Abstract
A new Cu(II) complex, [Cu2(1,3-BMIB)(OBA)2]n (1) (1,3-BMIB = 1,3-bis(2-methyl-1H-imidazol-1-yl)benzene, H2OBA = 4,4′-oxybis(benzoic acid)) was hydrothermally synthesized and has been structurally characterized. Complex 1 possesses a 3D 2-fold interpenetrating framework based on [Cu(OBA)]n layers with a Schläfli symbol {412.63}. Complex 1 displays excellent photocatalytic properties in the degradation of methylene blue (MB) and rhodamine B (RhB).
1 Introduction
Over recent decades, growing attention has focused on coordination polymers (CPs) owing to their diverse topological structures and potential applications, such as photocatalysis, gas storage, molecular magnets, luminescence sensors and others [1], [2], [3], [4]. There is no doubt that the final property of CPs rests with many factors, including but not limited to solvent, pH value, temperature and counter ions [5], [6], [7]. Generally, the reasonable design of organic bridging ligands plays an important role in constructing CPs [8]. 1,3-bis(2-methyl-1H-imidazol-1-yl)benzene (1,3-BMIB) as a V-shaped 2-methylimidazole ligand can form various novel polymer structures by using different geometrical conformations [9], [ 10]. 4,4′-Oxybis(benzoic acid) (H2OBA) as a multidentate O-donor ligand with its strong coordination ability and many coordination modes has also been used to construct multi-dimensional networks [11], [ 12]. In this study, H2OBA and 1,3-BMIB were selected as the organic carboxylate and auxiliary ligands to react with CuCl2·2H2O to obtain a novel coordination polymer, [Cu2(1,3-BMIB)(OBA)2]n (1). The photocatalytic properties of complex 1 for the degradation of MB and RhB were examined under UV irradiation in solvent suspensions.
2 Experimental
2.1 Chemicals and reagents
Reagents and solvents were purchased from the Jinan Camolai Trading Company and used directly without any further treatment. The synthetic method for the 1,3-BMIB ligand was based on literature [13], [ 14].
2.2 Physical measurements
The C, H and N elemental analysis were performed on a Vario EL III elemental analyzer. Thermal gravimetric analysis (TGA) was carried out under N2 atmosphere on a Perkin-Elmer Pyris 1 TGA analyzer in the temperature range 25–800 °C with a heating rate of 10 K min–1. FT-IR spectra ranging from 4000 to 400 cm−1 were recorded on a VECTOR 22 spectrometer by using KBr pellets. The fluorescence spectrum was obtained from a Fluoro Max-P spectrophotometer at room temperature.
2.3 Synthesis of [Cu2(1,3-BMIB)(OBA)2]n (1)
A mixture of H2OBA (25.8 mg, 0.1 mmol), 1,3-BMIB (23.8 mg, 0.1 mmol), CuCl2·2H2O (17.0 mg, 0.1 mmol), H2O (2 mL) and DMF (4 mL) was placed in a Teflon-lined stainless steel vessel, heated to 100 °C for two days, and then cooled to room temperature over 12 h. Blue crystals of 1 were obtained. Yield: 52% based on CuCl2·2H2O. Elemental analysis (%) calcd for C42H30Cu2N4O10 (877.800): C 57.47, H 3.44, N 6.38; found C 57.65, H 3.45, N 6.40. IR (cm−1): 3438 w, 1672 m, 1629 s, 1503 m, 1400 s, 1235 s, 1163 m, 1095 w, 1008 w, 878 m, 776 m, 741 w, 661 m, 460 w.
2.4 Photocatalytic measurement
Typical degradation experiments were done as follows: 30 mg of complex 1 and 10 μL of 30% H2O2 were mixed into 50 mL of the prepared aqueous solutions of MB and RhB (10 mg L−1) with magnetic stirring under irradiation by a 300 W medium-pressure mercury vapor lamp. To guarantee the adsorption-desorption equilibrium, the solutions were magnetically stirred in the dark for 30 min before irradiation. Later, 1 mL of the reaction solution was taken and analyzed with a UV/Vis spectrophotometer at specific intervals. The MB and RhB concentrations were measured by using the UV/Vis spectrophotometer at their maximum absorbance wavelength of 664 and 550 nm, respectively.
2.5 X-ray crystallography
Crystallographic data for complex 1 were collected on a Bruker Smart CCD diffractometer with MoKα radiation (λ = 0.71073 Å) using the ω − 2θ scan mode at T = 173(2) K. An absorption corrections was applied by the multi-scan program Sadabs. The structure was solved by Direct Methods and refined by full-matrix least-squares techniques based on F2 using the program package Shelxl-2014 [15]. All nonhydrogen atoms were treated anisotropically. The hydrogen atoms of the organic ligands were geometrically generated using a riding model and isotropically refined. Residual minor interstitial solvent molecules in crystals of 1 were highly disordered and removed from the diffraction data using the routine SQUEEZE of Platon [16]. The final crystal structure data as summarized in Table 1 refers to the complex 1 without additional crystal solvent molecules. Selected bond lengths and angles are listed in Table 2.
Crystal structure data of 1.
| Empirical formula | C42H30Cu2N4O10 |
|---|---|
| Crystal size/mm3 | 0.27 × 0.25 × 0.22 |
| Formula weight | 877.8 |
| Crystal system | Orthorhombic |
| Space group | Cmca (no. 64) |
| a/Å | 25.7247(17) |
| b/Å | 22.2653(13) |
| c/Å | 16.7381(10) |
| V/Å3 | 9587.0(10) |
| Z | 8 |
| Dcalcd/g cm−3 | 1.216 |
| μ (MoKα)/mm−1 | 0.940 |
| F(000), e | 3584 |
| Limits of data collection/deg | 3.045 ≤ θ ≤ 25.386 |
| Reflections collected | 17518 |
| Independent reflections; Rint | 4478; 0.0622 |
| Data; ref. parameters | 4478; 266 |
| R1; wR2 [I > 2 σ(I)] | 0.0795; 0.2152 |
| R1; wR2 (all data) | 0.1147; 0.2342 |
| GOF on F2 | 1.213 |
| Largest diff. peak and hole/e·A−3 | 1.16; −2.13 |
aR1 = Σ||Fo| – |Fc||/Σ|Fo|. bwR2 = [Σw(Fo2 – Fc2)2/Σw(Fo2)2]1/2, w = [σ2(Fo2) + (0.1184P)2]−1, where P = (Max(Fo2, 0) + 2Fc2)/3. cGoF = S = [Σw(Fo2 – Fc2)2/(nobs – nparam)]1/2.
Selected bond lengths (Å) and angles (deg) for 1
| Cu(1)–O(2) | 1.978(4) | Cu(1)–O(1)C | 1.975(4) | Cu(1)–N(1) | 2.141(5) |
|---|---|---|---|---|---|
| Cu(1)–O(5)A | 1.964(4) | Cu(1)–O(4)B | 1.967(4) | ||
| O(2)–Cu(1)–N(1) | 97.42(18) | O(2)–Cu(1)–O(1)C | 167.13(17) | O(5)A–Cu(1)–O(2) | 88.81(19) |
| O(5)A–Cu(1)–N(1) | 101.41(18) | O(4)B–Cu(1)–O(2) | 89.7(2) | O(1)C–Cu(1)–N(1) | 95.39(18) |
| O(5)A–Cu(1)–O(1)C | 89.83(17) | O(4)B–Cu(1)–N(1) | 91.61(19) | O(4)B–Cu(1)–O(1)C | 88.78(19) |
| O(5)A–Cu(1)–O(4)B | 166.98(18) |
Symmetry transformations used to generate equivalent atoms: A = x, y – 1/2, 1/2 – z; B = 1/2 – x, 1 – y, z – 1/2; C = 1/2 – x, 1/2 – y, –z.
CCDC 1982748 for 1 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre viawww.ccdc.cam.ac.uk/data_request/cif.
3 Results and discussion
3.1 Description of the structure
Crystal structure analysis revealed that complex 1 crystallizes in the orthorhombic space group Cmca and involves one Cu(II) ion, one OBA2– ligand and one-half 1,3-BMIB ligand in the asymmetric unit. As described in Figure 1, the Cu(II) center adopts square pyramidal {CuO4N} geometry encircled by four oxygen atoms (O2, O5A, O4B and O1C) from four different OBA2– ligands and one nitrogen atom (N1) from one 1,3-BMIB ligand (symmetry code: A = x, y – 1/2, 1/2 – z; B = 1/2 – x, 1 – y, z – 1/2; C = 1/2 – x, 1/2 – y, –z). The bond lengths around Cu(II) ions are in the region of 1.964(4)–2.141(5) Å. The coordination angles around the Cu(II) centers are in the range from 88.78(19) to 167.13(17)° (Table 2).

A view of the local coordination of the CuII cations in complex 1. The molecular entity shown has crystallographic mirror symmetry, the mirror plane passing through the atoms C(19) and C(21). Symmetry codes: A = x, y – 1/2, 1/2 – z; B = 1/2 – x, 1 – y, z – 1/2; C = 1/2 – x, 1/2 – y, –z; D = –x, y, z; E = x – 1/2, –y + 1/2, –z; F = x – 1/2, –y + 1, z – 1/2; G = –x, y – 1/2, –z + 1/2.
In complex 1, each H2OBA ligand is completely deprotonated and links four Cu(II) ions with (κ1-κ1)-(κ1-κ1)-μ4 coordination mode to generate a [Cu(OBA)]n layer (Figure 2b) based on paddle-wheel [Cu2(OCO)4] secondary building units (SBUs) with a Cu···Cu distance of 2.683 Å (Figure 2a). Moreover, these [Cu(OBA)]n layers are extended into a complicated 3D framework by 1,3-BMIB co-ligands acting as bridging pillars (Figure 3).
![Figure 2: View of the [Cu2(OCO)4] SBUs (a) and of a layer of complex 1 (b). The Cu dimer in (a) has crystallographic inversion symmetry, as have the large rings shown in (b). For symmetry codes see caption to Figure 1.](/document/doi/10.1515/znb-2020-0085/asset/graphic/j_znb-2020-0085_fig_002.jpg)
View of the [Cu2(OCO)4] SBUs (a) and of a layer of complex 1 (b). The Cu dimer in (a) has crystallographic inversion symmetry, as have the large rings shown in (b). For symmetry codes see caption to Figure 1.

View of the 3D structure of complex 1.
From the viewpoint of network topology, each [Cu2(OCO)4] SBU connects six adjacent [Cu2(OCO)4] SBUs and is 6-connected. The whole framework can be simplified as a 6-connected net with {412.63} topology. The three-dimensional coordination polymer of complex 1 leaves a large cavity. In order to stabilize the framework, the potential void space is filled by another identical network, leading to the two-fold mutually interpenetrating three-dimensional architecture (Figure 4).
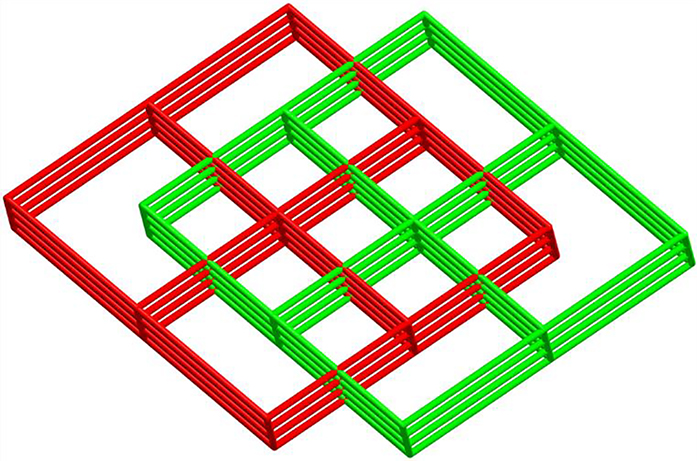
A schematic representation of the twofold interpenetrating framework in the structure of complex 1.
3.2 Thermal analysis
As shown in Figure 5, the weight of complex 1 is stable up to T = 120 °C and then the 1,3-BMIB and H2OBA ligands start to decompose in the temperature range 120–773 °C. Finally, the remaining product corresponds to CuO (found: 18.3%; calcd: 18.1%).

Thermogravimetric analysis of 1.
3.3 Photocatalytic properties
The photocatalysis activity of complex 1 was investigated by use of two organic model pollutants (MB and RhB). As shown in Figure 6, the characteristic absorption peaks obviously decreased as the reaction time increased. From Figure 7, the degradation rates of MB and RhB were 13 and 14% without complex 1, respectively. After adding complex 1, the rates increased to 96% (MB) and 91% (RhB) within 210 min, which indicated that complex 1 shows remarkable photocatalytic activity and may be a potential candidate for photocatalytic degradation of MB and RhB.

UV/Vis absorption spectra of the MB (a) and RhB (b) solution during the decomposition reaction in the presence of complex 1.

Photocatalytic decomposition rate of the MB and RhB solution under UV irradiation.
4 Conclusions
A coordination polymer, [Cu2(1,3-BMIB)(OBA)2]n (1) was synthesized from 1,3-bis(2-methyl-1H-imidazol-1-yl)benzene (1,3-BMIB), 4,4′-oxybis(benzoic acid) (H2OBA) and CuCl2·2H2O in a hydrothermal reaction. Complex 1 is a 3D 2-fold interpenetrating framework based on [Cu(OBA)]n layers. More importantly, complex 1 displays excellent good photocatalytic activity in the degradation of MB and RhB.
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Zhao, S. N., Song, X. Z., Song, S. Y., Zhang, H.-J. Coord. Chem. Rev. 2017, 337, 80–96; https://doi.org/10.1016/j.ccr.2017.02.010.Search in Google Scholar
2. Bar, A. K., Pichon, C., Coord, Sutter. J. P. Chem. Rev. 2016, 308, 346–380; https://doi.org/10.1016/j.ccr.2015.06.013.Search in Google Scholar
3. Dai, F., Fan, W., Bi, J., Jiang, P., Liu, D., Zhang, X., Lin, H., Gong, C., Wang, R., Zhang, L., Sun, D. Dalton Trans. 2016, 45, 61–65; https://doi.org/10.1039/c5dt04025f.Search in Google Scholar
4. Lustig, W. P., Mukherjee, S., Rudd, N. D., Desai, A. V., Li, J., Ghosh, S. K. Chem. Soc. Rev. 2017, 46, 3242–3285; https://doi.org/10.1039/c6cs00930a.Search in Google Scholar
5. Han, R.-M., Ma, J.-F., Liu, Y.-Y., Yang, J. CrystEngComm 2013, 15, 5641–5653; https://doi.org/10.1039/c3ce40528a.Search in Google Scholar
6. Lv, X., Liu, L., Huang, C., Guo, L., Wu, J., Hou, H., Fan, Y. Dalton Trans. 2014, 43, 15475–15481; https://doi.org/10.1039/c4dt02342k.Search in Google Scholar
7. Ramaswamy, P., Wong, N. E., Shimizu, G. K. H. Chem. Soc. Rev. 2014, 43, 5913–5932; https://doi.org/10.1039/c4cs00093e.Search in Google Scholar
8. Liu, H. Y., Wu, H., Ma, J. F., Liu, Y.-Y., Liu, B., Yang, J. Cryst. Growth Des. 2010, 10, 4795–4805; https://doi.org/10.1021/cg100688z.Search in Google Scholar
9. Chen, N.-N., Ni, J.-N., Wang, J., Tao, J.-Q. Acta Crystallogr. 2019, C75, 196–199; https://doi.org/10.1107/s2053229619000986.Search in Google Scholar
10. Wang, X. L., Li, J., Lin, H. Y., Hu, H.-L., Chen, B.-K., Mu, B. Solid State Sci. 2009, 11, 2118–2124; https://doi.org/10.1016/j.solidstatesciences.2009.08.017.Search in Google Scholar
11. Li, C. P., Chen, J., Mu, Y. H., Miao, D. Dalton Trans. 2015, 44, 11109–11118; https://doi.org/10.1039/c5dt00420a.Search in Google Scholar
12. Wu, L., Chigan, D., Yan, L., Chen, H. RSC Adv. 2017, 7, 5541–5548; https://doi.org/10.1039/c6ra26855b.Search in Google Scholar
13. Yin, C., Zhao, C., Xu, X.-J. Z. Naturforsch. 2019, 74b, 861–864; https://doi.org/10.1515/znb-2019-0134.Search in Google Scholar
14. Wang, J., Chen, N.-N., Zhang, C., Jia, L.-Y., Fan, L. CrystEngComm 2020, 22, 811–820; https://doi.org/10.1039/c9ce01474h.Search in Google Scholar
15. Sheldrick, G. M. Acta Crystallogr. 2015, C71, 3–8.Search in Google Scholar
16. Spek, A. L. Acta Crystallogr. 2009, D65, 148–155; https://doi.org/10.1107/s090744490804362x.Search in Google Scholar
© 2021 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this issue
- Synthesis and evaluation of α-glucosidase inhibitory activity of sulfonylurea derivatives
- Discovery of novel obovatol-based phenazine analogs as potential antifungal agents: synthesis and biological evaluation in vitro
- Fluorine analogs of dicamba and tricamba herbicides; synthesis and their pesticidal activity
- Synthesis and crystal structure analyses of tri-substituted guanidine-based copper(II) complexes
- Synthesis, cytotoxicity and in silico study of some novel benzocoumarin-chalcone-bearing aryl ester derivatives and benzocoumarin-derived arylamide analogs
- The guanidinium t-diaqua-bis(oxalato)chromate(III) dihydrate complex: synthesis, crystal structure, EPR spectroscopy and magnetic properties
- Synthesis of the scandium chloride hydrates ScCl3·3H2O and Sc2Cl4(OH)2·12H2O and their characterisation by X-ray diffraction, 45Sc NMR spectroscopy and DFT calculations
- Oxidative addition of a 8-bromotheobromine derivative to d10 metals
- A 3D 2-fold interpenetrating Cu(II) coordination polymer based on 4,4′-oxybis(benzoic acid) and 1,3-bis(2-methyl-imidazol-1-yl) benzene exhibiting photocatalytic properties
- Crystal structure of the new silicide LaNi11.8–11.4Si1.2–1.6
Articles in the same Issue
- Frontmatter
- In this issue
- Synthesis and evaluation of α-glucosidase inhibitory activity of sulfonylurea derivatives
- Discovery of novel obovatol-based phenazine analogs as potential antifungal agents: synthesis and biological evaluation in vitro
- Fluorine analogs of dicamba and tricamba herbicides; synthesis and their pesticidal activity
- Synthesis and crystal structure analyses of tri-substituted guanidine-based copper(II) complexes
- Synthesis, cytotoxicity and in silico study of some novel benzocoumarin-chalcone-bearing aryl ester derivatives and benzocoumarin-derived arylamide analogs
- The guanidinium t-diaqua-bis(oxalato)chromate(III) dihydrate complex: synthesis, crystal structure, EPR spectroscopy and magnetic properties
- Synthesis of the scandium chloride hydrates ScCl3·3H2O and Sc2Cl4(OH)2·12H2O and their characterisation by X-ray diffraction, 45Sc NMR spectroscopy and DFT calculations
- Oxidative addition of a 8-bromotheobromine derivative to d10 metals
- A 3D 2-fold interpenetrating Cu(II) coordination polymer based on 4,4′-oxybis(benzoic acid) and 1,3-bis(2-methyl-imidazol-1-yl) benzene exhibiting photocatalytic properties
- Crystal structure of the new silicide LaNi11.8–11.4Si1.2–1.6


