Abstract
Background
Many psychiatric disorders including schizophrenia, bipolar disorder and major depression convey an excess burden of cardiovascular morbidity and mortality. The medications used to treat these conditions may further adversely affect cardiovascular risk and exacerbate health disparities for vulnerable populations. There is a clinical need to appreciate the cardiometabolic adverse effects of psychotropic medications.
Methods
This paper reviews the most relevant cardiometabolic effects of psychotropic medications, organized around the components of metabolic syndrome. When known, the molecular and physiological mechanisms underlying any adverse cardiometabolic effects are detailed.
Results
Many commonly used psychotropic medications, particularly antipsychotics, mood stabilizers and some antidepressants, have been independently associated with cardiometabolic risk factors such as insulin resistance, obesity and dyslipidemia. Stimulants, antidepressants that inhibit reuptake of norepinephrine, some antipsychotics and valproic acid derivatives may also increase blood pressure.
Conclusion
Understanding, assessing and subsequently managing cardiometabolic complications of psychotropic medications are important to mitigate the excess cardiovascular morbidity and mortality in the clinical populations prescribed psychotropic medications. There is considerable variability in risk between medications and individuals. Timely management of iatrogenic cardiometabolic effects is critical.
Introduction
Metabolic syndrome also known as syndrome X or insulin-resistance syndrome is a cluster of conditions that occur together with insulin resistance as a common feature. The components of the syndrome include hypertension, central obesity, dyslipidemia [high triglycerides and low high density lipoprotein cholesterol (HDL-c)] and impaired glucose tolerance [1]. Many commonly used psychotropic medications adversely impact these components of metabolic syndrome as illustrated in Figure 1. These individual components of metabolic syndrome, which are risk factors for vascular disease, are the focus of this review.
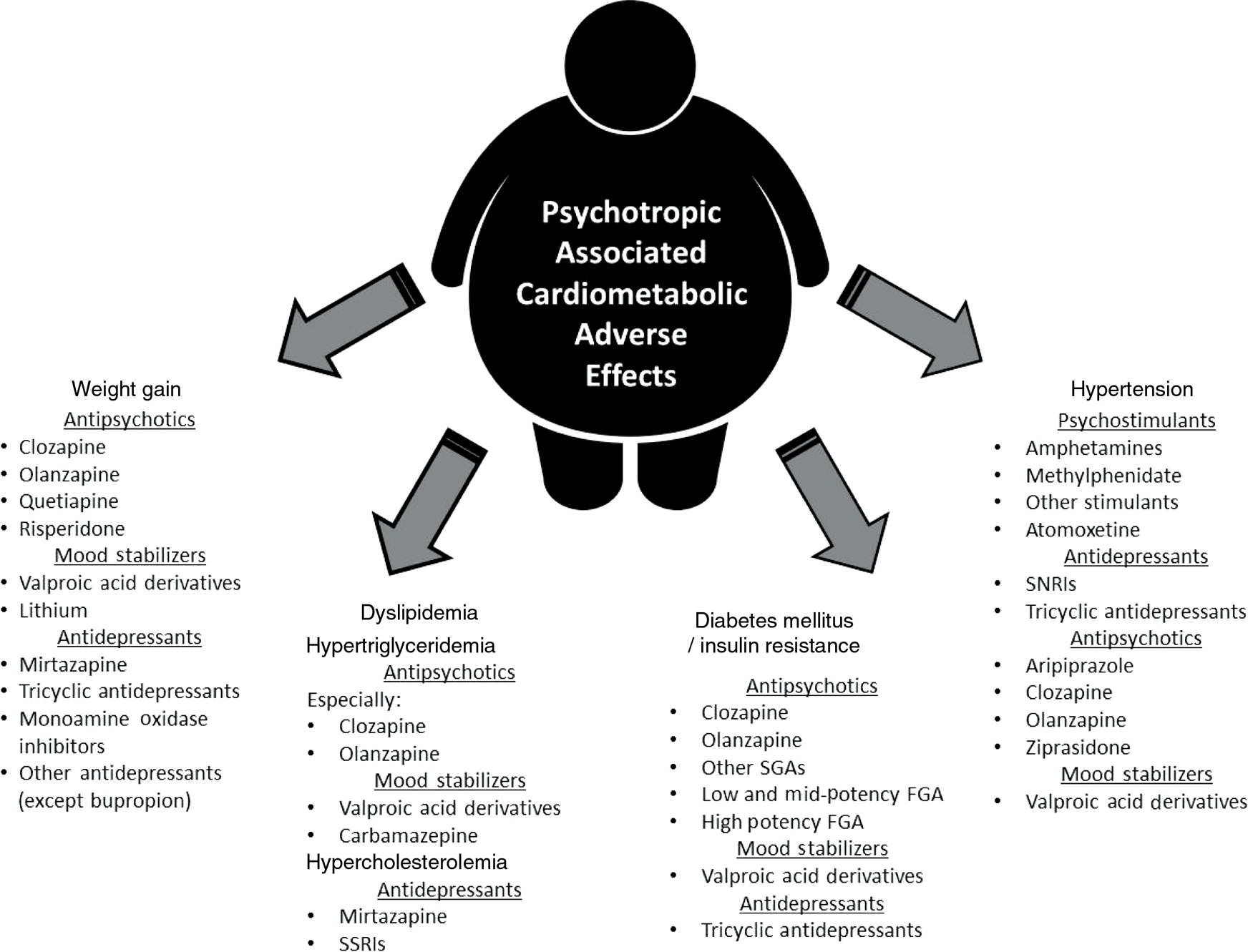
Summary of psychotropic medications associated with cardiometabolic iatrogenic effects.
This figure summarizes the medications or medication classes, where appropriate, associated with the cardiometabolic adverse effects that served as the focus of this review: weight gain dyslipidemia, insulin resistance or frank type 2 diabetes mellitus and hypertension.
Three broad classes of medications with the potential for cardiometabolic side effects are commonly used in the management of many psychiatric conditions: antidepressants, mood stabilizers and antipsychotics. Antidepressants are a mainstay in the treatment of anxiety and depressive disorders. While antidepressants may be combined with other agents, like fluoxetine (antidepressant) and olanzapine (antipsychotic) for bipolar depression [2], this review will focus on individual medications rather than combination products. Mood stabilizers include lithium, valproate (divalproex), carbamazepine and lamotrigine, many of which have anticonvulsant properties. These are the foundation for the treatment of bipolar disorder and are sometimes used in other mood disorders. Antipsychotics are another important class, commonly divided into the first-generation antipsychotics (FGAs) and second-generation antipsychotics (SGAs). Well-known FGAs include: fluphenazine, haloperidol and chlorpromazine. SGAs include agents such as aripiprazole, olanzapine, clozapine, quetiapine and risperidone. SGAs have become popular for treatment of more than just psychotic disorders and are even used off label for treatment of conditions such as insomnia, but these agents are associated with substantial risk of metabolic complications [3], [4], [5], [6], [7], [8]. Medication selection is dependent on several factors including, but not limited to, targeting acute psychiatric syndromes and the prevention of future episodes. Medication selection should also be driven by consideration of side-effects and there is evidence to suggest that physicians, particularly psychiatrists, underappreciate cardiometabolic adverse effects when prescribing psychotropic medications [9].
Weight gain
Large weight gains on the order of 3–5 kg/m2 over 2 years in those with a body mass index (BMI) ≥ 35 are associated with a 33%–53% increase in mortality independent of risk factors [10]. The clinical significance of gains in individuals in those within the normal weight range is less clear. Many psychiatric disorders are associated with weight gain after the onset of illness and institution of medication. A systematic review demonstrated increase in body weight by 10%–12% in the first 6–12 months following diagnosis and treatment of schizophrenia [11]. In a separate study, among 128 depressed patients prescribed imipramine over 33 weeks, 13% demonstrated increased weight of more than 10% over 5 months [12]. Most of the weight gain occurs in the first 6 months and is more prominent if the patient is already overweight [13], [14]. Some of the predictors for weight gain include young age, female gender, low BMI and family history of obesity, the dose and duration of treatment [15]. A long-term retrospective analysis (mean of 7 years) has clarified that a low BMI predicts higher acceleration of BMI change, but that a high premorbid BMI is associated with a greater total change in BMI [15]. Parents BMI is also predictive [15]. A strong predictor for long-term weight gain with antipsychotics is at least a 5% weight gain from baseline within the first month of treatment [16]. Therefore, the changes in weight for each patient must be monitored rigorously as they can have a great impact on the quality of life and importantly, early changes in treatment can prevent severe adverse effects [17]. There is substantial variability in weight gain across patients taking psychotropic medications. A 3-month study of 135 antipsychotic-naive children on risperidone demonstrated varied weight changes from weight loss in 4.4% of the cohort to weight gains of greater than 21% in 6.7% patients (Figure 2) [18]. This emphasizes the need for ongoing monitoring and assessment.
![Figure 2: Variability in observed weight gain.This figure illustrates the considerable individual variability in weight changes that can be seen with antipsychotic treatment. These data were from a Correll et al. [18] study of 135 antipsychotic-naïve children and adolescents treated with risperidone for 3 months. The mean weight gain in this study was 5.3 kg. Antipsychotic-naïve individuals gain more weight than those with prior treatment.](/document/doi/10.1515/hmbci-2017-0065/asset/graphic/j_hmbci-2017-0065_fig_002.jpg)
Variability in observed weight gain.
This figure illustrates the considerable individual variability in weight changes that can be seen with antipsychotic treatment. These data were from a Correll et al. [18] study of 135 antipsychotic-naïve children and adolescents treated with risperidone for 3 months. The mean weight gain in this study was 5.3 kg. Antipsychotic-naïve individuals gain more weight than those with prior treatment.
Antipsychotics
Antipsychotic medications commonly cause weight gain. Increased weight has been reported in 40%–80% of individuals on FGAs and SGAs [11]. Clozapine, olanzapine, and quetiapine, risperidone are most likely to produce severe weight gain. Comparison of five antipsychotic medications in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE), a publicly-funded comparative effectiveness study demonstrates relative differences in weight gain between the five antipsychotic agents studied (Figure 3) [19]. Over a quarter (28%) of the participants reported antipsychotic use at baseline. The olanzapine group demonstrated the greatest weight gain with participants randomized to olanzapine gaining an average of 2 lb (0.9 kg) per month above their baseline weight [19]. The effect of antipsychotics on weight has been compared most commonly to the effect that lithium has on weight. There was a greater than 15% weight gain more often in people prescribed olanzapine (HR = 1.84; p < 0.0001) or quetiapine (HR = 1.67; p < 0.001) compared to lithium [20]. Patients randomized to quetiapine for acute mania gained 3.3 kg in a 12-week efficacy clinical trial for acute mania vs. 1.0 kg on lithium [21]. In a separate meta-analysis comparing the effects of different antipsychotics on weight, the smallest weight gains were associated with ziprasidone, fluphenazine and haloperidol, [22] while molindone was actually associated with weight loss [23]. Aripiprazole is sometimes considered weight neutral, occasionally causing weight loss with acute use [24] or weight gain especially in the young [25]. However, even with aripiprazole, 8%–11% of patients may gain >7% of baseline weight after just 4 weeks of treatment [26]. All antipsychotics should be considered to carry the potential for extreme weight gain in vulnerable individuals [9]. Reviews of randomized placebo-controlled trials highlight the importance of individual variability and underscore the importance of ongoing monitoring [18]. The variability of reported changes in weight with antipsychotic medications is substantial. Rummel-Kluge and colleagues conducted a meta-analysis of randomized control trials contrasting weight gain across SGAs. There was heterogeneity in the degree of weight changes observed among studies with differences due to dosage of medication prescribed, duration of treatment and age of the samples, which across studies averaged in their mid-30s [27]. In head-to-head comparisons, clozapine produced significantly more weight gain (mean difference 2.9 kg) than risperidone. Olanzapine resulted in significantly more weight gain than amisulpride (2.1 kg), aripiprazole (3.9 kg), quetiapine (2.7 kg), and risperidone (2.4 kg) and ziprasidone (3.8 kg). Risperidone also yielded more weight gain than amisulpride (1.0 kg) while sertindole caused more weight gain than risperidone (1.0 kg) [27].
![Figure 3: Weight gain for antipsychotics used in the CATIE study.The CATIE study randomly assigned 1493 patients with schizophrenia to flexibly dosed olanzapine (7.5–30 mg/day), quetiapine (200–800 mg/day), risperidone (1.5–6.0 mg/day), perphenazine (8–32 mg/day) and, introduced in the latter portion of the study, ziprasidone (40–60 mg/day). The greatest weight gain was observed with olanzapine, followed by quetiapine and risperidone [19], where 1 pound (lb) equals approximately 0.45 kg.](/document/doi/10.1515/hmbci-2017-0065/asset/graphic/j_hmbci-2017-0065_fig_003.jpg)
Weight gain for antipsychotics used in the CATIE study.
The CATIE study randomly assigned 1493 patients with schizophrenia to flexibly dosed olanzapine (7.5–30 mg/day), quetiapine (200–800 mg/day), risperidone (1.5–6.0 mg/day), perphenazine (8–32 mg/day) and, introduced in the latter portion of the study, ziprasidone (40–60 mg/day). The greatest weight gain was observed with olanzapine, followed by quetiapine and risperidone [19], where 1 pound (lb) equals approximately 0.45 kg.
Mood stabilizers
Two of the more common mood stabilizers prescribed are valproate (divalproex) and lithium and both have been associated with weight gain. A 12-week randomized clinical trial by Bowden and colleagues [28] demonstrated significantly more weight gain with valproate than with lithium (1.1 vs. 0.2 kg; p = 0.04). Although, patients on valproate gained more weight than those on lithium, this did not appear to be as clearly dose dependent with valproate as with lithium [29]. Lithium-associated weight increases are less likely at plasma levels <0.8 mmol/L [30]. It is nonetheless not uncommon to observe a 4-kg weight gain during the first 2 years of treatment with lithium. Alternatively, other mood stabilizers such as lamotrigine or carbamazepine appear to have little effect on weight [31], [32]. Nonetheless, 11% of 173 subjects treated with lamotrigine, in a double blind phase of a randomized control trial, had weight gains of greater than 7% [31]. Most of the fat distribution seen with psychotropic drugs is located around the abdomen with a higher waist-to-hip ratio in patients taking valproate than in lean patients (p < 0.001) [33], associated with the metabolic syndrome.
Antidepressants
With antidepressants, weight gain may be due to improvements in depressive symptoms (i.e., resolution of decreased appetite) following treatment, or side-effect of antidepressants that presents as weight gain persisting after symptom remission [34]. Substantial weight gains have been reported for amitriptyline, mirtazapine and nortriptyline. In a meta-analysis of weight changes with antidepressants, weight gain of 1.52 kg was seen with amitriptyline, 1.74 kg with mirtazapine and 2.00 kg with nortriptyline over a 4–12-week period, while other non-tricyclic antidepressants were not associated with weight gain [35]. With medium- and long-term treatment, however, more antidepressants are associated with weight gain of more than 1 kg: paroxetine 2.73 kg, mirtazapine 2.59 kg, amitriptyline 2.24 kg, citalopram 1.69 kg and nortriptyline 1.24 kg. In the Netherlands Study of Depression and Anxiety comparing long-term side effects of all the antidepressants, the prevalence of self-reported weight gain was highest with mirtazapine (29%), followed by tricyclic antidepressants (TCAs) (22%) and selective serotonin reuptake inhibitors (SSRIs) (19%) after 1-year of follow-up [36]. Other antidepressant agents such as escitalopram, imipramine, fluvoxamine and trazodone showed no significant weight changes. Treatment with paroxetine, sertraline and fluoxetine resulted in mean changes in weight of 3.6%, 1% and −0.2%, respectively, at the end of 26–32 weeks. Extreme weight gain (>7%) was more prevalent in patients taking paroxetine (25.5%) than those taking sertraline (4.2%) or fluoxetine (6.8%). Risk for greatest weight gain was seen in those with a lower baseline BMI (short-term weight gain) and a family history of obesity [34]. A long-term follow-up of claims data did not show substantive differences between SSRIs in risk of weight gain with a pattern for no changes in weight in the first 3 months followed by steady increases in weight between 3 and 12 months [37]. Unlike other antidepressants, weight loss has been observed with bupropion [35], which tends to be proportional to the baseline BMI with only minimal decreases in weight observed amongst those who are underweight of normal weight [38].
A lack of head-to-head comparisons between psychotropic medications and heterogeneity in study design and clinical samples (e.g., treatment-naïve vs. those later in course of illness and treatment), renders it difficult to make precise determinations regarding relative liabilities for weight gain across medications. As already mentioned, there is also considerable variability in individual vulnerabilities in these adverse events. Figure 4 illustrates approximate propensity for weight gain of the agents discussed, although caution should be exercised in ascribing relative differences for any agents not assessed head-to-head in a randomized clinical trial.

Propensity for weight gain for selected psychotropic medications.
Medications and those who take them exhibit considerable variability in liability for weight gain. Aripiprazole, as an exemplar for this, shows variable propensity for weight gain across studies. The figure approximates risk across a variety of commonly prescribed psychiatric medications associated with weight gain. Relative position should not be used to infer clear differences between agents, particularly for agents from different classes where head-to-head data are less common.
Mechanisms of weight gain
A variety of mechanisms may explain the weight gain caused by psychotropic medications. Many psychotropic medications antagonize histamine-1 receptor (H1) [39], serotonin (5-HT2A, 5-HT2C) [40] and α1-adrenergic receptors [41]. The affinity of antipsychotics for these receptors correlates highly with weight gain [42]. Antagonism of serotonin and histamine receptors leads to increased central appetite and thus increased food intake. Additionally, H1 blockade increases carbohydrate craving and intake [41]. Monoamine oxidase inhibitors (MAOIs) and TCAs are thought to induce a greater weight gain than other antidepressants due to more potent H1 receptor antagonism [43]. SSRIs cause an initial reduction in 5-HT2C receptors promoting subsequent stimulation of presynaptic 5-HT1 receptors [44]. Fluoxetine has a high attraction for 5-HT2C receptors and antagonizes these receptors leading to increased appetite, yet it is associated with weight loss acutely. In a prospective study, 832 patients on fluoxetine, treated over 50 weeks were observed to experience a weight loss of <0.5 kg during the open-label acute non-randomized phase (4 weeks) whereas the double-blind randomized continuation phase (week 12–week 50) was associated with steady weight increases by 1.1 kg (at week 26), 2.2 kg (at week 38) and 3.1 kg (at week 50), respectively [45]. The reason for the differences between short-term and long-term weight effects with fluoxetine or other SSRIs has not been convincingly explained [46].
Conversely, weight loss is associated with bupropion [47], [48], [49]. Blocking dopamine and norepinephrine reuptake, as bupropion does, has been associated with weight loss. Bupropion has no effect on adrenergic, histamine or serotonergic receptors [50]. Comparisons between sertraline and bupropion in binge eating disorder showed no weight loss in the first 6 weeks but by week 14, 60% of those on bupropion had lost 5% of their weight [51]. Similarly, in a randomized, placebo-controlled trial by Croft and colleagues [38] involving 828 patients over 44 weeks, weight loss with bupropion was proportional to baseline BMI and this weight loss was dose dependent.
Psychotropic medications may adversely influence weight by interfering with the regulation of leptin and adiponectin. Leptin is a hormone responsible for controlling body weight through phosphorylation of protein kinase B (PKB). PKB in the presence of psychotropic medications such as lithium acts through a negative feedback to inhibit glycogen-synthase-kinase 3 beta (GSK3b), which blocks the ability of leptin to reduce food intake resulting in weight gain [52]. This same effect is seen with valproate. A systematic review of valproate-induced insulin resistance could not find compelling evidence to suggest hyperinsulinemia or hyperleptinemia is due to anything other than obesity, with more research needed to understand the mechanisms [53].
Adiponectin is an adipocyte-derived protein; its main role is to regulate insulin sensitivity and glucose homeostasis. Animal studies with mice and in vitro studies using mature adipocytes by Qiao et al. examined the effect of valproate on adiponectin gene expression. Valproate had a dose-dependent inhibitory effect on the gene expression of adiponectin in both mature fat cells and mice, this effect is also time dependent. By inhibiting an essential enzyme in deoxyribonucleic acid (DNA) transcriptional regulation, histone deacetylase, other essential enzymes are repressed leading to decreased adiponectin expression and obesity-associated insulin resistance [54].
An alternative explanation for weight gain by psychotropic medication involves the insulin-like effect of medications such as lithium. In a study on rats, following treatment with lithium, glucose administration was followed by decreased insulin levels and higher blood glucose levels [55]. Rose and Warms noted glucose 1,6-P2 synthase activity was lower in animals treated with lithium because lithium replaces zinc as an enzyme activator. This ultimately results in an insulin-like effect thus causing glucose tolerance [56]. The proposed mechanism involves cyclic adenosine monophosphate (cAMP) inhibition in the pancreas, leading to inhibition of insulin secretion and antagonism of sodium and calcium ions by lithium, which are responsible for insulin secretion [57].
Dyslipidemia
Dyslipidemia is another major adverse metabolic outcome associated with psychotropic medications and appears strongly related to medication-induced weight changes. Higher cholesterol levels are associated with substantially increased risk of cardiovascular mortality. The Multiple Risk Factor Intervention Trial (MRFIT) was a prospective trial that looked at mortality risks among young men over a 16-year follow-up period. The study found a two- to four-fold increased risk of deaths due to coronary heart disease in men with high cholesterol levels [>240 mg/dL (6.21 mmol/L)] compared to those with low cholesterol levels <200 mg/dL (<5.17 mmol/L) [58]. Additionally, men with better cholesterol levels (<200 mg/dL) had a greater life expectancy of 3.8–8.7 years compared to men with higher cholesterol levels [58]. Any low density lipoprotein cholesterol (LDL-c) level above 100 mg/dL is considered atherogenic and can increase the risk of cardiovascular disease [59].
Antipsychotics
The antipsychotics clozapine and olanzapine have been highlighted in several studies as being particularly predisposing to metabolic adverse effects [23], [60]. Olanzapine and clozapine are well known to cause significant hyperlipidemia and hypertriglyceridemia [61]. A Veterans’ Affairs analysis demonstrated the greater risk of dyslipidemia following treatment with olanzapine or quetiapine than with risperidone or haloperidol [61]. Hypertriglyceridemia with antipsychotics can be remarkably rapid; triglyceride levels may double as soon as in 2 weeks with risperidone use [62]. The magnitude of changes with most antipsychotics is equally substantial, especially in triglyceride levels. Overall, most common antipsychotics prescribed increase risk for lipid abnormalities in varying degree clozapine [odds ratio (OR) = 1.82, 95% confidence interval (CI) 1.61–2.05], olanzapine (OR = 1.56, 95% CI 1.47–1.67), FGAs (OR = 1.26, 95% CI 1.14–1.39) and aripiprazole (OR = 1.19, 95% CI 0.94–1.52) [61].
Mood stabilizers
Mood stabilizers are also associated with hyperlipidemia. Fifty-three children receiving carbamazepine, phenobarbital and valproic acid over a 12-month period had initial assessments that demonstrated a non-statistically significant increase in total cholesterol, HDL-c, LDL-c and triglycerides for all three agents which were persistent up to 1 year later [63]. Significant weight gain places additional risk for dyslipidemia. Lithium is not directly associated with dyslipidemia, [64] but its effects on lipids may occur secondary to lithium-induced hypothyroidism, which adversely impacts weight and lipids [65]. Several studies have shown an increase in triglyceride level in patients treated with valproate; however, no significant change in cholesterol levels were found [66], [67], [68]. Interestingly, there are reported reductions in total and LDL cholesterol in patients with schizophrenia and bipolar disorder treated with mood stabilizers [69], [70] despite increased triglycerides, weight gain and glucose and insulin abnormalities [71].
Antidepressants
Few studies and case reports are available to support the changes in lipid synthesis and storage with antidepressant use. Teitelbaum reported the case of a 46-year-old male with depression who had baseline triglyceride levels of 490 mg/dL. Following an initial treatment with fluoxetine for 3 years, he was switched to citalopram due to fluoxetine-induced side effects. Subsequently, his triglyceride levels increased to 846 mg/dL in a span of 3 months. Withdrawal of citalopram returned triglyceride to lower levels (223 mg/dL) [72]. In a small-randomized controlled trial, mirtazapine significantly increased cholesterol over 4 weeks compared to placebo (+7.6 mg/dL vs. −0.04 mg/dL) [73]. SSRI use has been cross-sectionally associated with hypercholesterolemia (OR = 1.36, p = 0.012) but not hypertriglyceridemia [74]. A case-control study conducted by Melledo and colleagues [75] showed an 11.5% statistically significant increase in mean LDL-c with paroxetine administered to both healthy adults and individuals suffering from panic disorder after 8 weeks of treatment.
Mechanisms of dyslipidemia
Although, the exact mechanism for changes in lipid profile following treatment is unknown; several plausible pathways have been proposed. Lipid abnormalities may follow observable weight gain associated with prescribed medications, increased lipid biosynthesis through induced gene expression of specific enzymes necessary for lipid metabolism [67] or altered lipid metabolism through different pathways. These pathways may include incorporation of 14 C-acetate into fatty acids and derived lipids, or enzyme inhibition and induction in cholesterol synthesis or through the synthesis of apolipoprotein B [76]. FGAs and SGAs are amphiphilic and act on cholesterol biosynthesis at the cellular level. Haloperidol inhibits the cholesterol biosynthetic reaction catalyzed by Δ-7 reductase, Δ-8, 7 isomerase and Δ-14 reductase. Clozapine inhibits Δ-24 reductase and/or Δ-8,7 isomerase reactions. Risperidone inhibits Δ-7 reductase, Δ-14 reductase and 14-α-demethylase, while ziprasidone inhibits Δ-7 and Δ-14 reductase [76]. This results in accumulation of different sterol intermediates in the cholesterol biosynthesis pathway leading to increased synthesis of complex lipids (phospholipids and triglycerides) [77]. Antidepressants activate sterol regulatory element-binding protein (SREBP) transcription factors, subsequently upregulating genes which are responsible for cholesterol and fatty acid biosynthesis [77]. TCAs such as clomipramine, imipramine and amitriptyline are the most potent activators of SREBP. Citalopram and mirtazapine activate it to a lesser extent, with fluoxetine and bupropion activating it only marginally. Mood stabilizers were not found to activate SREBP. Antiepileptic medications like carbamazepine and phenytoin are potent cytochrome P450 system (CYP450) inducers [78]. These medications induce CYP51A1, an enzyme responsible for catalyzing conversion of the precursor lanosterol to cholesterol in the latter part of the cholesterol synthetic pathway. The substrates on which it acts provide negative feedback inhibition on hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase, which is the rate-limiting enzyme for the pathway. Induction of this enzyme leads to increased conversion to cholesterol and loss of feedback inhibition on HMG-CoA reductase, which increases cholesterol production. Mintzer and colleagues [79] demonstrated statistically significant improvements in triglycerides (−47.1 mg/dL), total cholesterol (−24.8 mg/dL) and LDL-c (−19.9 mg/dL) after switching patients who were on carbamazepine or phenytoin (inducers) to levetiracetam or lamotrigine (non-inducing anti-epileptic medications) concluding that anti-convulsant medications may increase cardiovascular morbidity risk through effects on lipids.
Insulin resistance and the development of diabetes mellitus
Strong links exists between psychotropic medications and the development of insulin resistance, worsening of insulin resistance and development of overt diabetes [80], [81]. Data from the National Health and Nutrition Examination Survey (NHANES) demonstrate a 2.5 greater prevalence of diabetes in an age-matched sample taking antipsychotics and valproic derivatives than in the general population [80], [82].
Antipsychotics
While SGAs were promoted for having lower risk of extrapyramidal side effects and tardive dyskinesia than FGAs, evidence of worsening blood glucose with resolution of symptoms following withdrawal of SGAs was identified [83], [84]. Adolescents taking SGAs were found to have a 50% increased occurrence of type 2 diabetes mellitus relative to those not taking SGAs [85]. A retrospective cohort analysis of 484 patients on FGAs (haloperidol, fluphenazine, chlorpromazine and perphenazine) and olanzapine revealed a high plasma glucose level (>160 mg/dL) in 10.5% of patients on olanzapine, as compared to normal glucose levels in patients on FGAs [86]. Nonetheless, a retrospective study of 7139 subjects with schizophrenia from the Danish Central Psychiatric Research Registry suggests that initial treatment with mid-potency FGAs (zuclopenthixol, perhenazine) and current treatment with low-potency FGAs (chlorpromazine, levomepromazine) could lead to diabetes. SGAs tested in this cohort (olanzapine, OR: 1.44, CI 1.98–1.91) undoubtedly seem to be a risk factor for insulin resistance and diabetes mellitus and thus research has focused on their potential mechanisms [87]. The risk for development of diabetes in those taking risperidone and quetiapine was lower than olanzapine [88], [89]. A systematic analysis using the United States Food and Drug Administration data demonstrated that aripiprazole and ziprasidone are less likely to cause diabetes-related adverse events as compared to olanzapine, clozapine, risperidone and quetiapine [90].
Mood stabilizers
Valproic acid derivatives are another potential culprit for insulin resistance in those with bipolar disorder. A longitudinal cohort study in patients with epilepsy on valproate showed that increased insulin levels were found in the patient group who reported weight gain, possibly due to increased appetite [91]. In a systematic review of randomized trials that looked at various mood stabilizers, valproate users were at a greater risk of weight gain (RR = 2.04, p = 0.03), which could lead to insulin resistance [92]. Animal studies demonstrated no statistically significant differences in insulin levels between the lithium and placebo groups [93]. Lithium does exert an insulin-like effect on glucose transport in animal models. This has been a rationale for use of lithium to treat insulin resistance in non-insulin dependent diabetes mellitus [94], [95].
Antidepressants
With antidepressant medication use, depressive mood symptoms were associated with abnormalities in glucose metabolism and elevated insulin levels in a case-control study with 20 depressed patients and 13 matched healthy controls [91]. Although some of the decreased insulin sensitivity may be illness and not medication related, recent studies suggested abnormalities in insulin function with antidepressant medications may be centrally mediated at the level of the hippocampus through neurohormonal effects on metabolism [96]. In a review of randomized control trials of antidepressant medications, McIntyre and colleagues concluded that serotonergic antidepressants in general lower blood glucose levels. A 24-week placebo-controlled study examined glucose levels in 48 obese patients with type 2 diabetes mellitus. In the fluoxetine randomized group, hemoglobin A1c (HbA1c) levels were significantly lower (9.72% vs. 10% 10.76, p < 0.005) [97]. Fluoxetine was the most widely studied medication, venlafaxine and duloxetine were found to be metabolically neutral. TCAs are the only group of antidepressants that are associated with increased blood glucose levels [96].
Mechanisms for insulin resistance
Animal studies have elucidated many possible mechanisms on how insulin resistance may be induced by SGAs. Studies on rats have shown the SGA olanzapine increases serum glucose concentration in a dose-dependent manner. Olanzapine activates AMP-activated protein kinase (AMPK) in the hypothalamus, which causes hepatic gluconeogenesis via the sympathetic nervous system [98]. Another study on rats showed that olanzapine significantly increased saturated fatty acids and significantly decreased monounsaturated fatty acids in rat plasma. This change in the fatty acid profile may play a role in insulin resistance by unknown mechanisms. Olanzapine-induced hyperglycemia was prevented by the AMPK inhibitor compound C, providing further evidence that AMPK is involved [99]. Other evidence suggests the hyperglycemia is due to central insulin’s inability to suppress the hepatic glucose production [100]. Psychotropic medications may also predispose to insulin resistance through an epigenetic mechanism [101]. In humans, SGAs have been found to decrease global DNA methylation, and associations between SGA-induced insulin resistance and hypomethylation have been demonstrated [101]. A differentially methylated CpG site on the Fatty Acyl CoA Reductase 2 gene was associated with SGA-induced insulin resistance [102]. In aggregate, it appears that multiple pathways may be responsible for SGA-induced insulin resistance.
Hypertension
A less studied vascular adverse effect associated with psychotropic medications is increased blood pressure. Incremental elevations in blood pressure over 115/75 mm Hg have been associated with a greater risk of cardiovascular mortality in both men and women [103]. The risk of cardiovascular death in both women and men doubles with each increase of 20 mm Hg systolic and 10 mm Hg diastolic blood pressure [103]. Hence, the potentially clinically significant effects on blood pressure of these medications on the blood pressure should be kept in consideration when treating psychiatric conditions particularly in those who are also being managed for hypertension. A study by Goldstein and colleagues demonstrated higher blood pressures in individuals treated for bipolar disorder than healthy controls. Specific details regarding psychiatric medications prescribed was not obtained, precluding any differentiation by class [104]. In addition to efficacy trials of stimulants used in psychiatric managements [105], [106] animal models also demonstrate a dose-dependent statistical increase in blood pressure [107].
Orthostatic hypotension is a more common side effect of most psychiatric medications. As such, more reported evidence of orthostatic hypertension exists [108], [109]. However, in the spirit of the cardiometabolic focus of this review, this section highlights the cardiovascular impact of psychotropic medications through hypertension, which is a component of the metabolic syndrome.
Psychostimulants
Psychostimulants are well known to cause elevations in blood pressure. In a double-blind, randomized cross-over trial of children with attention deficit hyperactivity disorder on methylphenidate, amphetamine or dextroamphetamine, diastolic blood pressure elevations of 3–4 mm Hg were observed on 24-h ambulatory blood monitoring [110]. Methylphenidate has been associated with similar elevations in blood pressure in adults, 3.5 mm Hg in systolic and 4.0 mm Hg in diastolic blood pressure, with considerable individual variability [111]. A smaller systolic blood pressure increase of 2.0 mm Hg has been reported for the norepinephrine reuptake inhibitor and stimulant alternative, atomoxetine [112].
Antidepressants
Increases in blood pressure have also been demonstrated in the use of antidepressants such as venlafaxine, duloxetine and TCAs, which inhibit norepinephrine reuptake [113]. Depression is generally associated with a lower blood pressure but antidepressant use is associated with increased blood pressure. A case control study from the Netherlands Study of Depression and Anxiety demonstrated that a person on antidepressant was 2 times more likely to have elevated blood pressure [114]. In a meta-analysis of several clinical studies including 3744 patients with depression, venlafaxine (1.2 mm Hg) and imipramine (1.0 mm Hg) had statistically significant increases in supine diastolic pressures relative to placebo (−1.5 mm Hg) [115].
Antipsychotics
In 2013, Yasui-Furukori and Fujii recorded two unique cases documenting a steep increase in blood pressure following treatment with aripiprazole causing blood pressure readings of 200/110 mm Hg and 180/90 mm Hg, which improved after the medication was discontinued. Both patients reported headache while taking the medication, a symptom frequently associated with hypertension [116]. Clozapine, olanzapine and ziprasidone have been associated with hypertension, while quetiapine and risperidone appear to have little effect on blood pressure [117], [118]. Animal models similarly showed increased weight and blood pressure in those given olanzapine [107].
Mood stabilizers
With mood stabilizers like lithium, animal models demonstrated antihypertensive effects [119] through its similar renal effects in humans [120]. Valproate, on the other hand, has been associated with hypertension in 1%–5% of patients, [121] including a case report of a valproate-associated hypertensive urgency in an 8-year-old child which resolved with discontinuation of the medication [122].
Mechanisms of blood pressure elevation
While most SGAs antagonize 5HT2A, aripiprazole is a 5HT2A receptor partial agonist. 5HT2A causes contractions in the smooth muscles of vasculature, which increases vessel resistance. Aripiprazole acts as an agonist on the 5-HT2A receptor resulting in hypertension. Additionally, a possible antagonism of α1 adrenergic receptors by aripiprazole may be driving the changes observed in blood pressure [116], [123]. TCAs and FGAs have anticholinergic effects that may be responsible for increases in blood pressure [124]. Weight gain associated with antipsychotics or other psychotropic medications may also mediate changes in blood pressure. Individuals with obesity develop a selective resistance to the appetite suppression hormone, leptin. The hypothalamus becomes resistant to the anorexigenic effects of leptin, which ordinarily would decrease appetite and increase satiety. Resistance to leptin does not occur with regard to the regional sympathetic nerve activity response to leptin in the renal tissue and brown adipose and lumber tissues [125]. The increased renal sympathetic nerve activity results in obesity-induced hypertension due to selective leptin resistance.
Blood pressure elevation is more directly mediated through the effects on neurotransmitters. Psychostimulants such as amphetamines directly release norepinephrine, dopamine and serotonin into synapses, which increase the blood pressure through central dopaminergic and peripheral adrenergic effects [126], [127]. Studies of the norepinephrine reuptake inhibitor atomoxetine in those with central and peripheral autonomic failure suggest blood pressure increases occur through selective norepinephrine transporter blockade in peripheral sympathetic neurons. These effects are attenuated by central sympatholytic mechanisms and subsequently exaggerated in those with central autonomic failure [128]. TCAs similarly increase norepinephrine through reuptake inhibition, which leads to an elevation of blood pressure. FGAs have anticholinergic effects, which may elevate blood pressure [114]. Presynaptic dopamine-2 receptors (D2) can inhibit sympathetic nerve activity and this may acutely influenced by antipsychotics [129], whereas, with carbamazepine, elevations in blood pressure may be following increase in antidiuretic hormone [130] or antagonism of central noradrenergic receptors [131], although the exact mechanism still remains uncertain. Similarly, valproate is known to act through increasing the circulating gamma-aminobutyric acid (GABA), blocking sodium channels and inhibiting glutamate/N-methyl-D-aspartate (NMDA) receptor-mediated neuronal excitation [132]. Carbamazepine works in an analogous manner, which in turn may be due to its similarities in structure to imipramine (an antidepressant) [133].
Discussion
Several guidelines are available to help clinicians monitor side effects of commonly used psychotropic medications. The most prominent is the American Diabetic Association and American Psychiatric Association (ADA-APA) guidelines for monitoring the metabolic consequences of SGAs, which are summarized in Table 1 [83]. Two guidelines have suggested that HbA1c can be substituted for fasting glucose [134]. Where obtaining fasting labs might be a barrier, such as in patients who do not present to the clinic fasting and may not be reliable in obtaining a subsequent fasting lab draw, non-fasting lipids may be substituted [135].
ADA-APA guidelines for monitoring patients on second-generation antipsychotics.
| Baseline | 4 weeks | 8 weeks | 12 weeks | Quarterly | Every year | Every 5 years | |
|---|---|---|---|---|---|---|---|
| History of risk factorsa | ✓ | ✓ | |||||
| Body mass index | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Waist circumference | ✓ | ✓ | |||||
| Fasting blood glucose | ✓ | ✓ | ✓ | ||||
| Blood pressure | ✓ | ✓ | ✓ | ||||
| Lipid profile | ✓ | ✓ | ✓ |
aPersonal and family medical history of cardiovascular risk factors (obesity, hypertension, diabetes mellitus) or cardiovascular disease. This table highlights the recommendations from a consensus statement published by the American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists and the North American Association for the Study of Obesity. While these guidelines were drafted in response to mounting concerns about the adverse effects of second-generation antipsychotics, they provide a reasonable framework for the monitoring of other medications associated with similar cardiometabolic risk. The guidelines also recommend that patients, family and providers be aware of signs or symptoms of diabetes mellitus and diabetic ketoacidosis [83].
Despite available guidelines, studies have shown that recognition and treatment of the cardiometabolic adverse effects of psychotropic medications is inadequate [136], [137]. The ADA-APA guidelines also recommend selecting an agent with a lower propensity for weight gain and glucose intolerance in those with or at a higher risk of diabetes [83]. Unfortunately, clinicians, in general, do not appear to take preexisting diabetes mellitus, hyperlipidemia into consideration when selecting antipsychotics and only primary care physicians, not psychiatrists, appear to consider weight [138]. Only about a half or fewer of psychiatry residents report routinely screening for cardiometabolic risk factors and fewer still routinely treat these conditions [139]. A slight majority of psychiatry trainees view psychiatry as a primary care specialty and those that do are more likely to screen for diabetes and dyslipidemia [139]. While interest in primary care may vary, at least monitoring for the side effects of the very medications one is prescribing is expected [140], [141]. This has been attributed to a lack of knowledge among clinicians regarding the side effects of these medication, reported barriers to obtaining recommended measures (e.g., time or access to a scale or laboratory) and uncertainty about who is responsible for screening [140]. To resolve this, education about cardiometabolic effects of these medications and better integration of care are paramount. There is a growing movement to integrate other medical care into psychiatric teams, particularly those taking care of at-risk groups such as those with severe and persistent mental illness [142], [143].
The likelihood of early detection and institution of appropriate management for weight gain, dyslipidemia, insulin resistance and hypertension is increased if monitoring is incorporated into routine care. Those at higher risk for cardiometabolic syndrome will require closer monitoring. Side effects of psychotropic medications, especially weight gain, can lead to nonadherence. It is important, therefore, to monitor weight before and during treatment when any of the medications associated with weight gain are prescribed. If the patient is already overweight or obese, carefully consider all medication options. Early recognition is critical as weight gain within the first 4 weeks is a harbinger of continued weight gain. To manage treatment-emergent weight gain, clinicians must consider lowering doses, discontinuing medications or switching to alternative medications associated with fewer cardiometabolic side effects. Another option involves adding medications such as metformin and topiramate to either prevent or address weight gain [144]. Providers should also assess thyroid function and encourage lifestyle changes or dietary modification where applicable. Even prior to the onset of medication-induced cardiometabolic effects, primary preventive measures such as patient education, lifestyle modification and improved diet can also aid in averting their onset. This places the clinician in an active role in addressing excess cardiovascular morbidity and mortality in patients taking psychotropic medications.
Acknowledgments
The authors thank Jennifer Davis and Dr. Ambika Kattula for their assistance in initial literature review and drafting.
Author Statement
Research funding: Dr. Fiedorowicz was funded by the NIMH (R01 MH111578), NHLBI (P01HL014388) and the Institute for Clinical and Translational Science (ICTS) at the University of Iowa (U54TR001356 and UL1TR002345). Dr. Fiedorowicz receives funding (grant and consultation) from Myriad Genetics, Inc.
Conflict of interest: Authors state no conflict of interest. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of funding agencies.
Informed consent: Informed consent is not applicable.
Ethical approval: The conducted research is not related to either human or animals use.
References
[1] Fisher M. Cardiometabolic disease: the new challenge? Pract Diabetes Int. 2006;23:95–7.10.1002/pdi.909Search in Google Scholar
[2] Truman CJ, Goldberg JF, Ghaemi SN, Baldassano CF, Wisniewski SR, Dennehy EB, et al. Self-reported history of manic/hypomanic switch associated with antidepressant use: data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). J Clin Psychiatry. 2007;68:1472–9.10.4088/JCP.v68n1002Search in Google Scholar PubMed
[3] Henderson DC, Cagliero E, Gray C, Nasrallah RA, Hayden DL, Schoenfeld DA, et al. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: a five-year naturalistic study. Am J Psychiatry. 2000;157:975–81.10.1176/appi.ajp.157.6.975Search in Google Scholar PubMed
[4] Spivak B, Lamschtein C, Talmon Y, Guy N, Mester R, Feinberg I, et al. The impact of clozapine treatment on serum lipids in chronic schizophrenic patients. Clin Neuropharmacol. 1999;22:98–101.10.1097/00002826-199903000-00006Search in Google Scholar PubMed
[5] Simpson GM. Atypical antipsychotics and the burden of disease. Am J Manag Care. 2005;11(8 Suppl):S235–41.Search in Google Scholar
[6] Volavka J, Czobor P, Sheitman B, Lindenmayer J-P, Citrome L, McEvoy JP, et al. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry. 2002;159:255–62.10.1176/appi.ajp.159.2.255Search in Google Scholar PubMed
[7] Maglione M, Maher AR, Hu J, Wang Z, Shanman R, Shekelle PG, Off-label use of atypical antipsychotics: an update. 2011. https://www-ncbi-nlm-nih-gov.proxy.lib.uiowa.edu/pubmed/22973576.Search in Google Scholar
[8] Huang T-L, Chen J-F. Serum lipid profiles and schizophrenia: effects of conventional or atypical antipsychotic drugs in Taiwan. Schizophr Res. 2005;80:55–9.10.1016/j.schres.2005.05.001Search in Google Scholar PubMed
[9] De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2012;8:114–26.10.1038/nrendo.2011.156Search in Google Scholar PubMed
[10] Myrskylä M, Chang VW. Initial BMI, weight change, and mortality among middle- and older-aged adults. Epidemiology. 2009;20:840–8.10.1097/EDE.0b013e3181b5f520Search in Google Scholar PubMed PubMed Central
[11] Foley DL, Morley KI. Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch Gen Psychiatry. 2011;68:609–16.10.1001/archgenpsychiatry.2011.2Search in Google Scholar PubMed
[12] Schwartz TL, Nihalani N, Jindal S, Virk S, Jones N. Psychiatric medication-induced obesity: a review. Obes Rev. 2004;5:115–21.10.1111/j.1467-789X.2004.00139.xSearch in Google Scholar PubMed
[13] Sachs G, Bowden C, Calabrese JR, Ketter T, Thompson T, White R, et al. Effects of lamotrigine and lithium on body weight during maintenance treatment of bipolar I disorder. Bipolar Disord. 2006;8:175–81.10.1111/j.1399-5618.2006.00308.xSearch in Google Scholar
[14] Frank E, Kupfer DJ, Bulik CM, Levenson JA. Imipramine and weight gain during the treatment of recurrent depression. J Affect Disord. 1990;20:165–72.10.1016/0165-0327(90)90140-4Search in Google Scholar
[15] Gebhardt S, Haberhausen M, Heinzel-Gutenbrunner M, Gebhardt N, Remschmidt H, Krieg J-C, et al. Antipsychotic-induced body weight gain: predictors and a systematic categorization of the long-term weight course. J Psychiatr Res. 2009;43:620–6.10.1016/j.jpsychires.2008.11.001Search in Google Scholar PubMed
[16] Vandenberghe F, Gholam-Rezaee M, Saigí-Morgui N, Delacrétaz A, Choong E, Solida-Tozzi A, et al. Importance of early weight changes to predict long-term weight gain during psychotropic drug treatment. J Clin Psychiatry. 2015;76:e1417–23.10.4088/JCP.14m09358Search in Google Scholar PubMed
[17] Schorr SG, Slooff CJ, Bruggeman R, Taxis K. The incidence of metabolic syndrome and its reversal in a cohort of schizophrenic patients followed for one year. J Psychiatr Res. 2009;43:1106–11.10.1016/j.jpsychires.2009.03.002Search in Google Scholar PubMed
[18] Correll CU, Lencz T, Malhotra AK. Antipsychotic drugs and obesity. Trends Mol Med. 2011;17:97–107.10.1016/j.molmed.2010.10.010Search in Google Scholar PubMed PubMed Central
[19] Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–23.10.1056/NEJMoa051688Search in Google Scholar PubMed
[20] Hayes JF, Marston L, Walters K, Geddes JR, King M, Osborn DP. Adverse renal, endocrine, hepatic, and metabolic events during maintenance mood stabilizer treatment for bipolar disorder: a population-based cohort study. PLoS medicine. 2016;e100205813.10.1371/journal.pmed.1002058Search in Google Scholar PubMed PubMed Central
[21] Bowden C, Grunze H, Mullen J, Brecher M, Paulsson B, Jones M, et al. A randomized, double-blind, placebo-controlled efficacy and safety study of quetiapine or lithium as monotherapy for mania in bipolar disorder. J Clin Pharmacol. 2005;66:111–21.10.4088/JCP.v66n0116Search in Google Scholar
[22] Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–96.10.1176/ajp.156.11.1686Search in Google Scholar
[23] Allison DB, Casey DE. Antipsychotic-induced weight gain: a review of the literature. J Clin Psychiatry. 2001;62(Suppl7):22–31.Search in Google Scholar
[24] Fleischhacker WW, Heikkinen ME, Olié J-P, Landsberg W, Dewaele P, McQuade RD, et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol. 2010;13:1115–25.10.1017/S1461145710000490Search in Google Scholar PubMed
[25] Stigler KA, Potenza MN, Posey DJ, McDougle CJ. Weight gain associated with atypical antipsychotic use in children and adolescents. Paediatr Drugs. 2004;6:33–44.10.2165/00148581-200406010-00003Search in Google Scholar
[26] Gentile S. Long-term treatment with atypical antipsychotics and the risk of weight gain: a literature analysis. Drug Saf. 2006;29:303–19.10.2165/00002018-200629040-00002Search in Google Scholar
[27] Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Lobos CA, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123:225–33.10.1016/j.schres.2010.07.012Search in Google Scholar
[28] Bowden CL, Mosolov S, Hranov L, Chen E, Habil H, Kongsakon R, et al. Efficacy of valproate versus lithium in mania or mixed mania: a randomized, open 12-week trial. Int Clin Psychopharmacol. 2010;25:60–7.10.1097/YIC.0b013e328333ac1bSearch in Google Scholar
[29] Zuo S, Fries BE, Szafara K, Regal R. Valproic acid as a potentiator of metabolic syndrome in institutionalized residents on concomitant antipsychotics: fat chance, or slim to none? Pharm Ther. 2015;40:126–32.Search in Google Scholar
[30] Ackerman S, Nolan LJ. Bodyweight gain induced by psychotropic drugs. CNS Drugs. 1998;9:135–51.10.2165/00023210-199809020-00005Search in Google Scholar
[31] Calabrese JR, Bowden CL, Sachs G, Yatham LN, Behnke K, Mehtonen OP, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry. 2003;64:1013–24.10.4088/JCP.v64n0906Search in Google Scholar
[32] Chengappa KN, Chalasani L, Brar JS, Parepally H, Houck P, Levine J. Changes in body weight and body mass index among psychiatric patients receiving lithium, valproate, or topiramate: an open-label, nonrandomized chart review. Clin Ther. 2002;24:1576–84.10.1016/S0149-2918(02)80061-3Search in Google Scholar
[33] Pylvänen V, Knip M, Pakarinen A, Kotila M, Turkka J, Isojärvi Jouko IT. Serum insulin and leptin levels in valproate-associated obesity. Epilepsia. 2002;43:514–7.10.1046/j.1528-1157.2002.31501.xSearch in Google Scholar PubMed
[34] Fava M. Weight gain and antidepressants. J Clin Psychiatry. 2000;61(suppl 11):37–41.10.4088/JCP.v61n1109Search in Google Scholar PubMed
[35] Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259–72.10.4088/JCP.09r05346bluSearch in Google Scholar PubMed
[36] Bet PM, Hugtenburg JG, Penninx BW, Hoogendijk WJ. Side effects of antidepressants during long-term use in a naturalistic setting. Eur Neuropsychopharmacol. 2013;23:1443–51.10.1016/j.euroneuro.2013.05.001Search in Google Scholar
[37] Blumenthal SR, Castro VM, Clements CC, Rosenfield HR, Murphy SN, Fava M, et al. An electronic health records study of long-term weight gain following antidepressant use. JAMA Psychiatry. 2014;71:889–96.10.1001/jamapsychiatry.2014.414Search in Google Scholar
[38] Croft H, Houser TL, Jamerson BD, Leadbetter R, Bolden-Watson C, Donahue R, et al. Effect on body weight of bupropion sustained-release in patients with major depression treated for 52 weeks. Clin Ther. 2002;24:662–72.10.1016/S0149-2918(02)85141-4Search in Google Scholar
[39] Masand PS, Gupta S. Selective serotonin-reuptake inhibitors: an update. Harv Rev Psychiatry. 1999;7:69.10.3109/hrp.7.2.69Search in Google Scholar
[40] Deshmukh R, Franco K. Managing weight gain as a side effect of antidepressant therapy. Cleve Clin J Med. 2003;70:614–23.10.3949/ccjm.70.7.614Search in Google Scholar PubMed
[41] Baptista T, Kin N, Beaulieu S, De Baptista E. Obesity and related metabolic abnormalities during antipsychotic drug administration: mechanisms, management and research perspectives. Pharmacopsychiatry. 2002;35:205–19.10.1055/s-2002-36391Search in Google Scholar PubMed
[42] Wirshing DA, Wirshing WC, Kysar L, Berisford MA, Goldstein D, Pashdag J, et al. Novel antipsychotics: comparison of weight gain liabilities. J Clin Psychiatry. 1999;60:358–63.10.4088/JCP.v60n0602Search in Google Scholar
[43] Masand PS, Gupta S. Long-term side effects of newer-generation antidepressants: SSRIS, venlafaxine, nefazodone, bupropion, and mirtazapine. Ann Clin Psychiatry. 2002;14:175–82.10.3109/10401230209147454Search in Google Scholar
[44] Bouwer C, Harvey B. Phasic craving for carbohydrate observed with citalopram. Int Clin Psychopharmacol. 1996;11:273–8.10.1097/00004850-199612000-00009Search in Google Scholar PubMed
[45] Michelson D, Amsterdam JD, Quitkin FM, Reimherr FW, Rosenbaum JF, Zajecka J, et al. Changes in weight during a 1-year trial of fluoxetine. Am J Psychiatry. 1999;156:1170–6.10.1176/ajp.156.8.1170Search in Google Scholar
[46] Harvey BH, Colin D. Neuropharmacology of paradoxic weight gain with selective serotonin reuptake inhibitors. Clin Neuropharmacol. 2000;23:90–7.10.1097/00002826-200003000-00006Search in Google Scholar PubMed
[47] Arterburn D, Sofer T, Boudreau DM, Bogart A, Westbrook EO, Theis MK, et al. Long-term weight change after initiating second-generation antidepressants. J Clin Med. 2016;5:48.10.3390/jcm5040048Search in Google Scholar
[48] Jefferson JW, Rush AJ, Nelson JC, VanMeter SA, Krishen A, Hampton KD, et al. Extended-release bupropion for patients with major depressive disorder presenting with symptoms of reduced energy, pleasure, and interest: findings from a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2006;67:865–73.10.4088/JCP.v67n0602Search in Google Scholar
[49] Gadde KM, Parker CB, Maner LG, Wagner HR, Logue EJ, Drezner MK, et al. Bupropion for weight loss: an investigation of efficacy and tolerability in overweight and obese women. Obesity. 2001;9:544–51.10.1038/oby.2001.71Search in Google Scholar
[50] Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6:159–66.10.4088/PCC.v06n0403Search in Google Scholar
[51] Calandra C, Russo RG, Luca M. Bupropion versus sertraline in the treatment of depressive patients with binge eating disorder: retrospective cohort study. Psychiatr Q. 2012;83:177–85.10.1007/s11126-011-9192-0Search in Google Scholar
[52] Ricken R, Bopp S, Schlattmann P, Himmerich H, Bschor T, Richter C, et al. Leptin serum concentrations are associated with weight gain during lithium augmentation. Psychoneuroendocrinology. 2016;71:31–5.10.1016/j.psyneuen.2016.04.013Search in Google Scholar
[53] Verrotti A, La Torre R, Trotta D, Mohn A, Chiarelli F. Valproate-induced insulin resistance and obesity in children. Horm Res Paediatr. 2009;71:125–31.10.1159/000197868Search in Google Scholar
[54] Qiao L, Schaack J, Shao J. Suppression of adiponectin gene expression by histone deacetylase inhibitor valproic acid. Endocrinology. 2006;147:865–74.10.1210/en.2005-1030Search in Google Scholar
[55] Shah JH, Pishdad G. The effect of lithium on glucose and tolbutamide-induced insulin release and glucose tolerance in the intact rat. Endocrinology. 1980;107:1300–4.10.1210/endo-107-5-1300Search in Google Scholar
[56] Rose IA, Warms JV. Lithium action on glucose uptake in brain; role of glucose-1, 6-P2 as a regulator of hexokinase. Biochem Biophys Res Commun. 1980;92:1030–6.10.1016/0006-291X(80)90805-0Search in Google Scholar
[57] Caviezel F, Cattaneo A, Cetta G, Pozza G. Influence of short-term lithium carbonate administration on stimulated insulin secretion in normal man. Int J Clin Pharmacol Ther Toxicol. 1987;25:188–93.Search in Google Scholar
[58] Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. J Am Med Assoc. 2000;284:311–8.10.1001/jama.284.3.311Search in Google Scholar PubMed
[59] Grundy SM, Cleeman JI, Merz CN, Brewer HB, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Circulation. 2004;110:227–39.10.1161/01.CIR.0000133317.49796.0ESearch in Google Scholar PubMed
[60] Marder SR, Essock SM, Miller AL, Buchanan RW, Casey DE, Davis JM, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161:1334–49.10.1176/appi.ajp.161.8.1334Search in Google Scholar PubMed
[61] Olfson M, Marcus SC, Corey-Lisle P, Tuomari AV, Hines P, L’Italien GJ. Hyperlipidemia following treatment with antipsychotic medications. Am J Psychiatry. 2006;163:1821–5.10.1176/ajp.2006.163.10.1821Search in Google Scholar PubMed
[62] Kohen I, Manu P. Rapidly worsening hypertriglyceridemia during treatment with risperidone. Am J Ther. 2010;17:216–8.10.1097/MJT.0b013e318197eadfSearch in Google Scholar PubMed
[63] Yilmaz E, Dosan Y. Serum lipid changes during anticonvulsive treatment. Serum lipids in epileptic children. Acta Neurologica Belgica. 2001;101:217–20.Search in Google Scholar
[64] McIntyre RS, McElroy SL, Eudicone JM, Forbes RA, Carlson BX, Baker RA. A 52-week, double-blind evaluation of the metabolic effects of aripiprazole and lithium in bipolar I disorder. Prim Care Companion CNS Disord. 2011;13. DOI: 10.4088/PCC.11m01182.Search in Google Scholar PubMed PubMed Central
[65] Ezzaher A, Mouhamed DH, Mechri A, Neffati F, Douki W, Gaha L, et al. Thyroid function and lipid profile in bipolar I patients. Asian J Psychiatr. 2011;4:139–43.10.1016/j.ajp.2011.02.002Search in Google Scholar PubMed
[66] Herrán A, Ramírez ML, Carrera M, García-Unzueta MT, Sierra-Biddle D, Rodríguez-Cabo B, et al. Panic disorder, treatment with selective serotonin reuptake inhibitors, and cholesterol levels. J Clin Psychopharmacol. 2006;26:538–40.10.1097/01.jcp.0000237941.56107.b7Search in Google Scholar PubMed
[67] Vik-Mo AO, Birkenaes AB, Ferno J, Jonsdottir H, Andreassen OA, Steen VM. Increased expression of lipid biosynthesis genes in peripheral blood cells of olanzapine-treated patients. Int J Neuropsychopharmacol. 2008;11:679–84.10.1017/S1461145708008468Search in Google Scholar PubMed
[68] Schwarz E, Prabakaran S, Whitfield P, Major H, Leweke FM, Koethe D, et al. High throughput lipidomic profiling of schizophrenia and bipolar disorder brain tissue reveals alterations of free fatty acids, phosphatidylcholines, and ceramides. J Proteome Res. 2008;7:4266–77.10.1021/pr800188ySearch in Google Scholar PubMed
[69] Casey DE, Daniel DG, Wassef AA, Tracy KA, Wozniak P, Sommerville KW. Effect of divalproex combined with olanzapine or risperidone in patients with an acute exacerbation of schizophrenia. Neuropsychopharmacology. 2003;28:182.10.1038/sj.npp.1300023Search in Google Scholar PubMed
[70] Elmslie JL, Porter RJ, Joyce PR, Hunt PJ, Shand BI, Scott RS. Comparison of insulin resistance, metabolic syndrome and adiponectin in overweight bipolar patients taking sodium valproate and controls. Aust N Z J Psychiatry. 2009;43:53–60.10.1080/00048670802534341Search in Google Scholar PubMed
[71] Chang HH, Yang YK, Gean PW, Huang HC, Chen PS, Lu RB. The role of valproate in metabolic disturbances in bipolar disorder patients. J Affect Disord. 2010;124:319–23.10.1016/j.jad.2009.12.011Search in Google Scholar PubMed
[72] Teitelbaum M. Severe and moderate hypertriglyceridemia secondary to citalopram and fluoxetine. Psychosomatics. 2000;41:448–9.10.1176/appi.psy.41.5.448Search in Google Scholar PubMed
[73] Nicholas LM, Ford AL, Esposito SM, Ekstrom RD, Golden RN. The effects of mirtazapine on plasma lipid profiles in healthy subjects. J Clin Psychiatry. 2003;64:883–9.10.4088/JCP.v64n0805Search in Google Scholar PubMed
[74] Raeder MB, Bjelland I, Emil VS, Steen VM. Obesity, dyslipidemia, and diabetes with selective serotonin reuptake inhibitors: the Hordaland Health Study. J Clin Psychiatry. 2006;67:1974–82.10.4088/JCP.v67n1219Search in Google Scholar PubMed
[75] Le Melledo JM, Mailo K, Lara N, Abadia MC, Gil L, Van Ameringen M, et al. Paroxetine-induced increase in LDL cholesterol levels. J Psychopharmacol. 2008;23:826–30.10.1177/0269881108094320Search in Google Scholar PubMed
[76] Canfrán-Duque A, Casado ME, Pastor Ó, Sánchez-Wandelmer J, de la Peña G, Lerma M, et al. Atypical antipsychotics alter cholesterol and fatty acid metabolism in vitro. J Lipid Res. 2013;54:310–24.10.1194/jlr.M026948Search in Google Scholar PubMed PubMed Central
[77] Raeder MB, Fernø J, Glambek M, Stansberg C, Steen VM. Antidepressant drugs activate SREBP and up-regulate cholesterol and fatty acid biosynthesis in human glial cells. Neurosci Lett. 2006;395:185–90.10.1016/j.neulet.2005.10.096Search in Google Scholar PubMed
[78] Patsalos P, Duncan J, Shorvon S. Effect of the removal of individual antiepileptic drugs on antipyrine kinetics, in patients taking polytherapy. Br J Clin Pharmacol. 1988;26:253–9.10.1111/j.1365-2125.1988.tb05274.xSearch in Google Scholar PubMed PubMed Central
[79] Mintzer S, Skidmore CT, Abidin CJ, Morales MC, Chervoneva I, Capuzzi DM, et al. Effects of antiepileptic drugs on lipids, homocysteine, and C‐reactive protein. Ann Neurol. 2009;65:448–56.10.1002/ana.21615Search in Google Scholar PubMed
[80] Mangurian C, Keenan W, Newcomer JW, Vittinghoff E, Creasman JM, Schillinger D. Diabetes prevalence among racial-ethnic minority group members with severe mental illness taking antipsychotics: double jeopardy? Psychiatr Serv. 2017;68:843–6.10.1176/appi.ps.201600356Search in Google Scholar PubMed
[81] Bobo WV, Cooper WO, Stein C, Olfson M, Graham D, Daugherty J, et al. Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiatry. 2013;70:1067–75.10.1001/jamapsychiatry.2013.2053Search in Google Scholar PubMed
[82] Lambert BL, Chou CH, Chang KY, Tafesse E, Carson W. Antipsychotic exposure and type 2 diabetes among patients with schizophrenia: a matched case‐control study of California Medicaid claims. Pharmacoepidemiol Drug Saf. 2005;14:417–25.10.1002/pds.1092Search in Google Scholar PubMed
[83] American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596–601.10.2337/diacare.27.2.596Search in Google Scholar PubMed
[84] Rigalleau V, Gatta B, Bonnaud S, Masson M, Bourgeois ML, Vergnot V, et al. Diabetes as a result of atypical anti-psychotic drugs – a report of three cases. Diabetic Med. 2000;17:484–6.10.1046/j.1464-5491.2000.00296.xSearch in Google Scholar PubMed
[85] Rubin DM, Kreider AR, Matone M, Huang Y-S, Feudtner C, Ross ME, et al. Risk for incident diabetes mellitus following initiation of second-generation antipsychotics among Medicaid-enrolled youths. JAMA Pediatrics. 2015;169:e150285.10.1001/jamapediatrics.2015.0285Search in Google Scholar PubMed
[86] Dunlop B, Sternberg M, Phillips L, Andersen J, Duncan E. Disturbed glucose metabolism among patients taking olanzapine and typical antipsychotics. Psychopharmacol Bull. 2002;37:99–117.10.64719/pb.4176Search in Google Scholar
[87] Nielsen J, Skadhede S, Correll CU. Antipsychotics associated with the development of type 2 diabetes in antipsychotic-naive schizophrenia patients. Neuropsychopharmacology. 2010;35:1997–2004.10.1038/npp.2010.78Search in Google Scholar PubMed PubMed Central
[88] Koro CE, Fedder DO, Gilbert JL, Weiss S, Magder L, Kreyenbuhl J, et al. Assessment of independent effect of olanzapine and risperidone on risk of diabetes among patients with schizophrenia: population based nested case-control study. Br Med J. 2002;325:243.10.1136/bmj.325.7358.243Search in Google Scholar PubMed PubMed Central
[89] Ramaswamy K, Masand PS, Nasrallah HA. Do certain atypical antipsychotics increase the risk of diabetes? a critical review of 17 pharmacoepidemiologic studies. Ann Clin Psychiatry. 2006;18:183–94.10.1080/10401230600801234Search in Google Scholar PubMed
[90] Baker RA, Pikalov A, Tran Q-V, Kremenets T, Arani RB, Doraiswamy PM. Atypical antipsychotic drugs and diabetes mellitus in the US Food and Drug Administration Adverse Event database: a systematic Bayesian signal detection analysis. Psychopharmacol Bull. 2009;42:11–31.10.64719/pb.4092Search in Google Scholar
[91] Okamura F, Tashiro A, Utumi A, Imai T, Suchi T, Tamura D, et al. Insulin resistance in patients with depression and its changes during the clinical course of depression: minimal model analysis. Metabolism. 2000;49:1255–60.10.1053/meta.2000.9515Search in Google Scholar PubMed
[92] Cipriani A, Reid K, Young AH, Macritchie K, Geddes J. Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder. Cochrane Database Syst Rev. 2013;10:CD003196.10.1002/14651858.CD003196.pub2Search in Google Scholar PubMed PubMed Central
[93] Li H, Fang M, Xu M, Li S, Du J, Li W, et al. Chronic olanzapine treatment induces disorders of plasma fatty acid profile in Balb/c mice: a potential mechanism for olanzapine-induced insulin resistance. PLoS One. 2016;11:e0167930.10.1371/journal.pone.0167930Search in Google Scholar PubMed PubMed Central
[94] Tabata I, Schluter J, Gulve EA, Holloszy JO. Lithium increases susceptibility of muscle glucose transport to stimulation by various agents. Diabetes. 1994;43:903–7.10.2337/diab.43.7.903Search in Google Scholar PubMed
[95] Rodriguezgil J, Guinovart J, Bosch F. Lithium restores glycogen synthesis from glucose in hepatocytes from diabetic rats. Arch Biochem Biophys. 1993;301:411–5.10.1006/abbi.1993.1164Search in Google Scholar PubMed
[96] McIntyre RS, Soczynska JK, Konarski JZ, Kennedy SH. The effect of antidepressants on glucose homeostasis and insulin sensitivity: synthesis and mechanisms. Expert Opin Drug Saf. 2006;5:157–68.10.1517/14740338.5.1.157Search in Google Scholar PubMed
[97] Gray D, Fujioka K, Devine W, Bray G. A randomized double-blind clinical trial of fluoxetine in obese diabetics. Int J Obes Relat Metab Disord. 1992;16:S67–72.Search in Google Scholar
[98] Nagata M, Nakajima M, Ishiwata Y, Takahashi Y, Takahashi H, Negishi K, et al. Mechanism underlying induction of hyperglycemia in rats by single administration of olanzapine. Biol Pharm Bull. 2016;39:754–61.10.1248/bpb.b15-00842Search in Google Scholar PubMed
[99] Ikegami M, Ikeda H, Ohashi T, Ohsawa M, Ishikawa Y, Kai M, et al. Olanzapine increases hepatic glucose production through the activation of hypothalamic adenosine 5′-monophosphate-activated protein kinase. Diabetes Obes Metab. 2013;15:1128–35.10.1111/dom.12148Search in Google Scholar PubMed
[100] Kowalchuk C, Teo C. 217. Olanzapine abolishes the ability of central insulin to inhibit hepatic glucose production. Schizophr Bull. 2017;43(suppl_1):S110–S.10.1093/schbul/sbx021.295Search in Google Scholar
[101] Burghardt KJ, Goodrich JM, Dolinoy DC, Ellingrod VL. DNA methylation, insulin resistance and second-generation antipsychotics in bipolar disorder. Epigenomics. 2015;7:343–52.10.2217/epi.15.5Search in Google Scholar PubMed PubMed Central
[102] Burghardt KJ, Goodrich JM, Dolinoy DC, Ellingrod VL. Gene-specific DNA methylation may mediate atypical antipsychotic-induced insulin resistance. Bipolar Disord. 2016;18:423–32.10.1111/bdi.12422Search in Google Scholar
[103] Collaboration PS. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13.10.1016/S0140-6736(02)11911-8Search in Google Scholar
[104] Goldstein BI, Fagiolini A, Houck P, Kupfer DJ. Cardiovascular disease and hypertension among adults with bipolar I disorder in the United States. Bipolar Disord. 2009;11:657–62.10.1111/j.1399-5618.2009.00735.xSearch in Google Scholar PubMed PubMed Central
[105] Hammerness P, Wilens T, Mick E, Spencer T, Doyle R, McCreary M, et al. Cardiovascular effects of longer-term, high-dose OROS methylphenidate in adolescents with attention deficit hyperactivity disorder. J Pediatr. 2009;155:84–9. e1.10.1016/j.jpeds.2009.02.008Search in Google Scholar PubMed
[106] Godfrey J. Safety of therapeutic methylphenidate in adults: a systematic review of the evidence. J Psychopharmacol. 2009;23:194–205.10.1177/0269881108089809Search in Google Scholar PubMed
[107] Patil BM, Kulkarni NM, Unger BS. Elevation of systolic blood pressure in an animal model of olanzapine induced weight gain. Eur J Pharmacol. 2006;551:112–5.10.1016/j.ejphar.2006.09.009Search in Google Scholar PubMed
[108] Wagstaff AJ, Perry CM. Clozapine. CNS drugs. 2003;17:273–80.10.2165/00023210-200317040-00004Search in Google Scholar PubMed
[109] Glassman AH. Schizophrenia, antipsychotic drugs, and cardiovascular disease. J Clin Psychiatry. 2005;66:5–10.Search in Google Scholar
[110] Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92–5.10.1007/s00467-005-2051-1Search in Google Scholar PubMed
[111] Biederman J, Mick E, Surman C, Doyle R, Hammerness P, Harpold T, et al. A randomized, placebo-controlled trial of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2006;59:829–35.10.1016/j.biopsych.2005.09.011Search in Google Scholar PubMed
[112] Michelson D, Allen AJ, Busner J, Casat C, Dunn D, Kratochvil C, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry. 2002;159:1896–901.10.1176/appi.ajp.159.11.1896Search in Google Scholar PubMed
[113] Montgomery SA. Tolerability of serotonin norepinephrine reuptake inhibitor antidepressants. CNS spectrums. 2008;13(S11):27–33.10.1017/S1092852900028297Search in Google Scholar
[114] Licht CM, De Geus EJ, Seldenrijk A, Van Hout HP, Zitman FG, Van Dyck R, et al. Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension. 2009;53:631–8.10.1161/HYPERTENSIONAHA.108.126698Search in Google Scholar PubMed
[115] Thase ME. Effects of venlafaxine on blood pressure: a meta-analysis of original data from 3744 depressed patients. J Clin Psychiatry. 1998;59:502–8.10.4088/JCP.v59n1002Search in Google Scholar PubMed
[116] Yasui-Furukori N, Fujii A. Worsened hypertension control induced by aripiprazole. Neuropsychiatr Dis Treat. 2013;9:505–7.10.2147/NDT.S43950Search in Google Scholar PubMed PubMed Central
[117] Woo YS, Kim W, Chae J-H, Yoon B-H, Bahk W-M. Blood pressure changes during clozapine or olanzapine treatment in Korean schizophrenic patients. World J Biol Psychiatry. 2009;10:420–5.10.1080/15622970801910399Search in Google Scholar PubMed
[118] Khasawneh FT, Shankar GS. Minimizing cardiovascular adverse effects of atypical antipsychotic drugs in patients with schizophrenia. Cardiol Res Pract. 2014;2014:273060.10.1155/2014/273060Search in Google Scholar PubMed PubMed Central
[119] Das S, Bhargava H. Effect of lithium treatment on blood pressure and angiotensin-converting enzyme activity in normotensive Wistar-Kyoto and spontaneously hypertensive rats. Arch Int Pharmacodyn Ther. 1985;276:82–91.Search in Google Scholar
[120] Onusko E. Hypertension: pitfalls to prescribing for patients with high blood pressure. Curr Psychiatr. 2002;1:53–9.Search in Google Scholar
[121] Lexicomp. Valproic acid and derivatives. Hudson, Ohio, USA: Pediatric & Neonatal Lexi-Drugs; 2017.Search in Google Scholar
[122] Sivananthan M, Mohiuddin S. Valproate induced hypertensive urgency. Case Rep Psychiatry. 2016;2016:2.10.1155/2016/1458548Search in Google Scholar PubMed PubMed Central
[123] Davies MA, Sheffler DJ, Roth BL. Aripiprazole: a novel atypical antipsychotic drug with a uniquely robust pharmacology. CNS Drug Rev. 2004;10:317–36.10.1111/j.1527-3458.2004.tb00030.xSearch in Google Scholar PubMed PubMed Central
[124] Schwartz JT, Brotman AW. A clinical guide to antipsychotic drugs. Drugs. 1992;44:981–92.10.2165/00003495-199244060-00007Search in Google Scholar PubMed
[125] Morgan DA, Thedens DR, Weiss R, Rahmouni K. Mechanisms mediating renal sympathetic activation to leptin in obesity. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1730–6.10.1152/ajpregu.90324.2008Search in Google Scholar PubMed PubMed Central
[126] Heal DJ, Smith SL, Gosden J, Nutt DJ. Amphetamine, past and present–a pharmacological and clinical perspective. J Psychopharmacol. 2013;27:479–96.10.1177/0269881113482532Search in Google Scholar
[127] Volkow ND, Wang GJ, Fowler JS, Molina PE, Logan J, Gatley SJ, et al. Cardiovascular effects of methylphenidate in humans are associated with increases of dopamine in brain and of epinephrine in plasma. Psychopharmacology (Berl). 2003;166:264–70.10.1007/s00213-002-1340-7Search in Google Scholar
[128] Shibao C, Raj SR, Gamboa A, Diedrich A, Choi L, Black BK, et al. Norepinephrine transporter blockade with atomoxetine induces hypertension in patients with impaired autonomic function. Hypertension. 2007;50:47–53.10.1161/HYPERTENSIONAHA.107.089961Search in Google Scholar
[129] Linder JR, Sodhi SK, Haynes WG, Fiedorowicz JG. Effects of antipsychotic drugs on cardiovascular variability in participants with bipolar disorder. Hum Psychopharmacol. 2014;29:145–51.10.1002/hup.2380Search in Google Scholar
[130] Bo G, Cocito L, Maffini M, Perfumo P, Venturi S. Arterial hypertension caused by carbamazepine. Riv Neurol. 1986;57:333–5.Search in Google Scholar
[131] Mosqueda-Garcia R, Oates JA, Appalsamy M, Robertson D. Administration of carbamazepine in the nucleus of the solitary tract inhibits the antihypertensive effect of clonidine. Eur J Pharmacol. 1991;197:213–6.10.1016/0014-2999(91)90524-TSearch in Google Scholar
[132] Löscher W. Basic pharmacology of valproate. CNS Drugs. 2002;16:669–94.10.2165/00023210-200216100-00003Search in Google Scholar PubMed
[133] Jette N, Veregin T, Guberman A. Carbamazepine-induced hypertension. Neurology. 2002;59:275–6.10.1212/WNL.59.2.275Search in Google Scholar
[134] De Hert M, Vancampfort D, Correll CU, Mercken V, Peuskens J, Sweers K, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2011;199:99–105.10.1192/bjp.bp.110.084665Search in Google Scholar PubMed
[135] Vanderlip ER, Fiedorowicz JG, Haynes WG. Screening, diagnosis, and treatment of dyslipidemia among persons with persistent mental illness: a literature review. Psychiatr Serv. 2012;63:693–701.10.1176/appi.ps.201100475Search in Google Scholar PubMed
[136] Kilbourne AM, Post EP, Bauer MS, Zeber JE, Copeland LA, Good CB, et al. Therapeutic drug and cardiovascular disease risk monitoring in patients with bipolar disorder. J Affect Disord. 2007;102:145–51.10.1016/j.jad.2007.01.006Search in Google Scholar PubMed
[137] Kreyenbuhl J, Dickerson FB, Medoff DR, Brown CH, Goldberg RW, Fang L, et al. Extent and management of cardiovascular risk factors in patients with type 2 diabetes and serious mental illness. J Nerv Ment Dis. 2006;194:404.10.1097/01.nmd.0000221177.51089.7dSearch in Google Scholar PubMed PubMed Central
[138] Li C, Mittal D, Owen RR. Impact of patients’ preexisting metabolic risk factors on the choice of antipsychotics by office-based physicians. Psychiatr Serv. 2011;62:1477–84.10.1176/appi.ps.000882011Search in Google Scholar PubMed
[139] Wehr LM, Vanderlip ER, Gibbons PH, Fiedorowicz JG. Psychiatry residents’ perceptions and reported practices in providing primary care. J Grad Med Educ. 2017;9:237–40.10.4300/JGME-D-16-00351.1Search in Google Scholar PubMed PubMed Central
[140] Mitchell AJ, Delaffon V, Vancampfort D, Correll CU, De Hert M. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med. 2012;42:125–47.10.1017/S003329171100105XSearch in Google Scholar PubMed
[141] Vanderlip ER, Raney LE, Druss BG. A Framework for extending psychiatrists’ roles in treating general health conditions. Am J Psychiatry. 2016;173:658–63.10.1176/appi.ajp.2015.15070950Search in Google Scholar PubMed
[142] Vanderlip ER, Williams NA, Fiedorowicz JG, Katon W. Exploring primary care activities in ACT teams. Community Ment Health J. 2014;50:466–73.10.1007/s10597-013-9673-8Search in Google Scholar PubMed PubMed Central
[143] Vanderlip ER, Henwood BF, Hrouda DR, Meyer PS, Monroe-DeVita M, Studer LM, et al. Systematic literature review of general health care interventions within programs of assertive community treatment. Psychiatr Serv. 2017;68:218–24.10.1176/appi.ps.201600100Search in Google Scholar PubMed
[144] Fiedorowicz JG, Miller DD, Bishop JR, Calarge CA, Ellingrod VL, Haynes WG. Systematic review and meta-analysis of pharmacological interventions for weight gain from antipsychotics and mood stabilizers. Curr Psychiatry Rev. 2012;8:25–36.10.2174/157340012798994867Search in Google Scholar PubMed PubMed Central
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Original Articles
- Food addiction: is it a nosological category or a psychopathological dimension? Preliminary results of an Italian study
- Original Articles
- Cardiometabolic effects of psychotropic medications
- Direct and indirect effects of psychopharmacological treatment on the cardiovascular system
- Metabolic and neuroendocrine adaptations to undernutrition in anorexia nervosa: from a clinical to a basic research point of view
Articles in the same Issue
- Original Articles
- Food addiction: is it a nosological category or a psychopathological dimension? Preliminary results of an Italian study
- Original Articles
- Cardiometabolic effects of psychotropic medications
- Direct and indirect effects of psychopharmacological treatment on the cardiovascular system
- Metabolic and neuroendocrine adaptations to undernutrition in anorexia nervosa: from a clinical to a basic research point of view

