Abstract
A novel approach to the synthesis of 2-cyano-6-methoxybenzothiazole via the Cu-catalyzed cyanation of 2-iodo-6-methoxybenzothiazole was developed. K4[Fe(CN)6] was used as a source of cyanide, and a Cu/N,N,N′,N′-tetramethylethylenediamine (TMEDA) system was utilized as a catalyst. This approach is scalable and can be practiced with operational benign. The most stable conformation of 2-cyano-6-methoxybenzothiazole was delineated using the density functional theory (DFT)/B3LYP method with 6-311++G(d, p) basis set.
Introduction
Firefly luciferin is a common substrate in biological imaging [1] that originates from some insects [2]. It is widely applied in life sciences [[3], [4], [5]. The chemical preparation of firefly luciferin has been described previously [6], [7], [8], [9]. In particular, 2-cyano-6-methoxybenzothiazole has been converted to 2-cyano-6-hydroxybenzothiazole followed by the reaction with cysteine 6] (Scheme 1). Typical routes to 2-cyano-6-methoxybenzothiazole include the classical Rosenmund-von Braun [10] and Sandmeyer [11] reactions. These methods proceed with low atom economy and require toxic reagents such as KCN [12], NaCN [13], Zn(CN)2 or TMSCN [14] that are also difficult to handle in a large-scale synthesis [15], [16].

Synthesis of firefly luciferin.
The Cu-catalyzed cyanation of aryl halides to benzonitrile derivatives has been reported [13]. Various copper catalyst systems with bidentate ligands [17], [18], [19], [20], [21], [22], [23] have been developed. In 2004, Beller and co-workers introduced K4[Fe(CN)6] as a low-cost and eco-friendly source of cyanide [19]. Due to its strong CN bond, the catalyst deactivation was prevented through a slow release of cyanide ion [24]. Herein, a Cu-catalyzed cyanation of 2-iodo-6-methoxybenzothiazole for the synthesis of 2-cyano-6-methoxybenzothiazole was introduced. K4[Fe(CN)6] was applied as a source of cyanide, and CuI in the presence of N,N N′,N′-tetramethylethylenediamine (TMEDA) [25] was used as part of the catalyst system. Density functional theory (DFT) calculations were made with the structural parameters calculated using the B3LYP/6-311++G(d, p) method [26], [27], [28], [29].
Results and discussion
2-Amino-6-methoxybenzothiazole as a starting material was synthesized from p-anisidine as shown in Scheme 2 [30] and subsequently converted into 2-iodo-6-methoxybenzothiazole using a simple and efficient one-pot sequential diazotization-iodination method.
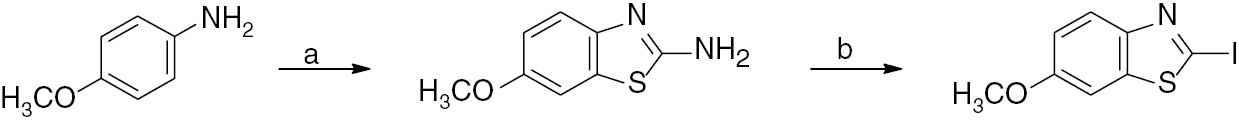
Synthesis of 2-iodo-6-methoxybenzothiazole from p-anisidine.
Reagents and conditions: (a) –CH3COOH, Br2, KSCN, 20 h, temperature <35°C, yield 85%; (b) –H2SO4, NaNO2, KI, 0°C, 30 min, yield 80%.
The one-pot cyanation of 2-iodo-6-methoxybenzothiazole to 2-cyano-6-methoxybenzothiazole was achieved using 0.25 mmol of K4[Fe(CN)6], 0.25 mmol of CuI and 3 mmol of TMEDA in acetonitrile at 160°C (Scheme 3). In addition, 1 mmol of mystril trimethyl bromide (MTMAB) was used as a phase transfer agent. The presence of a phase-transfer catalyst is essential for a successful cyanation reaction. Under these conditions, 2-cyano-6-methoxybenzothiazole was produced in a 90% yield. In the presence of 0.18 mmol of K4[Fe(CN)6], the cyanation still proceeded in a 90% yield, indicating that all six cyanide ions in the molecule are utilized in the reaction. On the other hand, increasing the amount of K4[Fe(CN)6] to more than 0.25 mmol resulted in a moderate yield of 60%, presumably due to the catalyst deactivation. In addition, a decrease in the CuI loading to 0.1 mmol from 0.25 mmol, resulted in a poor yield of 40%, even after an increase of the reaction time to 40 h.
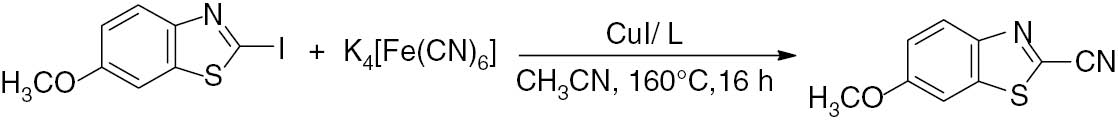
Cyanation reaction of 2-iodo-6-methoxybenzothiazole.
The optimized geometric structures of 2-cyano-6-methoxyoxybenzothiazole were calculated using the B3LYP 6-311++G(d, p) method. This molecule has C1 full point group and 51 fundamental modes of vibrations. It exists in two stable conformers, I and II. Conformer III is the transition state structure (Figure 1). Conformer I with the dihedral angle (C7-C6-O15-C16) of 1.4° is 0.17 kcal/mol more stable than conformer II and 2.02 kcal/mol more stable than conformer III. The dihedral angle (C7-C6-O15-C16) of the second stable structure is 179.98°.
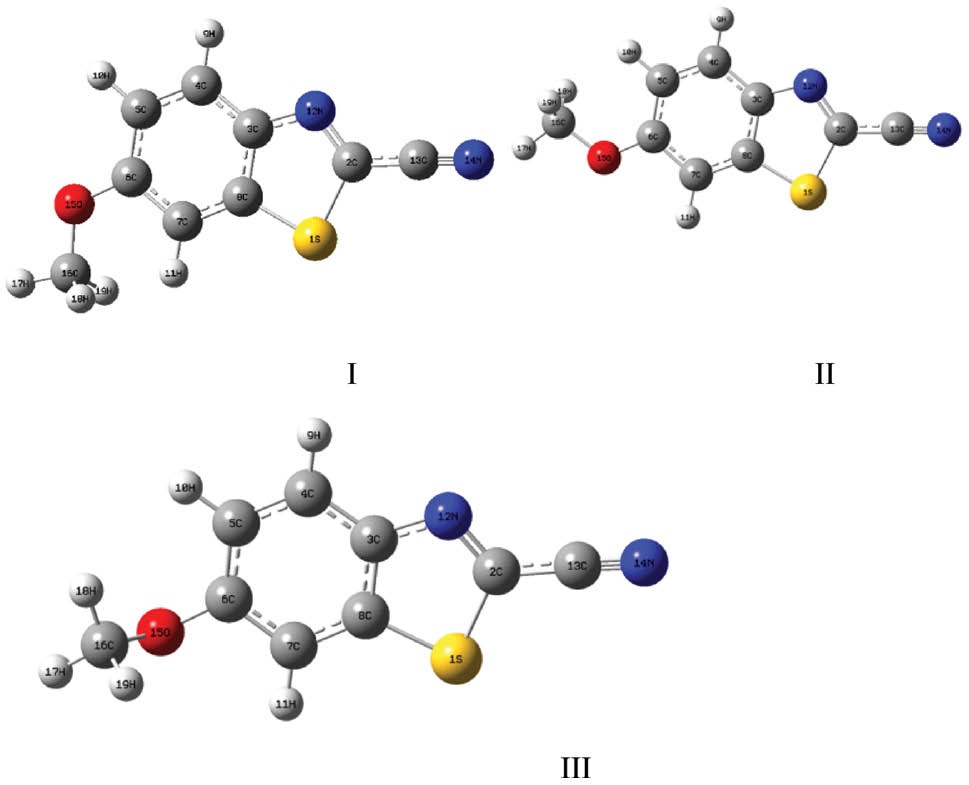
Conformers of 2-cyano-6-methoxybenzothiazole.
Conclusions
2-Cyano-6-methoxybenzothiazole, a synthetic intermediate of firefly luciferin, was prepared by a high-yield Cu-catalyzed cyanation of 2-iodo-6-methoxybenzothiazole. K4[Fe(CN)6] was applied as an eco-friendly cyanide source. The cyanation reaction was performed efficiently by applying the catalytic system of CuI/TMEDA that is non-toxic and environmentally friendly. The structure of 2-cyano-6-methoxybenzothiazole was characterized using computational studies.
Experimental
All chemicals and solvents were supplied by Merck (Germany) and used without purification. The Fourier transform infrared (FT-IR) spectra were recorded on an Alpha Centauri FT-IR (Bruker, Germany) spectrophotometer using KBr pellets. The 1H NMR (300 MHz) and 13C NMR (75 MHz) spectra were determined on a Mercury-300 MHz (Bruker, Germany) instrument. The mass spectra were obtained on an Agilent 575 mass spectrometer equipped with a quadruple analyzer. 2-Amino-6-methoxybenzothiazole was synthesized according to the method described by Stuckwisch [30].
Synthesis of 2-iodo-6-methoxybenzothiazole
A solution of 2-amino-6-methoxybenzothiazole (1.81 g, 0.01 mol) in water (3 mL) was stirred at 0°C and treated with acetic acid (3 mL) and sulfuric acid (6 m, 4.5 mL). The mixture was stirred for 1 h. The resultant clear solution was treated dropwise for 15 min at 0°C with an aqueous solution (3 mL) of NaNO2 (0.70 g, 0.01 mol). After stirring for 1 h, an aqueous solution (3 mL) of KI (1.65 mg, 0.01 mol) was added slowly for 30 min, until the evolution of nitrogen gas ceased. The product was extracted with EtOAc (3×12 mL), and the combined organic layers were washed with a 10% aqueous solution of Na2SO4, dried and concentrated. The residue of 2-iodo-6-methoxybenzothiazole was crystallized from ethanol; 1H NMR (300 MHz, DMSO-d6): δ 3.67 (s, 3H), 6.87 (m, 1H), 7.41 (m, 1H), 7.71 (m, 1H); IR: 2932, 1651, 1239, 1208 (C-N), 1053, 875 cm−1; MS: m/z 291.0 (M+). Anal. Calcd for C8H6INOS: C, 33.01; H, 2.15; N: 4.87. Found: C, 32.27; H, 2.08; N, 4.81.
Synthesis of 2-cyano-6-methoxybenzothiazole from 2-iodo-6-methoxybenzothiazole
A solution of CuI (47.6 mg, 0.25 mmol), K4[Fe(CN)6] (90 mg, 0.18 mmol) and TMEDA (348 mg, 3 mmol) in dry acetonitrile (5 mL) was stirred for 30 min and then treated with MTMAB (34 mg, 1 mmol) and 2-iodo-6-methoxybenzothiazole (290 mg, 1 mmol). The mixture was stirred under argon atmosphere at room temperature for 30 min and then under reflux at 160°C for 16 h. After cooling to room temperature, the mixture was extracted with diethyl ether (3×5 mL). The extract was dried with anhydrous MgSO4, filtered and concentrated. The red-brown solid residue was purified by column chromatography on silica gel eluting with hexane/CH2Cl2, 1:10, to give 2-cyano-6-methoxybenzothiazole in the form of pale yellow needles; yield 90%; mp 128–130°C; 1H NMR (300 MHz, CDCl3): δ 3.87 (s, 3H), 7.11 (m, 1H), 7.23 (m, 1H), 7.52 (m, 1H); 13C NMR (75 MHz, DMSO-d6): δ 160.6, 147.3, 141.3, 135.6, 125.6, 118.6, 113.1, 103.5, 55.6; IR: 3030, 2932, 2240, 1651, 1280, 1207, 1034, 872 cm−1; MS: m/z 190.1 (M+). Anal. Calcd for C9H6N2OS: C, 56.83; H, 3.18; N, 14.73. Found: C, 56.68; H, 3.01; N, 14.42.
References
[1] Wood, K. V. The chemical mechanism and evolutionary development of beetle bioluminescence. Photochem. Photobiol.1995, 62, 662–673.10.1111/j.1751-1097.1995.tb08714.xSearch in Google Scholar
[2] Ugarova, N. N.; Brovko, L. Y. Protein structure and bioluminescent spectra for firefly bioluminescence. Luminescence2002, 17, 321–330.10.1142/9789812776624_0013Search in Google Scholar
[3] Hamada, T.; Sutherland, K.; Ishikawa, M.; Miyamoto, N.; Honma, S.; Shirato, H.; Honma, K. I. In vivo imaging of clock gene expression in multiple tissues of freely moving mice. Nat. Commun.2016, 7, 11705.10.1038/ncomms11705Search in Google Scholar
[4] Li, J.; Chen, L.; Du, L.; Li, M. Cage the firefly luciferin – a strategy for developing bioluminescent probes. Chem. Soc. Rev.2013, 42, 662–676.10.1039/C2CS35249DSearch in Google Scholar
[5] Sellmyer, M. A.; Bronsart, L.; Imoto, H.; Contag, C. H.; Wandless, T. J.; Prescher, J. A. Visualizing cellular interactions with a generalized proximity reporter. Proc. Natl. Acad. Sci. USA2013, 110, 8567–8572.10.1073/pnas.1218336110Search in Google Scholar
[6] White, E. H.; McCapra, F.; Field, G. F. The structure and synthesis of firefly luciferin. J. Am. Chem. Soc. 1963, 85, 337–343.10.1021/ja00886a019Search in Google Scholar
[7] White, E. H.; Rapaport, E.; Seliger, H. H.; Hopkins, T. A. The chemi- and bioluminescence of firefly luciferin: an efficient chemical production of electronically excited states. Bioorg. Chem.1971, 1, 92–122.10.1016/0045-2068(71)90009-5Search in Google Scholar
[8] Seto, S.; Ogura, K.; Nishiyama, Y. A convenient synthetic method of 2-carbamoyl-6-methoxybenzothiazole, one of intermediates for the synthesis of firefly luciferin. Bull Chem. Soc. Jpn.1963, 36, 331–333.10.1246/bcsj.36.331Search in Google Scholar
[9] Toya, Y.; Takagi, M.; Nakata, H.; Suzuki, N.; Isobe, M.; Goto, T. A convenient synthetic method of 2-cyano-6-methoxybenzothiazole – a key intermediate for the synthesis of firefly luciferin. Bull Chem. Soc. Jpn.1992, 65, 392–395.10.1246/bcsj.65.392Search in Google Scholar
[10] Rosenmund, K. W.; Harms, H. Das am Ringkohlenstoff gebundene Halogen und sein Ersatz durch andere Substituenten. I. Mitteilung: Ersatz des Halogens durch die Carboxylgruppe. Eur. J. Inorg. Chem.1919, 52, 1749–1756.10.1002/cber.19190520840Search in Google Scholar
[11] Sandmeyer, T. Ueber die Ersetzung der Amidgruppe durch Chlor in den aromatischen Substanzen. Eur. J. Inorg. Chem.1884, 17, 1633–1635.10.1002/cber.18840170219Search in Google Scholar
[12] Ren, Y.; Liu, Z.; Zhao, S.; Tian, X.; Wang, J.; Yin, W.; He, S. Ethylenediamine/Cu (OAc)2 H2O-catalyzed cyanation of aryl halides with K4 [Fe(CN)6]. Catal. Commun.2009, 10, 768–771.10.1016/j.catcom.2008.11.034Search in Google Scholar
[13] Zanon, J.; Klapars, A.; Buchwald, S. L. Copper-catalyzed domino halide exchange-cyanation of aryl bromides. J. Am. Chem. Soc.2003, 125, 2890–2891.10.1021/ja0299708Search in Google Scholar
[14] Sundermeier, M.; Mutyala, S.; Zapf, A.; Spannenberg, A.; Beller, M. A convenient and efficient procedure for the palladium-catalyzed cyanation of aryl halides using trimethylsilylcyanide. J. Organomet. Chem.2003, 684, 50–55.10.1016/S0022-328X(03)00503-5Search in Google Scholar
[15] Velmathi, S.; Leadbeater, N. E. Palladium-catalyzed cyanation of aryl halides using K4[Fe(CN)6] as cyanide source, water as solvent, and microwave heating. Tetrahedron Lett.2008, 49, 4693–4694.10.1016/j.tetlet.2008.05.124Search in Google Scholar
[16] Grossman, O.; Gelman, D. Novel trans-spanned palladium complexes as efficient catalysts in mild and amine-free cyanation of aryl bromides under air. Org. Lett.2006, 8, 1189–1191.10.1021/ol0601038Search in Google Scholar PubMed
[17] Beletskaya, I. P.; Sigeev, A. S.; Peregudov, A. S.; Petrovskii, P. V. Catalytic Sandmeyer cyanation as a synthetic pathway to aryl nitriles. J. Organomet. Chem.2004, 689, 3810–3812.10.1016/j.jorganchem.2004.07.019Search in Google Scholar
[18] Schareina, T.; Zapf, A.; Maegerlein, W.; Mueller, N.; Beller, M. Copper-catalyzed cyanation of heteroaryl bromides: a novel and versatile catalyst system inspired by nature. Synlett.2007, 4, 0555–0558.10.1002/chin.200728100Search in Google Scholar
[19] Schareina, T.; Zapf, A.; Beller, M. An environmentally benign procedure for the Cu-catalyzed cyanation of aryl bromides. Tetrahedron Lett.2005, 46, 2585–2588.10.1016/j.tetlet.2005.02.106Search in Google Scholar
[20] Lang, H.; Jakob, A.; Milde, B. Copper (I) alkyne and alkynide complexes. Organometallics2012, 31, 7661–7693.10.1021/om300628gSearch in Google Scholar
[21] Evano, G.; Blanchard, N.; Toumi, M. Copper-mediated coupling reactions and their applications in natural products and designed biomolecules synthesis. Chem. Rev.2008, 108, 3054–3131.10.1021/cr8002505Search in Google Scholar
[22] Zhang, G.; Yi, H.; Zhang, G.; Deng, Y.; Bai, R.; Zhang, H.; Miller, J. T.; Kropf, A. J.; Bunel, E. E.; Lei, A. Direct observation of reduction of Cu (II) to Cu (I) by terminal alkynes. J. Am. Chem. Soc.2014, 136, 924–926.10.1021/ja410756bSearch in Google Scholar
[23] Schareina, T.; Beller, M. Copper-Catalyzed Cyanations of Aryl Halides and Related Compounds. In Copper-Mediated Cross-Coupling Reactions. Evano, G., Blanchard, N., Eds. Wiley: New Jersey, 2013; pp 313–334.10.1002/9781118690659.ch9Search in Google Scholar
[24] Schareina, T.; Zapf, A.; Cotte, A.; Mueller, N.; Beller, M. A bio-inspired copper catalyst system for practical catalytic cyanation of aryl bromides. Synthesis2008, 20, 3351–3355.10.1002/chin.200909081Search in Google Scholar
[25] Sigeev, A.; Beletskaya, I.; Petrovskii, P.; Peregudov, A. Cu (I)/Cu (II)/TMEDA, new effective available catalyst of sandmeyer reaction. Russ. J. Org. Chem.2012, 48, 1055–1058.10.1134/S1070428012080040Search in Google Scholar
[26] Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev.1988, 38, 3098.10.1103/PhysRevA.38.3098Search in Google Scholar
[27] Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision A. 02; Gaussian Inc.: Wallingford, CT, 2009.Search in Google Scholar
[28] Dega-Szafran, Z.; Katrusiak, A.; Szafran, M. Molecular structure of the complex of N-methylmorpholine betaine with 2,4-dinitrophenol. J. Mol. Struct.2005, 741, 1–9.10.1016/j.molstruc.2004.12.062Search in Google Scholar
[29] Tachikawa, M.; Mori, K.; Nakai, H.; Iguchi, K. An extension of ab initio molecular orbital theory to nuclear motion. Chem. Phys. Lett.1998, 290, 437–442.10.1016/S0009-2614(98)00519-3Search in Google Scholar
[30] Stuckwisch, C. Derivatives of 2-amino-6-methoxybenzothiazole. J. Am. Chem. Soc.1949, 71, 3417–3417.10.1021/ja01178a043Search in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Antioxidant, α-glucosidase inhibitory and in vitro antitumor activities of coumarin-benzothiazole hybrids
- Synthesis and properties of tetracyanoquinodimethane derivatives
- Research Articles
- Synthesis, characterization and computational studies of 2-cyano-6-methoxybenzothiazole as a firefly-luciferin precursor
- Synthesis of fluorine-containing phthalocyanines and investigation of the photophysical and photochemical properties of the metal-free and zinc phthalocyanines
- Copper-catalyzed synthesis of 2,3-disubstituted quinazolin-4(3H)-ones from benzyl-substituted anthranilamides
- Synthesis and mass spectrometric fragmentation pattern of 6-(4-chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines
- An efficient cascade synthesis of substituted 6,9-dihydro-1H-pyrazolo[3,4-f]quinoline- 8-carbonitriles
- Synthesis and antimicrobial evaluation of isoxazole-substituted 1,3,4-oxadiazoles
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Antioxidant, α-glucosidase inhibitory and in vitro antitumor activities of coumarin-benzothiazole hybrids
- Synthesis and properties of tetracyanoquinodimethane derivatives
- Research Articles
- Synthesis, characterization and computational studies of 2-cyano-6-methoxybenzothiazole as a firefly-luciferin precursor
- Synthesis of fluorine-containing phthalocyanines and investigation of the photophysical and photochemical properties of the metal-free and zinc phthalocyanines
- Copper-catalyzed synthesis of 2,3-disubstituted quinazolin-4(3H)-ones from benzyl-substituted anthranilamides
- Synthesis and mass spectrometric fragmentation pattern of 6-(4-chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines
- An efficient cascade synthesis of substituted 6,9-dihydro-1H-pyrazolo[3,4-f]quinoline- 8-carbonitriles
- Synthesis and antimicrobial evaluation of isoxazole-substituted 1,3,4-oxadiazoles

