Abstract
Ca12Ge17B8O58 was prepared by high-temperature solid state synthesis at 1100°C in a platinum crucible from calcium carbonate, boric acid, and germanium(IV) oxide. The compound crystallizes in the tetragonal crystal system in the space group P4̅ (No. 81) isotypically to Cd12Ge17B8O58. The structure was refined from single-crystal X-ray diffraction data: a = 15.053(8), c = 4.723(2) Å, V = 1070.2(2) Å3, R1 = 0.0151, and wR2 = 0.0339 for all data. The crystal structure of Ca12Ge17B8O58 consists of [Ge4O12]n chains composed of GeO4 tetrahedra and GeO6 octahedra. The chains are interconnected into a [Ge4O10.5]n network via corner sharing. By additional [Ge(B2O7)4]28– clusters, these units are connected to a three-dimensional [Ge17B8O58]24– framework. The open structure forms three types of tunnels with five-, six-, and seven-membered rings (MRs) along the c axis, where the Ca2+ are located.
1 Introduction
In the last few years, the interest in the class of metal borogermanates has steadily been increasing. Borogermanates are known for their interesting physical properties like luminescence, ferro-, pyro-, or piezoelectricity and nonlinear optical (NLO) effects [1], [2], [3], [4], [5], [6], [7], [8]. The compounds could be used as second harmonic generation (SHG) materials in laser applications, e.g. the SHG response of K2GeB4O9 · 2 H2O is two times higher than that of the well known reference KDP [6], [7]. Other compounds like Eu2GeB2O8 and Tb2GeB2O8 are luminescent materials for red and green light emission, respectively [8].
At the beginning of our project, a variety of alkali borogermanates such as A2GeB4O9 (A = Rb, Cs), AGeB3O7 (A = Rb, Cs), Rb4Ge3B6O17, K2GeB4O9 · 2 H2O [1], [3], [6], [7], and alkaline earth borogermanates like Ba3Ge2B6O16, Ba3[Ge2B7O16(OH)2](OH)(H2O), Ca10Ge16B6O51, SrGe2B2O8, and Sr3Ge2B6O16 were known [1], [2], [4]. In the year 2014, our group successfully synthesized the compound Sr3–x/2B2–xGe4+xO14 (x = 0.32) as the first boron containing member of the langasite family [9]. Furtheron, we focused our research on the system CaCO3-GeO2-H3BO3, where it was possible to synthesize a new colorless compound, which could not be identified using database reports. A single-crystal structure determination of the new unknown phase has revealed a novel calcium borogermanate with the composition Ca12Ge17B8O58, being isotypic to Cd12Ge17B8O58 [2]. In this work, we report the synthesis, the single crystal structure determination, and IR spectroscopic investigations of this new compound.
2 Experimental section
2.1 Synthesis
According to Eq. 1, a stoichiometric mixture of the starting materials CaCO3 (99.95%, Stream Chemicals, Newburyport, MA, USA), GeO2 (99.99%, ChemPur, Karlsruhe, Germany), and H3BO3 (99.5%, Merck, Darmstadt, Germany) was finely ground in an agate mortar and filled into a platinum FKS 95/5 crucible (feinkornstabilisiert, 95% Pt, 5% Au, Ögussa, Wien, Austria).
Calcination was performed in an electric resistance furnace (Nabertherm muffle furnace). The sample was heated up to 1100°C with a rate of 275°C h–1 and maintained at that temperature for 20 h. After that, the temperature was lowered to 500°C with a rate of 3°C h–1 before switching off the furnace. The product naturally cooled down to room temperature.
The new compound Ca12Ge17B8O58 could be obtained in form of colorless, air- and water-resistant crystals. The powder diffraction pattern (Fig. 1) showed reflections of Ca12Ge17B8O58 as the major phase. The eight reflections marked with asterisks could not be assigned up to now.
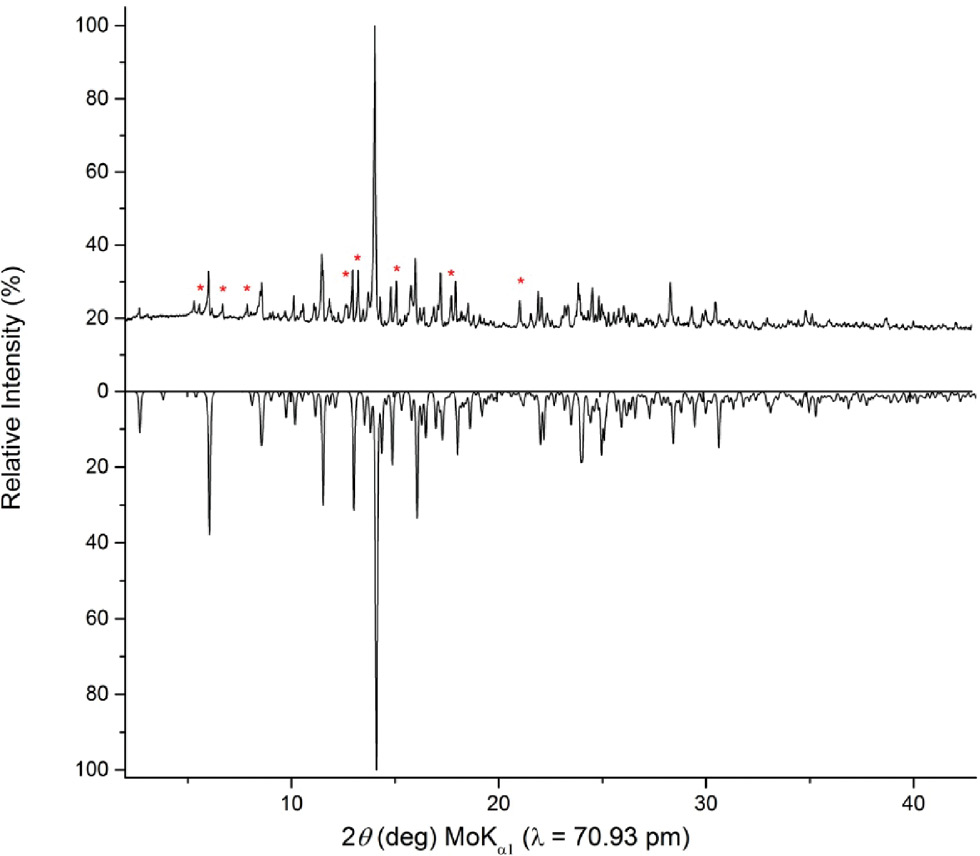
Top: experimental powder pattern of Ca12Ge17B8O58. The reflections marked with a red asterisk could not be assigned until now. Bottom: theoretical powder pattern of Ca12Ge17B8O58 based on single-crystal diffraction data.
2.2 Crystal structure analysis
The powder diffraction pattern of Ca12Ge17B8O58 was obtained in transmission geometry from a flat sample of the reaction product, using a Stoe Stadi P powder diffractometer with Ge (111)-monochromatized MoKα1 (λ = 70.93 pm) radiation. The comparison of the experimental powder pattern with the theoretical pattern simulated from the single-crystal data in Fig. 1 exhibited that they match well.
Small single crystals of Ca12Ge17B8O58 were selected by mechanical fragmentation using a polarization microscope. A Bruker D8 Quest Kappa diffractometer with Mo-Kα radiation (λ = 71.073 pm) was used to collect the single crystal intensity data at room temperature. A multi-scan absorption correction (Sadabs-2014 [10]) was applied to the intensity data sets. All relevant details of the data collection and the refinement are listed in Table 1. According to the systematic extinctions, the tetragonal space group P4̅ (No. 81) was derived for the crystal. Due to the fact that Ca12Ge17B8O58 is isotypic to Cd12Ge17B8O58 [2], the structural refinement was performed by using the positional parameters of Cd12Ge17B8O58 as starting values (Shelxl-13 [11], [12]). All atoms were refined anisotropically and the final difference Fourier synthesis did not reveal any significant residual peaks, leading to values of 0.0151 and 0.0339 for R1 and wR2, respectively. The refinement exhibited that the measured crystal was a twin, with the twin law (01̅0, 1̅00, 001̅). The atomic coordinates, anisotropic displacement parameters, and interatomic distances are listed in the Tables 2–4 . Graphical representations of the structure were produced with the program Diamond [13].
Crystal data and structure refinement of Ca12Ge17B8O58 (standard deviations in parentheses).
| Empirical formula | Ca12 Ge17B8O58 |
| Molar mass, g·mol–1 | 2730.27 |
| Crystal system | Tetragonal |
| Space group | P4̅ (No. 81) |
| Powder data | |
| Powder diffractometer | STOE Stadi P |
| Radiation | MoKα1 (λ = 70.93 pm) |
| a, Å | 15.005(6) |
| c, Å | 4.701(4) |
| V, Å3 | 1058.4(8) |
| Single crystal data | |
| Single crystal diffractometer | Bruker D8 Quest Kappa |
| Radiation | MoKα (λ = 71.073 pm) |
| a, Å | 15.053(8) |
| c, Å | 4.723(2) |
| V, Å3 | 1070.2(2) |
| Formula units per cell | 1 |
| Calculated density, g cm–3 | 4.24 |
| Crystal size, mm3 | 0.08 × 0.05 × 0.03 |
| Temperature, K | 275(2) |
| Absorption coefficient, mm–1 | 13.4 |
| F(000), e | 1288 |
| θ range, deg | 2.7–36.5 |
| Range in hkl | ±25, ±25, ±7 |
| Total no. of reflections | 54 998 |
| Independent reflections | 5232 |
| Reflections with I ≥ 2σ(I) | 5169 |
| Data/parameters | 5169/216 |
| Absorption correction | Multi-scan |
| Goodness-of-fit on Fi2 | 1.065 |
| Final R1/wR2 [I ≥ 2 σ(I)] | 0.0146/0.0338 |
| Final R1/wR2 (all data) | 0.0151/0.0339 |
| BASF | 0.5019 |
| Flack parameter | 0.002(4) |
| Largest diff. peak/hole, e Å–3 | 0.54/–1.07 |
Atomic coordinates and equivalent isotropic displacement parameters Ueq (Å2) of Ca12Ge17B8O58 with standard deviations in parentheses.
| Atom | Wyckoff position | x | y | z | Ueqa |
|---|---|---|---|---|---|
| Ca1 | 4h | 0.89082(3) | 0.85484(3) | 0.9925(2) | 0.00638(7) |
| Ca2 | 4h | 0.36340(3) | 0.75106(3) | 0.9949(2) | 0.00682(7) |
| Ca3 | 4h | 1.00858(3) | 0.67048(3) | 0.9908(2) | 0.00763(8) |
| Ge1 | 4h | 0.71772(2) | 0.81076(2) | 0.50096(11) | 0.00420(4) |
| Ge2 | 4h | 0.57951(2) | 0.78370(2) | 1.00144(11) | 0.00416(4) |
| Ge3 | 1b | 0 | 0 | 1/2 | 0.00412(7) |
| Ge4 | 4h | 0.53978(2) | 0.64481(2) | 0.50562(11) | 0.00460(4) |
| Ge5 | 4h | 0.48693(2) | 0.89366(2) | 0.50148(12) | 0.00443(4) |
| B1 | 4h | 0.81247(19) | 0.97612(18) | 0.5378(7) | 0.0059(6) |
| B2 | 4h | 0.70838(17) | 1.12165(17) | 0.5284(8) | 0.0047(5) |
| O1 | 4h | 0.77131(14) | 0.89579(14) | 0.6865(4) | 0.0059(3) |
| O2 | 4h | 0.90365(13) | 0.98363(14) | 0.7020(4) | 0.0059(3) |
| O3 | 4h | 0.81886(15) | 0.96965(13) | 0.2455(5) | 0.0070(3) |
| O4 | 4h | 0.76121(14) | 1.05043(14) | 0.6546(4) | 0.0063(3) |
| O5 | 4h | 0.61612(14) | 1.11002(15) | 0.6738(4) | 0.0078(3) |
| O6 | 4h | 0.74411(15) | 1.20473(13) | 0.6749(5) | 0.0072(4) |
| O7 | 4h | 0.70577(14) | 1.12664(14) | 0.2330(4) | 0.0068(3) |
| O8 | 2g | 1/2 | 0 | 0.3341(7) | 0.0098(5) |
| O9 | 4h | 0.56710(14) | 0.88733(15) | 0.7716(4) | 0.0065(3) |
| O10 | 4h | 0.44194(14) | 0.60329(15) | 0.3442(5) | 0.0097(4) |
| O11 | 4h | 0.60642(13) | 0.68580(13) | 0.2306(4) | 0.0062(3) |
| O12 | 4h | 0.48337(13) | 0.81459(14) | 0.2299(5) | 0.0065(3) |
| O13 | 4h | 0.50129(14) | 0.71818(14) | 0.7689(4) | 0.0063(3) |
| O14 | 4h | 0.67190(14) | 0.74558(14) | 0.7702(4) | 0.0061(3) |
| O15 | 4h | 0.65114(14) | 0.85616(13) | 0.2354(4) | 0.0065(3) |
aUeq is defined as one third of the trace of the orthogonalized Uij tensor.
Anisotropic displacement parameters Uij (Å2) of Ca12Ge17B8O58 with standard deviations in parentheses.
| Atom | U11 | U22 | U33 | U23 | U13 | U12 |
|---|---|---|---|---|---|---|
| Ge1 | 0.00402(8) | 0.00452(8) | 0.00404(8) | 0.00038(19) | –0.00017(18) | –0.00014(7) |
| Ge2 | 0.00382(8) | 0.00445(8) | 0.00422(8) | –0.00006(18) | 0.00016(18) | –0.00001(6) |
| Ge3 | 0.00379(10) | 0.00379(10) | 0.00479(17) | 0 | 0 | 0 |
| Ge4 | 0.00454(8) | 0.00432(8) | 0.00494(9) | 0.00012(19) | 0.00075(17) | 0.00009(6) |
| Ge5 | 0.00429(8) | 0.00466(8) | 0.00435(9) | –0.00002(19) | –0.00039(19) | 0.00017(7) |
| Ca1 | 0.00668(15) | 0.00592(15) | 0.00653(18) | 0.0002(3) | –0.0010(3) | 0.00090(13) |
| Ca2 | 0.00477(15) | 0.00818(16) | 0.00752(17) | –0.0007(4) | 0.0002(3) | –0.00097(12) |
| Ca3 | 0.00622(16) | 0.00886(16) | 0.00780(19) | 0.0003(3) | 0.0009(3) | 0.00160(13) |
| O1 | 0.0072(8) | 0.0037(7) | 0.0069(8) | 0.0008(7) | –0.0018(6) | –0.0035(6) |
| O2 | 0.0037(7) | 0.0070(8) | 0.0072(8) | 0.0003(6) | 0.0014(6) | –0.0002(6) |
| O3 | 0.0090(8) | 0.0065(8) | 0.0055(8) | –0.0006(7) | 0.0006(7) | 0.0009(6) |
| O4 | 0.0087(8) | 0.0053(8) | 0.0050(7) | 0.0000(6) | –0.0007(7) | 0.0030(6) |
| O5 | 0.0036(8) | 0.0122(9) | 0.0075(8) | 0.0019(7) | 0.0000(6) | –0.0006(7) |
| O6 | 0.0112(9) | 0.0034(8) | 0.0070(9) | 0.0000(6) | 0.0020(7) | –0.0024(6) |
| O7 | 0.0078(9) | 0.0068(8) | 0.0057(8) | 0.0000(7) | –0.0004(7) | 0.0013(6) |
| O8 | 0.0175(14) | 0.0032(11) | 0.0086(12) | 0 | 0 | 0.0010(10) |
| O9 | 0.0073(8) | 0.0067(8) | 0.0055(8) | 0.0013(7) | –0.0028(7) | –0.0006(6) |
| O10 | 0.0058(8) | 0.0134(9) | 0.0100(10) | –0.0010(7) | 0.0003(7) | –0.0063(7) |
| O11 | 0.0060(8) | 0.0062(8) | 0.0064(8) | 0.0025(6) | 0.0018(7) | –0.0012(7) |
| O12 | 0.0049(7) | 0.0072(8) | 0.0073(8) | –0.0036(7) | 0.0009(6) | –0.0003(6) |
| O13 | 0.0054(7) | 0.0072(7) | 0.0063(8) | –0.0036(7) | 0.0003(7) | 0.0007(6) |
| O14 | 0.0060(8) | 0.0061(7) | 0.0063(8) | 0.0025(7) | 0.0025(6) | 0.0006(6) |
| O15 | 0.0062(8) | 0.0064(8) | 0.0070(8) | 0.0012(7) | –0.0035(6) | –0.0003(6) |
| B1 | 0.0050(9) | 0.0055(9) | 0.0072(17) | –0.0004(9) | –0.0006(9) | –0.0002(7) |
| B2 | 0.0051(9) | 0.0032(8) | 0.0058(15) | 0.0003(10) | 0.0004(10) | 0.0007(6) |
Interatomic distances (Å) in Ca12Ge17B8O58 (standard deviations in parentheses).
| Ge1–O15 | 1.745(2) | Ge2–O14 | 1.859(2) | Ge3–O2 | 1.733(2) |
| Ge1–O14 | 1.748(2) | Ge2–O12 | 1.864(2) | Ge3–O2 | 1.733(2) |
| Ge1–O1 | 1.749(2) | Ge2–O11 | 1.873(2) | Ge3–O2 | 1.733(2) |
| Ge1–O6 | 1.749(2) | Ge2–O13 | 1.888(2) | Ge3–O2 | 1.734(2) |
| Ge2–O15 | 1.889(2) | ||||
| Ge2–O9 | 1.910(2) | ||||
| Ø | 1.748(2) | Ø | 1.881(2) | Ø | 1.733(2) |
| Ge4–O11 | 1.753(2) | Ge5–O12 | 1.751(2) | ||
| Ge4–O13 | 1.761(2) | Ge5–O5 | 1.753(2) | ||
| Ge4–O10a | 1.767(2) | Ge5–O9 | 1.759(2) | ||
| Ge4–O10b | 1.772(2) | Ge5–O8 | 1.796(2) | ||
| Ø | 1.763(2) | Ø | 1.765(2) | ||
| B1–O3 | 1.387(4) | B2–O7 | 1.398(4) | ||
| B1–O4 | 1.467(4) | B2–O4 | 1.462(4) | ||
| B1–O1 | 1.530(4) | B2–O6 | 1.527(4) | ||
| B1–O2 | 1.581(4) | B2–O5 | 1.559(4) | ||
| Ø | 1.491(4) | Ø | 1.487(4) | ||
| Ca1–O4 | 2.355(2) | Ca2–O14 | 2.311(2) | Ca3–O15 | 2.318(2) |
| Ca1–O3a | 2.364(2) | Ca2–O6 | 2.312(2) | Ca3–O4 | 2.337(2) |
| Ca1–O2a | 2.383(2) | Ca2–O12 | 2.326(2) | Ca3–O7 | 2.354(2) |
| Ca1–O1 | 2.388(2) | Ca2–O13 | 2.386(2) | Ca3–O9 | 2.477(2) |
| Ca1–O3b | 2.443(2) | Ca2–O7 | 2.395(2) | Ca3–O3 | 2.518(2) |
| Ca1–O2b | 2.489(2) | Ca2–O11 | 2.534(2) | Ca3–O5 | 2.523(2) |
| Ca1–O7 | 2.498(2) | Ca2–O5 | 2.602(2) | Ca3–O1 | 2.588(2) |
| Ca1–O6 | 2.705(2) | Ca2–O10 | 3.011(2) | Ca3–O8 | 2.993(2) |
| Ø | 2.453(2) | Ø | 2.485(2) | Ø | 2.514(2) |
Further details of the crystal structure investigation may be obtained from the Fachinformationszentrum Karlsruhe, D-76344 Eggenstein-Leopoldshafen, Germany (fax: +49-7247-808-666; e-mail: crysdata@fiz-karlsruhe.de, http://www.fiz-informationsdienste.de/en/DB/icsd/depot_anforderung.html) on quoting the deposition number CSD-431201.
2.3 Vibrational spectra
The transmission FT-IR spectrum of a single crystal of Ca12Ge17B8O58 was measured in the spectral range of 600–4000 cm–1 with a Vertex 70 FT-IR spectrometer (spectral resolution 4 cm–1), which is equipped with a KBr beam splitter, an LN-MCT (Mercury Cadmium Telluride) detector and a Hyperion 3000 microscope (Bruker, Vienna, Austria). 320 scans of the sample were acquired using a Globar (silicon carbide) rod as mid-IR source and a 15× IR objective as focus. During the measurement, the sample was positioned on a BaF2 window. A correction of atmospheric influences was performed with the software Opus 6.5.
3 Results and discussion
3.1 Crystal structure of Ca12Ge17B8O58
The new calcium borogermanate Ca12Ge17B8O58 crystallizes isotypically to Cd12Ge17B8O58 [2] in the tetragonal space group P4̅ (No. 81). The structure is composed of [Ge4O12]n chains (Fig. 2a). These chains are interconnected into a [Ge4O10.5]n network via corner sharing (Fig. 2b). Together with the [Ge(B2O7)4]28– clusters (Fig. 2c) a three-dimensional [Ge17B8O58]24– framework (Fig. 2d) is built, forming tunnels of five-, six-, and seven-membered rings along the c axis which are occupied by Ca2+ cations (Fig. 3).
![Fig. 2: Scheme showing the [Ge4O12]n chain (a); construction of the [Ge4O10.5]n network (b); [Ge(B2O7)4] unit (c); the three-dimensional [Ge17B8O58]24– anionic structure (d).](/document/doi/10.1515/znb-2016-0126/asset/graphic/j_znb-2016-0126_fig_002.jpg)
Scheme showing the [Ge4O12]n chain (a); construction of the [Ge4O10.5]n network (b); [Ge(B2O7)4] unit (c); the three-dimensional [Ge17B8O58]24– anionic structure (d).
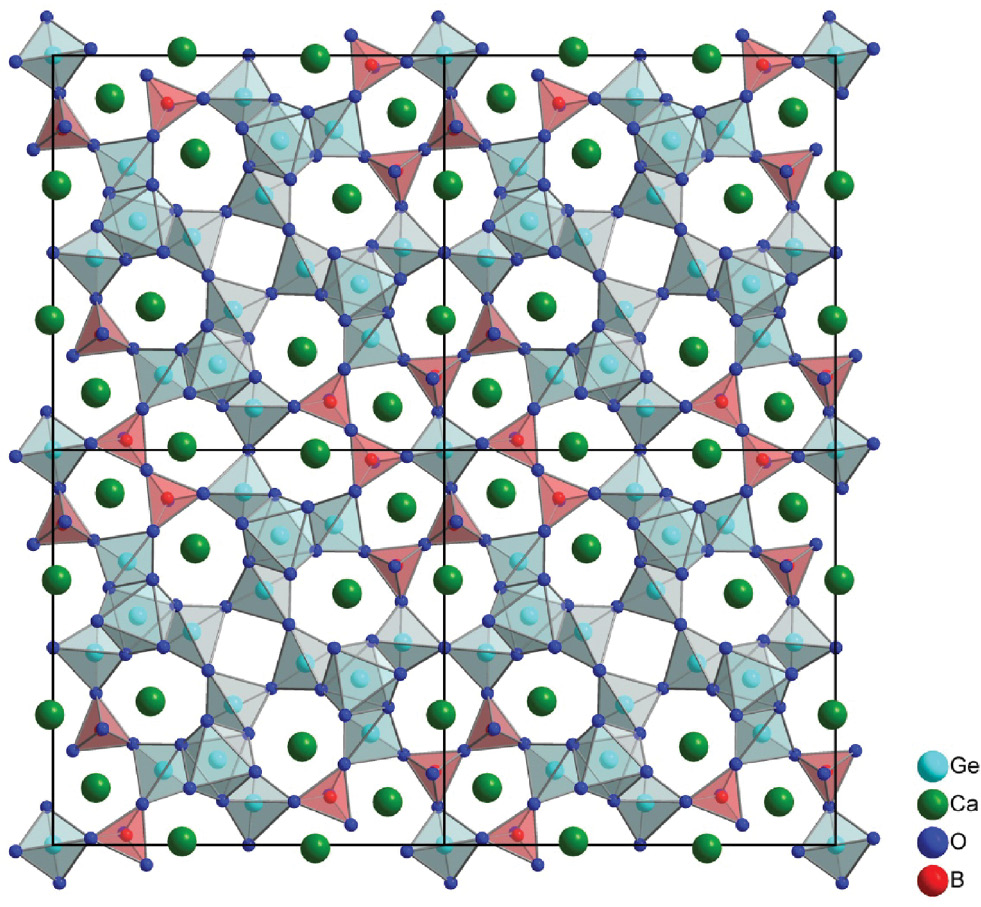
Crystal structure of Ca12Ge17B8O58 along the c axis.
The Ge(1)O4, Ge(4)O4, and Ge(5)O4 tetrahedra as well as the Ge(2)O6 octahedra are interconnected via vertex sharing building up the above mentioned [Ge4O12]n chains (Fig. 2a). Each of these chains is connected with three others to form a three-dimensional [Ge4O10.5]n network via corner sharing (Fig. 2b). The three-dimensional [Ge4O10.5]n network builds up small four-membered and large 24-membered rings forming tunnels along the c axis (Fig. 2b). A B2O7 dimer is formed by the B(1)O4 and B(2)O4 tetrahedra sharing a common oxygen atom. Four of those dimers are connected to the Ge(3)O4 tetrahedron via vertex sharing building up the [Ge(B2O7)4]28– clusters (Fig. 2c). The clusters are positioned in the large 24-membered ring tunnels of the three-dimensional [Ge4O10.5]n network via corner sharing, forming the three-dimensional [Ge17B8O58]24– anionic structure with tunnels of five-, six-, and seven-membered rings along the c axis (Fig. 2d) where the Ca2+ cations are located (Fig. 3). The five-membered ring is composed of three BO4 and two GeO4 tetrahedra. The six-membered ring consists of two BO4 tetrahedra, three GeO4 tetrahedra, and one GeO6 octahedron, whereas the seven-membered ring is formed by one BO4 tetrahedron, four GeO4 tetrahedra, and two GeO6 octahedra.
All three Ca atoms are eight-fold coordinated to oxygen anions with Ca–O distances ranging from 2.311(2) to 3.011(2) Å. The values correspond very well to the Ca–O distances of Ca10Ge16B6O51 ranging from 2.256(5) to 3.023(4) Å [2]. The two B atoms are coordinated by four oxygen atoms forming distorted tetrahedra with B–O distances ranging from 1.387(4) to 1.581(4) Å. Like in Cd12Ge17B8O58 [2], the B–O bond lengths exhibit a large variation, which stems from the different coordination environments of the oxygen atoms in the BO4 tetrahedra. The atom Ge3 is located on the four-fold axis, whereas all other Ge atoms are on general positions. Ge2 is octahedrally coordinated to oxygen anions with Ge–O distances ranging from 1.859(2) to 1.910(2) Å, whereas Ge1, Ge3, Ge4, and Ge5 are tetrahedrally coordinated by oxygen atoms with Ge–O distances between 1.733(2) and 1.796(2) Å. Table 4 shows the interatomic distances of Ca12Ge17B8O58. Table 5 compares the unit cell parameters of Ca12Ge17B8O58 and Cd12Ge17B8O58.
Comparison of the isotypic structures Ca12Ge17B8O58 and Cd12Ge17B8O58 (standard deviations in parentheses).
| Empirical formula | Ca12Ge17B8O58 | Cd12Ge17B8O58 |
| Reference | This work | [2] |
| Molar mass, g mol–1 | 2730.3 | 3598.3 |
| Unit cell dimensions | ||
| a, Å | 15.053(8) | 14.928(2) |
| c, Å | 4.723(2) | 4.698(1) |
| V, Å3 | 1070.2(2) | 1046.8(3) |
3.2 IR spectroscopy
Figure 4 displays the results of the IR spectroscopic measurement, which was performed on a single crystal of Ca12Ge17B8O58. The spectrum shows a series of different absorption bands with frequencies below 1500 cm–1 and from 2800–3000 cm–1, which can be associated with various vibrations of the different units in the crystal. The absorption bands between 645 and 875 cm–1 can be related to various modes within the different GeO4 tetrahedra, GeO6 octahedra and BO4 tetrahedra [14], [15], [16], [17], [18]. The region of 925–1200 cm–1 is due to bands of the symmetric stretching and asymmetric stretching modes of BO4 tetrahedra [18], [19], [20], [21]. The two small peaks between 1380 and 1450 cm–1 might represent a combinational tone of two vibration modes of Ge–O–Ge bonds [22]. Due to the overlap of the various bands, a precise assignment of the individual bands is impossible. The absorption bands at 2800–3000 cm–1 belong to the grease, which was used to fix the crystal on the class fiber. In summary, it can be stated that the IR spectrum confirms the existence of tetrahedrally and octahedrally coordinated germanium, and tetrahedrally coordinated boron atoms.
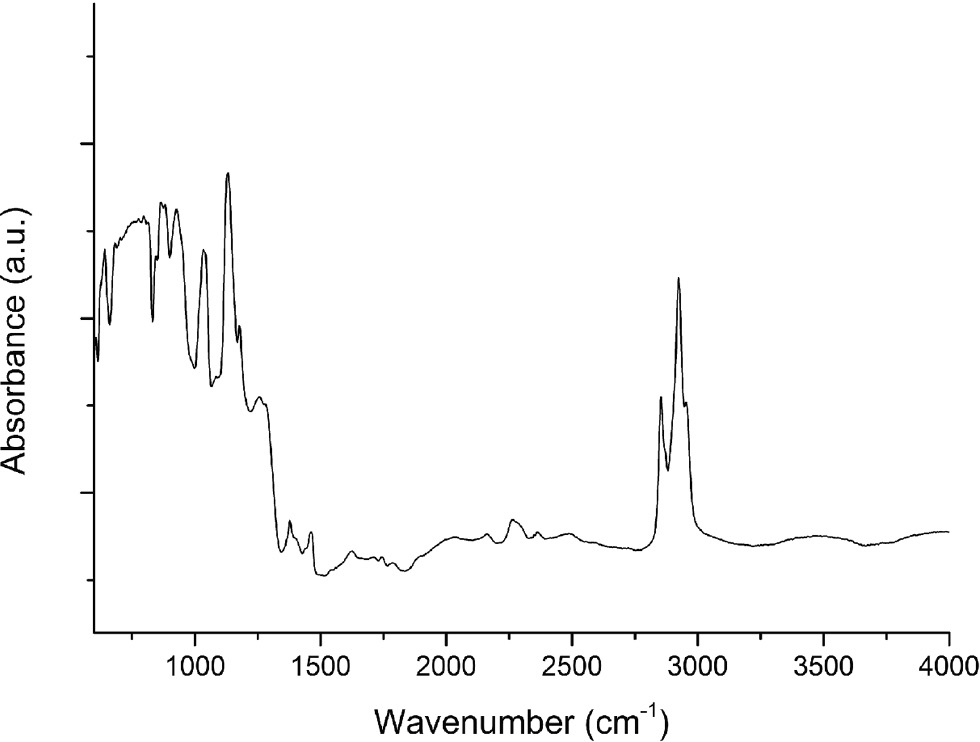
FT-IR reflectance spectrum of Ca12Ge17B8O58.
4 Conclusions
With the syntheses of Ca12Ge17B8O58, the list of known alkaline earth borogermanates could be extended to include an additional compound. It crystallizes in the tetragonal space group P4̅ (No. 81), being isotypic to Cd12Ge17B8O58 [2]. The main structural characteristics are [Ge4O12]n chains which are composed of GeO4 tetrahedra and GeO6 octahedra. The chains form a three-dimensional [Ge4O10.5]n network via corner sharing. Together with the [Ge(B2O7)4]28– clusters, the three-dimensional [Ge17B8O58]24– anionic structure with tunnels of five-, six-, and seven-membered rings is built. The tunnels are occupied by the Ca2+ cations. Our future research interests will be focused on the exploration of other alkaline earth and divalent transition metal borogermanates.
Acknowledgments
Special thanks go to Dr. G. Heymann for collecting the single crystal diffraction data, to D. Vitzthum for the measurements of the single crystal IR spectra, and to Univ.-Prof. Dr. R. Stalder, Institute for Mineralogy and Petrography, University of Innsbruck, for the access to the FTIR microscope.
References
[1] Y.-C. Hao, C.-L. Hu, X. Xu, F. Kong, J.-G. Mao, Inorg. Chem. 2013, 52, 13644.10.1021/ic402214pSearch in Google Scholar PubMed
[2] X. Xu, C.-L. Hu, F. Kong, J.-H. Zhang, J.-G. Mao, Inorg. Chem. 2011, 50, 8861.10.1021/ic2008254Search in Google Scholar PubMed
[3] J.-H. Zhang, C.-L. Hu, X. Xu, F. Kong, J.-G. Mao, Inorg. Chem. 2011, 50, 1973.10.1021/ic102451nSearch in Google Scholar PubMed
[4] J.-H. Zhang, F. Kong, J.-G. Mao, Inorg. Chem. 2011, 50, 3037.10.1021/ic1025697Search in Google Scholar PubMed
[5] J.-H. Zhang, F. Kong, X. Xu, J.-G. Mao, J. Solid State Chem.2012, 195, 63.10.1016/j.jssc.2011.12.045Search in Google Scholar
[6] H.-X. Zhang, J. Zhang, S.-T. Zheng, G.-M. Wang, G.-Y. Yang, Inorg. Chem. 2004, 43, 6148.10.1021/ic049071fSearch in Google Scholar PubMed
[7] F. Kong, H.-L. Jiang, T. Hu, J.-G. Moa, Inorg. Chem. 2008, 47, 10611.10.1021/ic801292pSearch in Google Scholar PubMed
[8] J.-H. Zhang, P.-X. Li, J.-G. Moa, Dalton Trans.2010, 39, 5301.10.1039/b927300jSearch in Google Scholar PubMed
[9] B. Petermüller, L. L. Petschnig, K. Wurst, G. Heymann, H. Huppertz, Inorg. Chem. 2014, 53, 9722.10.1021/ic5012296Search in Google Scholar PubMed
[10] Sadabs (version 5), Bruker Analytical X-ray Instruments Inc., Madison, Wisconsin (USA), 2014.Search in Google Scholar
[11] G. M. Sheldrick, Shelxs-13 and Shelxl-13, Program Suite for the Solution and Refinement of Crystal Structures, University of Göttingen, Göttingen (Germany), 2013.Search in Google Scholar
[12] G. M. Sheldrick, Acta Crystallogr.2008, A64, 112.10.1107/S0108767307043930Search in Google Scholar PubMed
[13] K. Brandenburg, Diamond (version 3.2i), Crystal and Molecular Structure Visualization, Crystal Impact – H. Putz & K. Brandenburg GbR, Bonn (Germany) 2012. See also: http://www.crystalimpact.com/diamond/.Search in Google Scholar
[14] E. Kamitsos, Y. Yiannopoulos, C. Varsamis, H. Jain, J. Non-Cryst. Solids1997, 222, 59.10.1016/S0022-3093(97)00389-XSearch in Google Scholar
[15] E. Culea, L. Pop, M. Bosca, J. Alloys Compd. 2010, 505, 754.10.1016/j.jallcom.2010.06.135Search in Google Scholar
[16] D. Di Martino, L. Santos, A. Marques, R. Almeida, J. Non-Cryst. Solids2001, 293, 394.10.1016/S0022-3093(01)00690-1Search in Google Scholar
[17] E. Mansour, G. El-Damrawi, R. Fetoh, H. Doweidar, Eur Phys. J. B2011, 83, 133.10.1140/epjb/e2011-20211-2Search in Google Scholar
[18] K. Blaszczak, A. Adamczyk, J. Mol. Struct. 2001, 596, 61.10.1016/S0022-2860(01)00686-XSearch in Google Scholar
[19] A. H. Reshak, X. Chen, F. Song, I. Kityk, S. Auluck, J. Phys.: Condens. Mater.2009, 21, 205402.10.1088/0953-8984/21/20/205402Search in Google Scholar
[20] E. Hinteregger, G. Heymann, T. S. Hofer, H. Huppertz, Z. Naturforsch.2012, 67b, 605.10.5560/ZNB.2012-0043Search in Google Scholar
[21] M. Ren, J. Lin, Y. Dong, L. Yang, M. Su, L. You, Chem. Mater.1999, 11, 1576.10.1021/cm990022oSearch in Google Scholar
[22] N. Terakado, K. Tanaka, J. Non-Cryst. Solids2008, 354, 1992.10.1016/j.jnoncrysol.2007.11.003Search in Google Scholar
©2016 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this Issue
- Li2Pt3Se4: a new lithium platinum selenide with jaguéite-type crystal structure by multianvil high-pressure/high-temperature synthesis
- RE4B4O11F2 (RE = Sm, Tb, Ho, Er): four new rare earth fluoride borates isotypic to Gd4B4O11F2
- Regioselective C-3 arylation of coumarins with arylhydrazines via radical oxidation by potassium permanganate
- Two new POM-based compounds containing a linear tri-nuclear copper(II) cluster and an infinite copper(II) chain, respectively
- Environmentally benign synthesis of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates using CeO2 nanoparticles as a reusable and robust catalyst
- Synthesis and structural characterization of Ca12Ge17B8O58
- Synthesis of some new hydrazide-hydrazones related to isatin and its Mannich and Schiff bases
- Purpureone, an antileishmanial ergochrome from the endophytic fungus Purpureocillium lilacinum
- Syntheses and crystal structures of two new silver–organic frameworks based on N-pyrazinesulfonyl-glycine: weak Ag···O/N interaction affecting the coordination geometry
Articles in the same Issue
- Frontmatter
- In this Issue
- Li2Pt3Se4: a new lithium platinum selenide with jaguéite-type crystal structure by multianvil high-pressure/high-temperature synthesis
- RE4B4O11F2 (RE = Sm, Tb, Ho, Er): four new rare earth fluoride borates isotypic to Gd4B4O11F2
- Regioselective C-3 arylation of coumarins with arylhydrazines via radical oxidation by potassium permanganate
- Two new POM-based compounds containing a linear tri-nuclear copper(II) cluster and an infinite copper(II) chain, respectively
- Environmentally benign synthesis of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates using CeO2 nanoparticles as a reusable and robust catalyst
- Synthesis and structural characterization of Ca12Ge17B8O58
- Synthesis of some new hydrazide-hydrazones related to isatin and its Mannich and Schiff bases
- Purpureone, an antileishmanial ergochrome from the endophytic fungus Purpureocillium lilacinum
- Syntheses and crystal structures of two new silver–organic frameworks based on N-pyrazinesulfonyl-glycine: weak Ag···O/N interaction affecting the coordination geometry

