Abstract
Nowadays, the polymer science has impact in practically all life areas. Countless benefits coming from the usage of materials with high mechanical and chemical resistance, variety of functionalities and potentiality of modification drive to the development of new application fields. Novel approaches of combining these synthetic substances with biomolecules lead to obtain multifunctional hybrid conjugates which merge the bioactivity of natural component with outstanding properties of artificial polymer. Over the decades, an immense progress in bioartificial composites domain allowed to reach a high level of knowledge in terms of natural-like systems engineering, leading to diverse strategies of biomolecule immobilization. Together with different available options, including covalent and noncovalent attachment, come various challenges, related mainly with maintaining the biological activity of fixed molecules. Even though the amount of applications that achieve commercial status is still not substantial, and is expanding continuously in the disciplines like “smart materials,” biosensors, delivery systems, nanoreactors and many others. A huge number of remarkable developments reported in the literature present a potential of bioartificial conjugates as a fabrics with highly controllable structure and multiple functionalities, serving as a powerful nanotechnological tool. This novel approach brings closer biologists, chemists and engineers, who sharing their effort and complementing the knowledge can revolutionize the field of bioartificial polymer science.
1 Introduction
Imitating the nature by researchers from all the fields of science is a common trend since decades and is continuously developing dynamically. The versatile applications of materials able to work like biological structures have made them a major interdisciplinary research focus. Since the discovery of macromolecules by the German scientist Herman Staudinger (Nobel Prize in 1953), the field of material sciences and technology reached countless life-changing developments, followed up with a drastic growth of plastic–polymer industry. The variety of possibilities offered by compounds chemically and mechanically stable with simultaneous potential of modification and functionalization opened a door for novel approaches that focus on designing materials able to adapt multiple functions, structures and capabilities – also biological like. The evolution of bioinspired polymeric materials leads to obtaining structures that combine the benefits coming from both sources – biological and synthetic ones. They can have advantages over the natural version, like improved mechanical robustness and reproducible chemistry, but at the same time operate according to biological mechanism, with the same or even higher efficiency. Those materials are developed by applying various strategies.
One of them is the synthesis of completely new chemical compounds that are able to adopt the same structure and function like pattern biomolecule. They are named biomimetic materials.
Within this approach, many stimuli-responsive polymers have been designed, like for instance a novel gel system developed by Yoshida from Tokyo University [1]. Over years of improvement, those polymeric gels are able to mimic autonomous oscillation movements present in living systems such as heartbeat or brain waves. This so-called biomimetic actuators convert a chemical oscillation process into a mechanical alteration in gels and can be also controlled by change of some parameters, like temperature or substrate concentration. This development provides a smart material that can generate self-propelled motions, pendulum motions, or directional motions and find variety of applications in biomedical sciences. Another example of the same concept is imitation of enzymes for widely understood sensing applications and biocatalysis [2, 3], which recently improved, overcomes the challenges concerning poor selectivity of synthesized sensors by application of molecular imprinted polymers [4]. It is reported that molecular imprinted polymers-based nitroreductase has been already successfully applied for efficient determination of a drug metronidazole or hexazinone herbicide – pervasive ground water contaminant [5]. A known example of natural polymer replacement by artificial one can be synthetic rubber; which it is used e. g. for car wheels production or artificial hair as a synthetic substitute of keratin [6, 7, 8, 9].
Another approach to create polymeric biomaterials is based on a concept of its biocompatibility and, thus, possibility to use them in living organisms. This is a common challenge in terms of application of many synthetic structures – to be safely used in vivo, polymers need to be biodegradable and its decomposition products cannot be toxic at all. Several synthetic polymers have been found to fulfil these requirements and are widely used, for instance polypropylene and polyurethane in breast implants, polyurethane in artificial heart or poly(glycolic acid) as absorbable sutures [10, 11, 12].
Besides, a big part of the research concerning the design of biological-like artificial systems is dedicated to the development of hybrid protein-synthetic polymer materials. The first attempts to obtain synthetic polymers containing biomolecule have been reported already in 1964 [13], and since this time the knowledge in this term has evolved significantly. This combination of synthetic and biological parts is a step forward to fabrication of hybrid bioinspired structures with unprecedented properties and applications which are discussed in this chapter.
2 Biomolecule immobilization techniques
This chapter is dedicated to specific kind of materials that contain both units – artificial polymer and biomolecule. The goal of this approach is to obtain materials, which would possess advantages of synthetic polymers, like their performing mechanical properties, reproducible chemistry and thus possible large-scale production, and also coming from biomolecule- biocompatibility and functionality of natural system. This establishment correlates to many challenges which depend on the type of protein incorporated into artificial medium and on the desired application of the material. However, the main defiance, present in all kinds of used protein, is maintaining their biological activity, which often is lost due to change in a conformation or incompatibility with polymer [14, 15, 16]. Thus, within this particular concept, different methods of protein incorporation can be applied and are usually classified as follows.
2.1 Adsorption
The simplest and very commonly used method is physical adsorption on a support material (Figure 1). If performed under suitable conditions, this method can efficiently bind biomolecule into a support via hydrophobic or van der Waals interactions. Although this strategy found application in immobilizing enzymes for e. g. medicine purification purposes [17, 18, 19, 20], it is usually too weak to cope industrial conditions; thus, in this field, it is preferable to employ other methods.
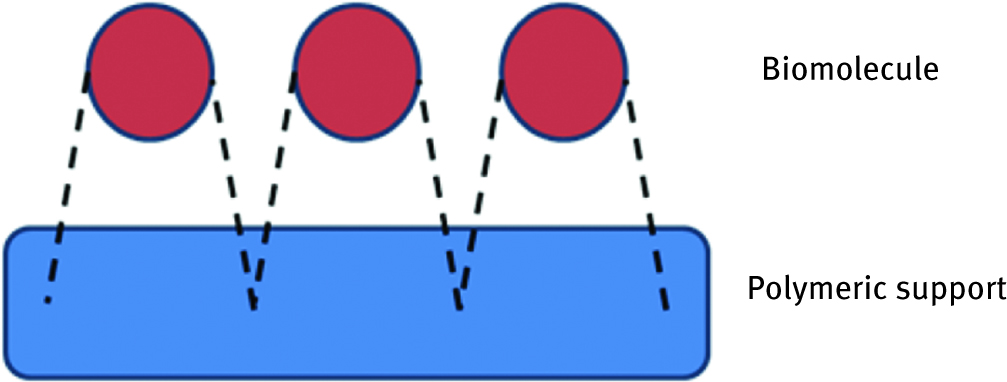
Scheme of physical adsorption of biomolecule on the polymeric support.
Adsorption is relatively easy to perform and due to the fact that it is free from chemical modification, it is a very efficient and non-invasive method, which maintains the biological activity of proteins. Also, since it is reversible, it facilitates the purification of used biomolecules and supports. However, the main handicap is requirement of mild working conditions and very careful adaptation of both molecules and carriers.
2.2 Covalent binding
This method provides much stronger linkages between polymeric support and biomolecule (Figure 2). Normally linkage occurs between functional groups present in the support surface and the groups of protein. Usually reactions used to create new bonds are 1,3-dipolar cycloadditions of terminal alkynes and azides, peptide bond formation, isourea linkage, [21] coupling via tresyl chloride, coupling via cyanogen bromide, coupling via cyanuric chloride or glutaraldehyde coupling [22].
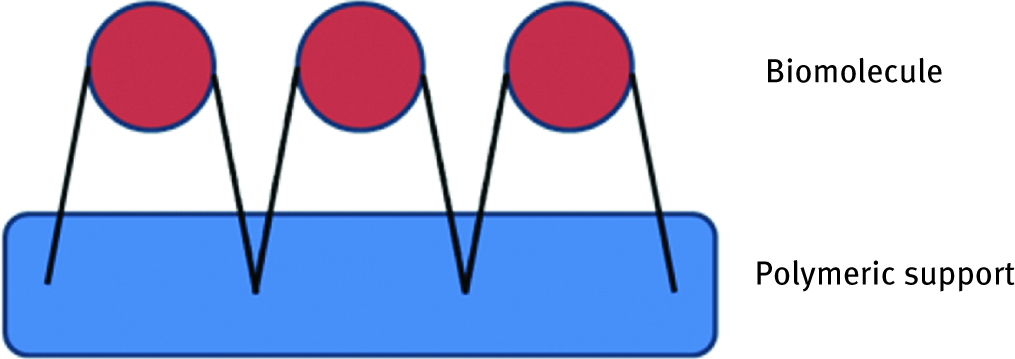
Scheme of chemical attachment of biomolecule to the polymeric support.
In this case, due to strong interactions, leakage of biomolecule is minimized. However; this is a much more expensive immobilization method in comparison to physical adsorption. Moreover, it is also connected with the use of organic solvents what, unfortunately, is very often related to the loss of biological activity.
2.3 Entrapment
Entrapment of biomolecules consists of suspending them within the polymeric gel which delimits the movements and stabilizes the protein/polymer structure (Figure 3). In this system, the gel acts also as a biomolecule protector, separating it from environment. As the polymer porosity can be controlled, it is possible to create a gel-net adjusted to the protein size in order to avoid leakage, with simultaneous permission to free moieties and active structure adaptation.
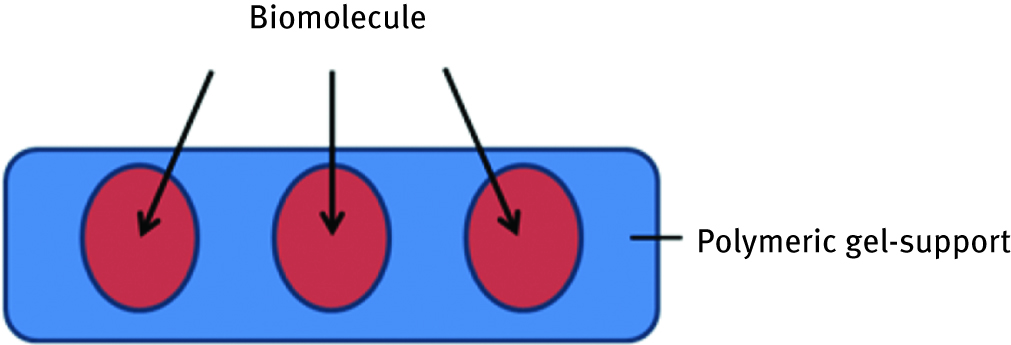
Scheme of entrapment of biomolecule in the polymeric gel.
Although protein loading achieved with this technique can be very high, there is a big disadvantage of this technique related to diffusion limitation through the gel: certain times, the gel does not allow the products of enzymatic reaction to release from it.
2.4 Encapsulation
Encapsulation is widely used in drug delivery system as well as in biocatalysis. Biomolecule closed in a polymeric semi-permeable shell has enough free space to freely move, but is isolated from environment (Figure 4). Possibility to create pH- or light-sensitive microcapsules allows to release the protein from its core under desirable conditions [23, 24]. Because of the capability to produce capsules in different sizes, it is possible to encapsulate even relatively big biomolecules. The limitations related to this method are similar to the one with gel entrapment and concern diffusion problems. Another important factor is sensitivity of polymer to external factors like temperature or pH. Depending on the biomolecule function, the properties of polymeric capsules needs to be adjusted in order to release it under the adequate condition in the target. The next chapter will discuss in more detail the function of nanospheres and nanocapsules for drug delivery systems.
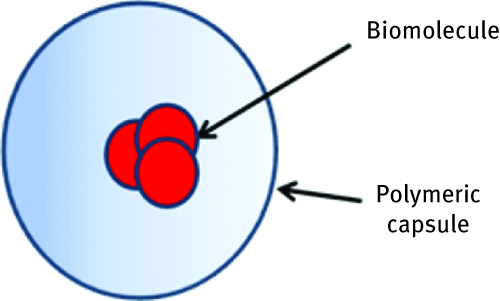
Scheme of biomolecule entrapment inside the polymeric capsule.
All these techniques have some disadvantages and the concrete design of the perfect system needs to take into account both types of limitation – from the side of polymeric support and from the site of biomolecule. However, development of new chemical strategies and production process optimization resulted in the immobilization of various biological elements on different polymers and induced many remarkable discoveries. Election of the method depends on the type of immobilized biocompound and application of the final product.
3 Polymer/biomolecule conjugates
Researchers from all over the world are struggling to face challenges connected with specificity of particular biomolecules and polymers, trying to overcome obstacles to obtain desirable, perfect, defect-free biomaterials with multiple functions. Biological/artificial conjugates found plenty of application in industry, technology, biosensing, tissue engineering and pharmacology [25, 26]. Those applications according to the type of used biomolecules and polymers are described in the following section, while application in drug delivery systems will be discussed specifically in the next chapter.
3.1 Enzymes
Enzymes are proteins that assist catalytic functions of biochemical processes. Their presence is crucial for living organisms because most of the reactions do not occur spontaneously at normal body temperature. The characteristic work done by enzymes is related to their extremely high specificity coming from its particular geometric configuration, which allows to bind only proper-shaped molecules (key and lock mechanism). The catalytic activity of enzymes can be affected by different factors like temperature, pH or concentration of enzyme or substrate. They are also sensitive to various chemicals which inhibit catalytic reactions in a reversible or irreversible way [27]. Thus, all those features need to be taken into account while designing immobilization conditions and method.
High efficiency and selectivity of enzymatic reactions has always been an inspiration for the development of novel bioreactors and biosensors. Recently, the industrial competence in biocatalysis is achieved due to common use of lipases, esterase or peptidases [18, 20, 28, 29, 30]. Polymer/enzyme conjugates are reaching significance also in the field of decontamination. As a trend in green chemistry, organophosphorus (OP) hydrolase was used [24, 25, 31]. This enzyme hydrolyzes OP compounds present in the soil, which are pesticide components. OPs are very toxic and can cause damage to the central nervous system. An example of hydrolase/polymer conjugate is reported in the literature and employs enzyme immobilization in the thermostable triblock copolymer micelles. This strategy overcomes low biomolecule stability under different working and storage conditions [32]. Methyl parathion hydrolase was entrapped, for the same purpose in the poly(gamma-glutamic-acid)/gelatin hydrogel and this even enhanced its catalytic efficiency. An interesting approach, published recently by Xiao-Yu Yan and coworkers [33], was the creation of nanofibrous membrane containing OP hydrolase which managed to fulfil both filtration and degradation functions for biochemical protective applications. In this approach, OP hydrolase is immobilized covalently via glutaraldehyde crosslinking on polyamide nanofibers. This novel design of the material makes it a potential tool for various civilian and military protection equipment and clothier production. Those fabrics would be able to protect human body via double mechanisms: by filtration of toxic products and converting it to nontoxic derivatives. This purpose is schematically demonstrated in Figure 5.
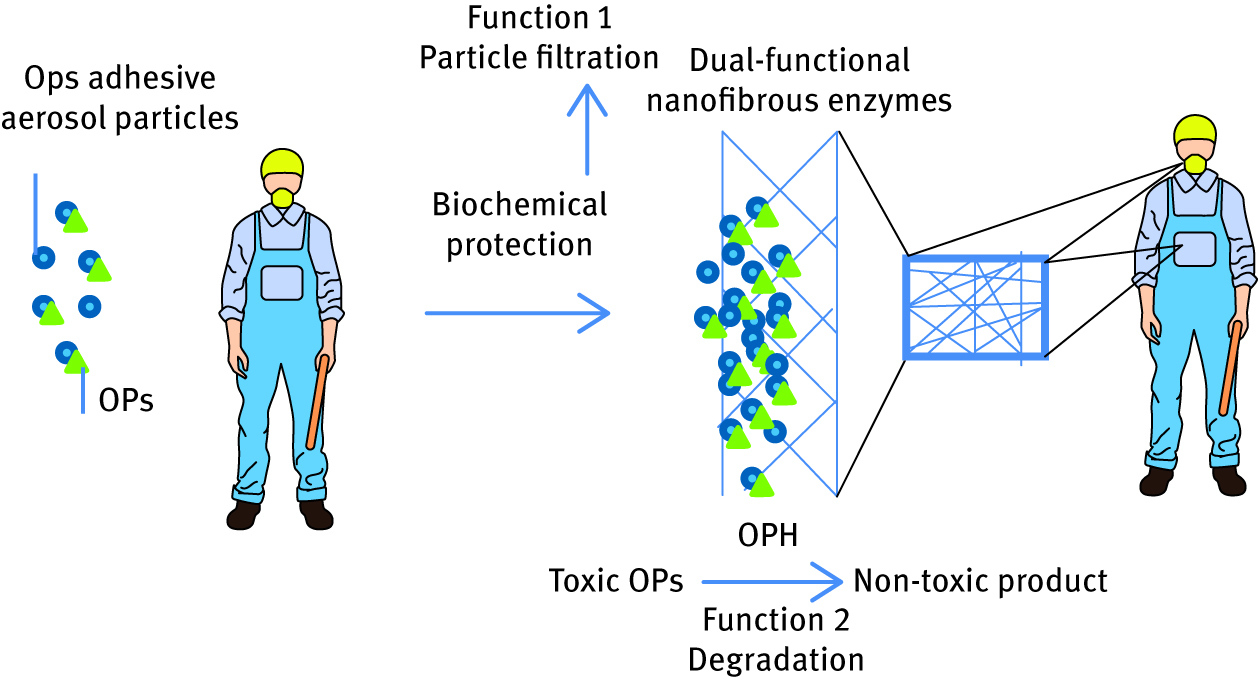
Schematic demonstration of double function of OP hydrolase-immobilized nanofibrous polymeric clothing.
Immobilization of OPH via the same technique – glutaraldehyde crosslinking – found also an application in bioremediation. Obtained in this way, OPH-polyester textiles are cheap in production and easy to handle. Immobilized enzyme has enhanced stability, has good activity in un-buffered water and is able to degrade even very low concentrations of organophosphate pesticides. Moreover, its reusability in batch or continuous processes demonstrates that this kind of polymer/enzyme structure has a potential in environmental bioremediation of contaminated water [34].
Noncovalent immobilization strategy was used to design a membrane that was able to immobilize even three enzymes at the same time [35]. This goal was reached by creation of flat sheet polypropylene membrane first coated with cellulose sublayer and application of simple pressure-driven filtration. By controlling of biofouling formation and driving it to form enzyme cake layers, obtained sheets maintain the activity of all three enzymes (formate dehydrogenase, formaldehyde dehydrogenase and alcohol dehydrogenase). This strategy provides a membrane that has an excellent potential application in multienzymatic cascade reactions, in this case bioconversion of carbon dioxide into methanol. The benefit coming from the strategy selected in this study is that there is no need to use organic solvents which very often are toxic and difficult to handle, and thus, may influence enzyme activity. To better understand the concept of performed immobilization, the new method was compared to sequential immobilization technique. Both options are shown in Figure 6.
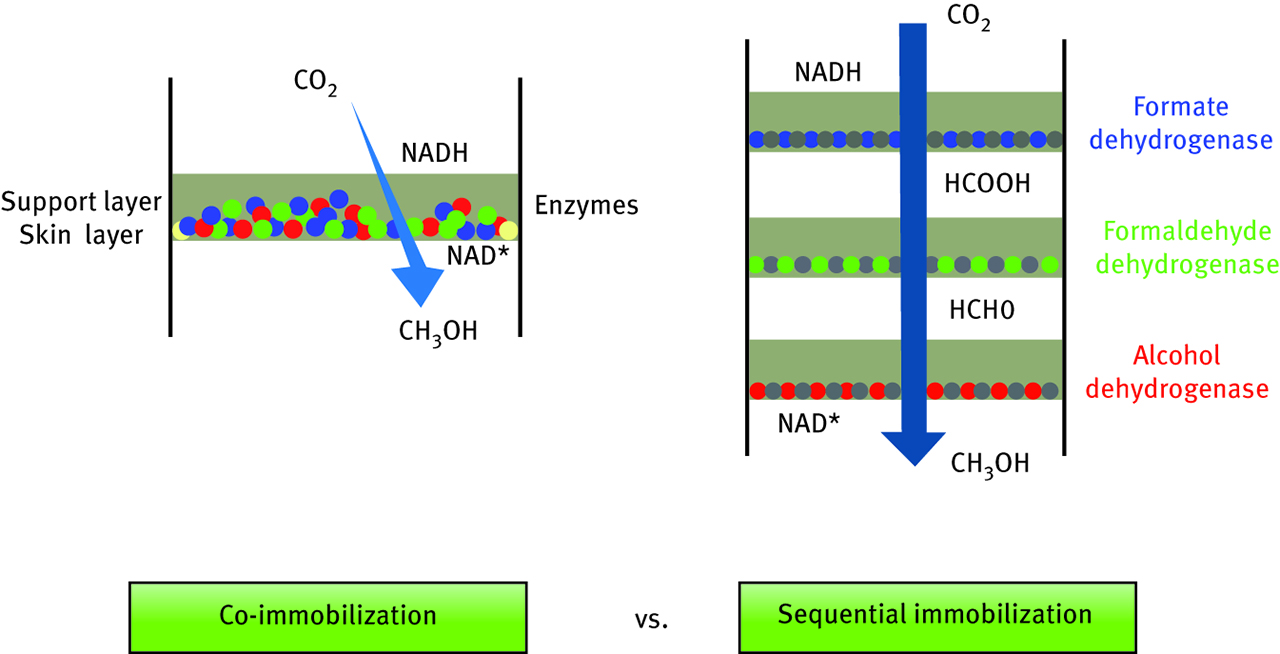
Schematic representation of two biomolecule immobilization mechanisms: pressure driven filtration-co-immobilization and sequential immobilization.
Another interesting point found by authors of this work is considering simultaneously two immobilization mechanisms. At first, enzymes are physically immobilized on a surface by adsorption, but some of the aggregates were also found inside of the membrane, which suggests also an entrapment of the biomolecules. Nevertheless, because of the fact that any of those techniques is not involved in the creation of new covalent bonds, enzymes are still maintaining their performance. However, results of CO2 conversion prove that the presented method requires additional modifications and improvements because of poor efficiency of carbon dioxide transformation, which is probably a result of insufficient space between the immobilized enzymes. Interferences coming from this fact cannot ensure proper reaction conditions for the following enzymatic reaction steps.
Most part of the research in this field is dedicated to enzyme immobilization for biosensing application, and the literature reports multiple examples of successful uses of this approach. The initiator of this branch of development was Thompson, who already in 1980 made a first step into novel material science expansion [36]. He used natural lipid bilayer with incorporated bioreceptors, which in living organisms play a role of biosensors. Thompson isolated this primal structure and immobilized on artificial polyamide support. By increasing the support stability, he made this sensitive structure handable and useful in biosensing applications. Since this moment, his idea was applied for immobilizing lipid bilayer on various supports and further modifying with proteins or artificial receptors, enzymes, antibodies, channel formers and carriers which play the role of transducers or analyte detectors. Possible determination of many psychoactive or toxic substances at very low concentration level makes biosensors have almost unlimited applications in our daily life. Very fast development of new bioelectronics makes a huge impact on progressing analytics, diagnostics, biomedicine and in all type of industries like agriculture and other industries. To draw an idea about how large is the application area of enzyme-based bioartificial polymers in biosensor production a few examples will be mentioned below.
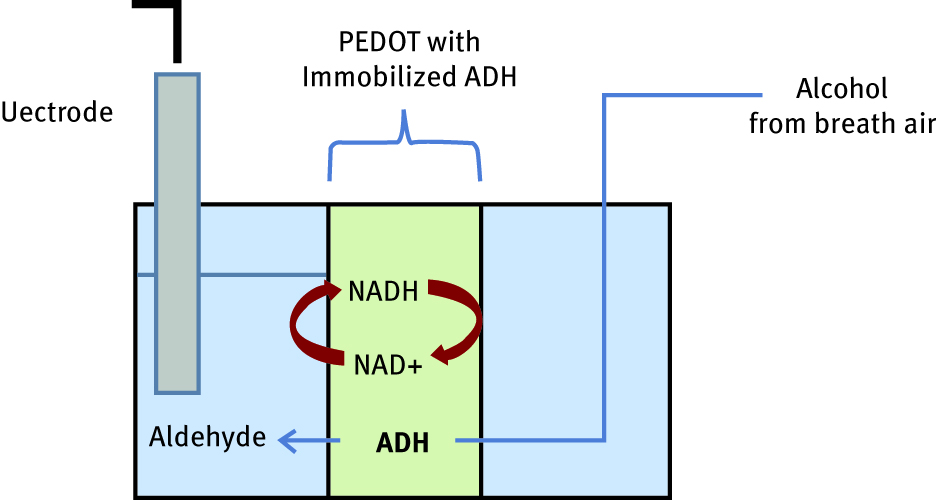
As suitable supports for enzyme immobilization are chosen electroconductive polymers which, thanks to its electronic properties, enhance the speed and sensitivity of biosensors [37]. Those kinds of conjugates are based on direct binding of biomolecule (biocatalyst) into the electronic device. Azak et al. and Ekiz [38, 39] e. g. developed a novel conducting polymer matrix electropolymerized onto the graphite electrode, to which glucose oxidase was covalently attached using glutaraldehyde crosslinking. Later, Ayranci used the same method to bond an enzyme to a metalo-polymer, ferrocenyldithiophosphonate [40]. These examples of hybrid materials found application in glucose detection biosensors. One interesting approach uses enzymatic degradation of ethanol by alcohol dehydrogenase, or alcohol oxidase, for the production of detectors for alcohol concentration in breath [41, 42]. In this kind of system, the reactions take place in the three-phase interface; thus, a perfect application in this field found breathable electrodes. To better understand the working principle of those equipment, a device developed by Winther-Jensen can be mentioned [43]. The group from Monash University immobilized alcohol dehydrogenaze [44] on a widely used biocompatible conductive polymer poly(3,4-ethylenedioxythiophene) PEDOT. Immobilization of biomolecule was performed by stuffing method, which is considered to be a gentle and noninvasive technique, potentially applicable for immobilization of biological elements. In the aforementioned method, biomolecule immobilization was enhanced also as an effect of shrinkage of material after additional post-polymerization coating. The schematic reactions occurring in this three-phase interface system and its application in the construction of breathable electrode are shown in Figure 7. Alcohol dehydrogenaze-immobilized PEDOT material is placed between two compartments – to the first one is collected the breath air, and the second is filled with a solution containing NADH (redox mediator), Ag/AgCl reference electrode and counter-electrode. As the alcohol vapour concentration in the first compartment increases, ADH in the presence of NADH converts it into the aldehyde and the reaction performance is amplified to a current response detectable by the electrode. Experiments show that enzyme maintained its activity and oxidizes alcohol efficiently.
Schematic representation of the breathable electrode based alcohol detector device.
Besides this fields, in a food industry alike agriculture and other life areas, gas sensors known as a electronic noses are also of great importance. They are devices that are designed to mimic human olfactory systems. This technology is a novel trend in “smell” sensors for a quick detection of vapour even at very low concentration. Possibility of using it in the determination of toxic gases in conditions that are dangerous for human health and life is an excellent and beneficial approach. An example of detector for formaldehyde (very harmful), widespread in environment volatile organic compound, was presented by Mitsubayashi et al. [45], who designed bio-sniffer stick based on a formaldehyde dehydrogenase enzymatic reaction. Dehydrogenase was mixed with functionalized poly(vinyl alcohol) and then immobilized on polytetrafluoroethylene (PTFE) chip. This chip was then placed on a PTFE/Pt electrode, which provides chemical stability and mechanical strength by maintaining flexibility of the device. Dehydrogenation of formaldehyde is performed at first by oxidized NAD+, next reduced NADH is oxidized as a result of reaction with PTFE/Pt electrode. Reactions occurring in the system are as follow:
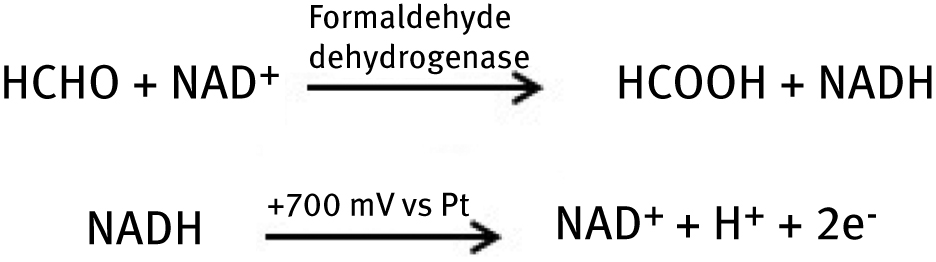
The fabricated biosensor exhibits high selectivity for formaldehyde recognition, and the detection limit is much lower than the human sense of smell; thus it presents a great perspectives for application in environmental monitoring. This is only one of many examples of the human sense reflection in electronic devices. Fast development of biosensor market allowed creating various apparatus like electronic tongue or stimuli-sensitive artificial skin. Fabrication of bioinspired enzyme-based materials is a revolution in nanotechnology and continuously delivers new tools with untold number of applications.
3.2 Polysaccharides
Polysaccharides are complex carbohydrates formed from at least two monosaccharides linked by glycosidic bond. In living organisms, they serve as energy storage and building functions. They are widespread and easy to obtain from natural sources. They possess a variety of structural and chemical properties, and they can be long chain or short chain, water soluble or insoluble and exhibit different functionalities. What is also the most important in terms of use it for bioconjugates synthesis, is their non-toxicity and environmental friendliness.
Polysaccharides found application in production of affinity membranes [46, 47]. The filtration mechanism through these kinds of membranes leads to adsorbate capturing (depending on the case; it might be for example viruses, endotoxins, bacteria or heavy metals) onto functionalized material surface, while the feed is passing through it. Rejected substance can be even smaller than the pores present in a membrane, but even though, due to big affinity to the “capturing” area, it will get adsorbed. For example, the pore surface might be functionalized with positively charged active group and within electrostatic interaction adsorb negatively charged ones from the feed [48] (Figure 8).
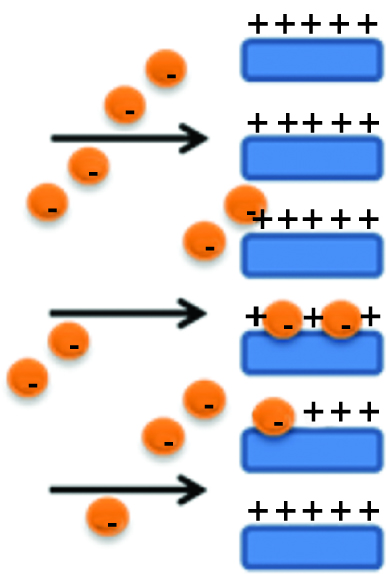
Scheme of affinity membrane separation mechanism.
These kind of membranes are characterized by low pressure drop and thus low energy consumption, high efficiency and high fluxes and can be easily regenerated. Various examples of the use of polysaccharide/polymer composite concern chitosan, a natural amino polymer extracted from chitin. As it is positively charged at low pH, it found application in development of materials which exhibit big affinity to removal of negatively charged species, such as heavy metals, across nanofibers or membrane contactors (chitosan//polyvinyl alcohol) reported by Prateek Khare et al. [49] or through membranes (PET/chitosan membrane) described by Li [50]. One of the interesting approaches of implementing this polysaccharide into artificial polymeric matrix is artificial liver development studied among others by Teotia [51]. They designed a system composed of polysulfone/tocopheryl polyethylene glycol succinate (Psf/TPGS) composite hollow fibre membrane, which is coated with chitosan. The coating procedure was performed by introducing first negatively charged groups into the polymer composite (sulfonation), which facilitated electrostatic interaction with amine groups of chitosan. The surface coated with polysaccharide exhibits biocompatibility and supports growth of cells (HepG2 cell line), thus mimicking liver-specific functions. This particular approach perfectly shows advantages of mechanical and chemical stability of synthetic Psf/TPGS membrane and biofunctionality of natural chitosan, which makes this polymer/polysaccharide conjugate a good potential material for artificial liver application.
Tissue engineering is another focus of scientists around the world who keep on looking for new ways to develop materials able to mimic biological bone tissues, facilitating its regeneration and simultaneously maintaining good mechanical stability. In this approach, polysaccharide-based composites are applied in a membrane configuration, like cellulose, alginate and chitosan [44, 52, 53], which supports tissue regeneration and makes permeable platform for new cells growth. Because of its ability to adopt different shapes and well adapt to hydrated environment, hydrogels are a type of polysaccharide composites that have been widely studied recently [54, 55, 56]. This group of highly hydrophilic polymeric materials is able to accumulate large amount of water and thus can form flexible, three-dimensional structures[57]. Examples of chitin/PCL and chitin/PCL/nHAp microgel developed by Arun Kumar et al. [58] explore the potentiality of its use in engineering of bone tissue in inaccessible places, like skull. Moreover, hydrogels due to its unique structure can incorporate drugs and other growth factors, which accelerate tissue regeneration and the most important – its degradation products are completely biocompatible. Nevertheless, although the easiness of handling and good rheological properties are remarkable, poor mechanical stability is undoubtedly the main disadvantage of hydrogels. However, this handicap is recently improving by mixing polysaccharides with natural minerals. This is the case of composite of less explored natural polymer which is pullulan. Developed by Amrita et al., hybrid fibres containing poly(β-hydroxybutyrate) (PHB) and hydroxyapatite (naturally occurred mineral), when combined with pullulan, enhance the mechanical stability of hydrogel even 10 times [59].
Chitosan has also shown potential as a flame retardant coating material. Laufer et al. applied its properties in coating polyurethane foams and polylactic acid (PLA) film [60] using layer-by-layer (LbL) assembly method. Positively charged chitosan was used to construct a multilayered film by convertible deposition of negatively charged mineral montmorillonite. The good performance of formed “nanobricks” presents a promising alternative for substituting halogenated flame retardant materials for environmentally friendly eco-coatings.
Another polysaccharide having big industrial importance is cellulose. This material found application not only in food packaging industry or as a biomedical device component but its interesting mechanical and optical properties give light on new potential functions. In the literature, various examples can be found of the use cellulose/synthetic polymer composites for construction of optoelectronic devices such as light diodes, flexible electrodes, biosensors or optical displays [4, 61, 62, 63, 64]. Shaukat Khan et al. prepared PEDOT/PPS/cellulose composite membranes by ex situ incorporation of those conductive polymers into bacterial cellulose pellicles. A similar approach has been achieved by Marins et al. who polymerized polyaniline doped with dodecyl benzene sulfonic acid onto bacterial cellulose sheets [65]. One year later, Müller et al. applied the same method by polymerizing polyaniline onto cellulose nanofibers [66]. Those novel conductive materials characterize outstanding stiffness and strength followed by biodegradability and renewability. Those properties make polymer/cellulose conjugates a material to be used for the production of intelligent clothes e. g. for health-monitoring system like heartbeat recording, body temperature control, body odour control, hemostatic materials and plenty of other tremendous possibilities [67, 68, 69, 70].
Cellulose is used also to produce other smart - shape memory materials, which deformed to temporary configuration are able to recover their original form as a result of response to some external stimulating factor. In the case of thermo-responsive shape memory materials, this factor is temperature. The original form of material can be first modified by applying external force and temperature above transition temperature and then, if the material is cooled down without releasing external force, a temporary shape will be fixed. To recover the original shape, the only thing needed is again heating up the device above transition temperature. Auad et al. [71] demonstrate reinforcement of thermoplastic polyurethane after confinement of small concentration of cellulose nanofibrils. Those kind of materials have potential application in medical devices; however, very often the temperature needed to induce shape change is not applicable for use in human body. This handicap has been overcome by Bai et al. [72], who applied ethyl cellulose/polycaprolactone (PCL) conjugate in order to adjust transition temperature to human body friendly (around 40°C). This interesting strategy lies in a design of water-activated rapidly switchable shape memory effect, reported by Zhu et al. [73] in cellulose nano-whiskers/thermoplastic polyurethane. They used the unique properties of a percolation network in nanocellulose which in combination with elasticity of a polymer lead to temporary shape fastening and original form quick recovery when wetted (Figure 9).
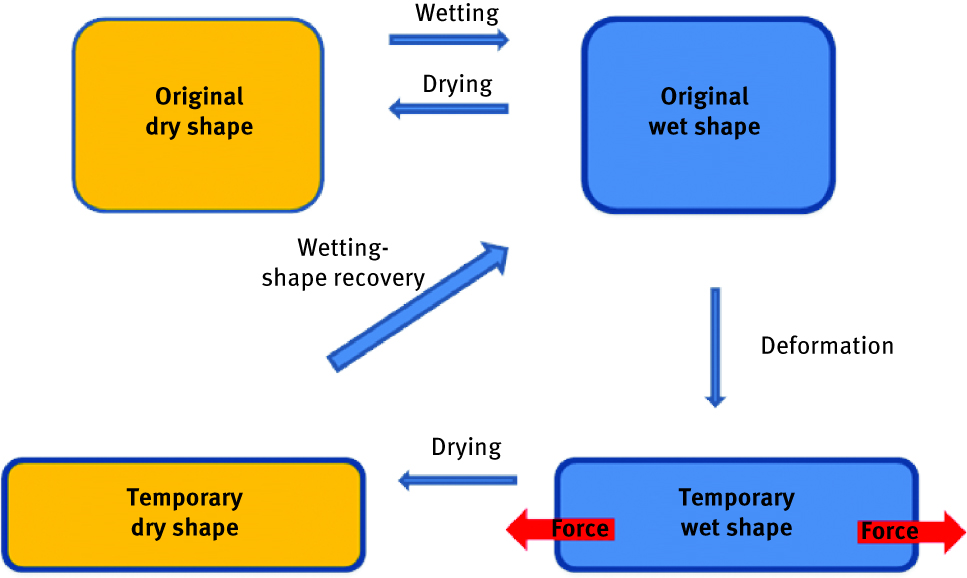
Schematic representation of water activated switchable shape memory effect mechanism.
Later, Liu et al. [74] applied a similar strategy in microcrystalline cellulose/poly(D,L-lactide) composite. This recent enhancement has an unprecedented impact on development of novel smart materials with shape memory functions with innumerable and inestimable potential. Moreover, polysaccharide availability and easiness of handling and treatment continuously force the researchers to explore new properties of natural polymers and find still new applications.
3.3 Channels
Other biomolecules widely used in the field of bioartificial composites are ionic channels. Ion channels are proteins that catalyse the diffusion of ions across the biological membranes and thus are responsible for maintaining electrochemical balance between cell and its surrounding. Since the cell membranes are electrical insulators, ionic channels function as a selective gate which opens for some of the molecules allowing them to pass into the cell and due to conformation change closes to the others [75]. Because of the type of factor which impact its “gating” motions, ion channels can be classified into different groups.
One of the transport mechanisms occurs when the ligand attached to the channel binds the ion and then, because of conformational change, allows it to pass through that formed door, according to the concentration gradient. That type of receptor is called extracellular ligand-gated ion channel and it is present in brain and muscles. Voltage-gated ion channels are activated due to the change in membrane electrical potential. Those proteins are well-known potassium and sodium ion channels and also calcium channel and proton channel. When the signal influencing conformational change in a protein structure comes from the interior of the cell, this channel is called intracellular ligand-gated ion channel, e. g. cystic fibrosis transmembrane conductance regulator which is ATP-gating anionic channel. Understanding completely all of these mechanisms leads to proper design of bioartificial conjugates with very specific functions and destiny. Ion channels are proteins with incredible importance in many biological processes that cause rapid changes in cells, like muscle contraction, selective transport of nutrients and ions or hormone release. They work very specifically and its characteristic selectivity is a result of responsive conformational changes which then regulate the ionic transport [75]. Because of its extraordinary properties, they found a huge interest in developing new polymeric hybrid materials, mainly for biomedical and biosensing applications. Since ion channel functionality is related to transportation, their properties are mostly applied in biomimetic membrane technology.
To incorporate this biomolecule into artificial polymeric matrix with maintaining its proper functionality and without affecting the structure, it is desirable to reflect its natural environment, the most possibly similar to the one in the cell membrane. To cope with this goal, scientists tended to synthetize materials able to mimic those present in living organism lipid bilayer. The most important criterion was obtaining materials which have high thermal and chemical long-term stability and mechanical durability but simultaneously exhibited specific flexibility which would provide a proper free space necessary to perform ionic channel conformational changes. The great alternative to cope with this challenge is synthesis of self-assembly block copolymers which, in combination with bioactive component, offer variety of possibilities to create highly organized supramolecular structures. Because of its dual nature, those synthetic amphiphiles provide large variability of possible architectures. They are built from hydrophobic and hydrophilic blocks which are organizing spontaneously in the solution, similar to the natural lipid bilayers present in cellular membranes. The layer binding occurs through weak noncovalent interactions like van der Waals forces, hydrophobic interactions or hydrogen bonds, and thus they are flexible and can adapt to diverse topologies. As an example, Zhang et al. [76] synthetized an amphiphilic block copolymer (poly(butadiene)52-blockpoly(ethylene oxide)) in which functionally incorporated model membrane channel protein alpha-haemolysin, responsible for transport of little molecules like ions, nutrients or antibiotics. This innovative approach coped with the challenge of incorporating biomolecule in a large area in a stable, supported artificial tethered solid-supported membrane, which provided a novel material with potential applications in drug screening, biosensing or trace analysing. Those kinds of active self-assemblies can be organized in a shape of membrane, micelles, tubes or vesicles. An important factor regulating successful protein ion channel incorporation into artificial surface was discussed by the research group of Meier [77], who studied in detail protein interaction with the support and events which can lead to deactivation or affect a structure. Their work concerning preparation of well-organized hybrid ion channel/artificial polymer structures resulted in the production of supramolecular assemblies. Hydrophobic polymer polydimethylsiloxane (PDMS) plays a role in the plasticity maintenance of structure in contact with solid support, while hydrophilic component – PMOXA – is separating ion channel from the direct contact with the base material. The same group from Basel proposed the modern concept of nano-machines, based on the combination of functionality and adjustability of amphiphilic copolymers and powerful biological activities [78]. Designed by them, nanoreactors connect enzymatic operation with function of ionic channel/polymer conjugate. Enzyme was encapsulated in amphiphilic poly(2-methyloxazoline)-block-poly(dimethylsiloxane)-block-poly(2-methyloxazoline) (PMOXA6−PDMS44−PMOXA6) copolymer with incorporated outer membrane protein F (OmpF) ion channel, which additionally modified, acts like a stimuli responsive gate- opening and closing dependently on the pH (Figure 10). This strategy is a big step leading to create robust and stable capsules, which can serve as a device to entrap various biological components, and apart from that, the concept of regulating membrane shell permeability by introducing stimuli responsive ion channel is a step forward to optimize nanoreactors in terms of the size of transported species and type of reaction performed.
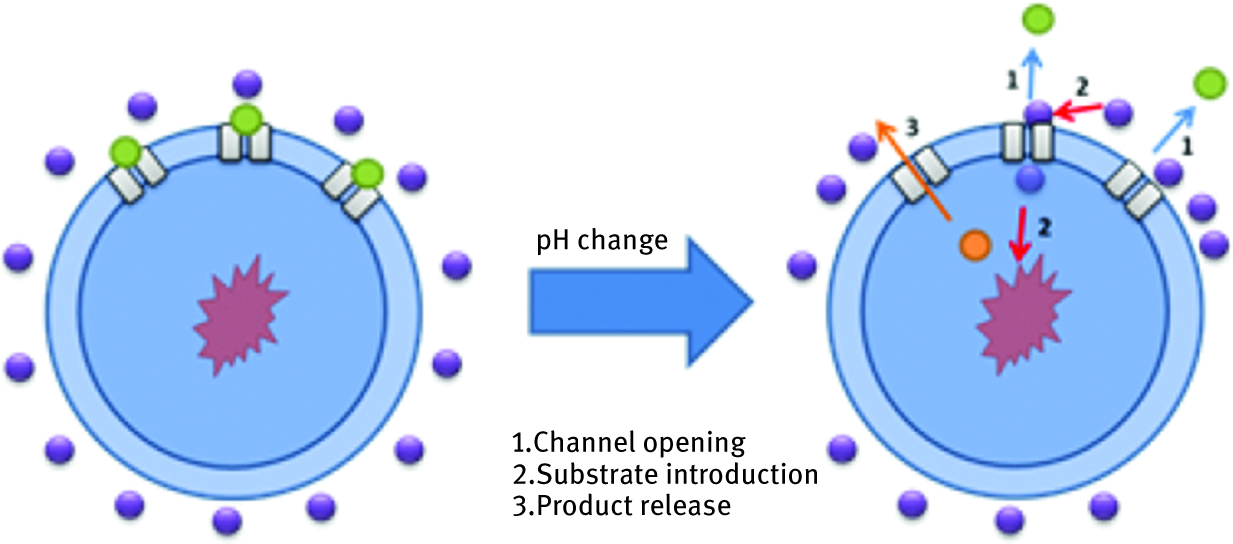
Scheme of the nanoreactor working mechanism.
Ion channels are perfect candidates to be used in applications related to ion exchange membranes and technologies as fuel cells or energy conversion systems. In the literature, we can find various examples of synthetizing polymers and copolymers with incorporated biological channels for this aim [79]. Aquaporins attract great interest in researchers because of its enhancing water flux properties [80]. This membrane water channel is bidirectional, which means that it regulates flow of water entering and going out of the cell. Aquaporins are composed of four monomers assembled in a way that the channel is narrow in the middle and wider at the ends, so allowing the passing of water molecule chain, but rejecting bigger solutes. Moreover, the internal local electrostatic field pushes the water molecules to rotate, which enhances the transport. These unusual properties depict aquaporin molecules as an excellent candidate for potential use for increasing water passage in water treatment membranes. Since aquaporin works in two directions, its orientation in a membrane film is not crucial for the membrane performance. This convenience allowed applying various techniques of its incorporation. In the literature, one can find examples of LbL immobilization [81], active layer formation [82, 83] or covalent bonding to lipid bilayer [84]. Improvements in gradient-driven water diffusion in ion channel containing membrane designs have been discussed wider in recent reviews [85, 86]. Since the incorporation of aquaporin focused mainly on the water flow increase, random organization of channels did not affect significantly the attainment of the goal. However, for selective ion transport, confinement of channels requires the highest possible organization of biomolecule. But still, together with increase of transport properties, it is difficult to maintain the high specificity of incorporated ionic gates, which is often related to the lack of control during the immobilization process. This challenge is continuously a main problem for researchers who face difficulties with assembling ion channels in the desired orientation along the membrane. The study on a well-controlled immobilization of biological channels within confinement in a precisely shaped and sized nanopores of polymers like PC and PET was performed by Balme et al. [87, 88, 89]. They studied the controlled incorporation of avidin, streptavidin and amphotericin B in track-etched membranes and also successfully incorporated gramicidin ion channel improving both flux and selectivity of polymers. Gramicidin was also reported as an biomolecule used for reverse osmosis desalination enhancement [90]. This protein, selective for monopositive ions in combination with polymeric membrane, increased water flux ameliorating desalination efficiency. In this approach, the most important factor, which decided the novelty of applying this particular molecule, was high resistance in the presence of big hydraulic pressure, which is standard operation condition during reverse osmosis process.
Apart from overcoming water scarcity problem, biological channel-based biomimetic filters have good potential in recently developing application which is membrane crystallization technique [91]. Crystallization is a widely used separation technique in a chemical industry in which a crucial parameter is the ability to reproduce crystals with the desired, strict characteristics. However, traditional methods assimilate many disadvantages which hamper reaching this goal. Not exact agitation and nonhomogenous solvent–nonsolvent distribution in traditional evaporators results in poor crystal recurrence and thus low efficiency of the process. Furthermore, high energy consumption related to this technology drives for looking for alternatives. Membrane crystallization is definitely a more economic approach, which applies membrane technology to produce well-controlled crystals that grow gradually from unsaturated solution. Aquaporin biomimetic membrane found an application in this technology [92]. Significantly enhanced water passage through the protein channels incorporated in the commercial polymeric membrane (AIM60 FO membrane from Aquaporin InsideTM) constitutes an interesting alternative for use in sodium carbonate crystallization systems in carbon dioxide capturing purpose.
Energy storage and conversion, solutions for energetic crisis and water scarcity, biosensing technology, tissue engineering, drug industry and models of understanding the mechanisms occurring in biological systems are invaluable gains from biomimetic ion channels/polymer materials. Continuous interest and growth of ideas, strategies and methods of functional channel immobilization make those biomimetic materials very special implement in membrane technology and other fields.
3.4 Nucleic acids
Nucleic acids, which include DNA and RNA, are composed of a pentose sugar, a phosphate group and a nitrogenous base. They fulfil a function of encoding and transmitting genetic information in all living organisms. Because of extremely specific interactions between nucleic acids in DNA helix (Watson–Crick base pairing AT and GC affinity), those components provide invention of highly programmable materials which are very useful biotechnological and biomedical tool. Development of amphiphilic bioinspired polymers which are able to self-organize in a form of various shapes, as mentioned before, is widely applied to separation of nucleotides, encapsulate biomolecules and drugs. Amphiphilic phenomenon is also used for fabrication micelles and solid lipid nanoparticles which have been broadly reported as efficient drug carriers (see the chapter “Recent advances in Bioartificial Polymeric Materials Based Nanovectors”). Combination of nucleic acids and synthetic elements allows production of materials which possess excellent information storage capability. Unusual properties of artificial matrix is an outset to create constructs with many possible assemblies and related to these functions. DNA can be conjugated with hydrophilic or hydrophobic polymers by covalent attachment. The strategies applied for this purpose is click chemistry coupling, Michael addition, amide bond formation, phosphoramidate linkage and others [93, 94, 95, 96]. The challenging factor of this tactics is the difficulty related to common DNA denaturation caused by harsh reaction condition. Even though covalent methods introduce some limitations and various strategies to overcome this fact were applied, many examples of covalently conjugated materials with variety of applications are reported [97, 98].
As described before, amphiphilic properties of synthetized conjugates lead to obtain specific properties which allow the formation of nanoscale components. In case of copolymers being a base to ion channel immobilization, the whole amphiphilic constituent was artificial. However, for DNA, the only artificial element needed to create amphiphile is hydrophobic polymeric tie, while the other part is made by nucleic acid itself. Properties of those designed composites permit engineering structures in various shapes, related to their particular and unique properties. They can be organized in the shape of micelles, when hydrophobic polymeric tie is directed to the interior of the sphere and hydrophilic DNA is turned to the exterior, forming a core. The same single amphiphilic layer can form elongated shape of tubes; however, double layer can be arranged in vesicles. In vesicles, both interior and exterior are hydrophilic, but the middle layer is built from hydrophobic polymeric chain. All of the morphologies are presented in Figure 11. Also, possibilities to engineer the structure in a very controlled way allow the design of a variety of architectures, e. g. so-called star polymers [99].
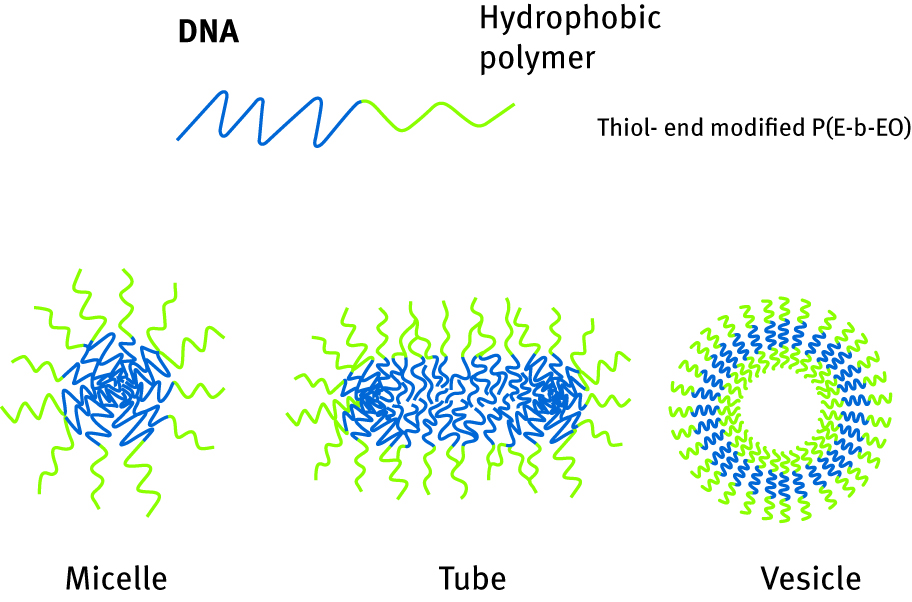
Scheme of DNA/hydrophobic polymer conjugates morphologies.
Even though polymers conjugated with DNA have found application mainly in drug delivery systems, a wide range of possibilities offered by those structures found other applications with a great impact on a technology expansion.
Because of the high organization level of nucleic acids, they may be used in the organization of other macromolecular constructions [100, 101, 102]. Going along with the capability of DNA/polymer conjugates to bind to other particles, the range of its applicability is widening, e.g. in combination with magnetic nanoparticles they could be used for magnetic resonance imaging [97]. With extremely advanced specificity of binding and the highest level of molecular recognition, they are also perfect tools for diagnostics and analytic assays, because nucleic acid sequence identification in real time allows recognizing genetic mutations or monitoring gene delivery.
DNA-grafted polymeric probe reported by Gaylord et al. has potential as a detector for DNA, proteins and other biological molecules. For this approach, properties of conjugated polymers were studied [103]. Conjugated polymers are the class of compounds, which, due to the presence of delocalized electrons in their structure are fluorescent and able to interact with light. In proposed assay, detection mechanism bases on a Forester energy transfer effect, which occurrs between two chromophores. Schematic representation of those interactions is presented in the Figure 12. Conjugated polymer (poly(9,9-bis(6-N,N,N-trimethylammonium)-hexyl)-fluorene phenylene)), called donor chromophore, is placed in a solution with peptide nucleic acid which has exactly determined sequence, called acceptor chromophore- previously labelled with a chromophore dye. When the donor chromophore enters in an excitation state, it transfers the energy to the acceptor chromophore within dipole–dipole interaction. Thus, the smallest is the distance between donor and acceptor, the more efficient is energy transfer and thus the optical properties of both polymer and peptide need to be adjusted in a way that they would favour FRET. When conjugated polymer is in the solution with noncomplementary nucleic acid strand, electrostatic interactions are too weak and the distance between strands is too large for efficient FRET effect. When a complementary single-strand DNA appears, occurs hybridization with present peptide nucleic acid and as a result, a donor chromophore gains multiple negative charges. This local higher charge density enables electrostatic interactions with conjugated polymer, which decrease the distance between donor and acceptor activating FRET.
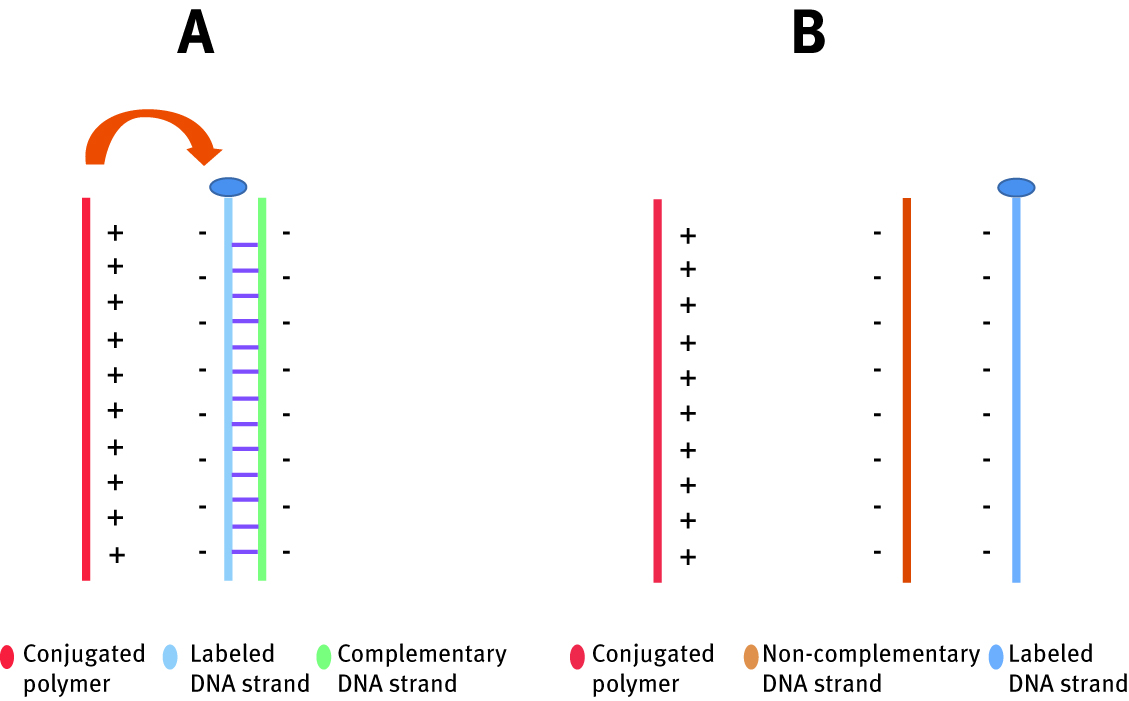
Schematic representation of interaction between conjugated polymer and DNA strand in the presence of complementary DNA strand (A) and non-complementary strand (B).
The accessibility of nucleic acid/polymer conjugate allows very high, characteristic specificity and can be used in DNA diagnostics even in very low concentrations. Moreover, recent progress in modification of DNA creates new perspectives for novel composite materials which exhibit biocompatibility, adopt various shapes and morphologies (capsules, vesicles, hydrogels etc.) and are important implement for biorecognition approaches.
3.5 Cells
The basic structural unit of an organism is a cell. This macromolecule can reproduce independently, is filled with cytoplasm, contains organelles and genetic material and is surrounded by semi-permeable membrane. Immobilization of cells is a proponent in the creation of so-called “living composites.” Those types of synthetic polymeric materials contain immobilized live microorganisms, and its applications vary from medicine to agriculture or food industry.
Use of free cells in technological spheres is quite widely described [104, 105, 106]. However, immobilized microorganisms have a lot of advantages over their nonimmobilized forms. Thanks to attachment to artificial polymers, it is possible to separate them from environment, protect its biological activity and control its release. For encapsulation of microorganisms, polyvinyl alcohol (PVA) is widely used. To better control or even stop release, different blocking strategies have also been studied [107]. There are also many examples of entrapping bacteria for tissue engineering or biosensing approaches in various types of hydrogels [108, 109, 110]. Their high water content and elasticity make them perfect candidates as a scaffold matrix for growing cells and entrapment of live or mobile microorganisms and also drugs (look at the chapter “RECENT ADVANCES IN BIOARTIFICIAL POLYMERIC MATERIALS BASED NANOVECTORS”). Those gel crosslinked cells are introduced to degraded tissue and, while growing, they induce polymer fibres to degradation, allowing the growth of new tissues [108] (Figure 13).
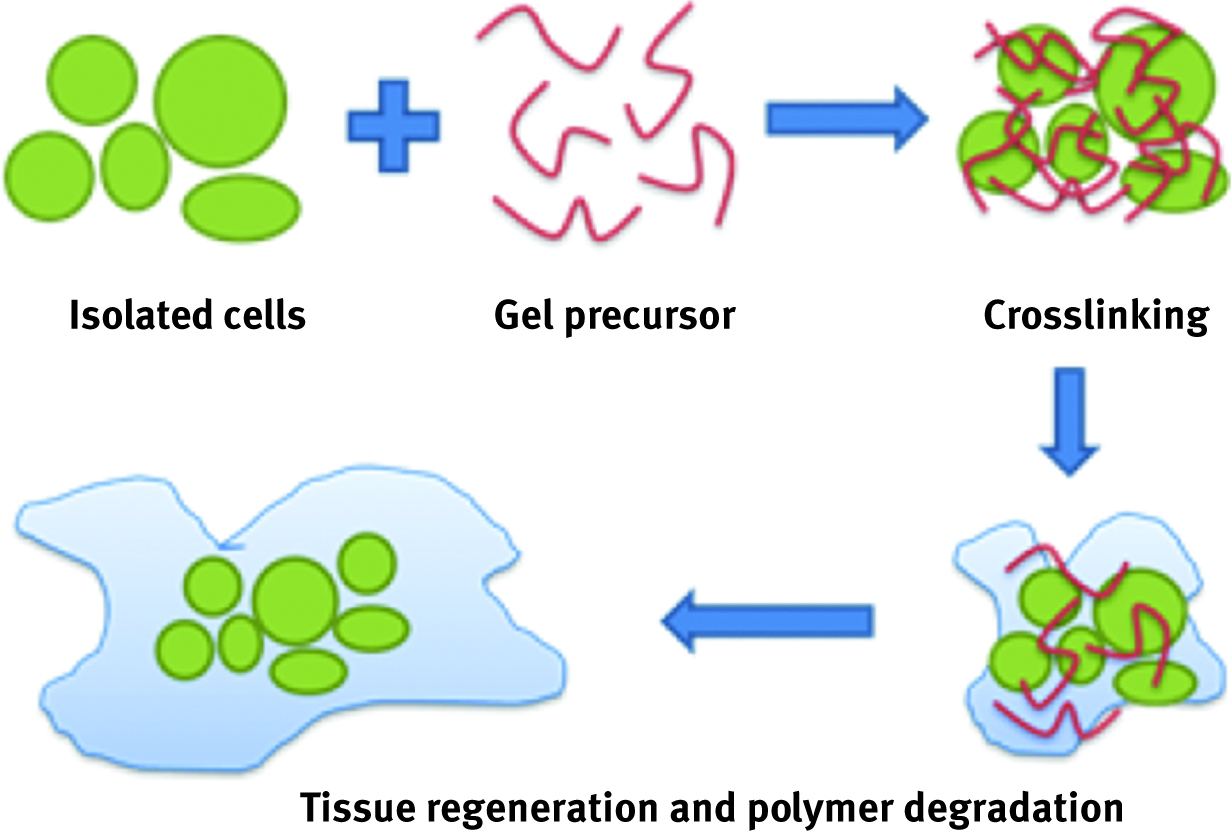
Schematic representation of a new tissue cell growth mechanism over the crosslinked gel.
Synthetic polymers that found application as a tissue engineering biodegradable matrix are, for example PEG or PLGA, used for entrapment of human bone and embryonic stem cells [111, 112, 113]. However, limited diffusion abilities of hydrogels put some restriction in their application in medicine and thus resulted in developing new research interests which are colloidal carriers called nanoaggregated (see the chapter “RECENT ADVANCES IN BIOARTIFICIAL POLYMERIC MATERIALS BASED NANOVECTORS”). PEG hydrogels are used also in cell biosensors for environmental monitoring, drug screening and diagnostics, as a captor of E. coli or murine fibroblasts [114, 115].
Cell immobilization, as in the case of other biomolecules, is related to some limitations, which in this case is construction barrier. Due to cultivation method and conditions, researchers perform usually two-dimensional growth of the culture. Nevertheless, this challenge has been overcome recently, again with the help of polymeric hydrogel. Flexibility of those provide creation of tridimensional cell-entrapping structures which are potential tool for progress in tissue engineering and biotechnology [109].
Immobilization of microbial cells is also considered to be a potential solution in terms of bioremediation and for removal of pollutants in soil and water. PDMS, PVA, latex copolymer, PLA, PU/PC and many other polymers are used as encapsulants for biomolecules. However, some of these materials, although commonly used, are poorly applicable in the bioremediation field because of its water solubility [116] or biomolecule activity limitations. Lately, another way to entrap cell biomaterial is the production of electrospun polymeric fibres [117, 118, 119]. In this approach, design of the cell/polymer composite provides significant improvement. In order to last longer, for a predetermined period of time, biomolecules need to be entrapped in a water-insoluble material which still allows for mobility of the cell. To do it, Klein et al. immobilized cells of Escherichia coli, Pseudomonas ADP and Pseudomonas putida S12 in an electrospun hollow fibre PCL fibres [119]. From the performance of the composed fibres, it can be seen that design needs to be enhanced even though the study shows that denitrification activity in the fibres is maintained. A similar approach was repeated by Knierim who uses a similar strategy to encapsulate bacteria in PVA and then cover the shell with hydrophobic poly-chloro-p-xylylene (PPX) [116] and letnic, closing yeast cells in PVA and cover it with PVDF [118]. This newly developing method is a potential way to support bioremediation material challenges, and the principle of the idea is presented in Figure 14.
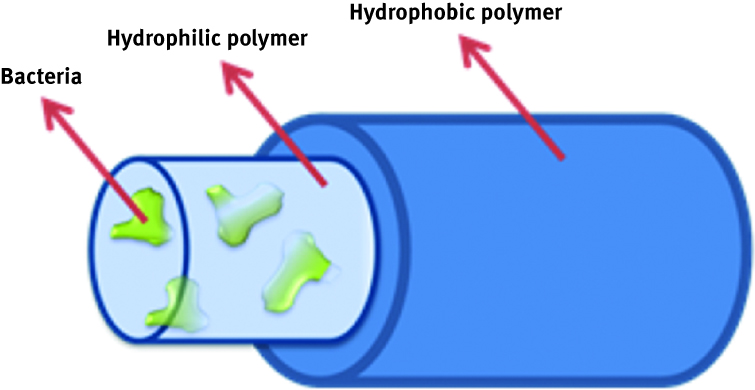
Scheme of a composite nanofiber.
Many examples of applying nanofiber technology (especially as blends with chitosan) in drug entrapment for tissue engineering approaches are mentioned in the following chapter. The immobilization of living cells is a challenge, but a variety of applications and benefits which it can bring over the “free cells” use approach drive researchers to find new methods for efficient entrapment with maintaining biological activity. Biodegradable, synthetic polymers with adjusted or modified properties are promising devices to settle numerous problems and inconveniences related to fixing microorganisms, and new methods are continuously developing.
4 Summary
Nowadays, the polymer science has an impact in practically all life areas. Countless benefits coming from the usage of materials with high mechanical and chemical resistance, a variety of functionalities and potentiality of modification drive to the development of new application fields. A novel approach of combining these synthetic substances with biomolecules leads to obtaining multifunctional hybrid conjugates which implement bioactivity of natural component with outstanding properties of artificial polymer. Over the decades, an immense progress in bioartificial composites domain allowed to reach a high level of knowledge in terms of natural-like systems engineering, leading to diverse strategies of biomolecule immobilization. Together with different available options, including covalent and noncovalent attachment, come various challenges, related mainly with maintaining the biological activity of fixed molecules. Even though the amount of applications that achieve commercial status is still not substantial, and is expanding continuously in disciplines like “smart materials,” biosensors, delivery systems, nanoreactors and many others. A huge number of remarkable developments reported in the literature present a potential of bioartificial conjugates as a fabrics with highly controllable structure and multiple functionalities, serving as a powerful nanotechnological tool. This novel approach brings closer biologists, chemists and engineers, who sharing their effort and complement the knowledge can revolutionize the field of bioartificial polymer science.
Acknowledgment
This article is also available in: Tylkowski, Polymer Engineering. De Gruyter (2017), isbn 978-3-11-046828-1.
References
1. Yoshida R. Self-oscillating polymer gels as novel biomimetic materials. In: Ngo TD, editors. Biomimetic technologies: principles and applications Vol. 82. Osaka, Japan: Woodhead Publishing, 2015:181–198.10.1016/B978-0-08-100249-0.00009-4Search in Google Scholar
2. Breslow R. Artificial enzymes. Wienheim: Wiley-VCH Verlag, 2005.10.1002/3527606645Search in Google Scholar
3. Qigang Wang ZY, Zhang X, Xiao X, Chang CK, Xu B. A supramolecular-hydrogel-encapsulated hemin as an artificial enzyme to mimic peroxidase. Angew Chem Int Ed. 2007;46(23):4285–4289.10.1002/anie.200700404Search in Google Scholar PubMed
4. Hu Weili, Shiyan Chen, Zhenhua Yang, Luting Liu, Huaping Wang, et al. Flexible electrically conductive nanocomposite membrane based on bacterial cellulose and polyaniline. J Phys Chem B. 2011;115(26):8453–8457.10.1021/jp204422vSearch in Google Scholar PubMed
5. Gu Y., et al. Biomimetic sensor based on molecularly imprinted polymer with nitroreductase-like activity for metronidazole detection. Biosens Bioelectron. 2016;77:393–399.10.1016/j.bios.2015.09.060Search in Google Scholar PubMed
6. Dudley MF. Process for the preparation of synthetic rubber latex 1966. Google Patents.Search in Google Scholar
7. Fryling CF. Production of adhesives and adhesive bases from synthetic rubber latex by causing phase inversion with a protective colloid and adding organic solvent 1956. Google Patents.Search in Google Scholar
8. Mielzynski Felix C, Zbikowski T. Hair darts for implanting in live or artificial media 1961. Google Patents.Search in Google Scholar
9. Okazaki M, Sone M, Ueyama Y. Synthetic fibers for artificial hair and production thereof 1974. Google Patents.Search in Google Scholar
10. Rodeheaver GT, Thacker JG, Edlich RF. Mechanical performance of polyglycolic acid and polyglactin 910 synthetic absorbable sutures. Surg Gynecol Obstet. 1981;153(6):835–841.Search in Google Scholar
11. Mohammad F. Specialty polymers materials and applications. New Delhi: I. K. International Publishing House Pvt. Ltd., 2007.Search in Google Scholar
12. Yu L.S., et al. New polyurethane valves in new soft artificial hearts. ASAIO Trans. 1989;35(3):301–304.10.1097/00002216-198907000-00040Search in Google Scholar
13. Khomyakov KP, Virnik AD, Rogovin ZZ. The prolongation of the action of pharmaceutical preparations by mixing or combining them with polymers. Russ Chem Rev. 1964;33(9):462–647.10.1070/RC1964v033n09ABEH001468Search in Google Scholar
14. Brady Dean JJ. Advances in enzyme immobilization. Biotechnol Lett. 2009;31:1639–1650.10.1007/s10529-009-0076-4Search in Google Scholar PubMed
15. Bowie JU. Solving the membrane protein folding problem. Nature. 2005;438(7068):581–589.10.1038/nature04395Search in Google Scholar PubMed
16. Jungbauer A, Kaar W. Current status of technical protein refolding. J Biotechnol. 2007;128(3):587–596.10.1016/j.jbiotec.2006.12.004Search in Google Scholar PubMed
17. Ying Liu, Fang Wang. Method for preparing S-ibuprofen and S-ibuprofen ester by biological catalysis 2008. Google Patents.Search in Google Scholar
18. Gekas VC. Artificial membranes as carriers for the immobilization of biocatalysts. Enzyme Microb Technol. 1986;8(8):450–460.10.1016/0141-0229(86)90046-3Search in Google Scholar
19. Nabeshima HHS, Fujimoto M, Tsuneyuki Nagase T. Enzyme immobilization with pullan gel. Osaka, Japan: Sumitomo Chemical Company, Limited, 1981.Search in Google Scholar
20. Sekhon S.S., et al. Immobilization of para-nitrobenzyl esterase-CLEA on electrospun polymer nanofibers for potential use in the synthesis of cephalosporin-derived antibiotics. Mol Cell Toxicol. 2014;10(2):215–221.10.1007/s13273-014-0023-xSearch in Google Scholar
21. Elnashar MMM. The art of immobilization using biopolymers, biomaterials and nanobiotechnology. In: Elnashar PM, editors. Biotechnology of biopolymers. United Kingdom, Germany: InTech Europe, 2011.10.5772/683Search in Google Scholar
22. Trevan MD. Enzyme immobilization by covalent bonding. In: Walker JM, editors. New protein techniques. Totowa, NJ: Humana Press, 1988:495–510.10.1385/0-89603-126-8:495Search in Google Scholar
23. Okhamafe A, Amsden B, Chu W, Goosen M. Modulation of protein release from chitosan-alginate microcapsules using the pH-sensitive polymer hydroxypropyl methylcellulose acetate succinate. J Microencapsul. 1996;13(5):497–508.10.3109/02652049609026035Search in Google Scholar
24. Gill I, Ballesteros A. Bioencapsulation within synthetic polymers (Part 2): non-sol–gel protein–polymer biocomposites. Trends Biotechnol. 2000;18(11):469–479.10.1016/S0167-7799(00)01493-1Search in Google Scholar
25. Sassolas A, Prieto-Simón B, Marty J. Biosensors for pesticide detection: new trends. Am J Anal Chem. 2012;3(3):210–232.10.4236/ajac.2012.33030Search in Google Scholar
26. Min K, Yoo YJ. Recent progress in nanobiocatalysis for enzyme immobilization and its application. Biotechnol Bioprocess Eng. 2014;19(4):553–567.10.1007/s12257-014-0173-7Search in Google Scholar
27. Harris TK, Keshwani MM. Chapter 7 measurement of enzyme activity. In: Richard RB, Murray PD, editors. Methods in enzymology. USA: Academic Press, 2009:57–71.Search in Google Scholar
28. Gitlesen T, Bauer M, Adlercreutz P. Adsorption of lipase on polypropylene powder. Biochimica Et Biophysica Acta (BBA) – Lipids Lipid Metab. 1997;1345(2):188–196.10.1016/S0005-2760(96)00176-2Search in Google Scholar
29. Singh R., et al. From protein engineering to immobilization: promising strategies for the upgrade of industrial enzymes. Int J Mol Sci. 2013;14(1):1232.10.3390/ijms14011232Search in Google Scholar PubMed PubMed Central
30. Li S.-F., et al. Immobilization of pseudomonas cepacia lipase onto the electrospun PAN nanofibrous membranes for transesterification reaction. J Mol Catal B Enzym. 2011;73(1–4):98–103.10.1016/j.molcatb.2011.08.005Search in Google Scholar
31. Kim M., et al. Enhanced activity and stability of organophosphorus hydrolase via interaction with an amphiphilic polymer. Chem Commun. 2014;50(40):5345–5348.10.1039/c3cc47675hSearch in Google Scholar
32. Suthiwangcharoen N, Nagarajan R. Enhancing enzyme stability by construction of polymer–enzyme conjugate micelles for decontamination of organophosphate agents. Biomacromolecules. 2014;15(4):1142–1152.10.1021/bm401531dSearch in Google Scholar
33. Yan XY, et al. Dual-functional OPH-immobilized polyamide nanofibrous membrane for effective organophosphorus toxic agents protection. Biochem Eng J. 2015;98:47–55.10.1016/j.bej.2015.02.022Search in Google Scholar
34. Gao Y., et al. Bioremediation of pesticide contaminated water using an organophosphate degrading enzyme immobilized on nonwoven polyester textiles. Enzyme Microb Technol. 2014;54:38–44.10.1016/j.enzmictec.2013.10.001Search in Google Scholar
35. Luo J., et al. Cascade catalysis in membranes with enzyme immobilization for multi-enzymatic conversion of CO2 to methanol. New Biotechnol. 2015;32(3):319–327.10.1016/j.nbt.2015.02.006Search in Google Scholar
36. Thompson M, Krull UJ, Worsfold PJ. The structure and electrochemical properties of a polymer-supported lipid biosensor. Anal Chim Acta. 1980;117:133–145.10.1016/0003-2670(80)87012-7Search in Google Scholar
37. Gerard M, Chaubey A, Malhotra BD. Application of conducting polymers to biosensors. Biosens Bioelectron. 2002;17(5):345–359.10.1016/S0956-5663(01)00312-8Search in Google Scholar
38. Azak H., et al. Synthesis of an amine-functionalized naphthalene-containing conducting polymer as a matrix for biomolecule immobilization. RSC Adv. 2013;3(42):19582.10.1039/c3ra42212gSearch in Google Scholar
39. Ekiz F., et al. Synthesis and application of poly-SNS-anchored carboxylic acid: a novel functional matrix for biomolecule conjugation. J Mater Chem. 2011;21(33):12337.10.1039/c1jm12048dSearch in Google Scholar
40. Ayranci R., et al. Ferrocene-functionalized 4-(2,5-Di(thiophen-2-yl)-1H-pyrrol-1-yl)aniline: a novel design in conducting polymer-based electrochemical biosensors. Sensors. 2015;15(1):1389–1403.10.3390/s150101389Search in Google Scholar
41. Park J.-K., et al. Determination of breath alcohol using a differential-type amperometric biosensor based on alcohol dehydrogenase. Anal Chim Acta. 1999;390(1–3):83–91.10.1016/S0003-2670(99)00135-XSearch in Google Scholar
42. Bidan G. Electroconducting conjugated polymers: new sensitive matrices to build up chemical or electrochemical sensors. A review. Sens Actuators, B. 1992;6(1):45–56.10.1016/0925-4005(92)80029-WSearch in Google Scholar
43. Winther-Jensen O, Kerr R, Winther-Jensen B. Alcohol vapour detection at the three phase interface using enzyme-conducting polymer composites. Biosens Bioelectron. 2014;52:143–146.10.1016/j.bios.2013.08.033Search in Google Scholar PubMed
44. Madhumathi K., et al. Preparation and characterization of novel β-chitin–hydroxyapatite composite membranes for tissue engineering applications. Int J Biol Macromol. 2009;44(1):1–5.10.1016/j.ijbiomac.2008.09.013Search in Google Scholar PubMed
45. Mitsubayashi K., et al. A bio-sniffer stick with FALDH (formaldehyde dehydrogenase) for convenient analysis of gaseous formaldehyde. Sens Actuators, B. 2008;130(1):32–37.10.1016/j.snb.2007.07.086Search in Google Scholar
46. Webster M., et al. Tunable thermo-responsive poly(N-vinylcaprolactam) cellulose nanofibers: synthesis, characterization, and fabrication. Macromol Mater Eng. 2013;298(4):447–453.10.1002/mame.201200081Search in Google Scholar
47. Barroso T., et al. Preparation and characterization of a cellulose affinity membrane for human immunoglobulin G (IgG) purification. J Memb Sci. 2010;348(1–2):224–230.10.1016/j.memsci.2009.11.004Search in Google Scholar
48. Angelo Basile CC. Integrated membrane systems and processes. United Kingdom: Wiley, 2016.10.1002/9781118739167Search in Google Scholar
49. Khare P., et al. Microchannel-embedded metal–carbon–polymer nanocomposite as a novel support for chitosan for efficient removal of hexavalent chromium from water under dynamic conditions. Chem Eng J. 2016;293:44–54.10.1016/j.cej.2016.02.049Search in Google Scholar
50. Li L, Li Y, Yang C. Chemical filtration of Cr (VI) with electrospun chitosan nanofiber membranes. Carbohydr Polym. 2016;140:299–307.10.1016/j.carbpol.2015.12.067Search in Google Scholar
51. Teotia R.S., et al. Bifunctional polysulfone-chitosan composite hollow fiber membrane for bioartificial liver. ACS Biomater Sci Eng. 2015;1(6):372–381.10.1021/ab500061jSearch in Google Scholar
52. Huang RYM, Pal R, Moon GY. Pervaporation dehydration of aqueous ethanol and isopropanol mixtures through alginate/chitosan two ply composite membranes supported by poly(vinylidene fluoride) porous membrane. J Memb Sci. 2000;167(2):275–289.10.1016/S0376-7388(99)00293-8Search in Google Scholar
53. Milella E., et al. Poly(L-lactide)acid/alginate composite membranes for guided tissue regeneration. J Biomed Mater Res. 2001;57(2):248–257.10.1002/1097-4636(200111)57:2<248::AID-JBM1165>3.0.CO;2-XSearch in Google Scholar
54. Kim YB, Kim GH. PCL/alginate composite scaffolds for hard tissue engineering: fabrication, characterization, and cellular activities. ACS Comb Sci. 2015;17(2):87–99.10.1021/co500033hSearch in Google Scholar
55. Croisier F, Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur Polym J. 2013;49(4):780–792.10.1016/j.eurpolymj.2012.12.009Search in Google Scholar
56. Hu J., et al. Visible light crosslinkable chitosan hydrogels for tissue engineering. Acta Biomater. 2012;8(5):1730–1738.10.1016/j.actbio.2012.01.029Search in Google Scholar
57. Ahmed EM. Hydrogel: preparation, characterization, and applications: a review. J Adv Res. 2015;6(2):105–121.10.1016/j.jare.2013.07.006Search in Google Scholar
58. Arun Kumar R., et al. Injectable chitin-poly(epsilon-caprolactone)/nanohydroxyapatite composite microgels prepared by simple regeneration technique for bone tissue engineering. ACS Appl Mater Interfaces. 2015;7(18):9399–9409.10.1021/acsami.5b02685Search in Google Scholar
59. Amrita A., Arora A., Sharma P., Katti D.S.. Pullulan-based composite scaffolds for bone tissue engineering: improved osteoconductivity by pore wall mineralization. Carbohydr Polym. 2015;123:180–189.10.1016/j.carbpol.2015.01.038Search in Google Scholar
60. Laufer G., et al. Clay–chitosan nanobrick walls: completely renewable gas barrier and flame-retardant nanocoatings. ACS Appl Mater Interfaces. 2012;4(3):1643–1649.10.1021/am2017915Search in Google Scholar
61. Nyholm L., et al. Toward flexible polymer and paper-based energy storage devices. Adv Mater. 2011;23(33):3751–3769.10.1002/adma.201004134Search in Google Scholar
62. Siró I, Plackett D. Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose. 2010;17(3):459–494.10.1007/s10570-010-9405-ySearch in Google Scholar
63. Chen X, Dong S. Sol-gel-derived titanium oxide/copolymer composite based glucose biosensor. Biosens Bioelectron. 2003;18(8):999–1004.10.1016/S0956-5663(02)00221-XSearch in Google Scholar
64. Mo Zl, et al. Heterogeneous preparation of cellulose–polyaniline conductive composites with cellulose activated by acids and its electrical properties. Carbohydr Polym. 2009;75(4):660–664.10.1016/j.carbpol.2008.09.010Search in Google Scholar
65. Khan S., et al. Bacterial cellulose–poly(3,4-ethylenedioxythiophene)–poly(styrenesulfonate) composites for optoelectronic applications. Carbohydr Polym. 2015;127:86–93.10.1016/j.carbpol.2015.03.055Search in Google Scholar PubMed
66. Müller D., et al. Electrically conducting nanocomposites: preparation and properties of polyaniline (PAni)-coated bacterial cellulose nanofibers (BC). Cellulose. 2012;19(5):1645–1654.10.1007/s10570-012-9754-9Search in Google Scholar
67. Rock M. Temperature and moisture responsive smart textile 2008. Google Patents.Search in Google Scholar
68. Filatov V.N., et al. Hemostatic textile material 2008. Google Patents.Search in Google Scholar
69. Hartmann M, Worley J, North M. Temperature regulating cellulosic fibers and applications thereof 2007. Google Patents.Search in Google Scholar
70. McNamara JJ, Ware W, Yu W. Modification of cellulosic substrates to control body odor 2014. Google Patents.Search in Google Scholar
71. Auad M.L., et al. Polyaniline-modified cellulose nanofibrils as reinforcement of a smart polyurethane. Polym Int. 2011;60(5):743–750.10.1002/pi.3004Search in Google Scholar
72. Bai Y., et al. A novel high mechanical strength shape memory polymer based on ethyl cellulose and polycaprolactone. Carbohydr Polym. 2013;96(2):522–527.10.1016/j.carbpol.2013.04.026Search in Google Scholar PubMed
73. Zhu Y., et al. Rapidly switchable water-sensitive shape-memory cellulose/elastomer nano-composites. Soft Matter. 2012;8(8):2509.10.1039/c2sm07035aSearch in Google Scholar
74. Liu Y., et al. Water-induced shape-memory poly(d,l-lactide)/microcrystalline cellulose composites. Carbohydr Polym. 2014;104:101–108.10.1016/j.carbpol.2014.01.031Search in Google Scholar
75. Aidley DJ, Stanfield PR. Ion channels: molecules in action. Cambridge: Cambridge University Press, 1996.Search in Google Scholar
76. Zhang X., et al. Natural channel protein inserts and functions in a completely artificial, solid-supported bilayer membrane. Sci Rep. 2013;3:2196.10.1038/srep02196Search in Google Scholar
77. Palivan C.G., et al. Bioinspired polymer vesicles and membranes for biological and medical applications. Chem Soc Rev. 2016;45(2):377–411.10.1039/C5CS00569HSearch in Google Scholar
78. Einfalt T., et al. Stimuli-triggered activity of nanoreactors by biomimetic engineering polymer membranes. Nano Lett. 2015;15(11):7596–7603.10.1021/acs.nanolett.5b03386Search in Google Scholar
79. Zhang Z., et al. A bioinspired multifunctional heterogeneous membrane with ultrahigh ionic rectification and highly efficient selective ionic gating. Adv Mater. 2016;28(1):144–150.10.1002/adma.201503668Search in Google Scholar
80. Agre P, Kozono D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett. 2003;555(1):72–78.10.1016/S0014-5793(03)01083-4Search in Google Scholar
81. Wang M., et al. Layer-by-layer assembly of aquaporin Z-incorporated biomimetic membranes for water purification. Environ Sci Technol. 2015;49(6):3761–3768.10.1021/es5056337Search in Google Scholar PubMed
82. Wagh P., et al. A new technique to fabricate high-performance biologically inspired membranes for water treatment. Sep Purif Technol. 2015;156:754–765.10.1016/j.seppur.2015.10.073Search in Google Scholar
83. Xie W., et al. An aquaporin-based vesicle-embedded polymeric membrane for low energy water filtration. J Mater Chem A. 2013;1(26):7592–7600.10.1039/c3ta10731kSearch in Google Scholar
84. Ding W., et al. Fabrication of an aquaporin-based forward osmosis membrane through covalent bonding of a lipid bilayer to a microporous support. J Mater Chem A. 2015;3(40):20118–20126.10.1039/C5TA05751ESearch in Google Scholar
85. Tang C.Y., et al. Desalination by biomimetic aquaporin membranes: review of status and prospects. Desalination. 2013;308:34–40.10.1016/j.desal.2012.07.007Search in Google Scholar
86. Habel J., et al. Aquaporin-based biomimetic polymeric membranes: approaches and challenges. Membranes. 2015;5(3):307.10.3390/membranes5030307Search in Google Scholar PubMed PubMed Central
87. Balme S., et al. Controlling potassium selectivity and proton blocking in a hybrid biological/solid-state polymer nanoporous membrane. Nanoscale. 2013;5(9):3961–3968.10.1039/c3nr00564jSearch in Google Scholar PubMed
88. Balme S., et al. New bioinspired membrane made of a biological ion channel confined into the cylindrical nanopore of a solid-state polymer. Nano Lett. 2011;11(2):712–716.10.1021/nl103841mSearch in Google Scholar PubMed
89. Bonhenry D., et al. Stability of the gramicidin-a channel structure in view of nanofiltration: a computational and experimental study. Soft Matter. 2011;7(22):10651–10659.10.1039/c1sm06277hSearch in Google Scholar
90. Saeki D., et al. Reverse osmosis membranes based on a supported lipid bilayer with gramicidin a water channels. Desalination. 2015;375:48–53.10.1016/j.desal.2015.07.030Search in Google Scholar
91. Drioli E, Di Profio G, Curcio E. Progress in membrane crystallization. Curr Opin Chem Eng. 2012;1(2):178–182.10.1016/j.coche.2012.03.005Search in Google Scholar
92. Ye W., et al. Enhanced performance of a biomimetic membrane for Na2CO3 crystallization in the scenario of CO2 capture. J Memb Sci. 2016;498:75–85.10.1016/j.memsci.2015.09.010Search in Google Scholar
93. Jeong JH, Kim SW, Park TG. Novel intracellular delivery system of antisense oligonucleotide by self-assembled hybrid micelles composed of DNA/PEG conjugate and cationic fusogenic peptide. Bioconjugate Chem. 2003;14(2):473–479.10.1021/bc025632kSearch in Google Scholar PubMed
94. Li M., et al. Responsive polymer-protein bioconjugates prepared by RAFT polymerization and copper-catalyzed azide-alkyne click chemistry. Macromol Rapid Commun. 2008;29(12-13):1172–1176.10.1002/marc.200800073Search in Google Scholar
95. He P, He L. Synthesis of surface-anchored DNA-polymer bioconjugates using reversible addition-fragmentation chain transfer polymerization. Biomacromolecules. 2009;10(7):1804–1809.10.1021/bm9002283Search in Google Scholar PubMed
96. Wilks TR. Synthesis of DNA-polymer conjugates using RAFT polymerisation, in department of chemistry. Coventry: University of Warwick, 2013.Search in Google Scholar
97. Peterson AM, Heemstra JM. Controlling self-assembly of DNA-polymer conjugates for applications in imaging and drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(3):282–297.10.1002/wnan.1309Search in Google Scholar PubMed
98. Peng L., et al. Engineering and applications of DNA-grafted polymer materials. Chem Sci. 2013;4(5):1928.10.1039/c2sc21198jSearch in Google Scholar
99. Wu W, Wang W, Li J. Star polymers: advances in biomedical applications. Prog Polym Sci. 2015;46:55–85.10.1016/j.progpolymsci.2015.02.002Search in Google Scholar
100. Li J., et al. Functional nucleic acid-based hydrogels for bioanalytical and biomedical applications. Chem Soc Rev. 2016;45(5):1410–1431.10.1039/C5CS00586HSearch in Google Scholar
101. Li Z., et al. Reversible and chemically programmable micelle assembly with DNA block-copolymer amphiphiles. Nano Lett. 2004;4(6):1055–1058.10.1021/nl049628oSearch in Google Scholar
102. Ding K., et al. Engineering the structural properties of DNA block copolymer micelles by molecular recognition. Angew Chem Int Ed. 2007;46(7):1172–1175.10.1002/anie.200603064Search in Google Scholar PubMed
103. Gaylord BS, Heeger AJ, Bazan GC. DNA detection using water-soluble conjugated polymers and peptide nucleic acid probes. Proc Natl Acad Sci. 2002;99(17):10954–10957.10.1073/pnas.162375999Search in Google Scholar PubMed PubMed Central
104. Ding WK, Shah NP. Acid, bile, and heat tolerance of free and microencapsulated probiotic bacteria. J Food Sci. 2007;72(9) M446-M450.10.1111/j.1750-3841.2007.00565.xSearch in Google Scholar PubMed
105. Kailasapathy K. Survival of free and encapsulated probiotic bacteria and their effect on the sensory properties of yoghurt. LWT Food Sci Technol. 2006;39(10):1221–1227.10.1016/j.lwt.2005.07.013Search in Google Scholar
106. Siripattanakul S, Pochant CJ, Khan E. Nitrate removal from agricultural infiltrate by bioaugmented free and alginate entrapped cells. Water Environ Res. 2010;82(7):617–621.10.2175/106143009X12529484816277Search in Google Scholar
107. Knierim C., et al. Blocked bacteria escape by ATRP grafting of a PMMA shell on PVA microparticles. Macromol Biosci. 2014;14(4):537–545.10.1002/mabi.201300398Search in Google Scholar PubMed
108. Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev. 2008;14(2):149–165.10.1089/ten.teb.2007.0332Search in Google Scholar PubMed PubMed Central
109. Zhou L., et al. Advances in cell-based biosensors using three-dimensional cell-encapsulating hydrogels. Biotechnol J. 2011;6(12):1466–1476.10.1002/biot.201100098Search in Google Scholar PubMed
110. Orive G., et al. Cell encapsulation: promise and progress. Nat Med. 2003;9(1):104–107.10.1038/nm0103-104Search in Google Scholar PubMed
111. Khademhosseini A., et al. Molded polyethylene glycol microstructures for capturing cells within microfluidic channels. Lab Chip. 2004;4(5):425–430.10.1039/b404842cSearch in Google Scholar PubMed
112. Xin X, Hussain M, Mao JJ. Continuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold. Biomaterials. 2007;28(2):316–325.10.1016/j.biomaterials.2006.08.042Search in Google Scholar PubMed PubMed Central
113. Partridge K., et al. Adenoviral BMP-2 gene transfer in mesenchymal stem cells: in vitro and in vivo bone formation on biodegradable polymer scaffolds. Biochem Biophys Res Commun. 2002;292(1):144–152.10.1006/bbrc.2002.6623Search in Google Scholar PubMed
114. Lee K.G., et al. Synthesis and utilization of E. coli-encapsulated PEG-based microdroplet using a microfluidic chip for biological application. Biotechnol Bioeng. 2010;107(4):747–751.10.1002/bit.22861Search in Google Scholar PubMed
115. Schulte V.A., et al. Microengineered PEG hydrogels: 3D scaffolds for guided cell growth. Macromol Biosci. 2013;13(5):562–572.10.1002/mabi.201200376Search in Google Scholar PubMed
116. Knierim C., et al. Living composites of bacteria and polymers as biomimetic films for metal sequestration and bioremediation. Macromol Biosci. 2015;15(8):1052–1059.10.1002/mabi.201400538Search in Google Scholar PubMed
117. Homaeigohar S, Elbahri M. Nanocomposite electrospun nanofiber membranes for environmental remediation. Materials. 2014;7(2):1017–1045.10.3390/ma7021017Search in Google Scholar PubMed PubMed Central
118. Letnik I., et al. Living composites of electrospun yeast cells for bioremediation and ethanol production. Biomacromolecules. 2015;16(10):3322–3328.10.1021/acs.biomac.5b00970Search in Google Scholar PubMed
119. Klein S., et al. Encapsulation of bacterial cells in electrospun microtubes. Biomacromolecules. 2009;10(7):1751–1756.10.1021/bm900168vSearch in Google Scholar PubMed
© 2017 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Applications of silver nanoparticles stabilized and/or immobilized by polymer matrixes
- Addressing the challenges of standalone multi-core simulations in molecular dynamics
- Virtually going green: The role of quantum computational chemistry in reducing pollution and toxicity in chemistry
- BioArtificial polymers
- Green analytical chemistry – the use of surfactants as a replacement of organic solvents in spectroscopy
- Membrane contactors for CO2 capture processes – critical review
- Elemental Two-Dimensional Materials Beyond Graphene
Articles in the same Issue
- Applications of silver nanoparticles stabilized and/or immobilized by polymer matrixes
- Addressing the challenges of standalone multi-core simulations in molecular dynamics
- Virtually going green: The role of quantum computational chemistry in reducing pollution and toxicity in chemistry
- BioArtificial polymers
- Green analytical chemistry – the use of surfactants as a replacement of organic solvents in spectroscopy
- Membrane contactors for CO2 capture processes – critical review
- Elemental Two-Dimensional Materials Beyond Graphene


