynthetic considerations and its specific rotation ([α]d +48 (c 0.05, MeOH)). It was reported that the configuration at C-4 could be assigned on the basis of the optical rotation′s sign. Butyrolactones with R-configured C-4 had positive optical rotation values such as butyrolactones I-VIII [20], [21]; however; those with negative optical rotation values possessed S-configured C-4 as in isobutyrolactone II and V [22]. Thus, the structure of2was assigned as methyl (R)-4-butoxy-2-(4-hydroxy-3-(3-hydroxy-3-methylbutyl)benzyl)-3-(4-hydroxyphenyl)-5-oxo-2,5-dihydrofuran-2-carboxylate and named aspernolide L.
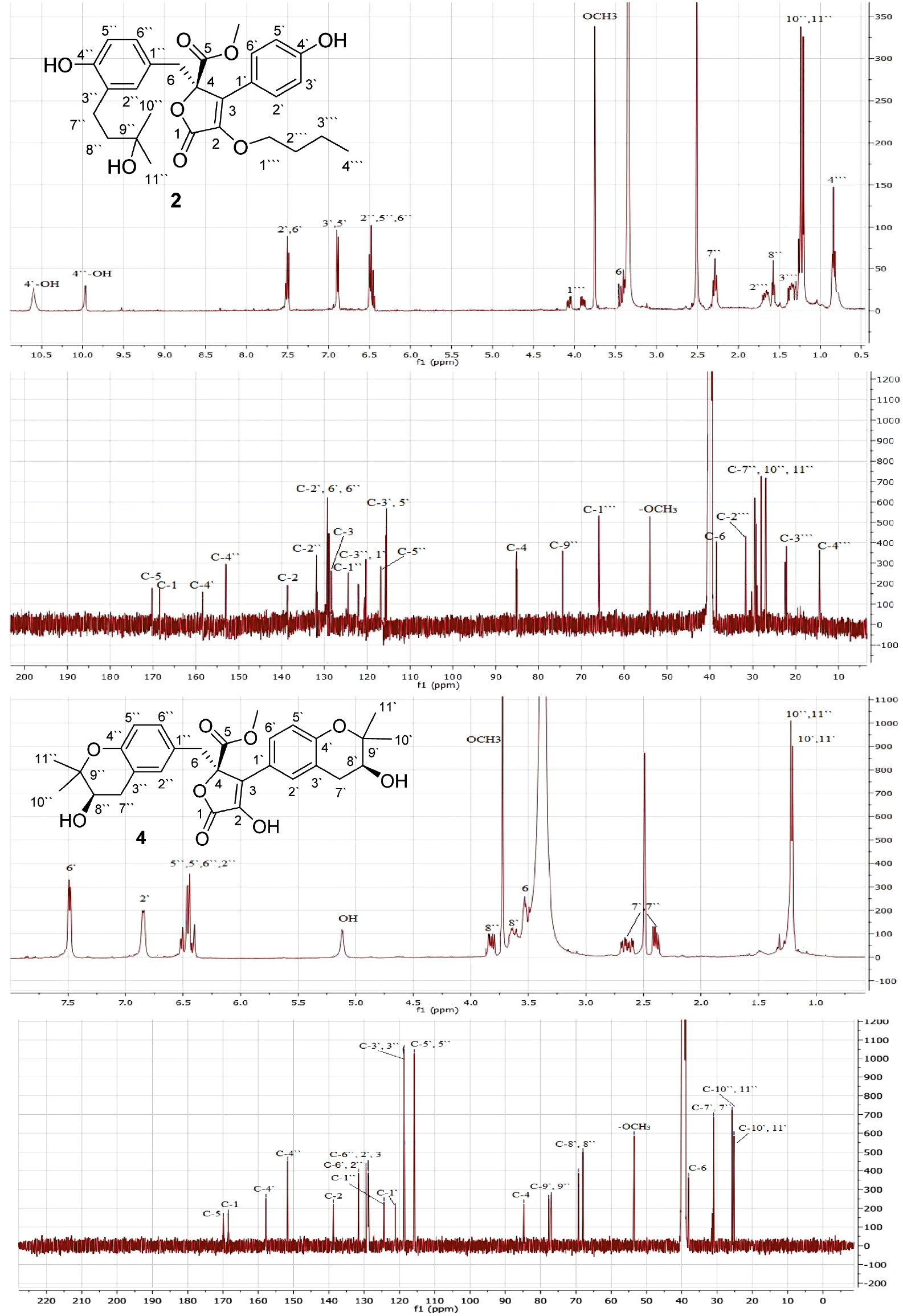
1H and 13C NMR spectra of compounds 2 and 4(DMSO-d6, 500 and 125MHz).
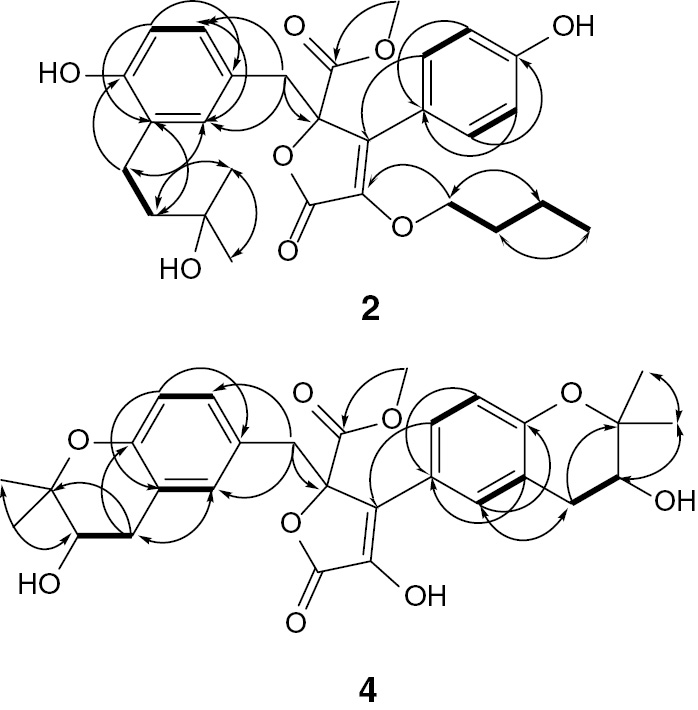
Some key 1H-1H COSY (―) and HMBC (H→C) correlations of 2and 4.
Compound 4 was also obtained as a yellow gum with a molecular formula C29H32O9 determined by its HRESIMS pseudo-molecular ion peak at m/z 525.2121 [M+H]+ (calculated C29H33O9, 525.2125), requiring 14 degrees of unsaturation. Compound 4 was 26 mass units and 2 degrees of unsaturation more than 2. The 1D and 2D NMR spectral data (Table 1) revealed that the structure of 4 was very similar to those of 2except the absence of the signals associated with the 3-hydroxy-3-methylbutyl, butyloxy, and 1,4-disubstituted benzene moieties. It had a 1,3,4-tri-substituted phenyl moiety at δH 6.87 (d, J=2.5 Hz, H-2′)/δC 128.8, 6.51 (d, J=7.5 Hz, H-5′)/115.8, and 7.49 (dd, J=7.5, 2.5 Hz, H-6′)/131.6 instead of the 1,4-disubstituted phenyl moiety in 2 (Figures S9 and S10). It was confirmed by the HMBC cross peaks of H-2′ to C-4′ and C-6′, H-5′ to C-1′, C-3′, and C-4′, and H-6′ to C-1′, C-4′, and C-5′. Its connectivity at C-3 was established by the HMBC cross peaks of H-2′ and H-6′ to C-3. Compared with 2, the two additional degrees of unsaturation, along with the observed 1H and 13C NMR signals, indicating the presence of two 2,2-dimethyltetrahydro-2H-pyran-3-ol rings fused to the two tri-substituted phenyl rings in 4, which was further confirmed by the HMBC correlations of H-7′ to C-2′, C-4′, and C-9′, H-8′ to C-3′, C-10′, and C-11′, H-10′ and H-11′ to C-8′ and C-9′, H-7″ to C-2″, C-4″, and C-9″, H-8″ to C-3″, C-10″, and C-11″, and H-10″ and H-11″ to C-8″ and C-9″, establishing their attachment at C3′-C4′ and C3″-C4″, respectively (Figure S12). The configuration of the secondary alcohols at C-8′ and C-8″ was assigned as S-configured based on the comparison of the 1H and 13C NMR chemical shifts as well as optical rotation value ([α]d +85 (c 0.03, MeOH)) with those of previously reported butyrolactones [1], [2], [23], [24]. This unambiguously led to the elucidation of 4 as methyl (R)-4-hydroxy-3-((S)-3-hydroxy-2,2-dimethylchroman-6-yl)-2-(((R)-3-hydroxy-2,2-dimethylchroman-6-yl)methyl)-5-oxo-2,5-dihydrofuran-2-carboxylate and named aspernolide M.
4 Conclusions
Six compounds (1–6) were isolated and characterized from the EtOAc extract of the Aspergillus versicolor isolated from the roots of Pulicaria crispa (Asteraceae), two of them are new natural products (2and 4). Compounds 1, 5, and 6 are reported for the first time from this fungus.
References
1. Ibrahim SR, Elkhayat ES, Mohamed GA, Khedr AI, Fouad MA, Kotb MHR, et al. Aspernolides F and G, new butyrolactones from the endophytic fungus Aspergillus terreus. Phytochem Lett 2015;14:84–90.10.1016/j.phytol.2015.09.006Search in Google Scholar
2. Elkhayat ES, Ibrahim SR, Mohamed GA, Ross SA, Terrenolide S. A new anti-leishmanial butenolide from the endophytic fungus Aspergillus terreus. Nat Prod Res 2016;30:814–20.10.1080/14786419.2015.1072711Search in Google Scholar PubMed
3. Ibrahim SR, Abdallah HM, Mohamed GA, Ross SA. Integracides H-J: New tetracyclic triterpenoids from the endophytic fungus Fusarium sp. Fitoterapia 2016;112:161–7.10.1016/j.fitote.2016.06.002Search in Google Scholar PubMed
4. Ibrahim SR, Mohamed GA, Ross SA. Integracides F and G: New tetracyclic triterpenoids from the endophytic fungus Fusarium sp. Phytochem Lett 2016;15:125–30.10.1016/j.phytol.2015.12.010Search in Google Scholar
5. Ibrahim SR, Mohamed GA, Moharram AM, Youssef DT. Aegyptolidines A and B: New pyrrolidine alkaloids from the fungus Aspergillus aegyptiacus. Phytochem Lett 2015;12:90–3.10.1016/j.phytol.2015.03.001Search in Google Scholar
6. Sanchez JF, Somoza AD, Keller NP, Wang CC. Advances in aspergillus secondary metabolite research in the post-genomic era. Nat Prod Rep. 2012;29:351–71.10.1039/c2np00084aSearch in Google Scholar PubMed PubMed Central
7. Wu QX, Crews MS, Draskovic M, Sohn J, Johnson TA, Tenney K, et al. Azonazine, a novel dipeptide from a hawaiian marinesediment-derived fungus, Aspergillus insulicola. Org Lett 2010;12:4458–61.10.1021/ol101396nSearch in Google Scholar PubMed PubMed Central
8. Furtado NAJC, Pupo MT, Carvalho I, Campo VL, Duarte MCT, Bastos JK. Diketopiperazines produced by an Aspergillus fumigates Brazilian Strain. J Braz Chem Soc 2005;16:1448–53.10.1590/S0103-50532005000800026Search in Google Scholar
9. Correa MJC, Nunes FM, Bitencourt HR, Borges FC, Guilhon GMSP, Arruda MSP, et al. Biotransformation of chalcones by the endophytic fungus Aspergillus flavus Isolated from Paspalum maritimum Trin. J Braz Chem Soc 2011;22:1333–38.10.1590/S0103-50532011000700019Search in Google Scholar
10. Li GY, Yang T, Luo Yi-Ga, Chen XZ, Fang D-M, Zhang GL. Brevianamide J, a new indole alkaloid dimer from fungus Aspergillus versicolor. Org Lett 2009;11:3714–17.10.1021/ol901304ySearch in Google Scholar PubMed
11. Lin WH, Brauers G, Ebel R, Wray V, Berg AS, Sudarsono, et al. Novel chromone derivatives from the fungus Aspergillus versicolor isolated from the marine sponge Xestospongia exigua. J Nat Prod 2003;66:57–61.10.1021/np020196bSearch in Google Scholar PubMed
12. Lee YM, Li H, Hong J, Cho HY, Bae KS, Kim MA, et al. Bioactive metabolites from the sponge-derived fungus Aspergillus versicolor. Arch Pharm Res 2010;33:231–5.10.1007/s12272-010-0207-4Search in Google Scholar PubMed
13. Fremlin LJ, Piggott AM, Lacey E, Capon RJ. Cottoquinazoline A and Cotteslosins A and B, metabolites from an australian marine-derived strain of Aspergillus versicolor. J Nat Prod 2009;72:666–70.10.1021/np800777fSearch in Google Scholar PubMed
14. Li XB, Zhou YH, Zhu RX, Chang WQ, Yuan HQ, Gao W, et al. Identification and biological evaluation of secondary metabolites from the endolichenic fungus Aspergillus versicolor. Chem Biodiv 2015;12:575–92.10.1002/cbdv.201400146Search in Google Scholar PubMed
15. Rekha C, Shridhar NB, Jagadeesh SS, Narayanaswamy HD. Isolation and identification of fungal isolates from contaminated meadow grass fodder. J Livestock Sci 2015;6:104–8.Search in Google Scholar
16. Yan HJ, Gao SS, Li CS, Li XM, Wang BG. Chemical constituents of a marine-derived endophytic fungus Penicillium commune G2M. Molecules 2010;15:3270–5.10.3390/molecules15053270Search in Google Scholar PubMed PubMed Central
17. Ye Y, Xia C, Yang J, Yang Y, Qin Y, Gao X, et al. Butyrolactones derivatives from the fermentation products of an endophytic fungus Aspergillus versicolor. Bull Korean Chem Soc 2014;35:3059–62.10.5012/bkcs.2014.35.10.3059Search in Google Scholar
18. Larsen TO, Frydenvang K, Frisvad JC, Christophersen C. UV-guided isolation of alantrypinone, a novel Penicillium Alkaloid. J Nat Prod 1998;61:1154–57.10.1021/np980056vSearch in Google Scholar PubMed
19. Ren-Yi G, Lei X, Yi K, Ming C, Jian-Chun Q, Li L, et al. Chaetominine, (+)-Alantrypinone, Questin, Isorhodoptilometrin, and 4-Hydroxybenzaldehyde Produced by the Endophytic Fungus Aspergillus sp. YL-6 Inhibit Wheat (Triticum aestivum) and Radish (Raphanus sativus) Germination. J Plant Interact 2015;10:87–92.10.1080/17429145.2015.1019742Search in Google Scholar
20. Zhou M, Lou J, Li Y, Wang Y, Zhou K, Ji B, et al. Butyrolactones from the endophytic fungus Aspergillus versicolor and their anti-tobacco mosaic virus activity. J Braz Chem Soc 2015;26:545–9.10.5935/0103-5053.20150008Search in Google Scholar
21. San-Martín A, Rovirosa J, Vaca I, Vergara K, Acevedo L, Viña D, et al. New butyrolactone from a marine-derived fungus Aspergillus sp. J Chil Chem Soc 2011;56:625–7.10.4067/S0717-97072011000100023Search in Google Scholar
22. Nong X, Wang Y, Zhang X, Zhou M, Xu X, Qi SH. Territrem and butyrolactone derivatives from a marine-derived fungus Aspergillus Terreus. Mar Drugs 2014;12:6113–24.10.3390/md12126113Search in Google Scholar PubMed PubMed Central
23. Li L-J, Li T-X, Kong L-Y, Yang M-H. Antioxidant aromatic butenolides from an insect-associated Aspergillus iizukae. Phytochem Lett 2016;16:134–40.10.1016/j.phytol.2016.03.014Search in Google Scholar
24. Haritakun R, Rachtawee P, Chanthaket R, Boonyuen N, Isaka M. Butyrolactones from the fungus Aspergillus terreus BCC 4651. Chem Pharm Bull 2010;58:1545–8.10.1248/cpb.58.1545Search in Google Scholar PubMed
Supplemental Material:
The online version of this article (DOI: https://doi.org/10.1515/znc-2016-0070) offers supplementary material, available to authorized users.
©2017 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Aspernolides L and M, new butyrolactones from the endophytic fungus Aspergillus versicolor
- Synthesis, in-vitro cytotoxicity of 1H-benzo[f]chromene derivatives and structure–activity relationships of the 1-aryl group and 9-position
- Biodegradation of micropollutant naproxen with a selected fungal strain and identification of metabolites
- A comparative proteomic analysis for adventitious root formation in lotus root (Nelumbo nucifera Gaertn)
- Curviflorside and curviflorin, new naphthalene glycoside and flavanol from Plicosepalus curviflorus
- Termiglaucescin, a new polyhydroxy triterpene glucoside from Terminalia glaucescens with antioxidant and anti-inflammatory potential
- Influence of structure of lactones with the methylcyclohexene and dimethylcyclohexene ring on their biotransformation and antimicrobial activity
- Effects of Hypericum perforatum extract on oxaliplatin-induced neurotoxicity: in vitro evaluations
- Bioenergetic strategy of microalgae for the biodegradation of tyrosol and hydroxytyrosol
Articles in the same Issue
- Frontmatter
- Aspernolides L and M, new butyrolactones from the endophytic fungus Aspergillus versicolor
- Synthesis, in-vitro cytotoxicity of 1H-benzo[f]chromene derivatives and structure–activity relationships of the 1-aryl group and 9-position
- Biodegradation of micropollutant naproxen with a selected fungal strain and identification of metabolites
- A comparative proteomic analysis for adventitious root formation in lotus root (Nelumbo nucifera Gaertn)
- Curviflorside and curviflorin, new naphthalene glycoside and flavanol from Plicosepalus curviflorus
- Termiglaucescin, a new polyhydroxy triterpene glucoside from Terminalia glaucescens with antioxidant and anti-inflammatory potential
- Influence of structure of lactones with the methylcyclohexene and dimethylcyclohexene ring on their biotransformation and antimicrobial activity
- Effects of Hypericum perforatum extract on oxaliplatin-induced neurotoxicity: in vitro evaluations
- Bioenergetic strategy of microalgae for the biodegradation of tyrosol and hydroxytyrosol


