Abstract
This chapter gives an introduction to the many practical uses of surfactants in analytical chemistry in replacing organic solvents to achieve greener chemistry. Taking a holistic approach, it covers some background of surfactants as chemical solvents, their properties and as green chemicals, including their environmental effects. The achievements of green analytical chemistry with micellar systems are reviewed in all the major areas of analytical chemistry where these reagents have been found to be useful.
1 Introduction
A review of micelles (i. e. surfactants, detergents, and soaps) as green chemical reagents seems to be an almost endless task as new results are constantly found in the literature. The effectiveness and properties of micelles will be illustrated through examples with numerous references to other articles and reviews that readers can explore on their own. Rather than give a complete review, which might have to be encyclopedic in nature, one hopes to give enough background that readers can investigate further and that they can understand the utility of surfactant systems as green chemical reagents. Because surfactants have been studied for an extended period, these mature chemical’s properties are well known, and with their widespread use in industry their safety and cost can be optimized.
2 General aspects of surfactants
In the discussion of surfactants, chemists often focus exclusively on the surfactant molecules and/or their relationship with the chemical(s) with which they react. Before we start that dialogue, briefly consider the surfactants’ solvent: water. If water were not so abundant, we would marvel at its unique properties. Although only 18 g/mole, the temperature range at which water is in the liquid state is much broader and much higher than would normally be expected. This phenomenon is of course due to hydrogen bonding illustrated in Figure 1. If one thinks of the crystal structure of a diamond with each tetrahedral carbon bonded to another, one can imagine why this macro-molecule is so hard; however, extremely pure water has a similar structure to a diamond since each molecule is connected to another through a similar tetrahedral matrix, this time held together by hydrogen bonding. Although not as strong as a covalent bond, this hydrogen bonding gives water its large liquid temperature range and cause it to be, in its pure form, a non-conducting liquid.
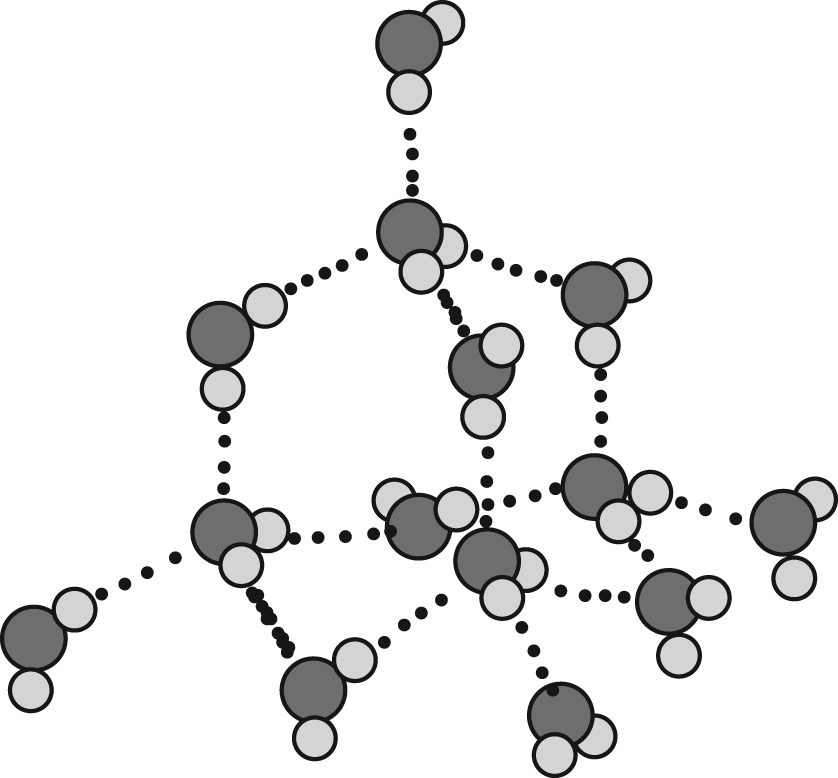
The diamond shape of pure water’s hydrogen bonding.
Adding other chemicals to water breaks up this diamond-like matrix and increases the entropy of the liquid, breaking up an ordered state into a more disordered state. The composition of these additional chemicals has a direct effect on the physical properties of the water–chemical mixture. For example, a soap surfactant is like two chemicals in one: a salt and an alkane. If the alkane length is short, then the surfactant is more soluble in water like ethanol compared to octanol. The salt functional group with an anionic charge can in the simplest terms be seen as a carbonate group. So, when one increases the alkaline earth concentration, one obtains a precipitate similar to if MgCO3, CaCO3 SrCO3, or BaCO3 were added to water. To think of it another way, in a separatory funnel with an organic layer and a cloudy aqueous layer, adding a soluble salt will clear the aqueous phase because the dielectric nature of water will increase with the salt and push the organic alkane out of the aqueous phase. This same reaction occurs when a micelle forms in water, lowering of the molarity where micelles form if a salt is added to an aqueous surfactant system [1].
The earliest version of soap may date back to Babylonian times, with soap still today the most used surfactant world-wide but has been replaced in areas with hard water and in developed countries by synthetic anionic surfactants. Several financial reports estimate that surfactants and detergents are an over 30-billion-dollar business. Their uses have been more varied than one might originally expect: during the Great War, Germany started using detergents due to a shortage of fats needed for the war effort because of the Allied blockade. More recently, chemists first considered surfactants as chemical reagents due to their useful major chemical properties. Surfactant chemistry has been so widespread due to their ability to separate nonpolar substances in water, but surfactants may also act as wetting agents (they lower the surface tension of water), emulsifiers, foaming agents, and dispersants, and have been used in aqueous and non-aqueous systems [2, 3, 4]. With the advent of Green Chemistry, their environmental friendly aspect has added a new reason to consider replacing organic solvents with surfactants. Much work has now been done in this field. For example, surfactants’ potential contribution to Green Chemistry is discussed in a 2001 review by Urata [5]. The type and amount of organic waste is tremendously reduced using aqueous surfactants systems that only contain 10−2 to 10−4 M surfactant in water compared to almost 100 % organic solvent.
Water, the most popular of solvents, will not dissolve molecules and complexes that are non-polar in nature, however, when mixed with surface active agents, (surfactants), oils and grease can be washed away. The solubilization occurs because the surfactants can form micelles, which are created at what is termed the critical micelle concentration (CMC). Below this value ionic surfactants act like electrolytes. The number of molecules needed to form an individual micelle is called the aggregation number. In water a dynamic three-dimensional ellipsoid or sphere occurs at the CMC as the hydrophilic head groups positioned outward in the aqueous solution and the hydrophobic long chain hydrocarbon are facing toward a center of the micelle, whose general structure is shown in Figure 2 [6]. The Stern layer consists of the hydrophilic groups that face outward toward the bulk water. The region adjacent to the Stern layer that contains a high density of counter-ions of the polar heads (Gouy–Chapman double layer) separates the hydrophobic interior of the micelle from the bulk aqueous phase outside. The center space is where the non-polar organic compounds can be solubilized. Fluorescence polarization experiments determined that the core of the micelle is hydrocarbon-like and fluid, microviscosities of 10 to 30 cP having been observed. The nature of the core has been extensively studied using Raman spectroscopy, fluorescence probes, positron annihilation and sound velocity measurements and excimer formation [7] and temperature dependence [8]. Micelles are distorted spheres, ellipsoidal in shape of small dimensions, with 1.5–3.0 nm radius. At higher surfactant concentrations much larger micelles may be formed which are rod like in character. Micelles are dynamic in nature with movement of the counter-ions and water on its surface and with the exchange of surfactant molecules from the micelle to the bulk solution. Generally, the type of polar head group indicates one of five surfactant types: nonionic, cationic, anionic, zwitterionic and gemini. Each of these groups has many examples and classes of surfactants. Examples of some common surfactants and their characteristics are given in Table 2.
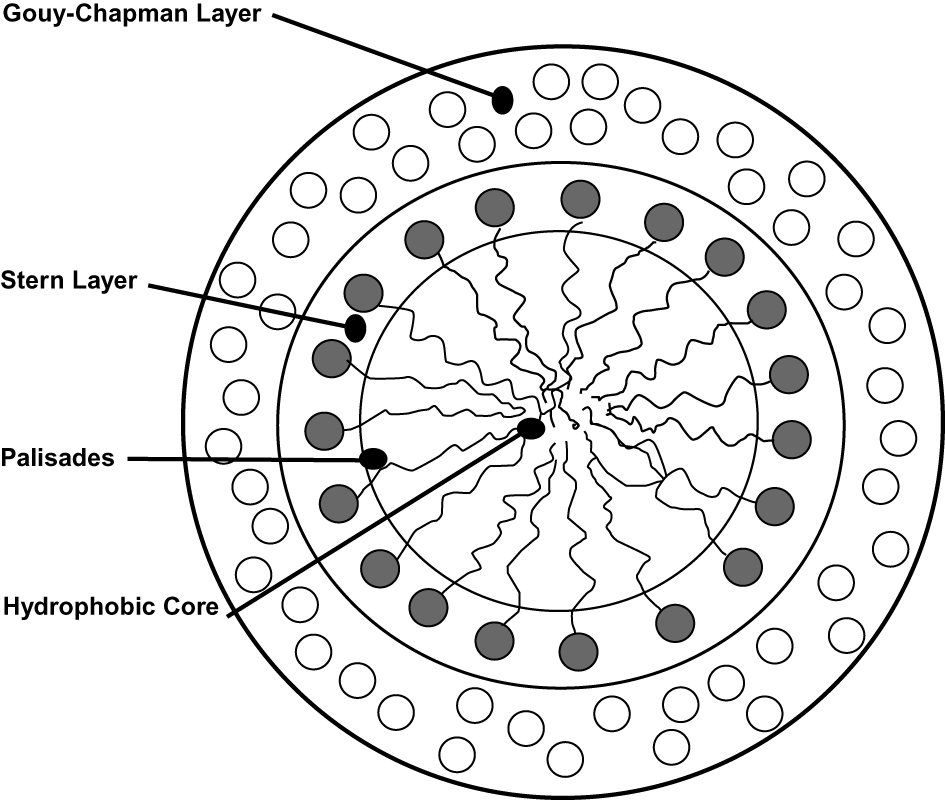
Micelle.
As an anionic surfactant, soap normally has a carboxylate group from the saponification of a fat by sodium hydroxide, but other anionic groups are possible: sulfate, sulfonate, dioctyl sulfosuccinate and phosphate. For laundry detergents the classes are alkylbenzene sulfonates, alkyl sulfates and alkyl ether sulfates, which are less susceptible to hard water but foam more than other detergents, secondary alkane sulfonates and soap.
For cationic surfactants, there are octenidine dihydrochloride, a variety of quaternary ammonium salts, esterified mono- or di-alkyl quaternary compounds, esterquats, and imidazoline derivatives. One of the first to achieve widespread use as a fabric softener was N,N-Dimethyl-N-octadecyloctadecan-1-aminium chloride, which has been phased out because of its low biodegradability [9]. This is an example of how the use of a mature industrial chemical can be chosen with a clear knowledge of its environmental properties compared to chemicals only used in academic research and they have not been fully studied.
The quaternary ammonium salt can also be the positive part of the zwitterionic (amphoteric) surfactant with the negative part containing a variety of functional groups such as sulfonates, phosphates (often found in nature), carboxylates and others. These surfactants are not used as widely because of their higher costs compared to other products. Zwitterionic surfactants include hydroxysultaines, alkyl betaine, and alkylamidopropyl betaine. Betaines are derived from imidazolines and alkylamphoacetates. These surfactants are constructed on salts of quaternary ammonium cations where the fatty acid is linked to the center of the molecule through ester linkages. This type of zwitterionic surfactant is commonly referred to as betaine-esters or esterquats and is more biodegradable than the early cationic surfactants that were also used as fabric softeners [9].
Gemini surfactants are dimeric surfactants with a spacer in between. These molecules have two hydrophilic groups and two tails per surfactant molecule. Gemini surfactants enjoy a number of superior properties when contrasted to the other single-headed, single-tailed surfactants. They exhibit smaller CMC values, have increased surface activity and less surface tension at the CMC, are more hard-water tolerant, and have superior wetting times as well as lower Krafft points. There have been several reviews of both cationic and anionic Gemini surfactants [10, 11, 12, 13].
There are several types of nonionic surfactants: polyethylene glycol alkyl ethers or alcohol ethoxylates, polyethylene glycol alkylphenyl ethers, fatty acid alkanolamides, alkylamine oxides, N-methylglucamides, polyoxyethylene glycol sorbitan alkyl esters and alkylpolyglycosides. The popular type that has polyoxyethylenated head group forms hydrated coils in the outer region of the micelle that act as the hydrophilic part of the micelle. A study of nonionic surfactants using Raman spectroscopy reported additional Raman lines were seen. These Raman peaks indicate the liquid-like nature of the micellar core. The Raman spectroscopy also denotes that the long ethylene oxide chains in some nonionic surfactants assume dihedral helical structures, typical in lengthy molecules. However, the nonionic surfactants with shorter ethylene oxide chains (Triton X-100) have a major portion of the ethylene oxide chain in an open coil structure (not a dihedral helical structure) and are hydrated instead of coiling on themselves [14].
2.1 Determination of the CMC
Numerous methods have been used to determine a surfactant’s CMC including spectroscopy, UV/Visible, IR, fluorescence, nuclear magnetic resonance, electrode potential/conductivity, voltammetry, also scattering techniques, calorimetry, surface tension, and foaming. This analysis is generally accomplished by plotting the surfactant’s concentration versus the physical property under investigation. Higher temperatures and pressure can make the CMC difficult to determine because the CMC may appear to occur at a wider range than would be the case in ambient conditions. Goodling et al. have characterized the CMC and aggregation number for sodium dodecylsulfate, SDS, by luminescence in an undergraduate experiment [15]. In a more recent study by Nakahara et al., the CMC values for both nonionic and anionic surfactants were assessed employing a photosensitive monoazacryptand–barium complex. Its fluorescence intensity is perceptively altered by environmental conditions established on the photo-induced electron transfer mechanism as a fluorescent probe, and this result was compared to the CMC’s previously reported in the literature that used 1,6-diphenyl-1,3,5-hexatriene, or 8-anilinonaphthalenesulfonic acid magnesium salt, or pyrene, as probes [16]. Besides being used to determine the CMC, luminescence has also been used to determine other surfactant characteristics: shape, size, aggregation number and microviscosity using not only fluorescence intensity but also fluorescence lifetime, anisotropy and quenching measurements [17]. The change in the added fluorescent dye’s color could be solely due to the change in the microenvironment from the formation of micelles at the CMC but other factors may also play a role. The dye may interact with the surfactant in the formation of the micelle, thereby altering the aggregation of the micelle. The dye may form an ion-pair or close ion-pair with the surfactant or a dimer with itself or form a special kind of micelle (mixed micelles) at concentrations far below the normal CMC characteristic of the surfactant as reported by Garcia et al. [18] These researchers further propose that the interaction of the surfactant with a –SO3– promotes electron withdrawing on the aromatic dye. Thus, with a lowering of the pKa of any –OH group, there is an ionization of easily dissociated groups and a change in the chromophore’s structure that is seen spectrophotometrically [18].
Electrolytes added to ionic surfactant solutions exhibit a lowering of the CMC, which has a linear dependence of log (CMC) on the concentration of added salt [19]. This phenomenon is not true for nonionic surfactants and their CMC values. When non-electrolytes are mixed with the surfactant media, the effects are reliant on the nature of the species added [18]. For polar non-electrolytes (e. g., n-alcohols), the CMC diminishes with increasing concentration of alcohol, whereas when urea is added to micellar solutions, this addition tends to increase the CMC and may even prevent micelle formation. Nonpolar additives are apt to have slight effects on the CMC. Usually, the occurrence of 20–40 % (v/v) of organic co-solvents (ethanol and acetonitrile) in water prevents micelle formation resulting in a reduction of fluorescence intensity [20].
2.2 The Krafft and cloud points
These two unique surfactant characteristics can be used for the effective separation of an analyte, which will be discussed later. The Krafft point is the minimum temperature at which surfactants form micelles. Below this value the surfactant remains in crystalline form, even in an aqueous solution. Above the Krafft point a fairly large amount of the surfactant can be dispersed in micelles, and solubility intensifies. Gu and Sjöblom have reviewed a series of surfactants and found that there is a linear relationship between the Krafft point and the logarithm of CMC for ionic surfactants [21]. These relationships have a continuous negative slope for different kinds of homologues of surfactants with the same kind of counter ion. For homologues of nonionic surfactants, a comparable linearity is seen; however, these surfactants exhibit a positive slope. Apparently this phenomenon reveals the subtle balance between the attraction and repulsion forces of surfactants, accountable both for phase separation and micelle formation [21].
The cloud point, the temperature where the mixture becomes cloudy as there is a phase separation and two phases appear, can occur with nonionic surfactants who do not exhibit Krafft points. Hinze published two excellent reviews on cloud point extractions (CPEs) in 1993 with Pramauro [22] and again in 1999 with Quina [23].
2.3 Probing the micelle environment
Several probe molecules that show altered spectroscopic behavior based on their environment have been used to study micelles. These investigations provide worthwhile information on the nature of diverse regions of micelles, that is, their degree of rigidity and polarity in the core and on the surface regions. The fluorescence emission spectra fine structure of pyrene changes with its solvent environment [24]. Specifically, the ratio of the third fluorescence peak to the first peak has been used in probe studies of micellar systems to compare them with both water and organic solvents like hexane and methanol [25]. This III/I ratio increases distinctly on altering the solvent from water to sodium dodecylsulfate, SDS, although the ratio was smaller than that detected in dodecane. This ratio change shows that the environment experienced by pyrene is somewhere between that of an alkane and water. The addition of pentanol which will go into the head group region of the micelle and thereby push the pyrene into the micelle core, this effect is seen by III/I ratio increase indicating it is in an organic environment [7]. Pyrene was used as a probe to determine the hydrophobicity of microenvironments and the partition coefficient in n-β-octylglucoside micelles [26].
Pyrene has also been used in the fluorescence quenching studies of SDS and CTAB micellar systems by changing the type and concentration of the counter ions and upon the addition of neutral molecules like benzyl alcohol [27, 28]. From the spectroscopic data of the polar molecule benzophenone (also acetophone and pyridinium ions) whose absorption spectrum is solvent dependent, one can conclude that this molecule is located on the micellar surface rather than the core. These molecules can also be employed to provide a measure of degree of local polarity for the surface of the micelle. Benzene and naphthalene were reported to have a hydrocarbon-like environment in micelles based on NMR studies [25]. A plot of the solvent’s dielectric constant versus wavelength maximum exhibited a line that showed a red shift with increased ε, molar absorptivity [29]. Drummond et al. used 2,6-diphenyl-4-(2,4,6-triphenyl-1-pyridinio)phenoxide {Reichardt’s dye, CAS No. 10081–39–7} to compile a table of effective interfacial dielectric constants in organized media and to measure the electrostatic surface potential for a series of cationic surfactants [30]. The explanation of this trend comes from the two differences in the same molecule: the aromaticity and the aldehyde group. In a nonpolar environment the fluorescence maximum is 400 nm (this is due to an n–π* transition). When the polarity of the micro-environment is increased so is the wavelength maximum, whereby the π–π* level (which lies close to the n–π * level), is brought below that of the n–π * by solvent interaction with the excited state [25]. The effect of quenching on the probe molecule was also investigated to further elucidate the nature of the micelle [25]. Prodan, 6-propionyl-2-(dimethylamino)naphthalene {CAS No. 70504-01-7} was used to determine the aggregation number of SDS micelles and showed that the aggregation number changed in the presence of sodium chloride: 91 (0.1 M NaCl), 105 (0.2 M NaCl and 129 (0.4 M NaCl), and several other interesting characteristics of micellar behavior as its excitation and emission wavelengths vary depending on its microenvironment [31]. 1-Naphthol {CAS No. 90-15-3} was used as a probe to study the difference between three different micellar systems: anionic–SDS, cationic–CTAB and nonionic–Triton X-100R [32]. In a study by Almgren et al. the kinetic equilibria between small neutral arenes and various ionic surfactants as the exit and reentry of the molecules in and out of the micelle were measured quantitatively using phosphorescence as a monitor for the processes in different deoxygenated aqueous micellar solutions and residence times of 1–100 microseconds were reported [33]. Conversely, a micelle will, typically, lose a monomer in 0.01–10 microseconds, so some monomers will be exchanged, during the time that the phosphorescence probe resides in the micelle. They also studied the solubility trends of some eleven aromatic compounds and found that in general that the solubility increases with increasing chain length of the hydrophobic portion of the surfactant with a noticeable difference between the cationic surfactants and the anionic surfactants studied. Their conclusion was that pyrene is preferentially solubilized at the surface of alkylammonium–surfactant micelles. Three different indoles (indole, 1-methylindole and 3-methylindole) were used to study the effects that Brij-35 had on their fluorescence since their fluorescence attributes in a different environment permits their association with the micelle to be quantified. In particular control of the odor caused by animal waste from 3-methylindole was address as being controlled and measured in surfactant systems [34]. This is seen as a possible solution to waste management.
In a study by Martens and Verhoeven, a SDS micellar solution exhibited a dramatic enhancement in the amount of ground-state electron donor–acceptor complexation between pyrene (donor) and N,N′-dimethyl-4,4′-bipyridinium dichloride, pq2+, (acceptor) when compared to homogeneous solutions or CTAB. The charge transfer absorptions observed evidence of a direct contact of the two species. Previous work hypothesized only a dynamic mechanism, but this work shows that a static mechanism also contributes to the overall mechanism in SDS micellar media because the pq2+ is held on the anionic surface of the micelle while the pyrene is in the interior facilitating the reaction by proximity [35]. A study of the fluorescence quenching effects of methylene iodide or nitromethane on aromatic compounds in the presence of both SDS and copper (II) ions with anthracene in CTAB were in agreement with the estimates of the kinetic equations and yield values for exit and entry rates of guest molecules in the micelle; the study demonstrated that the quencher is not disturbed by the excitation of the probe [36]. In a Journal of Chemical Education article, a kinetics demonstration shows the effect of micellar stabilization of excited state deprotonation of the 8-hydroxypyrene-1,3,6-trisulfonate ion {CAS No. 6358–69–6} being stabilized by the cationic surfactant CTAB [37].
These probe studies lead plainly to the direct conclusion that the movement of molecules in the micellar interior is affected by the micellar size, charge, counter ions, shape and viscosity regardless of whether the micelle is a spherical or rod-like shape. A review by Grieser et al. examines the many different spectral probe techniques that have been used to study micellar and vesicular systems [38].
2.4 Catalysis
Besides solubilization, surfactants have been used in catalysis, photolysis and extractions. The hydrophobic tail is most often a long chained hydrocarbon (linear, branched or aromatic) but it may consist of a fluorocarbon, a siloxane or even a double tail. The ability of organic compounds to be dissolved in water with the use of micelles can lead to the catalysis of the reaction between the reagents and the analyte. The pseudophase ion-exchange model proposes that reactions are primarily affected due to the proximity of reactants in a small micellar volume [39]. For example, when the cationic micelle attracts the anionic analyte, it facilitates a faster kinetic reaction by its proximity to the organic reagent inside the micelle. The reaction is no longer based on random collisions in a solution, as the reagents are closer together and in the proper orientation for the reaction to occur. For some organic reagents there are a combination of several factors that affect its solubility and reactivity in a micellar solution. The nonpolar solute may exhibit a deep penetration or a short penetration of the core at a shorter distance from the Stern layer. It is also possible that the ionic solutes or ionic portions of an organic solute may be absorbed or repelled by the polar micellar surface. This charge may also affect the kinetics of the desired chemical reaction. These effects entail the dynamics of the hydrophobic and the electrostatic interactions occurring in the micellar system with the reactants. Because of the different properties of each surfactant’s polar end, different surfactants are expected to behave differently as has been reviewed [7].
A kinetic study of the rate of fading of triphenylmethane dyes and of sulfonphthalein indicators were determined in alkaline solution in the presence of micelle-forming surfactants. The kinetics of a cationic dye like crystal violet was significantly accelerated by the addition of CTAB, and retarded by SDS but not as great effects were seen with the sulfonphthalein dyes [40]. Reeves studied the kinetics and nature of the aggregates (dyes complexing with or dimerizing with the surfactant) of the base-catalyzed hydrolysis of acetate and hexanoate esters of an azonaphthol sulfonate dye in the presence of two different cationic surfactants at various concentrations [41]. This study was instigated to help explore the different aggregation numbers determined when different dyes were used. In a companion study Reeves investigated the effects of diverse counter-ions and various concentrations on the base-catalyzed hydrolysis reaction of hexanoate esters of an azonaphthol sulfonate dye exhibited not only absorption wavelength shifts but also changes depending upon the CTAB concentration and the method of making the original species mixtures [42].
For example, for the hydrolysis of cytotoxic pyronins a threefold higher rate constant in an aqueous solution of cationic CTAB while the hydrolysis reaction was completely inhibited by anionic SDS [20]. Another example is the fluorescence analysis of cyanide ion using its reaction with 1,4-naphthaquinone-2-sulfonic acid; in the presence of CTAB micelles, the reaction takes 5 min as opposed to a reaction time of about 90 min without CTAB [20]. A most dramatic increase of 10,000 times is seen in the fluorescence analysis of thiols with 4-nitro-N-n-butyl-1,8-naphthalimide using a CTAC surfactant system [43].
A series of studies on the state and dynamics of electron transfer processes of exciplexes (a complex, existing in an excited state, that is dissociated in the ground state) has shown that micellar system alters the dynamics when compared to normal solutions. The exciplexes radical ions can be protected with success depending on expulsion from micelles directly after the initial electron transfer process. This micellar catalyzed ion-pair separation progression results in the formation of stable and long-lived ion radicals, which may well be detected both by transient absorption and by photoconduction methods. Alternatively, if the micellar surface “traps” the ion-pair owing to strong charge attraction, then a rapid ion recombination processes results. Such mechanisms are usually only observed in highly viscous systems but are readily observed in micelles and may help elucidate the catalytic activity of micellar systems [44]. Similarly, Alkaitis et al. studied the laser photolysis of phenothiazine in SDS micellar system compared to the photolysis in a methanol solution and found that the SDS leads to the formation of solvated electrons, cation radicals and triplets; therefore the yield of ions is much larger with an SDS system [45].
There are several possible routes for reactions to take in a micellar system. For a simple reaction of A + B → {C} → D, one must sometimes consider the transition state C and how it interacts in an aqueous micellar system. Both A and B could be inside the hydrophobic core of the micelle. There are three possible locations for A initially: partially in the micelle (Stern Layer, or Gouy–Chapman Layer or Palisade Layer), the surface of the micelle, or in the water. The same location options are true for B, D, and C. In fact, some reactions may be catalyzed by an anionic surfactant but quenched or retarded by a cationic one because of the charge attractions involved. One example is the free-radical reaction that is used for emulsion polymerization, a key industrial procedure that has a micellar system producing a proximity effect. The free radical that causes the polymerization and the reacting monomer are adjacent because of the micelle achieving an enhancement of the polymerization. Several other interesting systems are discussed by Thomas [7] and Fendler et al. [29] La Sorella presents a review in 2015 on the development of catalytic systems in water micellar systems [46].
Each of the characteristics of surfactants and micelles have not only been studied in their own right but have been put to use in many areas of chemistry, consumer products and in industry. Several reviews on the use of surfactants in analytical chemistry have been published previously [7, 19, 47, 48, 48, 50]. Not only does one expect the use of surfactants in water to replace organic solvents for greener chemistry, but also there is now an expectation that the micellar system will in many ways out preform its non-aqueous counterpart. Since the 1959 report of the catalyst of the phenol blue reaction with crystal violet in surfactant systems [40], there have been numerous investigations into the catalytic properties of micellar solutions and the mechanisms involved [51].
3 How environmentally safe are they? Concerns and beneficial uses
Green chemistry wishes to reduce or eliminate the generation of hazardous wastes. Surfactants replacing organic solvents can be a major aspect in this process by both replacement of a solvent and a reduction in the amount of chemicals used. However, one must continually try and look at updated literature on chemicals including their Safety Data Sheet to ascertain the viability of the current state of knowledge. Those of us who started our careers long enough ago remember the routine use of benzene, carbon tetrachloride and 2-naphthalamine in the laboratory. It took years of data and research to determine and understand the hazardous nature of these and other at one time common chemicals. Most anionic and nonionic surfactants are nontoxic and are widely used, and having a LD50 comparable to sodium chloride. Aquatic toxicity data are extensively available for the three major classes of surfactants: anionic, cationic and nonionic. It was found that the order of toxicity was cationic > anionic > nonionic surfactants [52].
Because of their widespread use in home and industry, surfactants commonly find their way into the environment [53]. The evaluation of environmental risk assessment and biodegradability of these organic substances is an important consideration for public health and environmental impact [54]. The toxicity data from laboratory and field studies are indispensable for us to evaluate the conceivable environmental risks from these useful chemical agents. The fate of the degradation of surfactants in the environment has been studied. They are readily degradable under aerobic conditions [55, 56] and also have been studied for biodegradability under anaerobic conditions [9]. Anaerobic conditions are found in the sludge digesters of wastewater treatment plants, sub-surface soil layers and the bottoms of rivers. After use, the surfactants are mainly expelled through a sewage treatment plant and then disseminated into the environment through the effluent released into surface waters and sludge disposal on agricultural lands. Surfactants have various behaviors and fates in the environment, which is exceptionally dependent upon whether their disposal is aerobic or anaerobic. Nonionic and cationic surfactants have been shown to have much greater sorption on soil and sediment than anionic surfactants [56]. An established initial step of wastewater treatment is the elimination of particulate matter in primary settling tanks; this particulate matter could include soap and detergents precipitated by calcium ions. Wastewater sludge is surfactant rich and is processed at elevated temperatures under anaerobic conditions [55]. Under these conditions, soap is readily biodegradable. Fatty alcohol sulfates, like sodium dodecylsulfate, and alcohol ether sulfates are readily biodegradable under both aerobic and anaerobic conditions [55].
3.1 Biodegradation
Biodegradation means the microbial breakdown of organic substances. The results of biodegradation for surfactants can be looked at on three levels. Primary biodegradation is where microorganisms cause the loss of surface-active properties that define the surfactant. Ultimate biodegradation is achieved when the surfactant is totally broken-down to inorganic end-products such as carbon dioxide, water and salts of any other elements, and consume these compounds and use them as energy and carbon sources. Ready aerobic biodegradability is an arbitrary classification of surfactants, involving certain specified screening tests for ultimate biodegradability. It is assumed that such surfactants in aquatic environment will then be able to rapidly and completely biodegrade under aerobic conditions.
The chemical structure of surfactants plays a major role in shaping their effect on the biotic and abiotic environment. This structure also influences the applicability of various analytical methods for analysis. Several factors must be considered: extraction and pre-concentration of the sample, qualitative and quantitative determination, and proper validation of the samples and method. It may occasionally be beneficial to separate anionic and nonionic surfactants simultaneously using solid phase extraction and to isolate them just prior to their quantitative analysis. A recent literature review provides an excellent starting point concerning the occurrence and concentrations of surfactants in different environmental samples [57]. The research summarizes the information on the analytical techniques and includes soil, street and indoor dust, bottom sediments, sewage sludge, and liquid samples, including precipitation, atmospheric deposits, aerosols, ground waters, surface waters, and sewage, along with their basic parameters of analysis, advantages and disadvantages of each method with numerous references [57]. Several classes of surfactants can be analyzed by potentiometric titrations including Epton’s two-phase titration method [19]. The endpoint for a titration can be determined by turbidity and refractive index changes. In another type of titration, barium chloride, which forms a charged complex with nonionic surfactants result in a “pseudo-cationic” molecule, is detectable by a surfactant-sensitive electrode [19, 58].
3.2 Reclamation
In an opposite role for surfactants, they can be used as soil and water decontamination agents. Another admirable review emphasizes the currently surfactant-based soil and wastewater treatment technologies that clean the environment with green surfactant chemistry [59]. Hydrophobic pollutants (such as pesticides, petroleum hydrocarbons, PCBs and PAHs) are 100–1000 times more soluble in micelles than bulk water. The aromatic hydrocarbons are encapsulated in the micelles’ hydrophobic interior and are removed through ultrafiltration since their size is larger than the pores of the filter [59]. Removal of heavy metals pollutants is possible by the micellar solution of surfactants [60, 61]. A flotation procedure using surfactants is normally used in mineral ore processing for separating hydrophobic and hydrophilic from one another, as in the purification of copper ore. This method can also be used as a remediation technique for polluted soils because it is effective in the removal of both organic and inorganic pollutants. The use of reverse micelles to remove ionic dyes like methyl orange and methylene blue was also discussed in this review paper [59].
Pharmaceuticals and personal care products have an increasing presence in the nation’s waterways. Their removal is becoming a major problem of concern. Surfactant-enhanced extraction has been reported for the removal of different personal care products. One surfactants method is emulsion liquid membrane, which has acquired ample attention for the removal of these pollutants from water. The extraction method is based on liquid membrane technology for selective permeability of solutes with the micelles acting as both extraction and stripping agents. Sequestering of pharmaceuticals can be accomplished by using the enhanced adsorption effects that surfactants impart to a solution [59]. Shah et al. also review the various methods that surfactants can be employed for the removal of toxic metals by using ultrafiltration, CPEs, activated carbon (having 2–4 increased capacity with surfactants), soil washing/desorption/extraction, adsorption onto soil and phytoremediation. The use of biosurfactants in heavy metals removal is reported to be more effective than their synthetic counterparts. Biosurfactants are regarded as having lower toxicity and better biodegradability with improved stability over a wide range of temperature and pH conditions, ionic strength/salinity and exhibiting enhanced foaming properties [59].
3.3 Linear alkylbenzenesulfonates in the environment
Among the anionic surfactants, the linear alkylbenzenesulfonates (LAS) are one of the most popular laundry detergents with billions of pounds produced each year. Naturally their environmental fate has been well studied. The linear chains are much more biodegradable and less toxic than those with branched chains. The fate of surfactants has allowed chemist to look at these chemical from a variety of ways. Besides their nature in forming micelles from their hydrophobic portion, they are ionic chemicals with electrostatic interactions. There are three types of interactions: hydrophobic interactions from the organic tail, chemical interactions of the sulfonate head group and electrostatic interactions from the charge on the sulfate group and the inorganic materials and humic material in a sediment. These options for interaction make the study of the exact mechanism of sorption more difficult because changing one parameter of an experiment actually changes the other types of interactions as well [62]. The solution’s pH and ionic strength (types of ions too) also affect the behavior of the surfactant in solution and its sorption onto solid material. A change in pH can affect surfactant and surface charge of the material. For example, under very low pH conditions the sulfate group would theoretically lose its charge by being protonated, and the surfactant would behave as an entirely nonpolar alkane.
The formation of complexes between LAS and cationic surfactants such as alkyltrimethylammonium chloride and dialkyldimethylammonium chloride unfortunately results in the complex adsorption onto river sediments, giving biodegradations rates that were two to three times longer than LAS alone [55]. Kruger et al. reported the rates of biodegradation improved with increasing dissolved oxygen concentrations. Results showed that there was preference for the biodegradation of the longer alkyl chain LAS homologs and external isomers (i. e., 2- and 3-phenyl), but that laboratory results were two to three times greater than those experienced in field tests. Kruger suggests based on his results that a supplementary increase in the injected dissolved oxygen concentration during the continuous field test would have caused an amplified biodegradation rate [63]. In a review article, McAvoy et al. reported that concentrations of LAS in anaerobically digested sludge (10,462 ± 5170 μg/g) were one to two orders of magnitude greater than those detected for aerobically digested sludge (152 ± 119 μg/g), demonstrating that LAS is degraded more expeditiously under aerobic conditions [64]. Anaerobic conditions are found in the sludge digesters of wastewater treatment plants, sub-surface soil layers and the bottoms of rivers.
Others have reported similar results with escalated levels of biodegradation (97–99 %) having been establish in some water treatment plants with aerobic processes [55, 65, 66]. By comparison, alkyl phenol ethoxylates are less biodegradable with values of 0–20 % having been cited [55]. The comprehensive biodegradation of surfactants necessitates a consortium of bacteria due to the partial metabolic capacities of different microorganisms [55]. The risk assessment of this class of surfactants to terrestrial plants and animals was described by Mieure et al. who also determined that there are satisfactory margins of safety in the use of wastewater for the irrigation of crops [67].
Once sludge from a treatment plant is applied on land, the LAS are promptly metabolized by aerobic bacteria and do not accumulate in soil as demonstrated by field experiments that showed that the application method, and whether the soil had been ploughed, or not, had no effect on degradation rates of the LAS [56].
Anionic surfactants can be found in soils as a result of sludge application to crop land and wastewater irrigation of farms. High concentrations of surfactants together with the metals associated with them can embody an environmental risk. However, at low concentrations, surfactant application to crop land and soil is unlikely to have a significant effect on trace metal mobility [68, 69]. Edwards et al. reported on the distribution of nonionic surfactant Triton X-100 and phenanthrene in a sediment/aqueous system and found that it can act either to heighten or to impede phenanthrene sorption from bulk solution [70]. Triton X-100 is a alkylphenol ethoxylate surfactant, these may not be appropriate for field remediation work, owing to these types of surfactants degrade into undesirable alkylphenol monoethoxylates and diethoxylates in addition to alkylphenols during the course of anaerobic biodegradation [71]. It is biodegradable under aerobic conditions, however.
Soap and other surfactants will react with Mg2+ and Ca2+ to form solids. These tend to adsorb with solid particles. Only those dissolved in water can be metabolized by microorganisms. Cationic surfactants are absorbed onto the anionic sludge at the bottom of landfill sediment, water treatment plants, bottom of septic tanks, river bottoms and lakes deeper than 10 m [9]. Anaerobic biodegradation requires the co-operation of different types of microorganisms like those found in a complex food chain to accomplish complete biodegradation. Merrettig-Bruns and Jelen reported in detail on the anaerobic biodegradation of a number of surfactants in all four classes. They concluded that heterogeneous atoms like ester bonds in the chemical structure improve the anaerobic biodegradability of those surfactants significantly. Esterquats, unlike quaternary ammonium compounds, are also ultimately biodegradable under anaerobic conditions, and this is one of the reasons esterquats have replaced quaternary ammonium compounds in common usage [9].
3.4 Perfluorooctanesulfonate in the environment
One exception is perfluorooctanesulfonate, PFOA, surfactants labeled as a persistent organic pollutant with the US EPA, Environmental Protection Agency, which established health advisories at the 70 parts per trillion level in 2016 for PFOA and PFOS and which had earlier a voluntarily agreement with industry to stop PFOA production in 2006 [72]. Water treatment with activated carbon or reverse osmosis can be used to clean the water. The two popular surfactants: linear alkylbenzene sulfonates and the alkyl phenol ethoxylates, APE, have recently been considered to be less desirable for widespread use also. Besides their use as detergents, and cosmetics, they find industrial uses in paints, pesticides, textile and petroleum recovery chemicals, metal working. Since they break down in the aerobic conditions found in sewage treatment plants and in soil to the metabolite nonylphenol (4-(2,4-dimethylheptan-3-yl)phenol), which is not readily biodegradable. The polyoxyethylene chain appears to be easily biodegradable, but the NP derivative looks more resilient [55]. Nonylphenol is alleged to be an endocrine disruptor owing to its capacity to mimic estrogen and subsequently to disrupt the natural balance of hormones (Figure 3) [73]. Prior to these studied APE’s accounted for about 55 % use in industry. The foremost alkylphenols consumed are nonylphenol and octylphenol. Nonylphenol ethoxylates cover about 80 % of the global market, and octylphenol ethoxylates account for the remainder [74]. In 1991 when Ana Soto of Tufts Medical School detected that breast cancer cells, which generally multiply only in the presence of an estrogen, showed the same behavior in plastic containers, apprehensions about potential nonylphenol estrogenic activity developed. Investigative work revealed that nonylphenol caused the growth [74]. The distribution of nonylphenolic compounds was found in the digested sludge of the Swiss sewage-waste treatment plants with 95 % nonylphenol and 5 % short-chain ethoxylates, partially since there is a hydrophobic nature in nonylphenol and partially because the anaerobic digestion of the sludge created nonylphenol [74]. These and similar results have resulted in regulations in the EU. On September 2014 the EPA recommended a Significant New Use Rule to oblige an EPA review before a manufacturer starts or resumes use of 15 different nonylphenols and nonylphenol ethoxylates. They have also used the Safer Detergents Stewardship Initiative to encourage a voluntary phase out of these products in industrial laundry detergents. The EPA has set that nonylphenol concentration should not exceed 6.6 μg/L in fresh water and 1.7 μg/L in saltwater [73, 75]. They are still used in much lesser quantities in the laboratory.
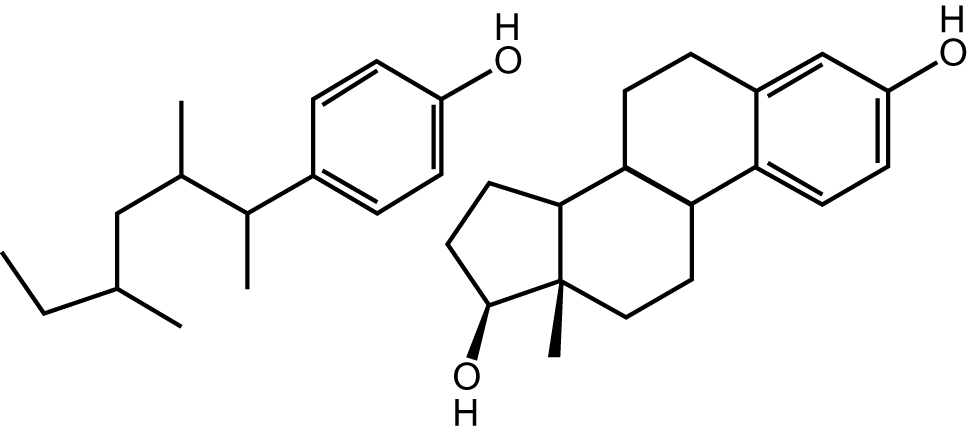
Structure of a nonylphenol (left) beside the estradiol hormone.
The toxicity of nonionic surfactants is contingent upon their structure: with increasing alkyl chain length there is generally an increase toxicity, while increasing ethylene oxide groups will usually reduce toxicity. These trends are comprehensible when one contemplates the toxicity mechanism of surfactants, specifically membrane disruption and protein denaturation, which are a function of the surface-active properties of surfactants [19].
There has also been some concern about how the solubilization effects of micelles would have on other organic compounds being introduced into the environment, specifically polyaromatic hydrocarbons and pesticides. Aronstein et al. studied the effect of low concentrations of surfactants on the biodegradation of sorbed aromatic compounds (<0.01 %) in soil, because of the possible effectiveness of surfactants for stimulating the microbial destruction of pollutants. Alfonic 810–60 (a linear alcohol ethoxylate nonionic surfactant) and Novel II 1412–56 (a similar linear alcohol ethoxylate nonionic surfactant of slightly longer length) increased the extent of desorption of phenanthrene from a mineral soil. Both surfactants at 10 μg/g of soil noticeably improved the amount of biodegradation of phenanthrene in both the mineral and the organic soil. Biphenyl mineralization in the mineral soil was not altered by either surfactant, but biodegradation in the organic soil was improved by Alfonic 810–60. Surfactants at low concentrations may allow for the mineralization of sorbed aromatic compounds in polluted soils was one of their major conclusions, but not all the surfactants studied were successful [76]. In a study of the solubility of DDT and trichlorobenzene, as anticipated, the solubility was improved when the surfactant was present at concentrations above their CMC [56].
3.5 Cationic surfactants in the environment
The major uses of cationic surfactants are as fabric softeners and antiseptic agents against bacteria and fungi, cosmetics, in laundry detergents, mouth wash, used in synthesis of gold nanoparticles, and in industry [56]. The situation for cationic surfactants is more troublesome environmentally. Dialkyldimethylammonium chlorides have very low acceptable LD–50’s, but alkylbenzyldimethylammonium chloride has an LD50 of 0.35 g/kg. Prolonged exposure of skin to surfactants can cause chafing as the surfactants disrupt the lipid coating that protects skin cells [77]. These surfactants are known to be toxic to animals, ecosystems, or humans and can also increase the diffusion of other environmental contaminants because of their unique properties [78]. Having a positive charge, cationic surfactants have a strong attraction for the surface of particulates in sewage sludge, which are principally negatively charged. Several studies have shown that in activated sludge 95 % of the cationic surfactants were adsorbed to the surface of particulate matter [55]. Investigations about alkylbenzyldimethylammonium chloride showed it to be ultimately biodegradable with >80 % of the carbon-14 labeled surfactants being released as 14CO2 [55]. Under aerobic conditions, the biodegradability of quaternary ammonium cationic surfactants generally exhibit reductions with the number of non-methyl alkyl groups, and substitution with a benzyl group can lower the biodegradability even more [56].
In a review by Scott et al. the data available suggest that raw sewage passing through a modern waste treatment plant has a substantial quantity of its surfactant load eliminated. Aerobic treatment processes seem to deliver the best conditions for prompt primary and ultimate biodegradation via a variety of bacteria. Wastewater effluent released into the environment appears to have had its surfactant load reduced to the extent that lethality on aquatic organisms is slight. Superfluous safety margins exist for over 25 varieties of organisms [55]. The biodegradability of surfactants in the environment was summarized by Yang et al. fatty acid esters, and cationic surfactants although were judged to be persistent under anaerobic conditions but were found to be biodegradable in aerobic conditions [56]. The breakdown of the cationic surfactants in coastal waters was reported with an associated increase in bacterio-plankton density, signifying that the degradation occurs because the compound is used as a growth substrate [56].
4 Surfactants in analytical chemistry
There are several reviews of micelles in analytical chemistry [47, 50]. Although solubilization is the property most often thought of for micelles, because there are several sites available within its structure, one must also consider kinetics, and changes in the spectral profile that can also occur. Most of the UV visible absorption methods that have been reported involve the determination of metal ions through complexation with of chelometric indicators. This includes the determination of metals with PAN {1-(2-pyridylazo)-2-naphthol, CAS No. 85–85–8} using Triton X-100 as the nonionic surfactant for the determination of cobalt at 620 nm. The absorptivity was 1.9 × 104 over the range of 0.4–3.2 ppm [79]. For zinc using Triton X-100 at 555 nm with an absorptivity of 5.6 × 104 over the range of 0–100 ppm [80]. With TAN, 1-(2-thiazolylazo)-2-naphthol, using Triton X-100 for the determination of nickel at 595 nm after a 5-min reaction time at a pH of 9.2 with an absorptivity of 4.0 × 104 over the range of 11–110 ppm [81]. Also TAM was used with Triton X-100 for the determination of nickel at 560 nm with an absorptivity of 6.5 × 104 over the range of 0.12–1.20 ppm in soil samples [82].
The solubilization property of micellar systems alleviates the need for organic or mixed aqueous–organic solvents and is indeed its major advantage in analysis. This solubilization ability can alone be utilized for sample preservation and storage. One example shows that Brij-35 surfactant in the sample solution was as effective as a 40% acetonitrile solution in inhibiting the loss of polycyclic aromatic compounds’ adsorption on the surface of borosilicate glass, or other containers [20].
4.1 UV–Visible spectroscopy
The use of surfactants to replace organic solvents in UV–Visible spectroscopy has been one of the major areas of success for this type of green analytical chemistry. There is often a 10-fold increase in sensitivity (molar absorptivity), as well as, a bathochromic shift reported with a change in solvents due to differences in solvent polarity. An early review by Hinze highlights many of the methods that had already been reported by 1979 [83]. It seems plausible that once the electrostatic forces have brought together the oppositely-charged molecules, hydrophobic interactions occur, dramatically altering the micro-environment experienced by the chromophore or complex. Nonionic surfactants exhibit a behavior that is similar to that of organic molecules, without the electrostatic nature seen with cationic and anionic surfactants that can parallel the behavior of electrolytes. A good example of this is the reaction of cobalt with thiocyanate which in water forms the pink complex [Co(H2O)6]2+ but in an alcohol or acetone mixture with water forms the familiar [Co(SCN)4]2− blue complex that absorbs at 625 nm. This was the basis for the visible detection of cobalt using a 3 % Tween-80 nonionic surfactant that eliminates the use of acetone, isoamyl alcohol or other organic solvents in a flow injection analysis method [84]. In many cases there have been reports that indicate that there is a significant bathochromic shift in the absorption wavelength for metal chelate complexes in the presence of micelles. These shifts were used to determine the CMC of some surfactants and the changes in an indicator’s pKa have also been widely studied [47]. Evidence supported by UV–Visible and fluorescence studies has caused speculation that a surfactant molecule can complex with the metal complex changing its character. Sometimes call ternary complexes, they have different spectral properties from the binary complex that is usually formed between the metal and the ligands. Rather than forming a separate ligand the “complexation” may occur at the surface of the micelle’s charged layer as was shown to happen with the π electrons of pyrene.
4.2 Cationic surfactants
A large number of metal cations have been analyzed with triphenylmethane dyes using the cationic surfactants CPC, CPB, CTAB, CTAC and tetradecyldimethylbenzylammonium chloride, zephiramine {CAS No. 139–08–2} including F, Ti, Be, Ga, V, Sc, Y and Al. Also in this group were a number of lanthanides using pyrocatechol violet {CAS No. 115–41–3} and a cationic surfactant media [49]. In some cases the surfactant only seems to be increasing the solubility, while in others it may also be altering the pKa of the dye and/or actually actively participating as a chelating agent in the metal–dye complexation. In a study by Marczenko and Jarosz the experimental conditions for the formation of ternary complexes of aluminum cation with Eriochrome Cyanine R, Chrome Azurol S or Pyrocatechol Violet, in the presence of the surfactants zephiramine, CTAB or CPC formed a tertiary complex with the metal that exhibited greater molar absorptivities than the binary system. Complexes with Pyrocatechol Violet were reported as not suitable for a spectrophotometric method of analysis, however [85].
Exactly how the surfactant interacts was the subject of a contemporary study in 2016 on the binding characteristics between Alizarin Red S {CAS No. 130–22–3} and cationic surfactants [86]. Another series organic dyes that were studied were the Alizarin green dyes used to determine a variety of cations including: vanadium, indium and uranyl [49, 87, 88]. The reaction for nitrite analysis which was determined by the coupling reaction of p-nitroaniline with 8-hydroxyquinaldine {CAS No. 826–81–3} to produce the purple azoxine dye if carried out in CTAB did not require an organic extraction, and had ε = 4.72 × 104 L/mol. cm which is 22 % larger than the earlier reported extraction method [89, 90]. There have been cases reported where the anion of the cationic surfactant used affects the analytical results of an experiment. This phenomenon is attributed to the reaction taking place or the product being physically located on the cationic surface of the micelle and the ion exchange properties of the anion in the solution being the driving force behind the observed data. The order of sensitivity was reported to be SO42− < Cl− < Br− ≤ NO3− [49]. When 3,3-dimethyl-2-phenyl-3H-indol {3,3-dimethyl-2-phenylindole, CAS No. 6636–32–4} was used in a study by Sarpal et al. as a fluorescence probe studying to pKa’s in SDS, CTAB and water, it was shown that CTAB offered a more hydrophobic environment for the probe molecule [91]. Cationic micellar systems generally enhance the acid dissociation and therefore decrease the pKa of organic compounds, and as expected an anionic surfactant will cause an increase in the pKa.
Cationic surfactants are known from catalysis studies to facilitate nucleophilic substitution reactions. There are several early examples of using this to enhance visible spectroscopy determinations. The reaction of cyanide ion with 5,5′-dithiobis(2-nitrobenzoic acid) to displace the corresponding absorbing thiol anion, using CTAB decreased the reaction time from 25 min to 1–3 min [92]. Micellar catalysis of nucleophilic reactions was used for determination of aromatic aldehydes, amines, and oximes with UV–Visible spectroscopy by catalytic acylation with p-nitrophenyl acetate in the presence of CTAB [93]. In 1979 Conners et al. reported the spectrophotometric determination of amino acids and peptides after CTAB‐catalyzed reaction with 1‐fluoro‐2,4‐dinitrobenzene. The more hydrophilic amino acid reactants exhibited larger relative rate enhancements, as projected for micellar reaction catalysis [94]. Recently the use of CTAB and Alizarin green were described in the analysis of benzalkonium bromide, used in eye drops, by the decrease in absorbance recorded in alkaline solutions [95].
4.3 Nonionic surfactants
The use of nonionic surfactant Triton X-100 has been used with the determination of metal complexes that normally use organic solvents like PAN, 1-(2-pyridylazo)-2-naphthol {CAS No. 85–85–8} and aqueous soluble reagents like PAR, 4-(2-pyridylazo) resorcinol {CAS No. 1141–59–9} [49]. The chromophore Cadion, 1-(4-nitrophenyl)-3-(4-phenylazophenyl)triazene, {Cas No. 5392–67–6} was used with p-nitrobenzenediazoaminobenzene-p-azobenzene in Triton X-100 for the dual wavelength determination of cadmium [96]. The naphthalene analog of Cadion, Cadion 2B, N-[(4-nitronaphthalen-1-yl)diazenyl]-4-phenyldiazenylaniline {CAS No. 6708–61–8} was used for the determination of silver in a Triton X-100 micellar system [97]. Cadion2B was also used to determine cyanide by the suppression of the absorbance of the copper and silver complexes by that anion in a Triton X-100 surfactant system [49].
4.4 Anionic surfactants
There have been hundreds of reports of metal ion complexing agents (chelometric indicators) being analyzed in cationic and nonionic surfactant system but few that have used anionic surfactants [47]. A spectrophotometric determination for uranium(VI) was based on formation of a red–violet complex from the reaction with 2-(3,5-dibromo-2-pyridylazo)-5-diethylaminophenol {CAS No. 14337–53–2} in a SDS micellar system [98]. The same chromophore was used for the determination of zirconium in aluminum and steel alloys with SDS at pH 4.6 [99] and silver [100] and zinc [101] in an SDS system. SDS was also used as the solubilization system for the spectrophotometric determination of Co (II), Ni (II), Cu (II), Pd (II), Ru (III) and Mo (VI) using sodium isoamylxanthate {CAS No.: 2540–36–5} as a reagent [102]. Ghaedi reported the interference-free spectrophotometric determination of Ni (II) ions based on the reaction between nickel and α-benzyl dioxime (N-[(E)-2-nitroso-1,2- diphenylethenyl]hydroxylamine) {CAS No. 23873–81–6} in a SDS micellar in 2007 [103]. The non-aqueous use of SDS (15 % in acetone) was used to analyze the fluoride ion concentration in bottled and sea water using the fluoride/lanthanum (III)/Alizarin fluorine blue {CAS No. 3952-78-1} ternary complex [104]. A new spectrophotometric method for hemoglobin analysis at 534 nm using SDS was reported, which unlike other methods avoids oxidative reagents and does not produce toxic wastes such as KCN and NaN3 that can cause environmental pollution [105]. The spectrophotometric determination of germanium with phenylfluorone, (2,6,7-trihydroxy-9-phenylxanthen-3-one) {CAS No. 975–17–7} at trace concentrations in a SDS surfactant system at 504 nm was reported by Dagar et al. [106]
4.5 Fluorescence spectroscopy
There are several reviews of fluorescence in micellar media [107, 108]. The Wandruszka review from 1992 covers the specific changes that occur in micellar media like Krafft point, changes in microviscosities and the use of fluorescence reagents to determine CMC and other attributes of micellar structure and would serve as a good introduction to the topics covered [17]. Although there had been many reports of fluorescence enhancement as part of a physical chemistry, probe characterization or catalysis study, many of these did not include any quantitative analysis of the analyte. The review by Hinze et al. in the 2008 Encyclopedia of Analytical Chemistry covers some of the same material but has a greater number of references (876) and the scope is more toward analytical applications and studies [20]. This reference includes several tables of applications: (1) drugs, vitamins, dyes and other organic substances, (2) determination of organic analytes, (3) determination of selected inorganic species and (4) micellar-enhanced lanthanide-sensitized determination of organic species.
In 1972 Ishibashi reported a six-fold enhancement in the determination of aluminum using lumogallion {CAS No. 4386–25–8} as the chelating agent combined with the nonionic surfactant IGEPAL CO 890 {polyoxyethylene (40) nonylphenyl ether, CAS No. 68412–54–4} [109]. San–Medel et al. studied the lumogallion niobium fluorescence with nonionic surfactant Triton X-100 and concluded that only when CMC is established can the ternary complex be accommodated in the surface of the micelles and fluorescence enhancement is observed. They concluded with the over-all idea of maximum fluorescence enhancements is detected when electrostatic and hydrophobic interactions can act concurrently for the complex and the micelles. They collated their results with the structure of the complex and that of the surfactants [110]. Some reports indicate that part of the fluorescence enhancement observed in micellar systems is not just due to increased solubility, encapsulation and protection of the fluorescence species from collisional deactivation inside the micelle, or interaction of the excited state with an ion-pair of the surfactant, but also the solubilization of the quenching impurities bound inside a separate micelle so that they cannot come in contact with the analyte which is in another micelle. The primary factor accountable for enhanced lifetimes in micellar media seems to be reduced quenching constants [20]. In 1987 San–Medel et al. investigated and reviewed several classes of metal chelating agents (flavonols, 8-hydroxyquinoline derivatives, azo dyes, and anthracene derivatives) for the solubilization in micellar media had fluorescence with the metal cations of Al, Nb and Ta. They observed that greater enhancement was observed when not only did the surfactant solubilize the complex at the CMC but when there were electrostatic interactions that occurred between the surfactant and the complex resulting in a more rigid structure. For some systems that exhibited fluorescence quenching in the presence of the cationic surfactant, they showed that this was due to inter-systems crossing being enhanced and phosphorescence occurring at the expense of the fluorescence [111]. Dominguez et al. also focused on the niobium–lumogallion–tartrate system in a 1989 paper that discussed the competition between the ligand’s and the metal complex’s interaction with the micelles for a variety of nonionic surfactants and CTAB where there was a noted red shift in the excitation wavelength and a blue shift in the emission wavelength from 630 nm in water to 600 nm in Triton X-100 [112].
The early work in 1982 of Singh and Hinze looked at the intensity of the pyrene fluorescence and its enhancement from 3 to 16 times in micellar systems of CTAC, SDS and Triton X-100 when compared to ethanol. The spectral parameters, fluorescence lifetimes, quantum yields, lower detection limits, and analytical figures of merit for pyrene all four systems were compared [113]. In a separate paper they investigated the 8- to 20-fold enhancement effects of different surfactant micellar systems upon the spectrofluorimetric method for the determination of amino acids by Roth’s method and the dansyl chloride procedure. They found that the fluorescence intensity of dansyl glycine was enhanced when in the presence of CTAC or dodecyl(trimethyl)azanium chloride {CAS No. 112–00–5} and the zwitterionic surfactant N-dodecyl-N,N-dimethylammonium-3-propane-1-sulfonic acid {CAS No. 14933–08–5} micellar systems. Similarly, the lysine derivative of o-phthaladehyde-2-mercaptoethanol exhibited increased fluorescence in the nonionic Brij-35 or Triton X-100 and SDS surfactants [114].
A study of the fluorescence of the Pb-morin system in the presence of 2 % the nonionic surfactant Genapol PF–20 (a poloxamer which is a nonionic triblock copolymers composed of a central hydrophobic chain of polyoxypropylene bordered by two hydrophilic chains of polyoxyethylene) was enhanced about 9-fold, at a pH of 3.3. The presence of a nonionic surfactant also gives greater stability with the system being stable for at least 3 hours. The excitation maximum occurs at 420 nm with fluorescence occurring at 495 nm and the detection limit was reported to be 0.06 µg mL−1 [115]. A 10-fold increase in the determination of aluminum with morin in the presence of a similar surfactant system was reported at a pH of 3.8 and a detection limit of 0.2 ppb. The number of interfering ions was also reduced significantly [49]. The pH can be a major factor in the enhancement and the type of surfactant that is best for the analysis. An anion that is the excitable molecular form would be expected to have the highest enhancement with a cationic or nonionic surfactant rather than an anionic surfactant which might produce a quenching effect. Fluorescent complexes of 8-hydroxy-7-iodoquinoline-5-sulfonic acid {CAS No. 547–91–1} with Al, Mg, and Zn cations in cationic surfactant systems exhibited enhancements in CTAB [20]. For the fluorescence analysis of Ga(III) with 1-(2-pyridylazo)-2-naphthol, PAN, {CAS No. 85–85–8} the anionic SDS micellar system increased sensitivity about 20 times better than that achieved in a 20:80 (v/v) ethanol-aqueous solvent system [20].
The fluorescence intensities of terbium, europium and samarium complexes with several β-diketone derivatives in the absence and presence of tri-n-octylphosphine oxide (TOPO) in micellar solution of nona-oxyethylene dodecyl ether [116]. Various analytical applications, including immunoassays, quantification of organic compounds has utilized lanthanide and actinide ions complexed with organic ligands for the sensitization of fluorescence analysis [20]. The presence of another f–block element can cause the fluorescence to become even more sensitive. Fluorescence intensities of Eu3+, Sm3+, Dy3+ and Tb3+ in the presence of a surplus of cations of Y, Lu, Gd or La, chelated with pivaloytrifluoroacetone in a Triton X-100-ethanol solution containing 1,10-phenanthroline, were increased by factors ranging from 61 to 1078 fold [117]. This can be referred to as the cofluorescence effect and has been reviewed by Xu et al. [118]
Fenproporex {3-(1-phenylpropan-2-ylamino)propanenitrile, CAS No. 16397-28-7} produces amphetamine as a metabolite and has been used as an appetite suppressant was enhanced by a factor of 2.6 when analyzed in the anionic surfactant SDS [119]. A similar study was conducted on benzodiazepines (a class of psychoactive drugs) using surfactants provided micellar enhancement factors for their fluorimetric analysis in the range 1.2–6.5 increase, depending on the nature of both the benzodiazepine and the surfactant used [120]. Tetracyclines form luminescence complexes with Eu3+ and it was established that the emission was enhanced by surfactants. Both CPC and Triton X-100 surfactants were studied, and enhancement by a factor of up to 34 was detected for some tetracyclines. Small structural variances between the different tetracyclines examined had a noticeable effect on the intensity of emission [121]. The analysis of EDTA, ethylenediaminetetraacetic acid, in various foods was accomplished with the fluorescence ternary complex Zr(IV), EDTA and Alizarin Red S in CTAB with a detection limit of 3.4 ng/mL [122].
4.6 Phosphorescence spectroscopy
The use of phosphorescence analysis has been limited despite the low detection limits possible because of the cryogenic temperatures that had previously been necessary and the numerous possibilities of quenching of the reaction. The unique feature of phosphorescence is the time delay due the triplet states which have long lifetimes. This was used to study probes of a micellar system from nanoseconds to seconds. Some early investigations on triplet anthracene indicated that the exit time of this molecule from a CTAB micelle was of the order of one millisecond [36].
Oxygen quenching of the triplet state has been a major difficulty in phosphorescence. It has a much lower solubility in water than in organic solvents, and one would expect a higher oxygen concentration in a micelle core than in the aqueous phase, a condition which is found experimentally. At pressures below 1 atm of oxygen the ratio of micelles with oxygen is about 1 in 7. Nevertheless, dissolved oxygen moves freely in and out of micelles, which results in the quenching of micelle–bound excited states [7]. Sanz–Medel et al. describe using sodium sulfite as an oxygen scavenger in surfactant systems for micelle–stabilized room temperature phosphorescence [123]. Other methods of oxygen removal were summarized by Hinze in his review [20].
Micelle-stabilized room temperature phosphorescence (MS-RTP) was first reported by Kalyanasundaram et al. [124] Some of the early reports of room temperature phosphorescence (RTP) utilized SDS with heavy atom cations of either silver or thallium (which is toxic) to increase the quantum yields in the determination of a number of polyaromatic hydrocarbons [125], and later of carbazole and its derivatives [126]. Because the micelle concentrates the interacting species in a far smaller volume about the micelle that has a heavy atom as its counter-ion, this effectively intensifies the heavy-atom concentration around the analyte and, the subsequent phosphorescence [48]. A series of licit and illicit drugs were investigated with RTP, sensitized phosphorescence and fluorescence using micellar media and cyclodextrens [127]. Thiabendazole (2-(4-thiazoly)-1H-benzimidazole), a fungicide widely used in agriculture, was determined in pineapple by RTP in a SDS micellar system using thallium as the heavy atom [128]. The investigation of 8-hydroxyquinollne and some of its derivatives as potential complexing reagents for the room temperature phosphorescence using CTAB for the determination of niobium with bromoform as the heavy atom source after oxygen removal was reported by Kalyanasundaram et al. in 1987 [129]. They concluded that electrostatic and hydrophobic forces acting simultaneously seem essential to secure micelle-stabilized room temperature phosphorescence. Similarly, Liu et al. used room temperature phosphorescence to determine a gallium(III)-7-iodo-8-hydroxyquinoline-5-sulfonic acid complex {Ferron} in the presence of CTAB and with sodium sulfite as an oxygen scavenger [130].
4.7 Atomic spectroscopy
The use of organic solvents in flame atomic absorption spectroscopy, FAAS, and emission methods of analysis has been less studied. To decide if an engine needs replacement bearings and rings it is a common procedure to use of organic solvents for the analysis of metals in engine oil to determine the wear characteristics of the engine. It is known that in the spray chamber organic solvents produce an aerosol that has a greater number of small droplets and the corresponding signals are usually increased compared to aqueous systems. However, there have been mixed results reported in the literature for surfactants as enhancement agents in FAAS. The anionic surfactant SDS exhibited enhancements in the FAAS absorption value while cationic and nonionic surfactants cause a depression or no effect in the signal [131]. Enhancement with SDS was also studied for several metals (Cr, Cu, Ga, In, Ge, Si, Sn, Te, Sb, As, Bi) and their effect on three electrode argon DC plasma spectrophotometer. The SDS enhancement effect was explained by a combination of earlier observed phenomena: effects of easily ionized elements, increased Penning ionization, thermal pinch and increased residence time in the plasma [132].
More consistent applications have been reported when the surfactants have been used for emulsifying agents to solubilize water-immiscible samples and avoid the use of organic solvents. This method has been used to determine lead in gasoline and lubricating oils, zinc in antifungal preparations and iron in lubricating oils [49]. Surfactants have been used successfully to sequester metal cations for their later determination by FAAS using CPEs and some other preconcentration steps discussed later in the flow injection analysis section of this survey.
4.8 Chromatography
In 1979 Armstrong first reported the use of surfactants in the mobile phase for TLC analysis for polynuclear aromatics [133] and pesticides [134]. This was followed with their use in HPLC [135, 136]. The retention times were dependent upon the concentration of the surfactant and if the analyte reacted with them. Another factor that could contribute to the selectivity of the micellar system if the solute was ionic or not, and if it was it could form an ion-pair with a surfactant molecule [137]. The elution order was reversed when one went from the anionic surfactant SDS to the cationic surfactant CTAB in the separation of phenol and acetophenone [50]. The added advantage that micellar systems have with HPLC is not only the separation characteristics but also the enhanced detection of surfactant systems that has already been documented using an UV–Visible or fluorescence HPLC detector [48]. Addition of small amounts of other organic solvents, like n-propanol, have been reported to have great effects on the separation characteristics of the chromatographic separation and using a column at 40 °C, and helps overcome some earlier problems with efficiency [50]. Changing the concentration of the surfactant in the mobile phase during a separation mimics gradient elution of a secondary solvent in HPLC. A review by Hinze in 1989 [138], and Esteve–Romero et al. in a 2016 review article looked at micellar liquid chromatography being used for the analysis of several drugs in serum and urine including: anticonvulsants, antiarrhythmics, tricyclic antidepressants, selective serotonin reuptake inhibitors, analgesics and bronchodilators [139]. Micellar chromatography results in the production of less toxic solvents and lower cost of reagents, and therefore should be of considerable use in the development of a green chemistry HPLC method of analysis.
4.9 Micellar electrokinetic chromatography
Terabe et al. first reported the use of micellar systems in 1984 in the electrokinetic separation of 14 different phenols using SDS allowing this technique’s samples to include water insoluble species. The theoretical plate count was between 210,000 to 400,000 for the 19-minute separation. The SDS was carried from the negative electrode to the positive electrode. When the cationic surfactant CTAB was used the electroosmotic flow was in the opposite direction [140]. The use of MEKC has been reported in varied field including pharmaceutical, clinical, environmental, and biochemistry for both organic and inorganic compounds. Its numerous advantages and usages were reviewed by Khaledi in 1997 [141], in 2012 by Sepaniak et al. [142], in toxicology [143], element speciation [144] and in the analysis of pharmaceuticals [145]. The review by Silva incorporates a review of instrumentation and analytical methodology including the use of micellar systems for online sample concentration techniques and detection [146].
4.10 Electrochemistry
In classical polarography surfactants were used to eliminate polarographic maxima. Since that early use there have been numerous physical chemistry studies using electrochemistry to help understand the fundamental nature of micelles. Although surfactant systems can fulfill their normal role of increasing solubility, they can also affect the characteristics of the double layer at the electrode and the diffusion of active species through a solution or even act as masking agents for some species [50]. The review by Rusling gives not only a historical review of surfactants in electrochemistry but also the background of these organized media [147]. Specific examples include the effect that adsorbed surfactants have on electrode behavior, including the lowering of the differential capacitance and reorganizing of the surfactant on the surface of the electrode due to changes in potential. Results discussed show evidence of adsorbed bilayers or hemimicelles on Pt and Hg electrodes [148]. The adsorbed surfactant can alter the double-layer arrangement, and the rate of electron transfer (by both acceleration and inhibition), and the apparent half-wave potential of an electroactive analyte [48]. In 1952 Proske used the solubilization power of micelle media to report the polarographic determination of anthraquinone in water using the surfactant Aerosol MA, dihexyl sodium sulfosuccinate {CAS No. 3006–15–3} [146]. Besides the solubilization ability of surfactants and the reaction intermediates, the diffusion process to the electrode is altered by their presence. Experiments produce a variety of results depending on the surfactant and the analyte. Like the alteration of the pKa in acid/base indicators the microenvironment of the micelle may cause a shift in the reaction potential. Generally, the half-wave potential has a negative shift but for some analytes there was no change and for the analysis of tetrathiofulvane, 2,2′-bis(1,3-dithiolylidene), in CTAB there was a positive shift [49]. In cyclic voltammetry studies the increased solubility of micellar systems can evince reversible reactions not seen in normal solvents. Micelles can stabilize anion radicals formed in the course of reduction reactions for some organic compounds. Such was the case for phthalonitrile and fluorenone and this behavior was attributed to the increased solubility of the anion radical in cationic micellar media [49] and with studies of nitrobenzene [149]. The micellar media has also been used because it allows for the solubilization of the electrochemically generated titrant to allow the electron transfer as discussed in the case of the ferrocinium ion being generated from ferrocene {Cas No. 102-54-5} [49].
In a more recent literature survey from 2006 by Vittal et al. which includes the modification of electrodes with metal hexacyanoferrates, their derivatized oxides and titanium oxide are reviewed [150] including the work they first reported of CTAB and nickel hexacyanoferrate films characterized by cyclic voltammetry [151]
4.11 Extractions
Surfactants have been used to form close ion-pairs that are soluble in organic liquids to achieve the separation of metal ions from aqueous solutions. Often this is then followed by the spectrophotometric determination of the species. Some metals form anionic complexes with other chelating agents so cationic surfactants may be used to form close ion-pairs with these species and extracted (in carbon tetrachloride, chloroform, xylene, or dichloroethane) and analyzed [49]. Other ions have also been used to form the ion-pair and then that is soluble in a nonionic surfactant [152]. This method has also been used for the determination of surfactants in water. Because many of these procedures use organic solvents we shall bypass a discussion of them but Hinze reviewed some of these in 1979 [83] and Paleologos in 2005 [153]. Reviews on extraction and pre-concentration of organic pollutants in environmental samples by Ferrera in 2004 [154] and extraction separation and preconcentration in metal analysis by Stalikas in 2002 are comprehensive guides to this area of analysis [155].
An alternative green extraction method uses the cloud point of the surfactant itself to effect the separation, known as CPE [22, 23, 156]. Upon changes in temperature, aqueous solutions of several nonionic and some zwitterionic surfactants develop a turbidity and a separation into two phases. This cloud point temperature occurs over a narrow temperature range where the large number of surfactants cause a scattering of light and make the solution appear cloudy. The process requires several easy steps. Add surfactant to solution to be analyzed making such its final concentration will be greater than its CMC value. After the species has dissolved raise the temperature in a temperature bath above the cloud point (or lowered in the instance of zwitterionic surfactants) and then phase separation occurs with nonionic surfactants in the lower layer or zwitterionic surfactants in the upper layer. The separation can be completed by gravity or centrifuging the same where the analyte has now been concentrated into the micellar layer. Some surfactants appear on the top layer and others on the bottom layer. Adjustment of the viscous surfactant rich layer may be desired before final analysis of the product [23]. The temperature at which a CPE happens can be changed by the addition of salts or other surfactants which may also alter the CMC [152]. The addition of sulfate increases the cloud point while urea and iodide lower it [22]. Extraction of metal ions requires smaller volumes and greener chemistry compared to traditional extractions. Watanabe pioneered this work and has written a review on the subject [157]. Extracted metal ions were then determined by a variety of analytical methods including fluorescence, visible and flame atomic absorption spectroscopy and others. Using the CPE technique as a sample preparation and preconcentration prior to analysis by HPLC or gas chromatography and an electron capture or flame ionization detection have been effectively used for the quantification of environmentally important compounds. This method produces fewer wastes and is less expensive than traditional extraction methods and more efficient is several incidences for a variety of organic and environmental samples [23]. The vast quantity of publications involving CPE involves the purification of membrane proteins following the work of Bordier [158]. A review by Quina and Hinze has several useful tables: (1) Some Extraction Parameters, (2) Recent CPE of Biomaterials/Clinical Analytes, and (3) Extraction and/or Preconcentration of Organic and Environmental Compounds (pesticides, fungicides, PCB’s, PAH’s phenols and others) and numerous applications in their article [23].
By combining CPE with fluorescence analysis one can achieve low detection limits. Using the nonionic surfactant Genapol X 80 {CAS No. 9043-30-5} the pesticides napropamide and thiabendazole were extracted and analyzed from water and soil samples. The fluorescence enhancement factors attained in the surfactant-rich phase were 1.2–2.0 for these two pesticides. Detection limits less than 0.2 μg/L and recovery rates of equal to 95 % were realized [20].
4.12 Titrations
There have been numerous investigations that use the ability of the surfactant to solubilize organic compounds that normally would have to be titrated in a non-aqueous media that can be titrated in a micellar environment in water using either a potentiometric or color indicator. This offers one the opportunity to use a greener solvent than the non-aqueous solvents normally used. Some examples of solvent substitutions are given in Table 1. As early as 1934 Hartley reported on the effect of surfactants on acid–base indicators studying some cationic surfactants such as CTAB [39]. The significant shifts in the apparent pKa values were also later reported to occur in nonionic micelle systems as well [49, 159]. For example methyl red has a pKa of 4.95 in water but the apparent pKa in a micellar system changes with the type of surfactant to 3.67 (cationic), 6.63 (anionic), and 5.20 (nonionic) [50]. Examination of the data and the low dielectric constants reported support the simple electrostatic theory for this phenomenon [159]. Fernandez et al. used two different fluorescent pH indicators, a hydroxycoumarin and an aminocoumarin dye, that were incorporated by means of long paraffinic chain substituents to neutral, anionic, and cationic micelles and comparisons were made with the pKa’s of aqueous solutions to determine if the electrical potential and/or a change of polarity were responsible for the apparent pKa shifts [160]. In their findings they concluded that the indicator’s physical location and behavior were similar in SDS and Triton X-100, but different for CTAB. The electrical potentials for SDS micelles (–134 mV) and CTAB (+148 mV) have a comparable shift to the potentials measured by the lipoid-pH indicator in charged monolayers of analogous charge density.
Comparison of solvents used in non-aqueous titrations and micellar systems.
| Types of compounds titrated in micellar systems [49] | Non-aqueous solvent replaced by micellar system [161] |
|---|---|
| Long chain amines | Acetic acid, dioxane, acetonitrile and others |
| Fatty acids | Acetone, methanol/benzene, DMF |
| Benzoic acids | CS2, acetonitrile, methanol |
| Sulfonamides | DMF |
| Sparingly soluble weak acids | Acetone, methanol/benzene, DMF |
| Sparingly soluble weak bases | Acetone, methanol/benzene, DMF |
| Halophenols | Pyridine, DMF, ethylene diamine, and others |
Underwood observed for the titration of carboxylic acids in CTAB with NaOH, phenolphthalein indicator, and amines in SDS with HCl, phenol red indicator, that the electrodes gave sluggish results compared to visual indicators [162]. Staroscik reported using both visual and potentiometric endpoint detection for the determination of some barbiturates in cationic micellar systems by acid-base titrimetry [163]. A study was made by Gerakis et al. using microcomputer-controlled titrations of pharmaceutical compounds: aspirin, naproxen, iopanoic acid {(RS)-2-[(3-Amino-2,4,6-triiodophenyl)methyl]butanoic acid, CAS No. 96-83-3} and benzoic acid and its derivatives using Tween-80, CTAB, CPC and SDS. Direct titrations in CTAB had good correlations with the non-aqueous titrations counterparts for the weak acids [164].
4.13 Flow injection analysis
Flow Injection Analysis, FIA, was first reported by Ruzicka and Hansen [165]. This method of analysis combines the speed of chromatography with numerous detection methods. In it a sample is injected into a stream of solvent that includes the chromophore and they are mixed in a reaction coil before passing through the appropriate detector. The benefits of FIA include easy variation of the parameters, low solvent and chromophore concentrations, conservation of reagents, and high sample throughput. The method has been used for automation of many different analytical determinations, there have been periodic reviews in the literature [165, 166, 167, 168].
A typical paper is the one by Resing and measures on fluorometric determination of aluminum in seawater by flow injection analysis with in-line preconcentration, using a resin, which employs the already existing lumogallion reaction in Brij-35 for the analysis [169]. The flow injection analysis of benzoyl peroxide in acne cream and flour using N,N,N,N-tetramethyl-p-phenylenediamine, Wurster’s reagent {CAS No. 637-01-4}, and a SDS surfactant system including cerium (IV) as a catalyst was measured by visible spectroscopy at 612 nm is another typical example of the use of micellar systems to act as a solvent and a catalyst for reactions. Other methods for benzoyl peroxide had heating baths and waiting times of 20–40 min [170].
The surfactant Triton X-100 illustrated the catalytic abilities of micelles in the FIA analysis of cobalt (II) and nickel (II) using PAR, {4-(2-pyridylazo) rescorcinol, CAS No. 13311–52–9}, in which the colored metal complex was formed without the need of a 15 min hot water bath. Detection was with a visible spectrophotometer at 510 nm [171]. Catalytic kinetic FIA spectrophotometric method for the determination of nanogram amounts of copper (II) was created using the catalytic effect of copper on the reduction of azure B, {3-Methylamino-7-dimethyl-aminophenothiazin-5-ium chloride, CAS No. 531–55–5} by sulfide in a CTAB surfactant system at 647 nm, with 50–1600 µg/L as the calibration range and 9.2 µg/L as the detection limit [172].
Zinc in seawater was analyzed by a FIA spectrophotometric method at 630 nm in a CTAB borax buffered solution using salicyl-fluorone {2,6,7-trihydroxy-9-(o-hydroxyphenyl)-3-fluorone, CAS No. 3569–82–2} as the metal complexation agent with a detection limit of 1.5 µg/L [173]. Lead determination was reported in the range of 1.0–12.0 µg/L and a detection limit of 0.027 µg/L employing 1,5-diphenylthiocarbazone {CAS No. 60–10–6} as the complexing agent in sulfuric acid and SDS for a FIA spectrophotometric analysis at 500 nm [174].
A very interesting use of surfactants is reported in FIA methods have been developed that will accomplish extractions and preconcentration of an analyte. The use of a complexation agent immobilized on a surfactant-coated alumina column acts as a separation and preconcentration step has been the basis for an analysis by Ahmadi et al., which involves column preparation by adding SDS just below its CMC to 1.5 g of alumina, then fine-tuning the pH to 2 with HCl before mixing for 10 min. The sample was washed with water then mixed with the organic ligand for 15 min., filtered and added to the column. The anion head group of the surfactant is bonded by charge attraction to the solid alumina, and the hydrophobic tail now becomes the external portion of the alumina particles giving a hydrophobic character to the phase and the organic ligand is adsorbed onto the treated alumina surface. In this fashion chromium (III) and total chromium were analyzed with an 8-hydroxyquinoline {CAS No. 134–31–6} as the immobilized organic reagent on a SDS-coated alumina column followed by flow injection atomic absorption spectrometry after elution with a 20 % ethanol solution in HCl. The linear calibration range was 1.0–100 µg/L and detection limit was 0.16 ng/L [175]. Silver was determined using a similar method with diethyldithiocarbamate {sodium (diethylcarbamothioyl)sulfanide, CAS No. 148–18–5} as the complexation agent immobilized on the SDS coated alumina column and analyzed by FAAS. An enrichment factor of 125 was achieved, and linear calibration was documented as 5–100 µg/L with a detection limit of 0.7 µg/L [176].
The analysis of pollutants, herbicides and pesticides has used chemiluminescence and fluorescence as the principle approaches for FIA investigations. The analysis of the phenylurea herbicide residues utilized FIA and fluorescence detection of the UV irradiated species of the herbicides in spiked tap water samples. Both SDS and CTAC were used at their CMC levels to solubilize the reaction products and fluorescence intensity enhancement, which exhibited linear calibration over two orders of magnitude with a detection limit of 0.33–0.92 mg/L [177]. The broadleaf herbicide metsulfuron-methyl, {2-{[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)amino]-oxomethyl]sulfamoyl}benzoic acid methyl ester, CAS No. 74223-64–6}, a sulfonylurea, was analyzed in environmental waters using a solid phase spectroscopy system and photochemical induced fluorescence. The irradiated product occurred was retained in a flow cell filled with ODS, octadecylsilane, silica gel coated with SDS had a detection limit of 0.14 µg/L. The solid phase elution was with a 30 % methanol–water solvent with 8 mM SDS between runs. The size of the injection volume of 1000 µL regulated the analytical range of 0.5–175 µg/L [178].
The very clever use of CPE has also been coupled with FIA [179]. Silva et al. sequestered and analyzed lead and cadmium by FAAS after CPE preconcentration in a FIA system. The metal ions were chelated with TAN, {1-[2-(2-thiazolyl)diazenyl]-2-naphthalenol, CAS No. 1147–56–4}, which migrated to the micelles of Triton X-114 and were subsequently concentrated onto a cotton or glass wool mini–column and flushed off for determination by FAAS by the addition of HCl. The enhancement factors were between 15.1 and 20.3, and detection limits were 4.5 μg/L for Pb and 0.75 μg/L for Cd [180]. A similar system had been used for the determination of benzo[a]pyrene using the peroxoxalate chemiluminescence detection system. A cotton filled mini-column collected Triton X-114 CPE phase that had its cloud point temperature depressed by using sodium sulfate in the mobile phase. Elution was achieved using pure acetonitrile. The method was based on the chemiluminescence product that resulted from the reaction of hydrogen peroxide oxidation and bis(2,4,6-trichlorophenyl)oxalate {CAS No. 1165–91–9}. The enrichment factors recounted as 40.2 for benzo[a]pyrene, 38.5 for benzo[k]fluoranthene and 41.3 for benzo[ghi]perylene [181]. The detection limits were brought down to the picomolar and femtomolar concentration levels in an ultrasensitive determination of silver, gold, and iron oxide nanoparticles in environmental samples that combines preconcentration using CPE and the chemical luminescent oxidation of luminol {5-amino-2,3-dihydrophthalazine-1,4-dione, CAS No. 521-31-3} [182]. Luminol was used recently in the determination of L-thyroxine in pharmaceutical preparations along with CTAB and KMnO4 that acted as enhancement factors for the chemiluminescence detection [183]. The FIA and chemiluminescence detection of gemifloxacin in pharmaceutical preparations and biological fluids used a CTAB surfactant system and the reaction of the analyte with diperiodatoargentate (III) [184].
5 Conclusions
This chapter has attempted to give an introduction to the many practical uses of surfactants and elucidate some background in our understanding of how they can be utilized to achieve green analytical chemistry. Seeing how a micellar system works can also give insight into new methods based on the solubilization, or catalytic properties of surfactant systems. The opportunity of both enhancement, and the replacement of organic solvents make micelles a solvent system to go to for cost and environmental purposes. A comprehensive look at micelles in analytical chemistry would require a separate encyclopedia and would be out of date right after it was written for this still very active field of research. All aspects of analytical chemistry are being explored, however, some fields such as, fluorescence and UV-Visible spectroscopy have been active since the 1970s. Other analytical methods offer new and exciting research uses for those same chemicals with which we wash our hands, our clothes and that we use every day inside and outside the chemistry laboratory (Table 2).
| Surfactant (CAS No.) | GMM g/mole | Aggregation number | CMC mMolar | Formula |
|---|---|---|---|---|
| Aerosol-22 (3401-73-8) | 653 | 0.70 [8] | C26H43NO16Na4S | |
| Aerosol-OT, AOT (577-11-7) | 444.56 | 3.2 [9] | C20H37O7NaS | |
| Brij 35 (9002-92-0) | 1225 | 40 | 0.09 | C12H25O(CH2CH2O)23H |
| Brij 96 (9009-91-0) | 709 | 0.94 [1] | HO(CH2CH2O)10C18H35 | |
| CPB, Cetylpyridinium bromide (140-72-7) | 402.47 | 0.90 [11] | C21H38NBr.H2O | |
| CPC, Cetylpyridinium chloride (6004-24-6) | 358.01 | 18-55 | 0.12 [6] | C21H38NCl.H2O |
| CTAB, Cetyl trimethylammonium bromide (9036-06-0) | 364.5 | 170 | 0.92 [3] | (C16H33)N(CH3)3Br |
| CTAC, Cetyl trimethylammonium chloride (79728-63-5) | 320.0 | 84 | 1.3 [3] | [(C16H33)N(CH3)3Cl |
| SDS, Sodium dodecyl sulfate (151-21-3) | 288.38 | 8.2 [11] | C12H25SO4Na | |
| Triton X-100 (9036-19-5) | 650 | 140 | 0.23 [12] | C8H17C6H4(OC2H4)10OH |
| Triton X-114 (9081-83-8) | 537 | C8H17C6H4O(CH2CH2O)7.5H | ||
| Tween-20 (93906-96-8) | 1228 | 0.059 [5] | C58H114O26 | |
| Tween-80 (9005-65-6) | 1310 | 58 | 0.012 [5] | C18H37-C6H9O5-(OC2H4)20OH |
Acknowledgment
This article is also available in: Benvenuto, Green Chemical Processes. De Gruyter (2017), isbn 978–3–11–044487–2.
References
1. Birdi KS, Singh HN, Dalsager SU. Interaction of ionic micelles with the hydrophobic fluorescent probe 1–anilino–8–naphthalenesulfonate. J Phys Chem. 1979;83(21):2733–2737.10.1021/j100484a010Search in Google Scholar
2. Rosen MJ. Surfactants and interfacial phenomena. New York, NY: John Wiley & Sons, 1978.Search in Google Scholar
3. Atwood D, Florence AT. Surfactants systems their chemistry, pharmacy and biology. New York, NY: Chapman and Hall, 1983.Search in Google Scholar
4. Tadros TF. Surfactants. Orlando, FL: Academic Press, 1984.Search in Google Scholar
5. Urata K, Takaishi N. A perspective on the contribution of surfactants and lipids toward “Green Chemistry”: present states and future potential. J Surfactants Deterg. 2001;4(2):191–200.10.1007/s11743-001-0174-4Search in Google Scholar
6. Aniansson GE. Dynamics and structure of micelles and other amphiphile structures. J Phys Chem. 1978; 82(26):2805–2808 .10.1021/j100515a011Search in Google Scholar
7. Thomas JK. Radiation–induced reactions in organized assemblies. Chem Rev. 1980;80(4):283–299.10.1021/cr60326a001Search in Google Scholar
8. Emert J, Behrens C, Goldenberg M. Intramolecular excimer–forming probes of aqueous micelles. J Am Chem Soc. 1979;101(3):771–772.10.1021/ja00497a065Search in Google Scholar
9. Merrettig-Bruns U, Jelen E. Anaerobic biodegradation of detergent surfactants. Materials. 2009;2(1):181–206.10.3390/ma2010181Search in Google Scholar
10. Rosen MJ, Tracy DJ. Gemini surfactants. J Surfactants Deterg. 1998;1(4):547–554.10.1007/s11743-998-0057-8Search in Google Scholar
11. Menger FM, Littau CA. Gemini surfactants: a new class of self–assembling molecules. J Am Chem Soc. 1993;115(22):10083–10090 and Menger FM, and Keiper JS. Gemini surfactants. Angewandte Chemie International Edition 2000, 39(11),1906–20.10.1021/ja00075a025Search in Google Scholar
12. Zana R. Dimeric (gemini) surfactants: effect of the spacer group on the association behavior in aqueous solution. J Colloid Interface Sci. 2002;248(2):203–220.10.1006/jcis.2001.8104Search in Google Scholar PubMed
13. Singh V, Tyagi R. Unique micellization and cmc aspects of gemini surfactant: an overview. J Dispersion Sci Technol. 2014;35(12):1774–1792.10.1080/01932691.2013.856317Search in Google Scholar
14. Kalyanasundaram K, Thomas JK. The conformational state of surfactants in the solid state and in micellar form A laser–excited Raman scattering study. J Phys Chem. 1976;80(13):1462–1473.10.1021/j100554a015Search in Google Scholar
15. Goodling K, Johnson K, Lefkowitz L, Williams BW. The modern student laboratory: luminescent characterization of sodium dodecyl sulfate micellar solution properties. J Chem Educ. 1994;71(1):A8.10.1021/ed071pA8Search in Google Scholar
16. Nakahara Y, Kida T, Nakatsuji Y, Akashi M. New fluorescence method for the determination of the critical micelle concentration by photosensitive monoazacryptand derivatives. Langmuir. 2005;21(15):6688–6695.10.1021/la050206jSearch in Google Scholar
17. Wandruszka RV. Luminescence of micellar solutions. Crit Rev Anal Chem. 1992;23(3):187–215.10.1080/10408349208050854Search in Google Scholar
18. Garcia MD, Sanz–Medel A. Dye–surfactant interactions: a review. Talanta. 1986;33(3):255–264.10.1016/0039-9140(86)80060-1Search in Google Scholar
19. Schramm LL, Stasiuk EN, Marangoni DG. 2 Surfactants and their applications. Annu Rep Sect “C” (Phys Chem). 2003;99:3–48.10.1039/B208499FSearch in Google Scholar
20. Memon N, Balouch A, Hinze WL. Fluorescence in organized assemblies. Encycl Anal Chem 2008;9:16-110.10.1002/9780470027318.a5409.pub2Search in Google Scholar
21. Gu T, Sjöblom J. Surfactant structure and its relation to the Krafft point, cloud point and micellization: some empirical relationships. Colloids Surf. 1992;64(1):39–46.10.1016/0166-6622(92)80160-4Search in Google Scholar
22. Hinze WL, Pramauro E. A critical review of surfactant–mediated phase separations (cloud–point extractions): theory and applications. Crit Rev Anal Chem. 1993;24(2):133–177.10.1080/10408349308048821Search in Google Scholar
23. Quina FH, Hinze WL. Surfactant–mediated cloud point extractions: an environmentally benign alternative separation approach. Ind Eng Chem Res. 1999;38(11):4150–4168.10.1021/ie980389nSearch in Google Scholar
24. Kalyanasundaram K, Thomas JK. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J Am Chem Soc. 1977;99(7):2039–2044.10.1021/ja00449a004Search in Google Scholar
25. Thomas JK. Effect of structure and charge on radiation–induced reactions in micellar systems. Acc Chem Res. 1977;10(4):133–138.10.1021/ar50112a005Search in Google Scholar
26. Itoh H, Ishido S, Nomura M, Hayakawa T, Mitaku S. Estimation of the hydrophobicity in microenvironments by pyrene fluorescence measurements: N–β–octylglucoside micelles. J Phys Chem. 1996;100(21):9047–9053.10.1021/jp953682zSearch in Google Scholar
27. Grätzel M, Thomas JK. Dynamics of pyrene fluorescence quenching in aqueous ionic micellar systems factors affecting the permeability of micelles. J Am Chem Soc. 1973;95(21):6885–6889.10.1021/ja00802a002Search in Google Scholar
28. Piñeiro L, Novo M, Al–Soufi W. Fluorescence emission of pyrene in surfactant solutions. Adv Colloid Interface Sci. 2015;215:1–2.10.1016/j.cis.2014.10.010Search in Google Scholar PubMed
29. Fendler JH, Fendler EJ, Infante GA, Shih PS, Patterson L. Absorption and proton magnetic resonance spectroscopic investigation of the environment of acetophenone and benzophenone in aqueous micellar solutions. J Am Chem Soc. 1975;97(1):89–95.10.1021/ja00834a016Search in Google Scholar
30. Drummond CJ, Grieser F, Healy TW. A single spectroscopic probe for the determination of both the interfacial solvent properties and electrostatic surface potential of model lipid membranes. Faraday Discuss Chem Soc. 1986;81:95–106.10.1039/dc9868100095Search in Google Scholar
31. Karukstis KK, Suljak SW, Waller PJ, Whiles JA, Thompson EH. Fluorescence analysis of single and mixed micelle systems of SDS and DTAB. J Phys Chem. 1996;100(26):11125–11132.10.1021/jp960993gSearch in Google Scholar
32. Mandal D, Pal SK, Bhattacharyya K. Excited–state proton transfer of 1–naphthol in micelles. J Phys Chem A. 1998;102(48):9710–9714.10.1021/jp982483nSearch in Google Scholar
33. Almgren M, Grieser F, Thomas JK. Dynamic and static aspects of solubilization of neutral arenes in ionic micellar solutions. J Am Chem Soc. 1979;101(2):279–291.10.1021/ja00496a001Search in Google Scholar
34. Ashby KD, Das K, Petrich JW. The effect of micelles on the steady–state and time–resolved fluorescence of indole, 1–methylindole, and 3–methylindole in aqueous media. Anal Chem. 1997;69(10):1925–1930.10.1021/ac9611632Search in Google Scholar
35. Martens FM, Verhoeven JW. Charge–transfer complexation in micellar solutions. Water penetrability of micelles. J Phys Chem. 1981;85(13):1773–1777.10.1021/j150613a001Search in Google Scholar
36. Infelta PP, Gratzel M, Thomas JK. Luminescence decay of hydrophobic molecules solubilized in aqueous micellar systems kinetic model. J Phys Chem. 1974;78(2):190–195.10.1021/j100595a021Search in Google Scholar
37. Marzzacco CJ. The effect of solvent and micelles on the rate of excited–state deprotonation. J Chem Educ. 1996;73(3):254.10.1021/ed073p254Search in Google Scholar
38. Grieser F, Drummond CJ. The physicochemical properties of self–assembled surfactant aggregates as determined by some molecular spectroscopic probe techniques. J Phys Chem. 1988;20:5580–5593.10.1021/j100331a012Search in Google Scholar
39. Hartly GS. The effect of long–chain salts on indicators: the valence–type of indicators and the protein error. Trans Faraday Soc. 1934;30:444–450.10.1039/tf9343000444Search in Google Scholar
40. Duynstw EFJ, Grunwald E. Organic reactions occurring in or on micelles I. Reaction rate studies of the alkaline fading of triphenylmethane dyes and sulfonphthalein indicators in the presence of detergent salts. J Am Chem Soc. 1959;81(17):4540–4542.10.1021/ja01526a025Search in Google Scholar
41. Reeves RL. Nature of mixed micelles from anionic dyes and cationic surfactants kinetic study. J Am Chem Soc. 1975;97(21):6019–6024.10.1021/ja00854a011Search in Google Scholar
42. Reeves RL. Effect of reacting and competing counterions on the hydrolysis kinetics of an anionic dye ester in mixed micelles with CTAB [hexadecyltrimethylammonium bromide]. J Am Chem Soc. 1975;97(21):6025–6029.10.1021/ja00854a012Search in Google Scholar
43. Triboni ER, Politi MJ, Cuccovia IM, Chaimovich H, Berci Filho P. Rate limiting step and micellar catalysis of the non‐classical nitro group nucleophilic substitution by thiols in 4 nitro N n butyl 1, 8 naphthalimide. J Phys Org Chem. 2003;16(6):311–317.10.1002/poc.618Search in Google Scholar
44. Katusin–Razem B, Wong M, Thomas JK. The effect of micellar phase on the state and dynamics of some excited state charge transfer complexes. J Am Chem Soc. 1978;100(6):1679–1686.10.1021/ja00474a007Search in Google Scholar
45. Alkaitis SA, Beck G, Grätzel M. Laser photoionization of phenothiazine in alcoholic and aqueous micellar solution electron transfer from triplet states to metal ion acceptors. J Am Chem Soc. 1975;97(20):5723–5729.10.1021/ja00853a015Search in Google Scholar
46. La Sorella G, Strukul G, Scarso A. Recent advances in catalysis in micellar media. Green Chem. 2015;17(2):644–683.10.1039/C4GC01368ASearch in Google Scholar
47. Hinze WL.. Mittal KL, editors. Use of surfactant and micellar systems in analytical chemistry. Solution Chem. Surfactants. Proc. Sect. 52nd Colloid Surf. Sci. Symp., 1979;79–127 1.10.1007/978-1-4615-7880-2_4Search in Google Scholar
48. Cline–Love LJ, Habarta JG, Dorsey JG. The micelle–analytical chemistry interface. J Anal Chem. 1984;56(11) 1132A–1148A.10.1021/ac00275a715Search in Google Scholar
49. Pelizzetti E, Pramauro E. Analytical applications of organized molecular assemblies. Anal Chim Acta. 1985;169:1–29.10.1016/S0003-2670(00)86203-0Search in Google Scholar
50. McLntire GL, Dorsey JG. Micelles in analytical chemistry. Crit Rev Anal Chem. 1990;21(4):257–278.10.1080/10408349008051631Search in Google Scholar
51. Fendler JH, Fendler EJ. Catalysis in micellar and micromolecular systems. New York: Academic Press, 1975.10.1016/B978-0-12-252850-7.50015-0Search in Google Scholar
52. Lewis M, Wee V. Aquatic safety assessment for cationic surfactants. Environ Toxicol Chem. 1983;2:105–108.10.1002/etc.5620020112Search in Google Scholar
53. Schramm LL. Emulsions, foams and suspensions: fundamentals and applications. Hoboken, NJ: John Wiley & Sons, 2006 May 12. May12 .Search in Google Scholar
54. Schramm LL. Surfactants: fundamentals and applications in the petroleum industry. Cambridge, UK: Cambridge University Press, 2000 Mar 23. Mar23 .10.1017/CBO9780511524844Search in Google Scholar
55. Scott MJ, Jones MN. The biodegradation of surfactants in the environment. Biochim Biophys Acta. 2000;1508(1–2):235–225.10.1016/S0304-4157(00)00013-7Search in Google Scholar
56. Ying GG. Fate, behavior and effects of surfactants and their degradation products in the environment. Environ Int. 2006;32(3):417–431.10.1016/j.envint.2005.07.004Search in Google Scholar
57. Olkowska E, Polkowska Z, Namieśnik J. Analytics of surfactants in the environment: problems and challenges. Chem Rev. 2011;111(9):5667–5700.10.1021/cr100107gSearch in Google Scholar
58. Oei HH, Mai I, Toro DC. Quantitative analysis of anionic or cationic surfactants using a surfactant electrode. J Soc Cosmet Chem. 1991;42(5):309–316.Search in Google Scholar
59. Shah A, Shahzad S, Munir A, Nadagouda MN, Khan GS, Shams DF, et al. Micelles as soil and water decontamination agents. Chem Rev. 2016;116(10):6042–6074.10.1021/acs.chemrev.6b00132Search in Google Scholar
60. Kim SO, Moon S–H, Kim KW. Removal of heavy metals from soils using enhanced electrokinetic soil processing. Water Air Soil Pollut. 2001;125 259−272.10.1023/A:1005283001877Search in Google Scholar
61. Jiang Y, Zhan H, Yuan J, Ma M, Chen H. Washing efficiency of heavy metals in soils with EDTA enhanced by surfactants. J Agric Res. 2006;25 119−123.Search in Google Scholar
62. Westall JC, Chen H, Zhang W, Brownawell BJ. Sorption of linear alkylbenzenesulfonates on sediment materials. Environ Sci Technol. 1999;33(18):3110–3118.10.1021/es9804316Search in Google Scholar
63. Krueger CJ, Radakovich KM, Sawyer TE, Barber LB, Smith RL, Field JA. Biodegradation of the surfactant linear alkylbenzenesulfonate in sewage–contaminated groundwater: a comparison of column experiments and field tracer tests. Environ Sci Technol. 1998;32(24):3954–3961.10.1021/es9803456Search in Google Scholar
64. McAvoy DC, Eckhoff WS, Rapaport RA. Fate of linear alkylbenzene sulfonate in the environment. Environ Toxicol Chem. 1993;12:977–987.10.1002/etc.5620120604Search in Google Scholar
65. Brunner PH, Capri S, Marcomini A, Giger W. Occurrence and behaviour of linear alkylbenzenesulphonates, nonylphenol, nonylphenol mono–and nonylphenol diethoxylates in sewage and sewage sludge treatment. Water Res. 1988;22(12):1465–1472.10.1016/0043-1354(88)90157-1Search in Google Scholar
66. Ruiz BF, Prats D, Rico C. Elimination of LAS (linear alkylbenzene sulfonate) during sewage treatment, drying and composting of sludge and soil amending processes. In: Quaghebeur D, Temmerman I, Angeletti G, editors. Organic contaminants in waste water, sludge and sediment. London: Elsevier Applied Science, 1989.Search in Google Scholar
67. Mieure JP, Waters J, Holt MS, Matthijs E. Terrestrial safety assessment of linear alkyl benzene sulphonate. Chemosphere. 1990;21:251–262.10.1016/0045-6535(90)90397-CSearch in Google Scholar
68. Hernández–Soriano MDC, Degryse F, Smolders E. Mechanisms of enhanced mobilisation of trace metals by anionic surfactants in soil. Environ Pollut. 2011;159(3):809–816.10.1016/j.envpol.2010.11.009Search in Google Scholar
69. Hernández–Soriano MDC, Peña A, Mingorance MD. Release of metals from metal–amended soil treated with a sulfosuccinamate surfactant: effects of surfactant concentration, soil/solution ratio, and pH. J Environ Qual. 2010;39(4):1298–1305.10.2134/jeq2009.0242Search in Google Scholar
70. Edwards DA, Adeel Z, Luthy RG. Distribution of nonionic surfactant and phenanthrene in a sediment/aqueous system. Environ Sci Technol. 1994;28:1550–1560.10.1021/es00057a027Search in Google Scholar
71. Brunner PH, Capri S, Marcomini A, Giger W. Occurrence and behavior of linear alkylbenzene sulphonates, nonylphenol, nonylphenol mono– and nonylphenol diethoxylates in sewage and sewage sludge treatment. Water Res. 1988;22:101–113.10.1016/0043-1354(88)90157-1Search in Google Scholar
72. EPA–factsheet PFOA & PFOS Drinking Water Health Advisories US Environmental Protection Agency May. July 2016. EPA 800–F–16–003 Available at: https://www.epa.gov/ground–water–and–drinking–water/drinking–water–health–advisories–pfoa–and–pfos. Accessed:July2016.Search in Google Scholar
73. Soares A, Guieysse B, Jefferson B, Cartmell E, Lester JN. Nonylphenol in the environment: a critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ Int. 2008;34(7):1033–1049.10.1016/j.envint.2008.01.004Search in Google Scholar
74. Renner R. European bans on surfactant trigger transatlantic debate. Environ Sci Technol. 1997;31(7) 316A–320A.10.1021/es972366qSearch in Google Scholar
75. David A, Fenet H, Gomez E. Alkylphenols in marine environments: distribution monitoring strategies and detection considerations. Mar Pollut Bull. 2009;58(7):953–960.10.1016/j.marpolbul.2009.04.021Search in Google Scholar
76. Aronstein BN, Calvillo YM, Alexander M. Effect of surfactants at low concentrations on the desorption and biodegradation of sorbed aromatic compounds in soil. Environ Sci Technol. 1991;25:1728–1731.10.1021/es00022a008Search in Google Scholar
77. Kosswig K. Surfactants. In: W. Gerhartz, editor. Ullmann’s encyclopedia of industrial chemistry Vol. A25. Weinheim, Germany: VCH Verlagsgesellschaft mbH, 1994:747–816. .Search in Google Scholar
78. Metcalfe TL, Dillon PJ, Metcalfe CD. Transport of toxic substances from golf courses into watersheds in the precambrian shield region of Ontario, Canada. Environ Toxicol Chem. 2008;27:811–818.10.1897/07-216.1Search in Google Scholar
79. Watanabe H. Spectrophotometric determination of cobalt with 1–(2–pyridylazo)–2–naphthol and surfactants. Talanta. 1974;21(4):295–302.10.1016/0039-9140(74)80007-XSearch in Google Scholar
80. Watanabe H, Sakai Y. Spectrophotometric determination of zinc with 1–(2–pyridylazo)–2–naphthol and surfactant. Bunseki Kagaku. 1974;23(4):396–402 Chem. Abstr. 1974, 81, 32939b.10.2116/bunsekikagaku.23.396Search in Google Scholar
81. Watanabe H, Miura J. Selection of masking agents in the spectrophotometric determination of nickel with 1–(2–thiazolylazo)–2–naphthol and nonionic surfactant. Bunseki Kagaku. 1976;25(10):667–670 Chem. Abstr. 1977, 87, 126585j.10.2116/bunsekikagaku.25.667Search in Google Scholar
82. Watanabe H, Ishii H. Spectrophotometric determination of nickel with 2–(2–thiazolylazo)–5–dimethylaminophenol and Triton X–100. Bunseki Kagaku. 1977;26(2):86–91 Chem. Abstr. 1977, 87, 126574e.10.2116/bunsekikagaku.26.86Search in Google Scholar
83. Hinze WL, Williams RW, Fu ZS, Suzuki Y, Quina FH. Novel chiral separation techniques based on surfactants. Colloids Surf. 1990;48:79–94.10.1016/0166-6622(90)80220-XSearch in Google Scholar
84. Ostlund SG, Pharr DY. Determination of cobalt(II) by flow injection analysis using a surfactant solvent system. J Undergraduate Chem Res. 2003;2(1):33–38.Search in Google Scholar
85. Marczenko Z, Jarosz M. Formation of ternary complexes of aluminium with some triphenylmethane reagents and cationic surfactants. Analyst. 1982;107(1281):1431–1438.10.1039/an9820701431Search in Google Scholar
86. Sharma R, Kamal A, Mahajan RK. A quantitative appraisal of the binding interactions between an anionic dye, Alizarin Red S, and alkyloxypyridinium surfactants: a detailed micellization, spectroscopic and electrochemical study. Soft Matter. 2016;12(6):1736–1749.10.1039/C5SM02667ASearch in Google Scholar
87. Šimek J, Son NT, Ružička E. Spectrophotometry study of the reaction between dyes of the Alizarin green series and vanadates in the presence of cetylpyridinium cation. Chem Pap. 1985;39(1):91–101.Search in Google Scholar
88. Šimek J, Son NT, Ružička E. Spectrophotometric study of the reaction of Alizarin green series dyes with uranyl ions in the presence of cationoid surfactants. Collect Czechoslov Chem Commun. 1985;50(3):611–620.10.1135/cccc19850611Search in Google Scholar
89. Tsao FP, Underwood AL. Spectrophotometric determination of nitrite with p–nitroaniline and 2–methyl–8–quinolinol in hexadecyl–trimethylammonium bromide solution. Anal Chim Acta. 1982;136:129–134.10.1016/S0003-2670(01)95371-1Search in Google Scholar
90. Nair J, Gupta VK. The spectrophotometric determination of nitrite in water with 8–quinolinol. Anal Chim Acta. 1979;111:311–314.10.1016/S0003-2670(01)93278-7Search in Google Scholar
91. Sarpal RS, Belletete M, Durocher G. Fluorescence probing and proton–transfer equilibrium reactions in water, SDS, and CTAB using 3, 3–dimethyl–2–phenyl–3H–indole. J Phys Chem. 1993;97(19):5007–5013.10.1021/j100121a025Search in Google Scholar
92. Spurlin S, Hinze W, Armstrong DW. Use of an aqueous micellar medium to improve the spectrophotometry determination of cyanide ion with 5, 5′–Dithiobis (2–Nitrobenzoic Acid). Anal Lett. 1977;10(12):997–1008.10.1080/00032717708059255Search in Google Scholar
93. Berezin IV, Yatsimirskaya NT, Yatsimirskii AK, Martinek K. Use of micellar catalysis in nucleophilic substitution and addition in organic analysis. Dokl Akad Nauk SSSR. 1986;287(3):643–647.Search in Google Scholar
94. Connors KA, Wong MP. Micellar catalysis of an analytical reaction: spectrophotometric determination of amino acids and peptides after cetrimonium bromide catalyzed reaction with 1 fluoro 2, 4 dinitrobenzene. J Pharm Sci. 1979;68(11):1470–1471.10.1002/jps.2600681141Search in Google Scholar
95. Liu Y, Wu H, Ma W. Spectrophotometric determination of benzalkonium bromide in pharmaceutical samples with Alizarin green. J Surfactants Deterg. 2013;16(2):265–269.10.1007/s11743-012-1391-7Search in Google Scholar
96. Watanabe H, Ohmori H. Dual–wavelength spectrophotometric determination of cadmium with cadion. Talanta. 1979;26(10):959–961.10.1016/0039-9140(79)80133-2Search in Google Scholar
97. Fu–Sheng W, Fang Y. Spectrophotometric determination of silver with cadion 2B and triton X–100. Talanta. 1983;30(3):190–192.10.1016/0039-9140(83)80049-6Search in Google Scholar
98. Hung SC, Qu CL, Wu SS. Spectrophotometric determination of uranium (VI) with 2–(3, 5–dibromo–2–pyridylazo)–5–diethylaminophenol in the presence of anionic surfactant. Talanta. 1982;29(7):629–631.10.1016/0039-9140(82)80015-5Search in Google Scholar
99. Zhang CP, Qi DY, Zhou TZ. Sensitive spectrophotometric determination of traces of zirconium with 2–(6–bromo–2–benzothiazolylazo)–5–diethylaminophenol in the presence of sodium lauryl sulphate. Talanta. 1982;29(12):1119–1121.10.1016/0039-9140(82)80229-4Search in Google Scholar
100. Hung SC, Qu CL, Wu SS. Spectrophotometric determination of silver with 2–(3, 5–dibromo–2–pyridylazo)–5–diethyl–aminophenol in the presence of anionic surfactant. Talanta. 1982;29(2):85–88.10.1016/0039-9140(82)80025-8Search in Google Scholar
101. Zhe T, Wu SS. Spectrophotometric determination of zinc with 2–(3, 5–dibromo–2–pyridylazo)–5–diethylaminophenol in the presence of anionic surfactant. Talanta. 1984;31(8):624–626.10.1016/0039-9140(84)80183-6Search in Google Scholar
102. Malik AK, Kaul KN, Lark BS, Faubel W, Rao AL. Spectrophotometric determination of cobalt, nickel palladium, copper, ruthenium and molybdenum using sodium isoamylxanthate in presence of surfactants. Turkish J Chem. 2001;25(1):99–105.Search in Google Scholar
103. Ghaedi M. Selective and sensitized spectrophotometric determination of trace amounts of Ni (II) ion using α–benzyl dioxime in surfactant media. Spectrochim Acta A Mol Biomol Spectrosc. 2007;66(2):295–301.10.1016/j.saa.2006.02.055Search in Google Scholar PubMed
104. Leon–Gonzalez ME, Santos–Delgado MJ, Polo–Diez LM. An improved method for the spectrophotometric determination of fluoride by addition of sodium dodecyl sulphate to the fluoride/lanthanum (III)/Alizarin fluorine blue system. Anal Chim Acta. 1985;178:331–335.10.1016/S0003-2670(00)86285-6Search in Google Scholar
105. Oshiro I, Takenaka T, Maeda J. New method for hemoglobin determination by using sodium lauryl sulfate (SLS). Clin Biochem. 1982;15(2):83–88.10.1016/S0009-9120(82)91069-4Search in Google Scholar
106. ThorburnáBurns D, Dagar D. Improvements to the spectrophotometric determination of germanium with phenylfluorone. Analyst. 1980;105(1246):75–79.10.1039/an9800500075Search in Google Scholar
107. Georges J. Molecular fluorescence in micelles and microemulsions: micellar effects and analytical applications. Prog Anal Spectrosc. 1990;13(1):27–45.Search in Google Scholar
108. Hinze WL, Singh HN, Baba Y, Harvey NG. Micellar enhanced analytical fluorimetry. Trac Trends Anal Chem. 1984;3(8):193–199 ,and Hinze WL, Srinivasan N, Smith TK, Igarashi S, and Hoshino H. Organized assemblies in analytical chemiluminescence spectroscopy: an overview. Advances in multidimensional luminescence 1990, 1, 149–206.10.1016/0165-9936(84)85006-2Search in Google Scholar
109. Ishibashi N, Kina K. Sensitivity enhancement of the fluorometric determination of aluminum by the use of surfactant. Anal Lett. 1972;5(9):637–641.10.1080/00032717208064341Search in Google Scholar
110. Sanz–Medel A, Fernandez Perez MM, De la Guardia Cirugeda M, Dominguez JC. Metal chelate fluorescence enhancement by nonionic micelles: surfactant and auxiliary ligand nature influence on the niobium–lumogallion complex. Anal Chem. 1986;58(11):2161–2166.10.1021/ac00124a012Search in Google Scholar
111. Sanz–Medel A, de la Campa RF, Alonso JI. Metal chelate fluorescence enhancement in micellar media: mechanisms of surfactant action. Analyst. 1987;112(4):493–497.10.1039/an9871200493Search in Google Scholar
112. Dominguez JC, de la Guardia Cirugeda M.. Some observations on the fluorometric determination of metallic elements in micellar media. Microchemical Journal. 1989;39(1):50–58.10.1016/0026-265X(89)90008-8Search in Google Scholar
113. Singh H, Hinze WL. Micellar enhanced spectrofluorometric methods: application to the determination of pyrene. Anal Lett. 1982;15(A3):221–243.10.1080/00032718208064379Search in Google Scholar
114. Singh HN, Hinze WL. Micellar enhanced fluorimetric determination of 1–NN–dimethylaminonaphthalene–5–sulphonyl chloride and o–phthalaldehyde–2–mercaptoethanol derivatives of amino acids. Analyst. 1982;107(1278):1073–1080.10.1039/an9820701073Search in Google Scholar
115. Medina J, Hernandez F, Marin R, Lopez FJ. Study of the fluorescence of the lead–morin system in the presence of nonionic surfactants. Analyst. 1986;111(2):235–237.10.1039/an9861100235Search in Google Scholar
116. Taketatsu T. Complex formation of europium with thenoyltrifluoroacetone and tri–n–octylphosphine oxide in micellar solution of nonionic surfactant. Chem Lett. 1981;10(8):1057–1058 .10.1246/cl.1981.1057Search in Google Scholar
117. Xu YY, Hemmilä IA. Co–fluorescence enhancement system based on pivaloyltrifluoroacetone and yttrium for the simultaneous detection of europium, terbium, samarium and dysprosium. Anal Chim Acta. 1992;256(1):9–16.10.1016/0003-2670(92)85319-2Search in Google Scholar
118. Xu YY, Hemmilä IA, Lövgren TN. Co–fluorescence effect in time–resolved fluoroimmunoassays – A review. Analyst. 1992;117(7):1061–1069.10.1039/AN9921701061Search in Google Scholar
119. Galdú MV, de la Guardia M, Braco L. Influence of anionic surfactants on the sensitisation of the fluorimetric determination of fenproporex. Analyst. 1987;112(7):1047–1050.10.1039/AN9871201047Search in Google Scholar
120. De la Guardia Cirugeda M, Soriano FR. Micellar enhancement of benzodiazepine fluorescence. Analyst. 1989;114(1):77–82.10.1039/an9891400077Search in Google Scholar
121. Jee RD. Study of micellar solutions to enhance the europium–sensitized luminescence of tetracyclines. Analyst. 1995;120(12):2867–2872.10.1039/an9952002867Search in Google Scholar
122. Campaña AM, Barrero FA, Ceba MR. Sensitive spectrofluorimetric method for the determination of ethylenediaminetetraacetic acid and its salts in foods with zirconium ions and Alizarin Red S in a micellar medium. Anal Chim Acta. 1996;329(3):319–325.10.1016/0003-2670(96)00180-8Search in Google Scholar
123. Diaz Garcia ME, Sanz–Medel A. Facile chemical deoxygenation of micellar solutions for room temperature phosphorescence. Anal Chem. 1986;58(7):1436–1440.10.1021/ac00298a036Search in Google Scholar
124. Kalyanasundaram K, Grieser F, Thomas JK. Room temperature phosphorescence of aromatic hydrocarbons in aqueous micellar solutions. Chem Phys Lett. 1977;51(3):501–505.10.1016/0009-2614(77)85410-9Search in Google Scholar
125. Love LC, Skrilec M, Habarta JG. Analysis of micelle–stabilized room temperature phosphorescence in solution. Anal Chem. 1980;52(4):754–759.10.1021/ac50054a035Search in Google Scholar
126. Skrilec M, Love LC. Micelle–stabilized room–temperature phosphorescence characteristics of carbazole and related derivatives. J Phys Chem. 1981;85(14):2047–2050.10.1021/j150614a019Search in Google Scholar
127. Love LC, Grayeski ML, Noroski J, Weinberger R. Room–temperature phosphorescence, sensitized phosphorescence and fluorescence of licit and illicit drugs enhanced by organized media. Anal Chim Acta. 1985;170:3–12.10.1016/S0003-2670(00)81720-1Search in Google Scholar
128. Carretero AS, Blanco CC, Fernández RE, Gutiérrez AF. Micellar–stabilized room–temperature phosphorimetric determination of the fungicide thiabendazole in canned pineapple samples. Fresenius’ J Anal Chem. 1998;360(5):605–608.10.1007/s002160050766Search in Google Scholar
129. Sanz–Medel A, Martinez Garcia PL, Diaz Garcia ME. Micelle–stabilized room–temperature liquid phosphorimetry of metal chelates and its application to niobium determination. Anal Chem. 1987;59(5):774–778.10.1021/ac00132a020Search in Google Scholar
130. Liu YM, de la Campa MR, García ME, Sanz–Medel A. Micelle–stabilized liquid room–temperature phosphorimetry for metals: the micellar reaction of gallium with 7–iodo–hydroxyquinoline–5–sulfonic acid and its application to the metal determination. Microchimica Acta. 1991;103(1–2):53–64.10.1007/BF01245052Search in Google Scholar
131. Fendler JH, Gilbert RD, Yen TF. Advances in the applications of membrane-mimetic chemistry. New York, NY: Springer Science & Business Media, 2012 Dec 6. 79–94.. Dec6 .Search in Google Scholar
132. Hickman A, Pharr DY. Enhancement effects of surfactants on the DC plasma analysis of P-block elements (in three parts). J Undergraduate Chem Res. 2012;11(2):54–58 and part II: 11(4),102–106;and part III: 2013, 12(2),28–32.Search in Google Scholar
133. Armstrong DW, McNeely M. Use of micelles in the TLC separation of polynuclear aromatic compounds and amino acids. Anal Lett. 1979;12(12):1285–1291.10.1080/00032717908067919Search in Google Scholar
134. Armstrong DW, Terrill RQ. Thin layer chromatographic separation of pesticides, decachlorobiphenyl, and nucleosides with micellar solutions. Anal Chem. 1979;51(13):2160–2163.10.1021/ac50049a025Search in Google Scholar
135. Armstrong DW, Henry SJ. Use of an aqueous micellar mobile phase for separation of phenols and polynuclear aromatic hydrocarbons via HPLC. J Liq Chromatogr. 1980;3(5):657–662.10.1080/01483918008060181Search in Google Scholar
136. Armstrong DW, Nome F. Partitioning behavior of solutes eluted with micellar mobile phases in liquid chromatography. Anal Chem. 1981;53(11):1662–1666.10.1021/ac00234a026Search in Google Scholar
137. Armstrong DW. Micelles in separations: practical and theoretical review. Sep Purif Methods. 1985;14(2):213–304.10.1080/03602548508068421Search in Google Scholar
138. Borgerding MF, Williams RL, Hinze WL, Quina FH. New perspectives in micellar liquid chromatography. J Liq Chromatogr. 1989;12(8):1367–1406.10.1080/01483918908049512Search in Google Scholar
139. Esteve–Romero J, Albiol–Chiva J, Peris–Vicente J. A review on development of analytical methods to determine monitorable drugs in serum and urine by micellar liquid chromatography using direct injection. Anal Chim Acta. 2016;926:1–6.10.1016/j.aca.2016.04.026Search in Google Scholar
140. Terabe S, Otsuka K, Ichikawa K, Tsuchiya A, Ando T. Electrokinetic separations with micellar solutions and open–tubular capillaries. Anal Chem. 1984;56(1):111–113.10.1021/ac00265a031Search in Google Scholar
141. Khaledi MG. Micelles as separation media in high–performance liquid chromatography and high–performance capillary electrophoresis: overview and perspective. J Chromatogr A. 1997;780(1):3–40.10.1016/S0021-9673(97)00610-9Search in Google Scholar
142. Grossman PD, Colburn JC. Capillary electrophoresis: theory and practice. San Diego, CA:Academic Press, 2012 Dec 2. 159–187.. Dec2 .Search in Google Scholar
143. Sieradzka E, Witt K, Milnerowicz H. The application of capillary electrophoresis techniques in toxicological analysis. Biomed Chromatogr. 2014;28(11):1507–1513.10.1002/bmc.3234Search in Google Scholar PubMed
144. Timerbaev AR. Element speciation analysis using capillary electrophoresis: twenty years of development and applications. Chem Rev. 2012;113(1):778–812.10.1021/cr300199vSearch in Google Scholar PubMed
145. Altria KD. Analysis of pharmaceuticals by capillary electrophoresis. New York and Philadelphia, PA:Springer Science & Business Media, 2013 Apr 17. Apr17 .Search in Google Scholar
146. Silva M. Micellar electrokinetic chromatography: a review of methodological and instrumental innovations focusing on practical aspects. Electrophoresis. 2013;34(1):141–158.10.1002/elps.201200349Search in Google Scholar PubMed
147. Rusling JF. Electrochemistry in micelles, microemulsions and related microheterogeneous fluids. Electroanalytical chemistry. 1993, 18, 1–80. In: Bard AJ, Decker M, editors. Electroanalytical chemistry a series of advances vol. 18. Boca Raton, FL: CRC Press 1994 .Search in Google Scholar
148. Proske GE. New technique for polarography of water–insoluble compounds. Anal Chem. 1952;24(11):1834–1836.10.1021/ac60071a037Search in Google Scholar
149. McIntire GL, Chiappardi DM, Casselberry RL, Blount HN. Electrochemistry in ordered systems 2 electrochemical and spectroscopic examination of the interactions between nitrobenzene and anionic, cationic, and nonionic micelles. J Phys Chem. 1982;86(14):2632–2640.10.1021/j100211a017Search in Google Scholar
150. Vittal R, Gomathi H, Kim KJ. Beneficial role of surfactants in electrochemistry and in the modification of electrodes. Adv Colloid Interface Sci. 2006;119(1):55–68.10.1016/j.cis.2005.09.004Search in Google Scholar
151. Vittal R, Gomathi H, Rao GP. Influence of a cationic surfactant on the modification of electrodes with nickel hexacyanoferrate surface films. Electrochimica Acta. 2000;45(13):2083–2093.10.1016/S0013-4686(99)00432-6Search in Google Scholar
152. Silva MF, Cerutti ES, Martinez LD. Coupling cloud point extraction to instrumental detection systems for metal analysis. Microchimica Acta. 2006;155(3–4):349–364.10.1007/s00604-006-0511-3Search in Google Scholar
153. Paleologos EK, Giokas DL, Karayannis MI. Micelle–mediated separation and cloud–point extraction. Trac Trends Anal Chem. 2005;24(5):426–436.10.1016/j.trac.2005.01.013Search in Google Scholar
154. Ferrera ZS, Sanz CP, Santana CM, Rodrıguez JJ. The use of micellar systems in the extraction and pre–concentration of organic pollutants in environmental samples. Trac Trends Anal Chem. 2004 Aug 31;23(7):469–479 Aug31.10.1016/S0165-9936(04)00732-0Search in Google Scholar
155. Stalikas CD. Micelle–mediated extraction as a tool for separation and preconcentration in metal analysis. Trac Trends Anal Chem. 2002;21(5):343–355.10.1016/S0165-9936(02)00502-2Search in Google Scholar
156. Mukherjee P, Padhan SK, Dash S, Patel S, Mishra BK. Clouding behaviour in surfactant systems. Adv Colloid Interface Sci. 2011;162(1):59–79.10.1016/j.cis.2010.12.005Search in Google Scholar
157. Tani H, Kamidate T, Watanabe H. Micelle–mediated extraction. J Chromatogr A. 1997;780(1):229–241.10.1016/S0021-9673(97)00345-2Search in Google Scholar
158. Bordier C. Phase separation of integral membrane proteins in Triton X–114 solution. J Biol Chem. 1981;256(4):1604–1607.10.1016/S0021-9258(19)69848-0Search in Google Scholar
159. Underwood AL. Dissociation of acids in aqueous micellar systems. Anal Chim Acta. 1982;140(1):89–97.10.1016/S0003-2670(01)95455-8Search in Google Scholar
160. Fernandez MS, Fromherz P. Lipoid pH indicators as probes of electrical potential and polarity in micelles. J Phys Chem. 1977;81(18):1755–1761.10.1021/j100533a009Search in Google Scholar
161. Siggia S, Hanna JG. Quantitative organic analysis via functional groups. New York, NY: John Wiley & Sons, 1979.Search in Google Scholar
162. Underwood AL. Acid–base titrations in aqueous micellar systems. Anal Chim Acta. 1977;93:267–273.10.1016/0003-2670(77)80031-7Search in Google Scholar
163. Starościk R, Maskiewicz E, Malecki F. Acid – Base titrations of barbiturates in aqueous micellar media. Fresenius’ Z Anal Chem. 1987;329(4):472–474.10.1007/BF00480090Search in Google Scholar
164. Gerakis AM, Koupparis MA, Efstathiou CE. Micellar acid – base potentiometric titrations of weak acidic and/or insoluble drugs. J Pharm Biomed Anal. 1993;11(1):33–41.10.1016/0731-7085(93)80146-RSearch in Google Scholar
165. Ruzicka J, Hansen EH. Retro-review of flow-injection analysis. Trac Trends Anal Chem. 2008;27(5):390–393.10.1016/j.trac.2008.03.004Search in Google Scholar
166. Růžička J. The second coming of flow-injection analysis. Anal Chim Acta. 1992;261(1):3–10.10.1016/0003-2670(92)80169-8Search in Google Scholar
167. Pharr DY. A review of the use of surfactants in flow injection analysis. Anal Lett. 2011;44(13):2287–2311.10.1080/00032719.2010.551689Search in Google Scholar
168. Memon N, D Tzanavaras P. Utilization of organized surfactant assemblies as solvents in flow injection analysis with emphasis to automated derivatization of organic analytes. Curr Anal Chem. 2014;10(3):338–348.10.2174/157341101003140521113053Search in Google Scholar
169. Resing JA, Measures CI. Fluorometric determination of Al in seawater by flow injection analysis with in–line preconcentration. Anal Chem. 1994;66(22):4105–4111.10.1021/ac00094a039Search in Google Scholar
170. Pharr DY, Tomsyck JA. Flow injection analysis of benzoyl peroxide using N, N, N, N-tetramethyl-p-phenylenediamine (TMPDA) and surfactants. Anal Lett. 2009;42(5):821–832.10.1080/00032710902721923Search in Google Scholar
171. Pharr DY, Sienerth KD, Tutor MJ. The analysis of nickel and cobalt by flow injection analysis using PAR, 4-(2-pyridylazo)-resorcinol, and Triton X-100. J Und Chem Res. 2004;3(2):57–63.Search in Google Scholar
172. Safavi AF, Mirzaee M, Hormozi Nezhad MR, Saghir N. Kinetic spectrophotometric determination of copper by flow injection analysis in cationic micellar medium. Spectrosc Lett. 2005;38(1):13–22.10.1081/SL-200042305Search in Google Scholar
173. Long F, Zhang X, Zou Y, Xia X, Jiang R, Zhang W. Determination of trace zinc in seawater using reverse FIA spectrophotometric method. Pige Kexue Yu Gongcheng. 2006;16(6):44–46.Search in Google Scholar
174. Ruengsitagoon W, Chisvert A, Liawruangrath S. Flow injection spectrophotometric determination of lead using 1, 5-diphenylthiocarbazone in aqueous micellar. Talanta. 2010;81(1):709–713.10.1016/j.talanta.2010.01.001Search in Google Scholar
175. Ahmadi SH, Shabani AM, Dadfarnia S, Taei M. On-line preconcentration and speciation of chromium by an 8-hydroxyquinoline microcolumn immobilized on surfactant-coated alumina and flow injection atomic absorption spectrometry. Turkish J Chem. 2007;31(2):191–199.Search in Google Scholar
176. Dadfarnia S, Shabani AH, Gohari M. Trace enrichment and determination of silver by immobilized DDTC microcolumn and flow injection atomic absorption spectrometry. Talanta. 2004;64(3):682–687.10.1016/j.talanta.2004.03.039Search in Google Scholar
177. Irace Guigand S, Leverend E, Seye MD, Aaron JJ. A new on line micellar enhanced photochemically induced fluorescence method for determination of phenylurea herbicide residues in water. Luminescence. 2005;20(3):138–142.10.1002/bio.817Search in Google Scholar
178. Flores JL, de Córdova ML, Am D. Flow-through optosensing device implemented with photochemically-induced fluorescence for the rapid and simple screening of metsulfuron methyl in environmental waters. J Environ Monit. 2009;11(5):1080–1085.10.1039/b818307dSearch in Google Scholar
179. Carabias-Martınez R, Rodrıguez-Gonzalo E, Moreno-Cordero B, Pérez-Pavón JL, Garcıa-Pinto C, Laespada EF. Surfactant cloud point extraction and preconcentration of organic compounds prior to chromatography and capillary electrophoresis. J Chromatogr A. 2000;902(1):251–265.10.1016/S0021-9673(00)00837-2Search in Google Scholar
180. Silva EL, dos Santos Roldan P. Simultaneous flow injection preconcentration of lead and cadmium using cloud point extraction and determination by atomic absorption spectrometry. J Hazard Mater. 2009;161(1):142–147.10.1016/j.jhazmat.2008.03.100Search in Google Scholar PubMed
181. Song GQ, Lu C, Hayakawa K, Lin JM. Comparison of traditional cloud-point extraction and on-line flow-injection cloud-point extraction with a chemiluminescence method using benzo[a]pyrene as a marker. Anal Bioanal Chem. 2006;384(4):1007–1012.10.1007/s00216-005-0224-1Search in Google Scholar PubMed
182. Tsogas GZ, Giokas DL, Vlessidis AG. Ultratrace determination of silver, gold, and iron oxide nanoparticles by micelle mediated preconcentration/selective back-extraction coupled with flow injection chemiluminescence detection. Anal Chem. 2014;86(7):3484–3492.10.1021/ac404071vSearch in Google Scholar PubMed
183. Cao J, Wang H, Liu Y. Determination of l-thyroxine in pharmaceutical preparations by flow injection analysis with chemiluminescence detection based on the enhancement of the luminol–KMnO4 reaction in a micellar medium. Spectrochim Acta A Mol Biomol Spectrosc. 2015;140:162–165.10.1016/j.saa.2014.12.105Search in Google Scholar PubMed
184. Zhao F, Zhao WH, Xiong W. Chemiluminescence determination of Gemifloxacin based on diperiodatoargentate (III) sulphuric acid reaction in a micellar medium. Luminescence. 2013;28(2):108–113.10.1002/bio.2347Search in Google Scholar
185. Perkowski J, Mayer J, Ledakowicz S. Determination of critical micelle concentration of non-ionic surfactants using kinetic approach. Colloids Surfaces A. 1995;101:103–106.10.1016/0927-7757(95)03203-PSearch in Google Scholar
186. Hait SK, Moulik SP. Determination of critical micelle concentration (CMC) of nonionic surfactants by donor-acceptor interaction with lodine and correlation of CMC with hydrophile-lipophile balance and other parameters of the surfactants. J Surf Det. 2001;4(3):303–309.10.1007/s11743-001-0184-2Search in Google Scholar
© 2017 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Applications of silver nanoparticles stabilized and/or immobilized by polymer matrixes
- Addressing the challenges of standalone multi-core simulations in molecular dynamics
- Virtually going green: The role of quantum computational chemistry in reducing pollution and toxicity in chemistry
- BioArtificial polymers
- Green analytical chemistry – the use of surfactants as a replacement of organic solvents in spectroscopy
- Membrane contactors for CO2 capture processes – critical review
- Elemental Two-Dimensional Materials Beyond Graphene
Articles in the same Issue
- Applications of silver nanoparticles stabilized and/or immobilized by polymer matrixes
- Addressing the challenges of standalone multi-core simulations in molecular dynamics
- Virtually going green: The role of quantum computational chemistry in reducing pollution and toxicity in chemistry
- BioArtificial polymers
- Green analytical chemistry – the use of surfactants as a replacement of organic solvents in spectroscopy
- Membrane contactors for CO2 capture processes – critical review
- Elemental Two-Dimensional Materials Beyond Graphene


