Abstract
Thermal expansion of the mineral soddyite, (UO2)2SiO4(H2O)2, and structurally related synthetic compound Na2(UO2)2SiO4F2 ( NAUSIF ) has been studied by means of high-temperature single-crystal and powder X-ray diffraction. The mineral is orthorhombic, Fddd, while NAUSIF is tetragonal, I41/amd. The framework structures of both compounds are comprised of either neutral [(UO2)2(SiO4)(H2O)2] or negatively charged [(UO2)2(SiO4)F2]2- chains of similar topology. In the structure of soddyite, the chains cross at the angle of 72°, while in NAUSIF of 90°. Upon increasing temperature, the acute inter-chain angles in soddyite increase due to hinge deformations, the overall symmetry approaching tetragonal. The mineral is stable below 325 ± 25 °С; between 325 and 640 °С, the decomposition products cannot be identified unambiguously and contain significant amount of amorphous phases; at higher temperatures, a mixture of U3O8 polymorphs is formed. NAUSIF is stable until its melting point of 625 ± 25 °С. The thermal expansion of both compounds is strongly anisotropic; for NAUSIF , it is due to difference in bond strength in the uranium and sodium polyhedra. Anisotropic thermal expansion of soddyite is controlled by shear deformations of the structure upon the temperature rise.
1 Introduction
Investigations of the oxidation products of uraninite UO2 1 , 2 , 3 are of essential importance for proper understanding the evolution of both mineral formation upon oxidation of uranium deposits and alteration of spent nuclear fuel. 4 Temperature is one of the key parameters affecting the stability of uranium compounds. Thermodynamic modeling of uraninite dissolution in silica-saturated groundwaters indicate that the process is controlled by formation of several uranyl compounds including soddyite (UO2)2SiO4(H2O)2. 5 Studies of mineral associations of uranium deposits oxidation areas demonstrate that interaction of fluids with rocks upon weathering produces several uranyl silicates including uranophane Ca[(UO2)(SiO3OH)]2(H2O)5, boltwoodite (K,Na)[(UO2)(SiO3OH)](H2O)1.5, haiweeite Ca[(UO2)2Si5O12(OH)2](H2O)3, and soddyite. 1 , 6 , 7 , 8
Soddyite was first described by Schoep in 1922; its first X-ray study was performed by Gorman in 1952. The mineral was found to be orthorhombic, а = 8.32 Å, b = 11.21 Å, с = 18.71 Å, space group Fddd. 9 Legros et al. 10 prepared 2UO3⋅GeO2⋅2Н2O and 2UO3⋅SiO2⋅2Н2O and demonstrated the silicate and the germanate to be isostructural; the structure of the latter was determined in 1975. 10 Later on, the crystals of synthetic soddyite were prepared by Kuznetsov et al. 11 , Moll 12 and Belokoneva et al. 13 In the latter work, the structure of the synthetic compound was solved to R1 = 0.021 and wR1 = 0.029 but hydrogen atoms were not localized. Attempts were also made to solve the structure from PND data. 14 Demartin et al. 15 Localized hydrogen positions and refined the structure down to R1 = 6.4 %. The latest study by Plášil et al. 16 confirmed the Fddd space group and the unit cell parameters were a = 8.3097(3) Å, b = 11.2205(4) Å, с = 18.6575(11) Å, R1 = 1.9 %.
Both synthetic and natural soddyite crystals were analyzed by a variety of techniques. Čejka 17 analyzed the IR spectroscopy data, while Frost 18 and Colmenero 19 provided the Raman spectra and DFT calculations. Particular attention was paid to the thermal properties of the mineral. The first decomposition stage of soddyite and its germanate analog at ca. 460 °C was found to correspond to the total dehydration and formation of amorphous products. 20 Formation of the latter was explained by the arising under-saturation of the uranyl cation due to the removal of coordinated water from the (UO2)O4(H2O) pentagonal bipyramid. 21 The second stage of soddyite decomposition at ca. 640 °C corresponds to the release of oxygen and uranium reduction to the tetravalent state with crystallization of coffinite analog, USiO4. Though the thermal behavior of soddyite is well studied, no reference data could be found concerning its thermal expansion. The data on polythermic studies of the synthetic soddyite were provided by Sureda et al. 22 and interpreted only as an illustration to the staged dehydration process. Synthetic soddyite was reported to lose water at 400 °С with subsequent melting at 470° С.
Blaton et al. 23 reported hydrothermal synthesis of Na2(UO2)2SiO4F2. Its crystal structure is derived from soddyite by replacing water molecules by the fluoride anions and filling the channels by the sodium cations; otherwise, the topologies of these frameworks are identical.
In the current study, the thermal behavior and particularly expansion of soddyite and Na2(UO2)2SiO4F2 ( NAUSIF further on) were probed by a set of variable-temperature single-crystal and powder diffraction methods.
2 Experimental
2.1 Samples and occurrence
Soddyite originates from Swambo Hill, Haut-Katanga, DR Congo. It occurs as canary-yellow blocky and platy crystals up to 0.3 mm across (Figure 1a and b) and associates with curite, goethite and kaolinite.
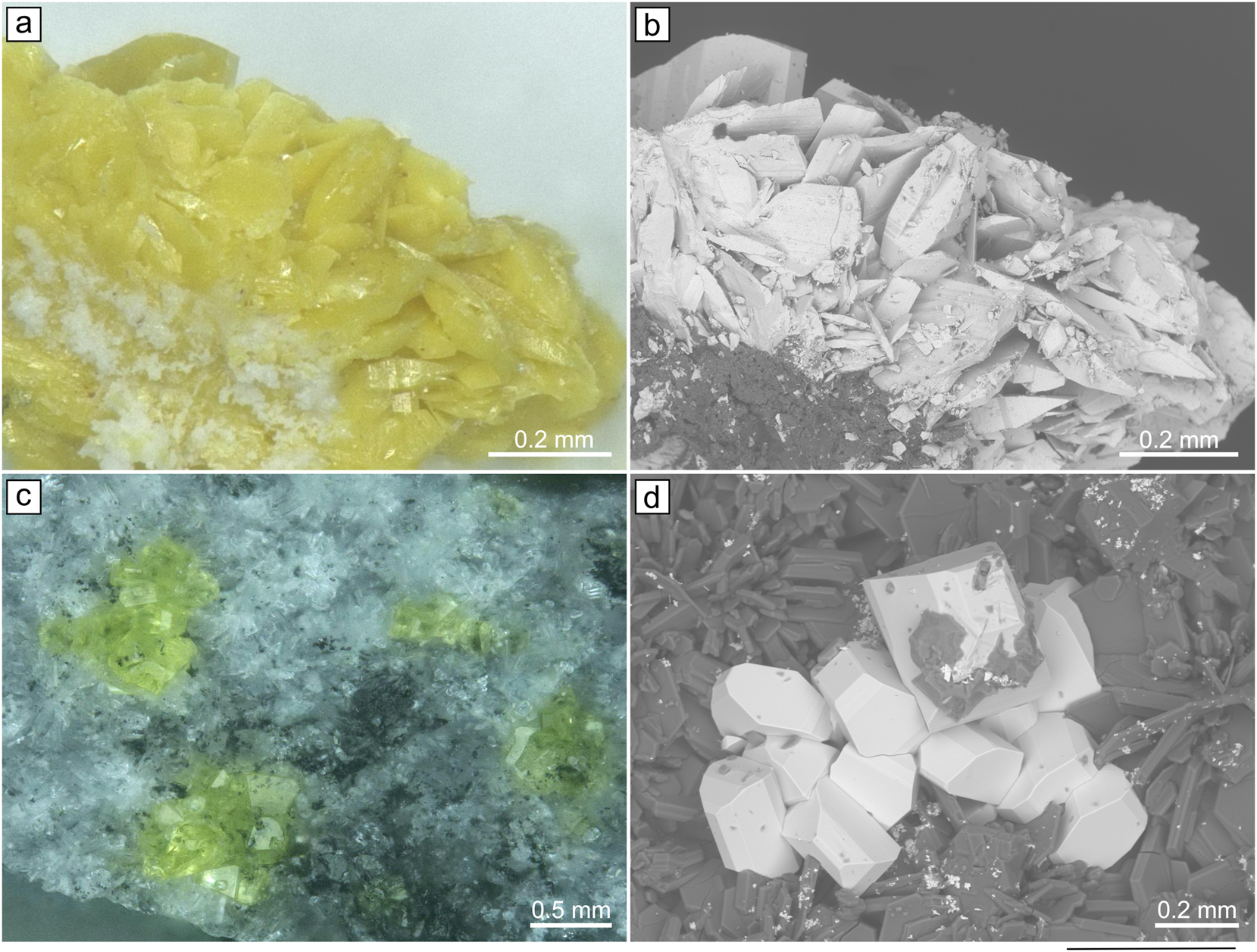
The images of yellow platy crystals of soddiyte (a,b) and yellow prismatic crystals of Na2(UO2)2(SiO4)F2 (c,d) under optical microscope and BSE.
2.2 Synthesis
Caution! Although the uranium precursors used contain depleted uranium, standard safety measures for handling radioactive substances must be followed.
As we had reported earlier, 24 single crystals of uranyl silicates can be readily prepared from uranium oxides and silica via “activation” by reactive fluorides. The crystals of NAUSIF (Figure 1c and d) were produced from a mixture of 22 mg of NaF (Vecton, 99.7 %) and 52 mg U3O8 (Vecton, 99.7 %) pre-dried at 80 °C. The reagents were additionally activated. This mixture was transferred to a silica tube (which served also as the source of silicon), then 30 µL of 40 % hydrofluoric acid was injected. After 1 min, the tube was attached to a vacuum line, evacuated, and sealed. The silica tube was heated to 950 °С at a rate of 70 °С/h, soaked for 99 h, and cooled to room temperature at the rate of 5 °С/h.
2.3 Single-crystal X-ray studies
Single-crystal X-ray data of soddyite and NAUSIF were collected using a Rigaku XtaLAB Synergy-S diffractometer equipped with a PhotonJet-S detector operating with MoKα radiation at 50 kV and 1 mA. A single crystal was chosen and more than a hemisphere of data collected with a frame width of 0.5° in ω, and 25 s spent counting for each frame. The data were integrated and corrected for absorption applying a multi-scan type model using the Rigaku Oxford Diffraction programs CrysAlis Pro 1.1.11.
The NAUSIF crystal was studied in the temperature range of 25–725 °С using a «Hot Air Gas Blowers » heating system. The structures were successfully refined with the use of SHELX software package. 25 Atom coordinates and thermal displacement parameters for each temperature are collected in the corresponding cif files (Supplement 1); experimental parameters are provided in Table 1. Unit-cell parameters are given for 25 °С. The procedure of variable-temperature single-crystal X-ray study is in that the crystal is placed in a silica capillary and thermostated before each data collection. Therefore, temporal restrictions and essential lowering of reflection intensities due to extinction by capillary walls require the use of relatively large crystals. Unfortunately, due to hydration and intergrowths twinning, just two acceptable soddyite crystals could be picked out of the natural samples; ever those were so small that full data collection would require more than 14 h. Hence, PXRD studies were the only possible way for investigating the thermal evolution of soddyite. In the meantime, the NAUSIF crystals provided by high-temperature technique were quite large and well-grained crystals excellent for the capillary experiments.
Crystallographic data and refinement parameters for soddiyte and Na2[(UO2)2(SiO4)F2].
| soddyite | Na2[(UO2)2(SiO4)F2] | |
|---|---|---|
| Temperature (°С) | 25 MoKα, 0.71073 Å |
|
| Radiation | ||
| Crystal system | Orthorhombic | Tetragonal |
| Space group | Fddd | I41/amd |
| a (Å) | 8.3246(4) | 6.9749(2) |
| b (Å) | 11.2245(5) | |
| c (Å) | 18.6734(6) | 18.2807(12) |
| Volume (Å3) | 1744.83(13) | 889.34(8) |
| dcalc (g/cm3) | 5.057 | 5.348 |
| μ (mm−1) | 37.246 | 36.658 |
| Crystal size (mm) | 0.05 × 0.06 × 0.14 | 0.03 × 0.5 × 0.11 |
| θ Range (°) | 4.24–27.37 | 4.44–27.89 |
| h, k, l ranges | −9 → 10, −14 → 11, −24 → 23 |
−8 → 8, −6 → 9, −24 → 12 |
| Total reflections collected | 2042 | 1256 |
| Unique reflections (R int ) | 0.024 | 0.020 |
| R1[F > 4σF], wR1[F > 4σF] | 0.022, 0.054 | 0.014, 0.030 |
| Rall, wRall | 0.026, 0.055 | 0.018, 0.031 |
| Goodness-of-fit | 1.133 | 1.088 |
The effect of thermal motion on the bond-length values from single-crystal X-ray diffraction experiments is well-known. 26 Corrections for all bonds in the studied compounds were calculated by using a formula for the rigid-body motion:
Where L and l0 are corrected and observed A1–A2 bond lengths, respectively; Beq(A1) and Beq(A2) are equivalent temperature factors of A1 (cation, i.e. U, Si, Na) and A2 (anion i.e. O, F) atoms, respectively.
2.4 Chemical composition
The chemical analysis of soddyite was carried out with a Hitachi FlexSEM 1,000 scanning electron microscope equipped with EDS Xplore Contact 30 detector and Oxford AZtecLive STD system of analysis. Analytical conditions were: accelerating voltage 20 kV and beam current 5 nA. Only U, Si and O were recorded, contents of other elements with atomic numbers higher than that of beryllium were below detection limits. The following standards and X-ray lines were used: Si–SiO2, Kα; U–UO2, Mβ.
The chemical composition of soddyite is (wt%, H2O content calculated by stoichiometry): SiO2 8.87, UO3 85.93, H2O 5.39, total 100.19. The empirical formula based on 8 O apfu and 2 Η2Ο molecules is U2·01Si0·99O8(H2O)2.
Qualitative electron microprobe analysis of NAUSIF carried out using TM3000 (Hitachi, Tokyo, Japan) revealed no other elements, except U, Si, F and Na, with an atomic number greater than 11 (Na).
2.5 Powder X-ray analysis
The PXRD of Na2[(UO2)2(SiO4)F2] and soddyite were studied in air by means of a Rigaku Ultima X-ray diffractometer (Cu-Kα radiation). The samples were prepared using heptane. The speed of the experiment is 2°/min. Unit-cell parameters were refined by least-square methods.
2.6 High-temperature X-ray powder diffraction study
Thermal behavior of soddyite was studied in air by means of a Rigaku Ultima X-ray diffractometer (Cu-Kα radiation) with a high-temperature camera Rigaku HTA 1600. The samples were prepared from heptane’s suspension on a Pt–Rh plate. The temperature step was 20 °C in the range of 25–1,000 °C. Unit-cell parameters at different temperatures were refined by least-square methods. Main coefficients of the thermal expansion tensor were determined using linear approximation of temperature dependences by the ThetaToTensor program. 27
2.7 Infrared spectroscopy
In order to obtain infrared (IR) absorption spectra (Figure S1), powdered samples of NAUSIF and soddyite have been mixed with dried KBr, pelletized, and analyzed using an ALPHA FTIR spectrometer (Bruker Optics) with a resolution of 4 cm−1. 10 Scans were obtained. The IR spectrum of an analogous pellet of pure KBr was used as a reference.
3 Results
3.1 Crystal structure of Na2(UO2)2SiO4F2 and soddyite
In the both soddyite and NAUSIF structures, a single symmetry unique uranium atom contributes to a typical uranyl cation (Ur; <U-Oap> = 1.773 Å). In soddyite (Figure 2a), it is coordinated, in the equatorial plane, by four oxygen atoms from the silicate tetrahedra (<U-Oeq> = 2.250 Å) and water molecule (U–H2O = 2.415 Å) with formation of a UrO4(H2O) pentagonal bipyramid. In NAUSIF , water molecule is replaced by a fluoride anion (<U-Oeq> = 2.373 Å, U–F = 2.211 Å) and an UrO4F polyhedron is formed (Figure 2b). The silicon atoms center the typical SiO4 tetrahedra (<Si–O> = 1.634 and 1.611 Å for soddyite and NAUSIF , respectively). In the latter structure, the sodium cations reside in the trans-NaO4F2 octahedra (<Na–O> = 2.290 Å, <Na–F> = 2.777 Å) (Figure 2c).

Coordination environments of atoms in soddyite and NAUSIF . U6+ and Si4+ coordination in the structure of soddyite (a) and NAUSIF (b) coordination of Na+ in chains in NAUSIF (с) uranyl silicate chains in soddyite (d) and NAUSIF (e).
The bond-valence sums, calculated using the parameters from Gagne and Hawthorne 28 correlate well to the formal valences of the atoms (Table S1). The slight overbonding for the silicon atoms is rather commonly observed among the structures of uranyl silicates. 29 , 30
In both structures, the UO7 polyhedra share edges to form chains (Figure 2d and e) rather common for uranium minerals. 8 The silicate tetrahedra decorate these chains sharing edges with the UO7 bipyramids so that the latter feature a single terminal vertex occupied by water molecule in the structure of soddyite (Figure 2d), and by F- in NAUSIF (Figure 2e). As a result, either neutral [(UO2)2(SiO4)(H2O)2] or negatively charged [(UO2)2(SiO4)F2]2- chains are formed. These chains link via the opposite edges of the silicate tetrahedra to form microporous frameworks with channels of 3.84 × 4.51 Å and 3.80 × 4.55 Å, respectively (Figure 3). In NAUSIF , the channels are occupied by sodium cations.
![Figure 3:
Projections of the soddyite structure along [112] (a) and onto ab plane (b); projections of
NAUSIF
structure onto bc (c) and ab (d) planes. Angles between the directions of chain propagation are also indicated.](/document/doi/10.1515/zkri-2024-0091/asset/graphic/j_zkri-2024-0091_fig_003.jpg)
Projections of the soddyite structure along [112] (a) and onto ab plane (b); projections of NAUSIF structure onto bc (c) and ab (d) planes. Angles between the directions of chain propagation are also indicated.
Soddyite is orthorhombic (Fddd), while NAUSIF is tetragonal (I41/amd). In the former structure the chain propagation directions intersect at the acute angle of 73° (Figure 3b), while in the latter, this angle is 90° (Figure 3d). Note that the soddyite structure, after weeksite, 31 is just a second example of a microporous framework among uranyl silicate minerals.
3.2 Single crystal X-ray HT study of NAUSIF
A polythermal single-crystal X-ray experiment for NAUSIF was conducted in the 25–725 °C range with an increment of 50 °C, and the structure was refined at each step (see the cif files in Supplement). The compound is stable until its melting point of 625 ± 25 °С. Thermal dependences of its unit-cell parameters can be satisfactorily described by linear functions, a(T) = 6.957 + 0.052 × 10−3T, c(T) = 18.283 + 0.009 × 10−3 T, V(T) = 885.1 + 13.8 × 10−3 T (Figure 4)

Projection of the NAUSIF onto ac, thermal dependences of the unit-cell parameters and sections of thermal expansion tensors. Arrows indicate the changes in bond lengths in the polyhedra upon heating (red = expansion; green = nearly constant). The error bars are smaller than the size of markers.
The thermal expansion of NAUSIF is strongly anisotropic (α11 = 7.0, α33 = 1.0, αV = 15 × 10−6 K−1). It is underpinned by the anisotropy of thermal evolution of bond lengths and angles in the coordination polyhedra. In UO7, upon heating from 25 to 625 °C, the elongation of uranyl bonds is within the standard deviation of 0.003 Å; the U1–O1 bonds, aligned along а, expand twice as less (Δd = 0.014 Å) compared to U1–O1, aligned nearly along с (Δd = 0.027 Å). In the meantime, the U–F1 bond lengths remain nearly invariable (Δd = 0.005 Å). The same is true for the strong covalent Si–O bonds (Δd = 0.002 Å). The thermal expansion of the NaO4F2 octahedra is strongly anisotropic. Their main axes form a 50° angle towards c. The elongation rate for the Na–F bonds (Δd = 0.069 Å) is nearly thrice above that of Na–O2 (Δd = 0.025 Å). Between 25 and 625 °C, the O2–O2 edge lengths increase from 3.380 to 3.442 Å. The edge lengths in the UO6F polyhedra also increase anisotropically. The O1–O1 edge, shared by the UO6F and SiO4 polyhedra, increases from 2.45 to 2.54 Å. The О1-U1-O1 changes from 162.89 to 163.85°.
3.3 Thermal expansion of soddyite studied by powder X-ray diffraction
The mineral is stable until 325 ± 25 °C. Between 325 and 640 °C, the PXRD pattern contain just several weak reflections; above 640 °C, crystallization of U3O8 is observed (Figure 5a). Upon heating, the positions of soddyite reflections shift essentially which is caused mainly by the dehydration.

Thermal evolution of PXRD pattern of soddyite (а) and thermal dependence of its unit-cell parameters (b). The U3O8 reflections are highlighted in red. The error bars are smaller than the size of markers.
Thermal dependences of unit cell parameters are satisfactorily described by second-order polynomials (Figure 5b). Upon heating, the b and c decrease while a increases. The thermal expansion is also strongly anisotropic; the structure shrinks along b and expands along а. In fact, the orthorhombic (Fddd) a call parameters b tend to approach the same value of ca. 9 Å, which is the tetragonal cell parameter for NAUSIF . Yet, the symmetry remains orthorhombic in the whole temperature interval (Figure 6). Increase of the symmetry upon heating is a commonly observed phenomenon, 32 frequently due to hinge of shear deformations of the structure.
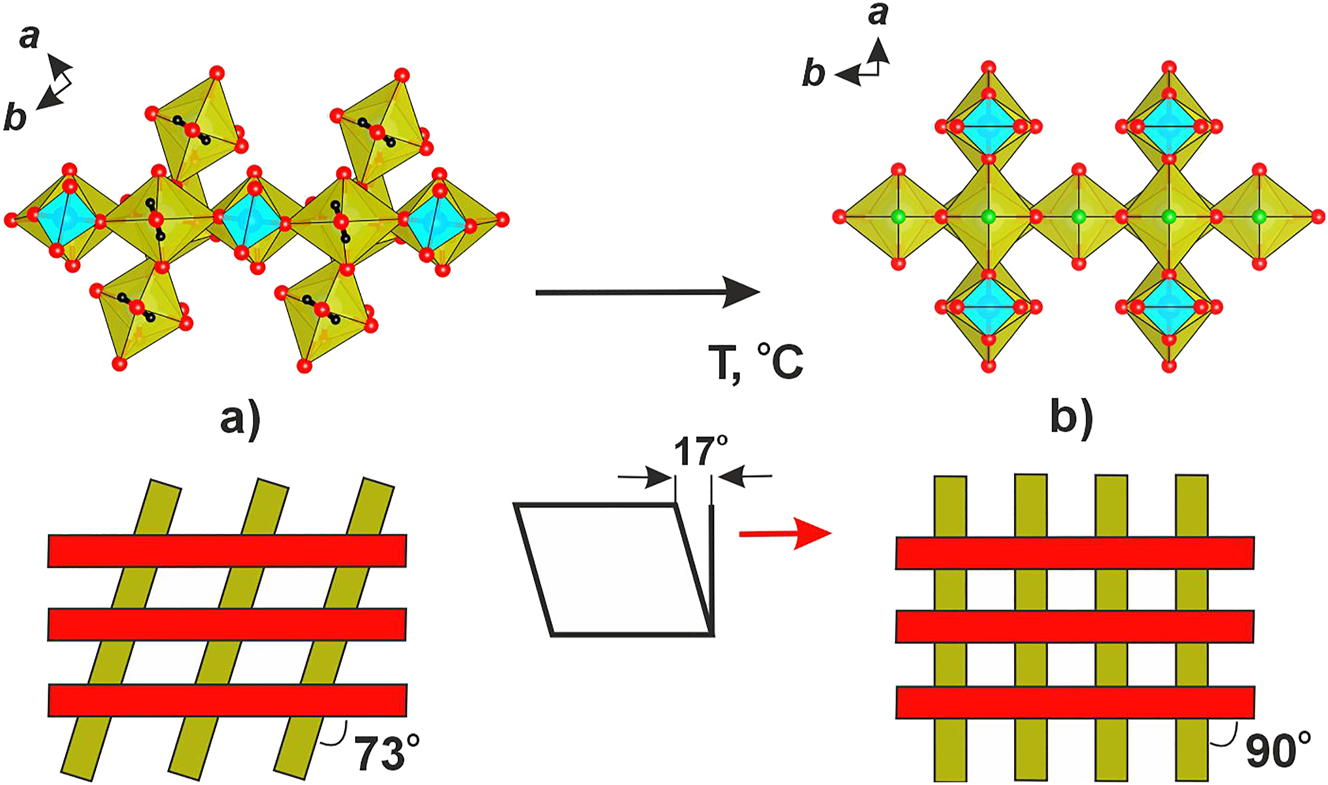
Chain linkage in the structures of soddyite (а) and NAUSIF (b). The hinge deformation is represented schematically below.
As noted above, the frameworks in the structures of soddyite and
NAUSIF
exhibit the same topology yet the angles between chain propagation directions are different. It is reasonable to suggest that upon increasing temperature, the orthorhombic framework of soddyite tends towards tetragonal symmetry with this angle increasing from 72 to 90°. Note that at elevated temperatures, the a and b unit-cell parameters of soddyite are related to the tetragonal a parameter of
NAUSIF
approximately by factors of
4 Concluding remarks
In the current work, we investigated, for the first time, the thermal behavior of soddyite (via powder XDR) and a related compound NAUSIF (via single-crystal X-ray diffraction). The former is stable until 325 ± 25 °С, while the latter, until 625 ± 25 °С. Based on the structural data, we suggest a way of approximating anisotropy of thermal expansion. Despite the topological similarity of the frameworks, the thermal stability and expansion parameters of soddyite and NAUSIF are essentially different. For the latter, thermal expansion is not so anisotropic as for the former. Thermal evolution of the mineral suggests the presence of hinge or shear deformations so that the structure approaches a higher symmetry; that of NAUSIF exhibits a moderate anisotropy of thermal expansion. These differences can be explained considering change of chemical composition of the mineral due to water release and presence of sodium cations in the channels in NAUSIF . Reference data on soddyite revealed two steps of mass loss upon heating, water release at 300–460 °С, and oxygen evolution at 640–740 °С. In general, our data agree to these observations except that some weak reflections persist in the 325–640 °C range which may indicate stepwise transformation. Formation of U3O8 above 640 °С agrees to the previous reports.
Studies on oxidation of uraninite, as well as of SNF 4 , 33 indicate that UO2 is unstable under acidic conditions and the rate of its transformation can be quite high. The most common oxidation products are schoepite (UO2)8O2(OH)12(H2O)12, uranophane Ca[(UO2)(SiO3OH)]2(H2O)5, boltwoodite (K,Na)[(UO2)(SiO3OH)](H2O)1.5, soddyite (UO2)2(SiO4)(H2O)2, and compreignacite K2(UO2)6O4(OH)6(H2O)7. Abundant formation of soddyite upon oxidation of uraninite was observed in Nevada (Yucca Mountain) with intermediate formation of becquerelite. 2 , 3
Certain nuclides like 237Np, 34 , 35 135Cs, 137Cs, 36 and 90Sr 37 can accumulate in uranyl compounds. For their immobilization, minerals with microporous structures are considered to be more preferable. 34 , 35 , 36 , 37 Among uranyl silicates, these are weeksite and soddyite. Klingensmith et al. 35 reported incorporation of NpO2+ into synthetic soddyite with concomitant introduction of Na+ into the channels, to keep the charge balance: (UO2)2-x(NpO2)xNaxSiO4·2H2O.
According to Weck et al. 38 soddyite is unstable under oxidizing conditions and transforms into studtite. One can suggest that the electroneutral framework containing only relatively weakly bound water molecules is prone to transformations, possible via an exchange route. Our polythermic studies demonstrate that soddyite decomposes above 325 °C. Some analogies can be traced to the thermal behavior of certain zeolites. 39 On the other hand, NAUSIF is stable until 625 °C. Substitution of readily exchangeable (and expellable upon heating) neutral water molecules in the uranium coordination sphere by more strongly bound fluorine atoms and further linkage of the structure by the sodium cations in the channels essentially stabilizes the structure motif. Note that the synthesis conditions for the artificial soddyite and NAUSIF are similar, and both compounds can crystallize during one experiment (Wochten et al., 1997). Considering simultaneous presence of soddyite and boltwoodite, (K,Na)[(UO2)(SiO3OH)](H2O)1.5, one could suggest formation of NAUSIF during oxidation of uranium deposits in the presence of F−. Comparison of soddyite and NAUSIF suggests that the latter compound is more promising for the immobilization of radionuclides.
Acknowledgments
Technical support by the X-Ray Diffraction and Geomodel Resource Centers of Saint-Petersburg State University is gratefully acknowledged.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: This work was financially supported by the Russian Science Foundation through the grant 23-27-00153.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Plášil, J. Oxidation-hydration Weathering of Uraninite: the Current State-Of-Knowledge. J. Geosci. 2014, 59, 99–114; https://doi.org/10.3190/jgeosci.163.Search in Google Scholar
2. Wronkiewicz, D. J.; Bates, J. K.; Gerding, T. J.; Veleckis, E.; Tani, B. S. Uranium Release and Secondary Phase Formation during Unsaturated Testing of UO2 at 90 °C. J. Nucl. Mater. 1992, 190, 107–127; https://doi.org/10.1016/0022-3115(92)90081-u.Search in Google Scholar
3. Wronkiewicz, D. J.; Bates, J. K.; Wolf, S. F.; Buck, E. C. Ten-year Results from Unsaturated Drip Tests with UO2 at 90 °C: Implications for the Corrosion of Spent Nuclear Fuel. J. Nucl. Mater. 1996, 238, 78–95; https://doi.org/10.1016/s0022-3115(96)00383-2.Search in Google Scholar
4. Finch, R. J.; Buck, E. C.; Finn, P. A.; Bates, J. K. Oxidative Corrosion of Spent UO2 Fuel in Vapor and Dripping Groundwater at 90 °C. Mater. Res. Sympos. Proc. 1999, 556, 431–438; https://doi.org/10.1557/proc-556-431.Search in Google Scholar
5. Trocellier, P.; Cachoir, C.; Guilbert, S. Dissolution of Uranium Dioxide in Granitic Groundwater by Secondary Phase Formation. J. Nucl. Mater. 1998, 256, 197–206; https://doi.org/10.1016/s0022-3115(98)00068-3.Search in Google Scholar
6. Gob, S.; Guhring, J. E.; Bau, M.; Markl, G. Remobilization of U and REE and the Formation of Secondary Minerals in Oxidized U Deposits. Am. Mineral. 2013, 98, 530–548; https://doi.org/10.2138/am.2013.4275.Search in Google Scholar
7. Belova, L. N.; Doynikova, O. A. Conditions of Formation of Uranium Minerals in the Oxidation Zone of Uranium Deposits. Geol. Ore Depos. 2003, 45, 148–152.Search in Google Scholar
8. Krivovichev, S. V.; Plášil, J. Mineralogy and Crystallography of Uranium. In Uranium: From Cradle to Grave; Burns, P. C., Sigmon, G. E., Eds.; Mineralogical Association of Canada Short Courses: Quebec, QC, Canada, Vol. 43, 2013; pp 15–119.Search in Google Scholar
9. Gorman, D. H. Studies of Radioactive Compounds: V – Soddyite. Am. Mineral. 1952, 37, 386–393.Search in Google Scholar
10. Legros, J. P.; Jeannin, Y. Coordination de l’uranium par l’Ion germanate. II. Structure du germanate d′uranyle dihydraté (UO2)2GeO4(H2O)2. Acta Crystallogr. 1975, 31, 1140–1143; https://doi.org/10.1107/s0567740875004670.Search in Google Scholar
11. Kuznetsov, L. M.; Tsvigunov, A. N.; Makarov, E. S. Hydrothermal Synthesis and Physico-Chemical Study of the Synthetic Analog of Soddyite. Geochimiya 1981, 10, 1493.Search in Google Scholar
12. Moll, H.; Matz, W.; Schuster, G.; Brendler, E.; Bernhard, G.; Nitsche, H. Synthesis and Characterization of Uranyl Orthosilicate (UO2)2SiO4·2H2O. J. Nucl. Mater. 1995, 227, 40–49; https://doi.org/10.1016/0022-3115(95)00148-4.Search in Google Scholar
13. Belokoneva, E. L.; Mokeeva, V. I.; Kuznetsov, L. M.; Simonov, M. A.; Makarov, E. S.; Belov, N. V. Crystal Structure of Synthetic Soddyite, (UO2)2[SiO4](H2O)2. Dokl. Akad. Nauk SSSR 1979, 246, 93–96.Search in Google Scholar
14. Nozik, Y. Z.; Kuznetsov, L. M. Neutron Diffraction Study of Synthetic Soddyite by the Full-Profile Analysis Technique, Kristallografiya 1990, 35, 1563–1564.Search in Google Scholar
15. Demartin, F.; Gramaccioli, C. M.; Pilati, T. The Importance of Accurate Crystal Structure Determination of Uranium Minerals. II. Soddyite (UO2)2(SiO4)·2H2O. Acta Crystallogr. 1992, 48, 1–4; https://doi.org/10.1107/s0108270191004481.Search in Google Scholar
16. Plášil, J. Mineralogy, Crystallography and Structural Complexity of Natural Uranyl Silicates. Minerals 2018, 8, 551; https://doi.org/10.3390/min8120551.Search in Google Scholar
17. Čejka, J Infrared Spectroscopy and Thermal Analysis of the Uranyl Minerals. Rev. Mineral. Geochem. 1999, 38, 521–622.10.1515/9781501509193-017Search in Google Scholar
18. Frost, R. L.; Čejka, J.; Weier, M. L.; Martens, W.; Kloprogge, J. T. A Raman and Infrared Spectroscopic Study of the Uranyl Silicates - Weeksite, Soddyite and Haiweeite. Spectrochim. Acta 2006, A64, 308–315; https://doi.org/10.1016/j.saa.2005.07.028.Search in Google Scholar PubMed
19. Colmenero, F.; Bonales, L. J.; Cobos, J.; Timóna, V. Structural, Mechanical and Vibrational Study of Uranyl Silicate Mineral Soddyite by DFT Calculations. J. Solid State Chem. 2017, 253, 249–257; https://doi.org/10.1016/j.jssc.2017.06.002.Search in Google Scholar
20. Chernorukov, N. G.; Knyazev, A. V.; Sergacheva, I. V.; Ershova, A. V. Synthesis and Physicochemical Study of Compounds in UO3-AkOk/2 (Ak = B, Si, Ge)-H2O Systems. Radiochemistry 2004, 46, 218–223; https://doi.org/10.1023/b:rach.0000031675.23613.22.10.1023/B:RACH.0000031675.23613.22Search in Google Scholar
21. Serezhkin, V. N.; Rasshchepkina, N. A.; Serezhkina, L. B. Thermal Decomposition of Zinc Uranyl Sulfate and Zinc Selenito Uranylate Hydrates. Radiokhimiya 1980, 22, 49–52.Search in Google Scholar
22. Sureda, R.; Casas, I.; Gimenez, J.; de Pablo, J.; Quinones, J.; Zhang, J.; Ewing, R. C. Effects of Ionizing Radiation and Temperature on Uranyl Silicates: Soddyite (UO2)2(SiO4)(H2O)2 and Uranophane Ca(UO2)2(SiO3OH)2 3-5H2O. Environ. Sci. Technol. 2011, 45, 2510–2515; https://doi.org/10.1021/es1041496.Search in Google Scholar PubMed
23. Blaton, N.; Vochten, R.; Peeters, O. M.; van Springel, K. The Crystal Structure of Na2(UO2)2SiO4F2, a Compound Structurally Related to Soddyite, and Formed during Uranyl Silicate Synthesis in Teflon-Lined Bombs. Neues Jahrbuch Mineral., Monatsh. 1999, 6, 253–264.Search in Google Scholar
24. Nazarchuk, E. V.; Siidra, O. I.; Charkin, D. O.; Tagirova, Y. G. Framework Uranyl Silicates: Crystal Chemistry and a New Route for the Synthesis. Materials 2023a, 16, 4153; https://doi.org/10.3390/ma16114153.Search in Google Scholar PubMed PubMed Central
25. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8, https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
26. Downs, R. T. Analysis of Harmonic Displacement Factors. In High-Temperature and High-Pressure Crystal Chemistry; Hazen, R. M., Downs, R. T., Eds.; Mineralogical Society of America, Geochemical Society: Washington, DC, Vol. 41, 2000; pp. 61–87.10.1515/9781501508707-007Search in Google Scholar
27. Bubnova, R. S.; Firsova, V. A.; Filatov, S. K. Software for Determining the Thermal Expansion Tensor and the Graphic Representation of its Characteristic Surface (Theta to Tensor-TTT). Glass Phys. Chem. 2013, 39, 347–350; https://doi.org/10.1134/s108765961303005x.Search in Google Scholar
28. Gagné, O. C.; Hawthorne, F. C. Bond-length Distributions for Ions Bonded to Oxygen: Results for the Transition Metals and Quantification of the Factors Underlying Bond-Length Variation in In-Organic Solids. Acta Crystallogr. 2016, B72, 602–625.10.26434/chemrxiv.5410921Search in Google Scholar
29. Nazarchuk, E. V.; Siidra, O. I.; Charkin, D. O.; Tagirova, Y. G. Uranyl Silicate Nanotubules in Rb2[(UO2)2O(Si3O8)]: Synthesis and Crystal Structure. Z. Kristallogr. 2023, 238, 349–354, https://doi.org/10.1515/zkri-2023-0019.Search in Google Scholar
30. Nazarchuk, E. V.; Siidra, O. I.; Charkin, D. O.; Tagirova, Y. G. U. (VI) Coordination Modes in Complex Uranium Silicates: Cs[(UO6)2(UO2)9(Si2O7)F] and Rb2[(PtO4)(UO2)5(Si2O7)]. Chemistry 2022, 4, 1515–1523; https://doi.org/10.3390/chemistry4040100.Search in Google Scholar
31. Fejfarová, K.; Plášil, J.; Yang, H.; Čejka, J.; Dušek, M.; Downs, R. T.; Barkley, M. C.; Škoda, R. Revision of the Crystal Structure and Chemical Formula of Weeksite, K2(UO2)2(Si5O13)·4H2O. Am. Mineral. 2012, 97, 750–754; https://doi.org/10.2138/am.2012.4025.Search in Google Scholar
32. Filatov, S. High-temperature Crystal Chemistry. In Theory, Methods and Research Results; Nedra: Leningrad, 1990; p. 288. (in Russian).Search in Google Scholar
33. Wilson, C. N. Results From Nevada Nuclear Waste Storage Investigations (NNWSI) Series 3 Spent Fuel Dissolution Tests; Pacific Northwest Laboratory Report PNL-7170: Richland, Washington, 1990.10.2172/137809Search in Google Scholar
34. Burns, P. C.; Klingensmith, A. L. Uranium Mineralogy and Neptunium Mobility. Elements 2006, 2, 351–356; https://doi.org/10.2113/gselements.2.6.351.Search in Google Scholar
35. Klingensmith, A. L.; Burns, P. C. Neptunium Substitution in Synthetic Uranophane and Soddyite. Am. Mineral. 2007, 92, 1946–1951; https://doi.org/10.2138/am.2007.2542.Search in Google Scholar
36. Burns, P. C. Cs Boltwoodite Obtained by Ion Exchange from Single Crystals: Implications for Radionuclide Release in a Nuclear Repository. J. Nucl. Mater. 1999, 265, 218–223; https://doi.org/10.1016/s0022-3115(98)00646-1.Search in Google Scholar
37. Burns, P. C.; Li, Y. The Structures of Becquerelite and Sr-Exchangedbecquerelite. Am. Mineral. 2002, 87, 550–557; https://doi.org/10.2138/am-2002-0418.Search in Google Scholar
38. Weck, P. F.; Kim, E.; Buck, E. C. On the Mechanical Stability of Uranyl Peroxide Hydrates: Implications for Nuclear Fuel Degradation. RSC Adv. 2015, 5, 79090–79097; https://doi.org/10.1039/c5ra16111h.Search in Google Scholar
39. Bish, D. L.; Carey, C. B. Thermal Behavior of Natural Zeolites. In Book: Natural Zeolites: Occurrence, Properties, Applications; Bish, D. L., Ming, D. W., Eds.; Mineralogical Society of America: Washington, DC, Vol. 45, 2000; pp. 403–452.10.1515/9781501509117-015Search in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/zkri-2024-0091).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- In this issue
- Inorganic Crystal Structures (Original Paper)
- SrAl8Rh2 – the first phase in the Sr/Al/Rh system and new representative of the CeAl8Fe2 type structure
- Plagioclase feldspars (Ca1-x Na x )(Al2-x Si2+x )O8: synthesis and characterizations of mechanical weathering relevant to Martian regolith
- Thermal evolution of soddyite, (UO2)2SiO4(H2O)2 and structurally related Na2(UO2)2SiO4F2
- Synthesis and crystallographic characterization of Cu[SeCN]
- Organic and Metalorganic Crystal Structures (Original Paper)
- Synthesis, structure and fluorescence of a novel zinc(II) polymer based on N-[(3-pyridine)-3-sulfonyl]-threonine
- Crystallographic Computing (Original Paper)
- How many symmetry operations are needed to generate a space group?
Articles in the same Issue
- Frontmatter
- In this issue
- Inorganic Crystal Structures (Original Paper)
- SrAl8Rh2 – the first phase in the Sr/Al/Rh system and new representative of the CeAl8Fe2 type structure
- Plagioclase feldspars (Ca1-x Na x )(Al2-x Si2+x )O8: synthesis and characterizations of mechanical weathering relevant to Martian regolith
- Thermal evolution of soddyite, (UO2)2SiO4(H2O)2 and structurally related Na2(UO2)2SiO4F2
- Synthesis and crystallographic characterization of Cu[SeCN]
- Organic and Metalorganic Crystal Structures (Original Paper)
- Synthesis, structure and fluorescence of a novel zinc(II) polymer based on N-[(3-pyridine)-3-sulfonyl]-threonine
- Crystallographic Computing (Original Paper)
- How many symmetry operations are needed to generate a space group?

