Synthesis, optical and electrochemical properties of 2-[(9H-fluoren-2-yl)aryl]-1H-benz[d]imidazole and 2,7-bis[(1H-benz[d]imidazol-2-yl)aryl]- 9H-fluorene derivatives
Abstract
A series of 2-[(9H-fluoren-2-yl)aryl]-1H-benzo[d]imidazoles 11–13 and 2,7-bis[(1H-benzo[d]imidazol-2-yl)aryl]-9H-fluorenes 14–16 containing different linking aromatic units were synthesized in good yields. Their absorption and fluorescence properties were investigated in solution and in the solid state. Most compounds possess good fluorescence-emitting ability with φFL values in the region of 0.31–0.99 in solution and display strong blue emission. Structure–optical behavior characteristics and further details of the electronic properties from cyclic voltammetry measurements and theoretical calculations are discussed.
Introduction
Fluorescent heterocyclic compounds are of interest as emitters for electroluminescence devices, molecular probes for biochemical research, dyes for textiles and polymers, fluorescent whitening agents and photo-conducting materials [1–9] among other things. In particular, imidazole derivatives including phenylbenzo[d]imidazoles [10–21] and 1,3,5-tris(1-phenylbenzo[d]imidazol-2-yl)benzene (TPBI) have found applications in electrical and optical materials [22–24].
Due to their rigid oligophenyl unit within the molecular backbone and the possibility of facile functionalization at the methylene bridge, fluorene-based derivatives, including polymers and oligomers, are important as luminescent materials [19, 25–28], as well as carrier transport materials for field effect transistors (FETs) [29–31]. Their fluorescent characteristics rely largely on molecular structure and molecular assembly. Changes in the substitution pattern, conjugation, and molecular electronic structure can bring about very different optical and physical properties for such materials. There is presently a great interest to increase the structural or spatial dimensions of π conjugated molecules in order to acquire more favorable physical properties. In continuation of our efforts in synthesizing various fluorescent small molecules [32–35], in this paper we describe molecular combinations of fluorene and benzo[d]imidazole linked by π-conjugated moiety. We report the structure-optical behavior characteristics and further details of the electronic properties from cyclic voltammetry measurements and theoretical calculations of novel 2-[(9H-fluoren-2-yl)aryl]-1H-benzo[d]imidazole derivatives 11a–c, 12a,b, 13a,b and 2,7-bis[(1H-benzo[d]imidazol-2-yl)aryl]-9H-fluorene derivatives 14a–c, 15a,b, 16a,b. Extended conjugation is believed to result in intermolecular stacking interactions which are detrimental to the emission characteristics. Therefore, two n-hexyl or two methyl groups were introduced to the C-9 position of the fluorene moiety to increase solubility as well as to hinder intermolecular π-π stacking.
Results and discussion
The key aldehyde intermediates 3–5 and 7–9 were prepared as shown in Scheme 1. The bromo precursors 2 and 6 were conveniently converted to the arylated aldehyde derivatives 3–5 and 7-9 in 70–80% yields by treatment with the corresponding boronic acids in the presence of PdCl2(PPh3)2 and K2CO3 under the Suzuki reaction conditions [36]. Condensation of 3–5 or 7–9 with the diaminobenzene derivatives 10 in dioxane at 110°C for 2-5 h afforded the target compounds 11a–13b and 14a–16b in 60–80% yields (Scheme 2). All target compounds were purified by column chromatography on silica gel and their molecular structures were confirmed by 1H NMR, 13C NMR, and HR-MS.

Synthesis of the aldehyde intermediates.

Synthesis of 11a–16b.
The ORTEP view of the structure of 11b in Figure 1A indicates that the moieties of fluorene, phenylene and 2-phenyl-1H-benzo[d]imidazole are non-coplanar with dihedral angles of 7.829o and 33.613°, respectively. In compound 13b, as shown in Figure 2A, the fluorene moiety forms torsion angle of 13.100° with the adjacent thiophene ring ii, and the thiophene ring ii forms a torsion angle of 18.325° with the imidazole ring iii. The crystal packings of 11b and 13b are shown in Figures 1B and 2B. The molecules are stacked in the overlapping manner and face-to-face π-π intermolecular interactions between molecules can be regarded as the stacking force.

Molecular structures of compound 11b.
The H atoms are omitted for clarity: (A) ORTEP drawing, (B) a view of the molecule packing structure.

Molecular structures of compound 13b.
The H atoms are omitted for clarity: (A) ORTEP drawing, (B) a view of the molecule packing structure.
The photophysical properties of compounds 11a–13b were examined using UV-vis absorption and fluorescence spectra in dilute solutions and in solid films. The absorption peak wavelengths (λabs) and the emission wavelengths (λem) are listed in Experimental. The emission spectra in the solid state were obtained for films spin-coated onto quartz from chloroform solution. All compounds show similar absorption profiles, principally owing to π-π* transitions of the molecular backbone. Compounds 11a, 12a, 13a, 14a, 15a and 16a exhibit the absorption maxima at 337 nm, 368 nm, 378 nm, 360 nm, 394 nm, and 400 nm in diluted CH2Cl2 solutions, respectively. The absorption maxima of 11a–16b follow the order: 11a–c (benzene) <12a,b (furan) <13a,b (thiophene) (Figure 3), 14a–c (benzene) <15a,b (furan) <16a,b (thiophene). This result demonstrates that the heterocyclic ring plays an important role in the UV-vis absorption spectra due to the lone pair electrons which can cause n-π* transitions with lower energy in the heteroatom (O, S). Compounds 14a–c, 15a,b, and 16a,b have longer absorption wavelengths than 11a–c, 12a,b, and 13a,b due to the elongation of conjugation. Compounds 15a,b and 16a,b exhibit the longest absorption wavelengths. Interestingly, compounds 11b,c, 12b, 13b, 14b,c, 15b and 16b with the phenyl or para-MeO substituted phenyl group in N-1 positions have almost the same absorption maxima as those of N-H compounds 11a, 12a,13a, 14a, 15a and 16a, indicating that arylation of benz[d]imidazole has a limited effect on the energy levels of these compounds.

Normalized absorption spectra of 11a–13b (1×10-5 M) recorded in CH2Cl2.
Theoretically, the absorption spectra caused by π-π* transitions exhibit red shifts with the increase in solvent polarity. However, compounds 11a–16b show blue-shifted absorption maxima by 5–26 nm in diluted MeOH solutions in comparison with the dilute CH2Cl2 solutions. The blue-shifted emission in MeOH may be ascribed to hydrogen-bonding interactions, which probably inhibits the reorientation of the benzo[d]imidazole in the excited state by hindering the rotation of the benzene, furan and thiophene subunits.
All compounds 11a–16b display blue or blue-green emissions in dilute CH2Cl2 solutions (Figure 4). Compounds 11a, 12a, 13a, 14a, 15a and 16a show emission peaks at 452 nm, 456 nm, 461 nm, 457 nm, 463 nm and 496 nm, respectively. In contrast to the UV-vis absorption properties, the heteroatom (O, S) and the length of conjugation have less significant effects on the emission spectra of these compounds. A slight red-shift is observed for compounds 11c and 14c compared with their corresponding N-H analogues 11a and 14a. This result attests the partial involvement of the N-substituted chromophores in the electronically excited state. By comparison with the dilute CH2Cl2 solutions, the emission spectra of all compounds in MeOH solutions show shifts to shorter wavelengths by 37–68 nm, probably due to the hydrogen-bonding effects in MeOH solutions [37].

Normalized emission spectra of 11a–13b (1×10-5 M) recorded in CH2Cl2 solutions; excitation at λex at room temperature.
In comparison with the dilute CH2Cl2 solution, the emission spectra of compounds 11b,c, 12b, 13b, 14b,c and 15a,b in solid state are shifted to shorter wavelengths by 10–53 nm. These compounds probably form H-aggregates, which decreases the π-conjugation significantly, in turn, resulting in the observed blue shift [38]. However, compounds 11a, 12a and 14a show shift to longer wavelengths by 5–8 nm. The red shift of the emission observed in the solid state is probably due to the strong intermolecular forces, which drive the molecules to pack at high density to form J-aggregates with restricted molecular rotation and an increased π-conjugation, in turn, resulting in the red shift [39].
Most compounds show high fluorescence in CH2Cl2 solution with fluorescence quantum yields φFL in the range of 0.77–0.99 (Table 1). The relatively low quantum yields observed for compounds 13a,b and 15a,b with heteroatoms suggest a fluorescence quenching caused by the heteroatom.
Optical and electrochemical properties of compounds 11a–15b.
| Dye | φFLa | HOMOb | LUMOc | Egd | Dye | φFLa | HOMOb | LUMOc | Egd |
|---|---|---|---|---|---|---|---|---|---|
| 11a | 0.78 | 5.47 | 2.59 | 2.91 | 13b | 0.60 | 5.82 | 3.14 | 2.68 |
| 11b | 0.84 | 5.74 | 2.83 | 2.91 | 14a | 0.54 | 5.84 | 3.36 | 2.48 |
| 11c | 0.81 | 5.43 | 2.62 | 2.92 | 14b | 0.99 | 5.75 | 2.94 | 2.81 |
| 12a | 0.70 | 5.75 | 3.02 | 2.73 | 14c | 0.99 | 5.72 | 2.90 | 2.82 |
| 12b | 0.71 | 5.72 | 2.99 | 2.73 | 15a | 0.31 | 5.59 | 3.00 | 2.51 |
| 13a | 0.59 | 5.64 | 2.95 | 2.69 | 15b | 0.38 | 5.43 | 2.81 | 2.62 |
aFluorescence quantum yields measured in CH2Cl2 solution using a 0.1 M H2SO4 solution of quinine sulfate (φFL = 0.55) as a reference.
bHOMO energy levels derived from the oxidation potential using EHOMO = 4.6+Eox, the redox potentials were measured with cyclic voltammetry system.
cLUMO energy levels were deduced using the formula ELUMO = EHOMO-Eg.
dEg = 1240/UV(onset). UVs(onset) were estimated from the onset of the absorption spectra.
The redox potentials of all compounds were determined by cyclic voltammetry (CV) measurements which were carried out in a three-electrode cell setup with 0.1 M tetrabutylammonium perchlorate (Bu4NClO4) as a supporting electrolyte in anhydrous CH2Cl2 to probe the electrochemical behavior of the materials. The HOMO values were calculated as follows: HOMO = [Eonset]red/ox (vs SCE) + 4.6, Eg = 1240/UV (onset) [40, 41]. As can be seen from Table 1, the band gaps (Eg) of about 2.51–2.92 eV were estimated from the optical edge. Compounds 12a–15b exhibit relatively smaller band gaps by comparison with compounds 11a–c. The HOMO energies of these compounds were calculated by using the ferrocene/ferrocenium redox couple as a reference (4.6 eV) and were within the range of 5.43–5.84 eV. The LUMO energies were calculated from their corresponding HOMO energies and the optical band gap as estimated from the intersection of the absorption and emission peaks, which ranged from 2.59 eV to 3.36 eV. Compounds 14a–c show relatively higher HOMO and LUMO energies than 11a–c, probably due to the extended length of π-conjugation. However, in the case of 15a,b, the HOMO and LUMO are lower in energy than those of 12a,b; the ability of the heterocyclic ring (furan) to donate electrons is a possible explanation [42].
Density functional theory (DFT) calculations were also used to characterize the three-dimensional geometries and the frontier molecular orbital energy levels of the representative compounds 11b, 12b, 13b and 14b, 15b, 16b at the B3LYP/6-31G* level by using the Gaussian 03 program [40, 41]. The orbital plots of the HOMO-LUMO are illustrated in Figure 5. In the case of compounds 11b, 12b, 14b, 15b and 16b, the HOMO and LUMO are mainly localized on the fluorene and benzene (11b, 14b), furan (12b, 15b), and thiophene (16b) subunits, indicating that the absorption and emission processes are mostly attributed to the π-π* transitions centered at the fluorene and aromatic moieties. Interestingly, compound 13b spreads HOMO and LUMO through the thiophene subunit, which can explain the relatively high energy level. The LUMO of all compounds are not localized on the benzo[d]imidazole, which can explain the shorter-wavelength absorptions in polar solutions. The trends in the experimentally observed longer-wavelength absorptions for compounds 14b and 15b matches well the computed low-energy excitations.
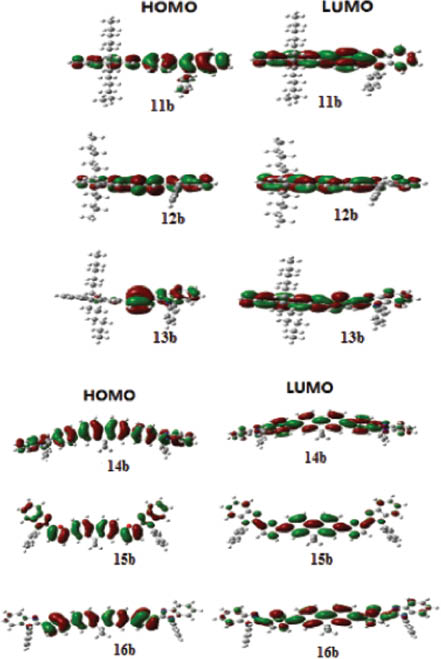
HOMO and LUMO electronic density distributions of 11b, 12b, 13b and 14b, 15b, 16b.
Conclusions
2-[(9H-Fluoren-2-yl)aryl]-1H-benzo[d]imidazole and 2,7-bis [(1H-benzo[d]imidazol-2-yl)aryl]-9H-fluorene derivatives containing central aromatic or heteroaromatic subunits were conveniently synthesized in good yields. These compounds were thoroughly characterized by spectral and computational methods. Most compounds display emission in a blue region with φFL values in the range of 0.31–0.99. The HOMO energy levels of about 5.43–5.84 eV and the LUMO energy levels of about 2.59–3.36 eV were calculated. Further research regarding application of these systems as organic light-emitting diodes and as fluorescent probes is under way in our laboratory.
Experimental
Commercially available reagents were purchased and used without further purification. 1H NMR (500 MHz) and 13C NMR (125 MHz) spectra were recorded on a Bruker Avance spectrometer in CDCl3 or DMSO-d6. Chemical shifts in CDCl3 were referenced to CHCl3 (7.26 ppm) for 1H NMR, and to CDCl3 (77.16 ppm) for 13C NMR. The center line of the multiplet of DMSO-d6 was taken as δ 2.50 for 1H NMR. Silica gel plates GF254 were used for thin layer chromatography (TLC), and silica gel H or 300–400 mesh was used for flash column chromatography. Melting points were measured on a digital melting point apparatus without correction. The absorption and fluorescence spectra were recorded using a UV-2501Pc spectrophotometer and a RF-5301 fluorescence spectrophotometer, respectively. High-resolution mass spectra were recorded on a Bruker APEX III 7.0 instrument. The redox potentials of compounds were determined with cyclic voltammetry (CV) using a Base 2000 CV system. The HOMO values were recalculated from the reported literature values as HOMO = [Eonset]red/ox (vs SCE) + 4.6; Eg = 1240/UV (onset).
General procedure for synthesis of 3–5
To a mixture of 2-bromo-9,9-dihexylfluorene (2) [34] (1 mmol), an aryl boronic acid (1.2 mmol), potassium carbonate (4 mmol) and PdCl2(PPh3)2 (0.1 mmol), was added toluene (9 mL) and water (1 mL) at room temperature. The mixture was heated to 90°C under nitrogen atmosphere for 8 h. After cooling, the mixture was extracted with ethyl acetate (10 mL). The organic extract was washed with saturated brine and dried over Na2SO4. After removing the solvent, the crude product was purified by column chromatography on silica gel using ethyl acetate (1%–2%) in petroleum ether as eluent to afford the final product 3–5.
4-(9,9-Dihexyl-9H-fluoren-2-yl)benzaldehyde (3)
This compound was obtained from 2 and (4-formylphenyl)boronic acid; yellow oil; yield 80%; 1H-NMR (CDCl3): δ 10.11 (s, 1H), 8.02 (d, J = 7.8 Hz, 2H), 7.74 (m, 4H), 7.67 (s, 2H), 7.41 (m, 3H), 2.04–2.11 (m, 4H); 13C-NMR (CDCl3): δ 191.9, 151.7, 151.1, 147.7, 141.8, 140.4, 138.4, 135.1, 130.3, 129.0, 127.7, 127.5, 127.0, 126.4, 126.6, 120.2, 120.0, 55.3, 40.4, 31.5, 29.7, 23.8, 22.6, 14.0. HR-MS. Calcd for C32H38O ([M + H]+): m/z 439.3001. Found: m/z 439.2995.
5-(9,9-Dihexyl-9H-fluoren-2-yl)furan-2-carbaldehyde (4)
This compound was obtained from 2 and (5-formylfuran-2-yl)boronic acid; yellow solid; yield 76%; 1H-NMR (CDCl3): δ 9.68 (s, 1H), 7.86 (d, J = 1.3 Hz,1H), 7.77 (m, 3H), 7.37 (m, 4H), 6.91 (d, J = 3.3 Hz,1H), 2.05 (m, 4H), 1.11 (m, 15H), 0.76 (t, J = 7.3 Hz, 6H), 0.59-0.68 (m, 4H); 13C-NMR (CDCl3): δ 177.0, 160.4, 151.9, 151.6, 143.0, 140.2, 127.9, 127.6, 127.0, 124.6, 123.0, 120.1, 119.5, 107.6, 55.4, 40.4, 31.5, 29.7, 29.7, 23.7, 22.6, 14.0. HR-MS. Calcd for C30H36O2 ([M + H]+): m/z 429.2793. Found: m/z 429.2895.
5-(9,9-Dihexyl-9H-fluoren-2-yl)thiophene-2-carbaldehyde (5)
This compound was obtained from 2 and (5-formylthiophen-2-yl)boronic acid; brown yellow solid; yield 71%; 1H-NMR (CDCl3): δ 9.92 (s, 1H), 7.79 (d, J = 4.4 Hz, 1H), 7.75 (m, 2H), 7.68–7.71 (m, 2H), 7.49 (d, J = 4.3 Hz, 1H), 7.37 (m, 3H), 2.02 (m, 4H), 1.01–1.17 (m, 15H), 0.77 (t, J = 7.5 Hz, 6H), 0.66 (m, 4H);13C-NMR (CDCl3): δ 182.7, 155.3, 151.8, 151.1, 142.0, 140.1, 129.5, 127.8, 127.0, 126.4, 125.5, 124.1, 123.8, 123.0, 120.7, 120.3, 120.1, 55.3, 40.3, 31.4, 29.6, 23.7, 22.5, 14.0. HR-MS. Calcd for C30H36OS ([M + H]+): m/z 445.2565. Found: m/z 445.2535.
General procedure for synthesis of 7–9
To a mixture of 2,7-dibromo-9,9-dimethyl-9H-fluorene (6, 1 mmol), an aryl boronic acid (2.4 mmol), potassium carbonate (4 mmol), PdCl2(PPh3)2 (0.2 mmol) was added toluene (9 mL) and water (1 mL) at room temperature. The mixture was heated to 90°C under nitrogen atmosphere for 24 h, then cooled and extracted with ethyl acetate (10 mL) and water (10 mL). The organic layer was washed with saturated brine and dried over Na2SO4. After removing the solvent, the crude product was purified by column chromatography on silica gel using ethyl acetate (2%–3%)/petroleum ether as eluent to afford the final purified product 7–9.
4,4′-(9,9-Dimethyl-9H-fluorene-2,7-diyl)bisbenzaldehyde (7)
This compound was obtained from 6 and (4-formylphenyl)boronic acid; yellow solid; yield 70%; 1H-NMR (CDCl3): δ 10.10 (s, 2H), 8.01 (d, J = 8.8 Hz, 3H), 7.87 (m,5 H), 7.71 (m, 5H), 7.46–7.52 (m, 1H), 1.64 (s, 6H); 13C-NMR (CDCl3): δ 191.9, 154.8, 147.4, 139.9, 135.1, 132.1, 128.6, 127.7, 121.6, 120.8, 47.2, 27.2. HR-MS. Calcd for C29H22O2 ([M + Na]+): m/z 425.1518. Found: m/z 425.1516.
5,5′-(9,9-Dimethyl-9H-fluorene-2,7-diyl)bis(furan-2-carbaldehyde) (8)
This compound was obtained from 6 and (5-formylfuran-2-yl)boronic acid; yellow solid; yield 62%; 1H-NMR (DMSO-d6): δ 9.68 (s, 2H), 7.95 (s, 2H), 7.80-7.86 (m, 5H), 7.67–7.75 (m, 2H), 7.66–7.11 (m, 1H), 7.48 (m, 2H), 7.38 (d, J = 4.4 Hz, 2H), 6.94 (d, J = 4.4 Hz, 2H), 1.61 (s, 6H); 13C-NMR (DMSO-d6): δ 177.1, 159.8, 154.9, 152.0, 139.9, 132.1, 132.0, 131.9, 131.9, 128.5, 128.4, 128.4, 124.9, 120.9, 119.5, 107.8, 47.3, 26.9. HR-MS. Calcd for C25H18O4 ([M + Na]+): m/z 405.1103. Found: m/z 405.0640.
5,5′-(9,9-Dimethyl-9H-fluorene-2,7-diyl)bis(thiophene-2-carbaldehyde) (9)
This compound was obtained from 6 and (5-formylthiophen-2-yl)boronic acid; yellow solid; yield 60%; 1H-NMR (DMSO-d6): δ 9.98 (s, 2H), 7.86 (s, 2H), 7.67 (m, 4H), 7.59 (m, 3H), 7.50 (m, 1H), 7.41 (m, 2H), 7.30 (d, J = 4.2 Hz, 2H), 6.95 (d, J = 4.4 Hz, 2H), 1.59 (s, 6H); 13C-NMR (DMSO-d6): δ 179.1, 163.6, 158.5, 156.0, 149.9, 145.1, 136.0, 135.6, 131.7, 126.5, 123.4, 123.4, 121.2, 119.9, 117.5, 107.1, 45.5, 23.3. HR-MS. Calcd for C25H18O2S2 ([M + Na]+): m/z 437.0646. Found: m/z 437.0640.
General procedure for synthesis of 11a–c
To a mixture of 4-(9,9-dihexyl-9H-fluoren-2-yl)benzaldehyde (3, 0.7 mmol), benzene-1,2-diamine, N-phenylbenzene-1,2-diamine or N-(4-methoxyphenyl)benzene-1,2-diamine (10a–c, 1.0 mmol), and TsOH (0.1 mmol), was added dioxane (7 mL) at room temperature. The mixture was heated under reflux for 9 h, then concentrated, and the residue was purified by column chromatography on silica gel using ethyl acetate (5%–10%)/petroleum ether as eluent to afford the final product.
2-[4-(9,9-Dihexyl-9H-fluoren-2-yl)phenyl]-1H-benz[d]imidazole (11a)
This compound was obtained from 3 and 10a; yellow solid; yield 80%; mp > 400 °C; 1H-NMR (CDCl3): δ 8.20 (d, J = 8.3 Hz, 2H), 7.59-7.83 (m, 8H), 7.29-7.40 (m, 5H), 2.03 (m, 4H), 1.01–1.16 (m, 12H), 0.77 (t, J = 7.4 Hz, 6H), 0.68 (m, 4H); 13C-NMR (CDCl3): δ 151.5, 151.5, 151.0, 143.5, 138.7, 128.1, 127.7, 127.2, 126.8, 125.9, 122.9, 121.2, 120.0, 119.8, 55.2, 40.4, 47.2, 23.7, 22.5, 13.9; λabs (CH2Cl2) 337 nm, λabs (MeOH) 332 nm; λem (CH2Cl2) 452 nm, λem (MeOH) 400, 397 nm, λem (film) 460 nm. HR-MS. Calcd for C38H43N2 ([M + H]+): m/z 527.3426. Found: m/z 527.3426.
2-[4-(9,9-Dihexyl-9H-fluoren-2-yl)phenyl]-1-phenyl-1H-benz[d]imidazole (11b)
This compound was obtained from 3 and 10b; white solid; yield 75%; mp 147–150°C; 1H-NMR (CDCl3): δ 7.96 (d, J = 7.7 Hz, 2H), 7.51-7.78 (m, 11H), 7.27-7.43 (m, 8H), 2.02 (t, J = 8.6 Hz, 4H), 1.09 (m, 12H), 0.77 (t, J = 7.3 Hz, 6H), 0.68 (m, 4H); 13C-NMR (CDCl3): δ 152.1, 151.5, 150.9, 143.0, 142.5, 140.9, 140.6, 138.8, 137.4, 137.1, 129.9, 128.6, 128.5, 127.5, 127.2, 126.9, 126.8, 125.9, 123.3, 122.9, 119.9, 119.8, 110.4, 55.2, 40.4, 31.4, 29.7, 23.7, 22.5; λabs (CH2Cl2) 332 nm, λabs (MeOH) 328 nm; λem (CH2Cl2) 468 nm, λem (MeOH) 405 nm and 425 nm, λem (film) 444.9 nm. HR-MS. Calcd for C44H46N2 ([M + H]+): m/z 603.3739. Found: m/z 603.3738.
2-[4-(9,9-Dihexyl-9H-fluoren-2-yl)phenyl]-1-(4-methoxyphenyl)-1H-benz[d]imidazole (11c)
This compound was obtained from 3 and 10c; white solid; yield 70%; mp 128–130°C; 1H-NMR (CDCl3): δ 7.93 (d, J = 8.5 Hz, 2H), 7.75 (m, 4H), 7.65 (d, J = 9.3 Hz, 2H), 7.59 (t, J = 8.5 Hz, 2H), 7.22–7.39 (m, 9H), 7.07 (d, J = 8.0 Hz, 2H), 3.92 (s, 3H), 2.01 (t, J = 8.5 Hz, 2H), 1.03 (m, 12H), 0.77 (t, J = 7.4 Hz, 6H), 0.62-0.70 (m, 4H); 13C-NMR (CDCl3): δ 159.6, 152.2, 151.4, 150.9, 142.4, 140.9, 140.6, 138.8, 137.7, 129.7, 128.7, 126.8, 126.8, 125.9, 123.2, 122.9, 122.9, 121.2, 119.9, 119.8, 119.6, 115.1, 110.4, 55.5, 55.1, 40.4, 31.4, 29.6, 23.7, 22.5, 13.9; λabs (CH2Cl2) 333 nm, λabs (MeOH) 326 nm; λem (CH2Cl2) 462 nm, λem (MeOH) 401 nm and 425 nm, λem (film) 452 nm. HR-MS. Calcd for C45H48N2O ([M + H]+): m/z 633.3845. Found: m/z 633.3833.
General procedure for synthesis of 12a,b and 13a,b
To a mixture of 5-(9,9-dihexyl-9H-fluoren-2-yl)furan-2-carbaldehyde (4, 0.7 mmol) or 5-(9,9-dihexyl-9H-fluoren-2-yl)thiophene-2-carbaldehyde (5, 0.7 mmol), benzene-1,2-diamine (10a, 1.0 mmol) or N-phenylbenzene-1,2-diamine (10b, 1.0 mmol), and TsOH (0.1 mmol) was added dioxane (7 mL) at room temperature. The mixture was heated under reflux for 9 h. The solvent was removed under reduced pressure and the crude product was purified by column chromatography on silica gel using ethyl acetate (10%–14%)/petroleum ether as eluent to afford the final product 12 or 13.
2-[5-(9,9-Dihexyl-9H-fluoren-2-yl)furan-2-yl]-1H-benz[d]imidazole (12a)
This compound was obtained from 4 and 10a; yellow solid; yield 65%; mp 203-206°C; 1H-NMR (CDCl3): δ 7.74 (m, 5H), 7.34 (m, 6H), 6.90 (d, J = 3.6 Hz, 2H), 2.01 (m, 4H), 1.08 (m, 12H), 0.76 (t, J = 8.5 Hz, 6H), 0.62 (m, 4H); 13C-NMR (CDCl3): δ 156.1, 151.4, 150.9, 143.8, 141.5, 140.4, 128.4, 127.4, 126.8, 123.3, 123.2, 122.8, 120.0, 119.8, 118.3, 113.3, 107.6, 55.2, 40.4, 31.5, 29.7, 23.7, 22.5, 13.9; λabs (CH2Cl2) 368 nm, λabs (MeOH) 364 nm; λem (CH2Cl2) 456 nm, λem (MeOH) 394 nm and 417 nm, λem (film) 464 nm. HR-MS. Calcd for C36H40N2O ([M + H]+): m/z 517.3219. Found: m/z 517.3217.
2-[5-(9,9-Dihexyl-9H-fluoren-2-yl)furan-2-yl]-1-phenyl-1H-benz[d]imidazole (12b)
This compound was obtained from 4 and 10b; yellow solid; yield 60%; mp 92–96°C; 1H-NMR (CDCl3): δ 7.94 (d, J = 9.5 Hz, 2H), 7.69 (m, 4H), 7.64 (d, J = 9.4 Hz, 1H), 7.56 (d, J = 7.6 Hz, 2H), 7.37 (m, 5H), 7.26 (m, 2H), 7.15 (d, J = 8.8 Hz, 1H), 6.77 (d, J = 3.4 Hz, 1H), 1.95-2.10 (m, 4H), 1.02-1.20 (m, 12H), 0.80 (t, J = 7.4 Hz, 6H), 0.57-0.65 (m, 4H); 13C-NMR (CDCl3): δ 156.2, 151.2, 150.8, 144.1, 143.1, 141.2, 140.5, 137.3, 137.1, 129.8, 129.3, 128.5, 128.1, 127.3, 126.8, 123.4, 123.2, 123.2, 122.8, 119.8, 119.7, 119.5, 118.0, 115.2, 110.2, 106.2, 106.8, 55.2, 40.4, 31.5, 29.7, 23.6, 22.6, 14.0; λabs (CH2Cl2) 371 nm, λabs(MeOH) 368 nm; λem (CH2Cl2) 469 nm, λem (MeOH) 426 nm, λem (film) 462 nm. HR-MS. Calcd for C42H44N2O ([M + H]+): m/z 593.3532. Found: m/z 593.3538.
2-[5-(9,9-Dihexyl-9H-fluoren-2-yl)thiophen-2-yl]-1H-benz[d]imidazole (13a)
This compound was obtained from 5 and 10a; yellow solid; yield 65%; mp 189–192°C; 1H-NMR (CDCl3): δ 7.72 (m, 3H), 7.65 (m, 2H), 7.60 (m, 2H), 7.33 (m, 4H), 7.28 (m, 2H), 1.99 (t, J = 8.6 Hz, 4H), 1.08 (m, 12H), 0.76 (t, J = 7.4 Hz, 6H), 0.66 (m, 4H); 13C-NMR (CDCl3): δ 151.6, 150.9, 148.2, 147.1, 141.5, 140.3, 132.1, 130.9, 127.9, 127.3, 126.8, 124.8, 123.6, 123.2, 123.2, 122.9, 120.1, 119.8, 55.1, 40.3, 31.4, 29.7, 29.6, 23.7, 22.5, 13.9; λabs (CH2Cl2) 378 nm, λabs (MeOH) 362 nm; λem (CH2Cl2) 461 nm, λem (MeOH) 425 nm, λem (film) 450 nm. HR-MS. Calcd for C36H40N2S ([M + H]+): m/z 533.2990. Found: m/z 533.2988.
2-[5-(9,9-Dihexyl-9H-fluoren-2-yl)thiophen-2-yl]-1-phenyl-1H-benz[d]imidazole (13b)
This compound was obtained from 5 and 10b; green solid; yield 80%; mp 95–97°C; 1H-NMR (CDCl3): δ 7.93 (d, J = 8.8 Hz, 1H), 7.64 (m, 9H), 7.35 (m, 4H), 7.26 (d, J = 8.3 Hz, 1H), 7.18 (d, J = 4.8 Hz, 1H), 7.11 (d, J = 8.4 Hz, 1H), 6.76 (s, 1H), 2.01 (t, J = 16.6 Hz, 4H), 1.10 (m,12H), 0.79 (t, J = 7.5 Hz, 6H), 0.64-0.73 (m, 4H); 13C-NMR (CDCl3): δ 151.6, 151.0, 148.0, 147.0, 147.2, 141.4, 140.4, 137.7, 136.4, 132.2, 130.2, 129.7, 129.0, 128.3, 127.3, 126.8, 124.6, 123.4, 123.2, 123.2, 122.9, 120.3, 120.1, 119.8, 119.4, 110.1, 55.1, 40.4, 31.4, 29.7, 29.7, 23.7, 22.6, 14.0; λabs (CH2Cl2) 376 nm, λabs (MeOH) 364 nm; λem (CH2Cl2) 457 nm, λem (MeOH) 421nm and 442 nm, λem (film) 450 nm. HR-MS. Calcd for C42H42N2S ([M+H]+): m/z 609.3303. Found: m/z 609.3295.
General procedure for synthesis of 14a–16b
To a mixture of an aldehyde 7-9 (0.7 mmol), a benzene-1,2-diamine 10a-c (1.0 mmol), and TsOH (0.1 mmol), was added dioxane (7 mL) at room temperature. The mixture was heated under reflux for 9 h, then concentrated under reduced pressure, and the residue was purified by column chromatography on silica gel using ethyl acetate (16%-20%)/petroleum ether as eluent to afford the final product.
2,2′-[(9,9-Dimethyl-9H-fluorene-2,7-diyl)bis(4,1-phenylene)]bis(1H-benz[d]imidazole) (14a)
This compound was obtained from 7 and 10a; yellow solid; yield 65%; mp > 400°C; 1H-NMR (DMSO-d6): δ 8.31 (d, J = 9.5 Hz, 4H), 8.04 (m,8H), 7.82 (dd, J1 = 1.4 Hz, J2 = 2.6 Hz, 2H), 7.69 (br, 2H), 7.56 (br, 2H), 7.23 (br, 2H), 1.63 (s, 6H); 13C-NMR (DMSO-d6): δ 155.1, 151.4, 142.0, 139.0, 138.4, 129.4, 127.6, 127.4, 126.4, 121.6, 121.3, 79.7, 79.4, 79.1, 47.3, 27.3; λabs (CH2Cl2) 360 nm, λabs (MeOH) 348 nm; λem (CH2Cl2) 457 nm, λem (MeOH) 401 nm and 426 nm, λem (film) 462 nm. HR-MS. Calcd for C41H30N4 ([M + H]+): m/z 579.2548. Found: m/z 579.2530.
2,2′-[(9,9-Dimethyl-9H-fluorene-2,7-diyl)bis(4,1-phenylene)]bis(1-phenyl-1H-benz[d]imidazole) (14b)
This compound was obtained from 7 and 10b; white solid; yield 75%; mp 318–32°C; 1H-NMR (CDCl3): δ 7.94 (d, J = 8.3 Hz, 2H), 7.80 (d, J = 8.4 Hz, 2H), 7.51-7.72 (m, 19H), 7.39 (m, 6H), 7.30 (m, 3H), 1.58 (s, 6H); 13C-NMR (CDCl3): δ 154.6, 152.0, 142.8, 142.3, 139.3, 138.4, 137.3, 137.0, 130.0, 129.9, 129.8, 128.6, 128.5, 127.5, 126.9, 126.2, 123.4, 123.1, 121.2, 120.5, 119.7, 110.4, 47.1, 27.3; λabs (CH2Cl2) 360 nm, λabs (MeOH) 340 nm; λem (CH2Cl2) 493 nm, λem (MeOH) 402 nm and 425 nm, λem (film) 450 nm. HR-MS. Calcd for C53H38N4 ([M + H]+): m/z 731.3174. Found: m/z 713.3169.
2,2′-[(9,9-Dimethyl-9H-fluorene-2,7-diyl)bis(4,1-phenylene)]bis(1-(4-methoxyphenyl)-1H-benz[d]imidazole) (14c)
This compound was obtained from 7 and 10c; white solid; yield 70%; mp 294–296°C; 1H-NMR (CDCl3): δ 7.92 (d, J = 7.2 Hz, 2H), 7.81 (d, J = 8.3 Hz, 2H), 7.73 (d, J = 8.5 Hz, 4H), 7.64 (m, 8H), 7.23-7.39 (m, 10H), 7.07 (d, J = 9.4 Hz, 4H), 3.92 (s, 6H), 1.59 (s, 6H); 13C-NMR (CDCl3): δ 159.6, 154.6, 152.1, 142.2, 139.3, 138.4, 137.7, 129.8, 129.6, 128.6, 126.9, 126.2, 123.3, 123.0, 121.2, 120.5, 119.7, 115.1, 110.4, 55.5, 29.7, 27.3; λabs (CH2Cl2) 350 nm, λabs (MeOH) 346 nm; λem (CH2Cl2) 463 nm, λem (MeOH) 402,425 nm, λem (film) 452 nm. HR-MS. Calcd for C55H42N4O2 ([M + H]+): m/z 791.3386. Found: m/z 791.3346.
2,2′-[(9,9-Dimethyl-9H-fluorene-2,7-diyl)bis(furan-5,2-diyl)]bis(1H-benz[d]imidazole) (15a)
This compound was obtained from 8 and 10a; yellow-brown solid; yield 68%; mp > 400°C; 1H-NMR (CDCl3): δ 8.16 (s, 2H), 8.00 (q, J = 27.7 Hz, 4H), 7.63 (br, 4H), 7.37 (d, J = 3.3 Hz, 2H), 7.30 (d, J = 3.3 Hz), 7.23 (q, J = 9.3 Hz, 4H), 1.63 (s, 6H); 13C-NMR (CDCl3): δ 155.1, 154.9, 145.3, 144.0, 138.5, 129.3, 121.4, 118.8, 113.3, 108.8, 47.2, 27.3; λabs (CH2Cl2) 394 nm, λabs (MeOH) 390 nm; λem (CH2Cl2) 463 nm, λem(MeOH) 403 nm and 425 nm, λem (film) 427 nm. HR-MS. Calcd for C37H26N4O2 ([M + H]+): m/z 559.2134. Found: m/z 559.2119.
2,2′-[(9,9-Dimethyl-9H-fluorene-2,7-diyl)bis(furan-5,2-diyl)]bis(1-phenyl-1H-benz[d]imidazole) (15b)
This compound was obtained from 8 and 10b; brown solid; yield 67%; mp 242–250°C; 1H-NMR (DMSO-d6): δ 7.90 (d, J = 9.3 Hz, 2H), 7.72 (m, 6H), 7.64 (d, J = 8.5 Hz, 2H), 7.55 (m, 4H), 7.26–7.43 (m, 8H), 7.15 (d, J = 8.4 Hz, 2H), 6.93 (d, J = 4.2 Hz, 2H), 6.76 (d, J = 3.1 Hz, 2H), 1.51 (s, 6H); 13C-NMR (DMSO-d6): δ 155.9, 154.4, 144.1, 142.9, 138.5, 137.0, 129.8, 129.3, 128.8, 128.1, 123.5, 123.5, 123.2, 120.2, 119.5, 118.0, 115.2, 110.2, 107.0, 47.1, 29.7; λabs (CH2Cl2) 420 nm, λabs (MeOH) 394 nm; λem (CH2Cl2) 470 nm and 500 nm, λem (MeOH) 402 nm and 427 nm, λem (film) 437 nm. HR-MS. Calcd for C49H44N2O2 ([M + H]+): m/z 711.2760. Found: m/z 711.2755.
2,2′-[(9,9-Dimethyl-9H-fluorene-2,7-diyl)bis(thiophene-5,2-diyl)]bis(1H-benz[d]imidazole) (16a)
This compound was obtained from 9 and 10a; yellow solid; yield 62%; mp > 400°C; 1H-NMR (DMSO-d6): δ 7.99 (d, J = 1.5 Hz, 2H), 7.96 (d, J = 8.8 Hz,2H), 7.87 (d, J = 4.3Hz, 2H), 7.76 (dd, J1 = 1.5 Hz, J2 = 2.4 Hz, 2H), 7.74 (d, J = 4.3 Hz, 2H), 7.59 (br, 4H), 7.22 (s, 4H), 1.59 (s, 6H); 13C-NMR (DMSO-d6): δ 155.1, 147.2, 146.3, 138.5, 133.0, 128.2, 125.2, 121.3, 120.4, 47.3, 29.4, 27.2; λabs (CH2Cl2): 400 nm, λabs (MeOH): 393 nm; λem (CH2Cl2): 496 nm, λem (MeOH): 436 nm. HR-MS. Calcd for C37H26N4S2 ([M + H]+): m/z 591.1677. Found: m/z 591.1669.
2,2′-[(9, 9-Dimethyl-9H-fluorene-2,7-diyl)bis(thiophene-5,2-diyl)] bis(1-phenyl-1H-benz[d]imidazole (16b)
This compound was obtained from 9 and 10b; yellow solid; yield 60%; mp 112–116°C; 1H-NMR (CDCl3): δ 7.91 (s, 2H), 7.43–7.72 (m, 16H), 7.05–7.41 (m, 9H), 6.88 (s, 1H), 1.53 (s, 6H); 13C-NMR (CDCl3): δ 154.6, 147.9, 146.9, 138.7, 137.5, 136.1, 132.7, 130.6, 130.3, 130.1, 129.8, 129.6, 128.3, 128.2, 125.1, 123.9, 123.5, 123.4, 120.6, 120.2, 119.1, 114.0, 110.1, 47.0, 29.7, 27.1; λabs (CH2Cl2): 418 nm, λabs (MeOH): 393 nm; λem (CH2Cl2): 482 nm, λem (MeOH): 445,472 nm. HR-MS. Calcd for C49H44N2S2 ([M + H]+): m/z 743.2303. Found: m/z 743.2293.
X-ray structural analysis of 11b and 13b
Suitable single crystals of 11b and 13b for X-ray structural analysis were obtained by slow concentration of solution in chloroform at room temperature. The selected crystals with approximate dimensions of 0.19 × 0.15 × 0.03 mm for 11b and 0.211 × 0.089 × 0.076 mm for 13b were mounted on a four-circle single crystal X-ray diffractometer (CAD4 DIFFACTIS 586). A graphite monochromated MoKα radiation (λ = 0.071073 nm) was used and the data were collected at 296 (2) K. The structure was solved by direct methods and refined by full-matrix least-squares method on Fobs2 using the SHELXTL 97 software package. The non-hydrogen atoms were refined anisotropically. The hydrogen atoms bound to carbon were located by using geometrical calculations, with their position and thermal parameters being fixed during the structure refinement. A summary of the crystallographic data and structure refinement details are given in Table 2.
Crystal data and structure refinement for 11b and 13b.
| 11b | 13b | |
|---|---|---|
| Empirical formula | C44H46N2 | C42H44N2S |
| Formula weight | 602.83 | 608.85 |
| Temperature | 296(2) K | 296(2) K |
| Wavelength | 0.71073 Å | 0.71073 Å |
| Crystal system, space group | Triclinic, P1̅ | Triclinic, P1̅ |
| a (Å) | 8.8196(12) | 8.849(2) |
| b (Å) | 11.3574(14) | 11.872(3) |
| c (Å) | 18.332(2) | 17.588(4) |
| α (°) | 79.002(3) | 101.232(7) |
| β (°) | 85.340(3) | 90.384(7) |
| γ (°) | 85.257(4) | 102.640(6) |
| Volume (Å3) | 1792.3(4) | 1766.0(7) |
| Z | 2 | 2 |
| Density (calc.) (mg/m3) | 1.117 | 1.145 |
| Absorption coefficient (mm-1) | 0.064 | 0.122 |
| F (000) | 648 | 652 |
| Crystal size (mm) | 0.175 × 0.158 × 0.079 | 0.211 × 0.089 × 0.076 |
| θ range for data collection (°) | 1.831–25.049 | 1.795–25.495 |
| Limiting indices | -10 ≤ h ≤ 10, -11 ≤ k ≤ 13, -21 ≤ l ≤ 21 | -10 ≤ h ≤ 10, -14 ≤ k ≤ 10, -18 ≤ l ≤ 21 |
| Reflections collected/unique | 10157/6296 [R(int) = 0.0397] | 10410/6549 [R(int) = 0.0395] |
| Completeness to theta = 25.05 | 97.5% | 99.8% |
| Absorption correction | Semi-empirical from equivalents | Semi-empirical from equivalents |
| Max. and min. transmission | 0.7457 and 0.6580 | 0.7457 and 0.6015 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 6331/89/483 | 6549/98/465 |
| Goodness-of-fit on F2 | 1.038 | 0.996 |
| Final R indices [I > 2σ (I)] | R1 = 0.0853, wR2 = 0.2188 | R1 = 0.0691, wR2 = 0.1699 |
| R indices (all data) | R1 = 0.1569, wR2 = 0.2548 | R1 = 0.1290, wR2 = 0.2030 |
| Absolute structure parameter | 0.004(2) | 0.008(4) |
| Largest diff. peak and hole (eÅ-3) | 0.275 and -0.243 | 0.380 and -0.219 |
Acknowledgments
The authors are grateful for supports from the National Natural Science Foundation of China (Project No. 81202402 and 21272154). The authors also thank Drs. H. Deng and M. Shao, The Instrumental Analysis & Research Center of Shanghai University, for structural analysis.
References
[1] Tao, S.; Li, L.; Yu, J.; Jiang, Y.; Zhou, Y.; Lee, C. S. Bipolar molecule as an excellent hole-transporter for organic-light emitting devices. Chem. Mater.2009, 21, 1284–1287.10.1021/cm803087cSearch in Google Scholar
[2] Rouxel, C.; Charlot, M.; Mir, Y.; Frochot, C.; Mongin, O.; Blancharddesce, M. Banana-shaped biphotonic quadrupolar chromophores: from fluorophores to biphotonic photosensitizers. New J. Chem.2011, 35, 1771–1780.10.1039/c1nj20073aSearch in Google Scholar
[3] Moura, G. L. C.; Simas, A. M. Two-photon absorption by fluorene derivatives: systematic molecular design. J. Phys. Chem. C2010, 114, 6106–6116.10.1021/jp100314cSearch in Google Scholar
[4] Andrade, C. D.; Yanez, C. O.; Rodriguez, L.; Belfield, K. D. A series of fluorene-based two-photon absorbing molecules: synthesis, linear and nonlinear characterization, and bioimaging. J. Org. Chem.2010, 75, 3975–3982.10.1021/jo1005075Search in Google Scholar PubMed PubMed Central
[5] Wang, H. L.; Li, Z.; Shao, P.; Qin, J. G.; Huang, Z. L. Two-photon absorption property of a conjugated polymer: influence of solvent and concentration on its property. J. Phys. Chem. B2010, 114, 22–27.10.1021/jp906264xSearch in Google Scholar PubMed
[6] Grimsdale, A. C.; Chan, K. L.; Martin, R. E.; Jokisz, P. G.; Holmes, A. B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev.2009, 109, 897–1091.10.1021/cr000013vSearch in Google Scholar PubMed
[7] Muroga, T.; Sakaguchi, T.; Hashimoto, T. Synthesis and photoluminescence properties of heterocycle-containing poly(disubstituted acetylene)s. Polymer2012, 53, 4380–4387.10.1016/j.polymer.2012.08.009Search in Google Scholar
[8] Cao, X. Y.; Zhang, W.; Pei, J. π -Conjugated twin molecules based on truxene: synthesis and optical properties. Org. Lett.2004, 6, 4845–4848.10.1021/ol048144wSearch in Google Scholar PubMed
[9] Zhao, Z. J.; Xu, X. J.; Jiang, Z. T.; Lu, P.; Yu, G.; Liu, Y. Q. Oligo(2,7-fluorene ethynylene) with pyrene moieties: synthesis, characterization, photoluminescence, and electroluminescence. J. Org. Chem.2007, 72, 8345–8353.10.1021/jo7013388Search in Google Scholar PubMed
[10] Liao, Y. L.; Lin, C. Y.; Wong, K. T.; Hou, T. H.; Hun, Y. W. A novel ambipolar spirobifluorene derivative that behaves as an efficient blue-light emitter in organic light-emitting diodes. Org. Lett. 2007, 9, 4511–4514.10.1021/ol701994kSearch in Google Scholar PubMed
[11] Lee, J. F.; Chen, Y. C.; Lin, J. T.; Wu, C. C.; Chen, C.Y.; Dai, C. A.; Chao, C. Y.; Chen, H. L.; Liau, W. B. Blue light-emitting and electron-transporting materials based on dialkyl-functionlized anthracene imidazophenanthrolines. Tetrahedron2011, 67, 1696–1702.10.1016/j.tet.2010.12.059Search in Google Scholar
[12] Zhang, Y.; Lai, S. L.; Tong, Q. X.; Chan, M. Y.; Ng, T. W.; Wen, Z. C.; Zhang, G. Q.; Lee, S. T.; Kwong, H. L.; Lee, C. S. Synthesis and characterization of phenanthroimidazole derivatives for applications in organic electroluminescent devices. J. Mater. Chem.2011, 21, 8206–8214.10.1039/c1jm10326aSearch in Google Scholar
[13] Wang, C. L.; Dong, H. L.; Hu, W. P.; Liu, Y. Q.; Zhu, D. B. Semiconducting pi-conjugated systems in field-effect transistors: a material odyssey of organic electronics. Chem. Rev.2012, 112, 2208–2267.10.1021/cr100380zSearch in Google Scholar PubMed
[14] He, G. S.; Tan, L. S.; Zheng, Q. D.; Prasad, P. Multiphoton absorbing materials: molecular designs, characterizations, and applications. Chem. Rev.2008, 108, 1245–1330.10.1021/cr050054xSearch in Google Scholar PubMed
[15] Hung, L. S.; Chen, C. H. Recent progress of molecular organic electroluminescent materials and devices. Mater. Sci. Eng. R.2012, 39, 143–222.10.1016/S0927-796X(02)00093-1Search in Google Scholar
[16] Snaith, H. J.; Greenham, N. C.; Friend, R. H. The origin of collected charge and open-circuit voltage in blended polyfluorene photovoltaic devices. Adv. Mater.2004, 16, 1640–1645.10.1002/adma.200305766Search in Google Scholar
[17] Werts, M. H. W.; Gmouh, S.; Mongin, O.; Pons, T.; Desce, M. Strong modulation of two-photon excited fluorescence of quadripolar dyes by (de)protonation. J. Am. Chem. Soc.2004, 126, 16294–16295.10.1021/ja0446606Search in Google Scholar PubMed
[18] Zhou, X. H.; Yan, J. C. Exploiting an imidazole-functionalized polyfluorene derivative as a chemosensory material. J. Macromolecules2004, 37, 7078–7080.10.1021/ma049057mSearch in Google Scholar
[19] Zhang, Y.; Lai, S. L.; Tong, Q. X.; Lo, M. F.; Ng, T. W.; Chan, M. Y.; Wen, Z. C.; He, J.; Jeff, K. S.; Tang, X. L. High efficiency nondoped deep-blue organic light emitting devices based on imidazole-π-triphenylamine derivatives. Chem. Mater. 2012, 24, 61–70.10.1021/cm201789uSearch in Google Scholar
[20] Li, W. J.; Liu, D. D.; Shen, F. Z.; Ma, D. G.; Wang, Z. M.; Feng, T.; Xu, Y. X.; Yang, B.; Ma, Y. G. A twisting donor-acceptor molecule with an intercrossed excited state for highly efficient, deep-blue electroluminescence. Adv. Funct. Mater. 2012, 22, 2797–2803.10.1002/adfm.201200116Search in Google Scholar
[21] Kulkarni, A. P.; Tonzola C.J.; Babel A.; Jenekhe S.A. Electron transport materials for organic light-emitting diodes. Chem. Mater.2004, 16, 4556–4573.10.1021/cm049473lSearch in Google Scholar
[22] Kuo, C. J.; Li, T. Y.; Lien, C. C.; Liu, C. H.; Wu, F. I.; Huang, M. J. Bis(phenanthroimidazolyl)biphenyl derivatives as saturated blue emitters for electroluminescent devices. J. Mater. Chem.2009, 19, 1865–1871.10.1039/b816327hSearch in Google Scholar
[23] Wang, Z. M.; Lu, P.; Chen, S. M.; Gao, Z.; Shen, F. Z.; Zhang, W. S.; Xu, Y. X.; Kwok, H. S.; Ma, Y. G. Phenanthro[9,10-d]imidazole as a new building block for blue light emitting materials. J. Mater. Chem.2011, 21, 5451–5456.10.1039/c1jm10321kSearch in Google Scholar
[24] Lai, M.Y.; Chen, C. H.; Huang, W. S.; Lin, J. T.; Ke, T. H.; Chen, L. Y.; Tsai, M. H.; Wu, C. Benzimidazole/amine-based compounds capable of ambipolar transport for application in single-layer blue-emitting OLEDs and as hosts for phosphorescent emitters. Angew. Chem. Int. Ed.2008, 47, 591–595.10.1002/ange.200704113Search in Google Scholar
[25] Jenekhe, S. A.; Osaheni, J. A. Excimers and exciplexes of conjugated polymers. Science1994, 265, 765–768.10.1126/science.265.5173.765Search in Google Scholar PubMed
[26] Han, J.; An, J.; Im, C.; Cho, N. S.; Shim, H. K.; Majima, T. Comparing electroluminescence efficiency and photoluminescence quantum yield of fluorene based pi-conjugated copolymers with narrow band-gap comonomers. J. Photochem. Photobiol. A.2009, 205, 98–103.10.1016/j.jphotochem.2009.02.020Search in Google Scholar
[27] Setayesh, S.; Grimsdale, A. C.; Weil, T.; Enkelmann, V.; Müllen, K.; Meghdadi, F.; List, E. J. W.; Leising, G. Polyfluorenes with polyphenylene dendron side chains: toward non-aggregating, light-emitting polymers. J. Am. Chem. Soc.2001, 123, 853–946.10.1021/ja0031220Search in Google Scholar PubMed
[28] Marsitzky, D.; Scott, J. C.; Chen, J. P.; Lee, V. Y.; Miller, R. D.; Setayesh, S.; Mullen, K. Poly-2,8-(indenofluorene-co-anthracene) colorfast blue-light-emitting random copolymer. Adv. Mater.2001, 13, 1096–1100.10.1002/1521-4095(200107)13:14<1096::AID-ADMA1096>3.0.CO;2-ISearch in Google Scholar
[29] Li, Z. H.; Wong, M. S.; Fukutani, H.; Tao, Y. Full emission color tuning in bisdipolar diphenyl amino-endcapped oligoaryl fluorenes. Chem. Mater.2005, 17, 5032–5040.10.1021/cm051163vSearch in Google Scholar
[30] Yasuda, T.; Fujita, K.; Tsutsui, T. Carrier transport properties of monodisperse glassy-nematic oligofluorenes in organic field-effect transistors. Chem. Mater.2005, 17, 264–268.10.1021/cm048532sSearch in Google Scholar
[31] Lai, W. Y.; Zhu, R.; Fan, Q. L.; Hou, L. T.; Cao, Y.; Huang, W. Monodisperse sixarmed triazatruxenes: microwave-enhanced synthesis and highly efficient pure-deep-blue electroluminescence. Macromolecules2006, 39, 3707–3970.10.1021/ma060154kSearch in Google Scholar
[32] Chen, H. F.; Cui, Y. M.; Guo, J. G.; Lin, H. X.; Synthesis and optical properties of 6,10-dihydrofluoreno[2,3-d:6,7-d,]diimidazole derivatives. Dyes Pigm.2012, 94, 583–591.10.1016/j.dyepig.2012.03.004Search in Google Scholar
[33] Guo, J. G., Cui, Y. M.; Lin, H. X.; Xie, X. Z.; Chen, H. F. New fluorene derivatives based on3,9-dihydrofluoreno [3,2-d]imidazole (FI): Characterization and influence of substituents on photoluminescence. J. Photochem. Photobiol. A: Chem.2011, 219, 42–49.10.1016/j.jphotochem.2011.01.014Search in Google Scholar
[34] Hao, Z. S.; Li, M. J.; Lin, H. X.; Gu, Z. B.; Cui, Y. M. Synthesis, optical and electrochemical properties of 2,3-diphenyl-10H-indeno[1,2-g]quinoxaline, 15H-dibenzo[a,c]indeno[1,2-i]phenazine and 15H-indeno[1,2-i]phenanthro[4,5-abc]phenazine derivatives. Dyes Pigm. 2014, 109, 54–66.10.1016/j.dyepig.2014.04.042Search in Google Scholar
[35] Gu, Z. B.; Lin, H. X.; Cui, Y. M.; Li, M. J.; Hao, Z. S. Synthesis and characterization of 2′,7′-diarylspiro[cyclopentane-1,9’-fluorene] derivatives. Monatsh. Chem.2015, 146, 1519–1527.10.1007/s00706-015-1432-9Search in Google Scholar
[36] Jiang, Y.; Wang J. Y.; Ma Y.; Cui Y. X.; Zhou Q. F.; Pei J. Large rigid blue-emitting π-conjugated stilbenoid-based dendrimers: synthesis and properties. Org. Lett.2006, 8, 4287–4290.10.1021/ol0616283Search in Google Scholar PubMed
[37] Kumar, D.; Thomas, K. R. J.; Lin, C. C.; Jou, J. H. Pyrenoimidazole-based deep-blue-emitting materials: optical, electrochemical, and electroluminescent characteristics. Chem. Asian J.2013, 8, 2111–2124.10.1002/asia.201300271Search in Google Scholar PubMed
[38] Mulhern, K.; Detty, M.; Watson, D. Aggregation-induced increase of the quantum yield of electron injection from chalcogenorhodamine dyes to TiO2. J. Phys. Chem. C.2011, 115, 6010–6018.10.1021/jp111438xSearch in Google Scholar
[39] Adachi, K.; Mita, T.; Yamate, T.; Yamazaki, S.; Takechi, H.; Watarai, H. Controllable adsorption and ideal H-aggregation behaviors of phenothiazine dyes on the tungsten oxide nanocolloid surface. Langmuir.2010, 26, 117–125.10.1021/la902174sSearch in Google Scholar PubMed
[40] Chen, S.; Raad, F. S.; Ahmida, M.; Kaafarani, B. R.; Eichhorn, S. H. Columnar mesomorphism of fluorescent board-shaped quinoxalino-phenanthrophenazine derivatives with donor–acceptor structure. Org. Lett. 2013, 15, 558–561.10.1021/ol303375xSearch in Google Scholar PubMed
[41] Feng, X.; Iwanaga, F.; Hu, J. Y.; Tomiyasu, H.; Nakano, M.; Redshaw, C.; Elsegood, M. R. J.; Yamato, T. An efficient approach to the synthesis of novel pyrene-fused azaacenes. Org. Lett. 2013, 15, 3594–3597.10.1021/ol401438aSearch in Google Scholar PubMed
[42] Thomas, J.; Tyag, P. Synthesis, spectra, and theoretical investigations of the triarylamines based on 6H-indolo[2,3-b]quinoxaline. J. Org. Chem.2010, 75, 8100–8111.10.1021/jo1016663Search in Google Scholar PubMed
©2016 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Preliminary Communication
- Design, synthesis, and anticancer activity of novel aryl/heteroaryl chalcone derivatives
- Research Articles
- A simple and convenient method for the synthesis of 1,3,5-triazine-nitrolic acids. The first X-ray investigation of Z-isomeric nitrolic acid
- Pot, atom and step-economic (PASE) synthesis of medicinally relevant spiro[oxindole-3,4′-pyrano[4,3-b]pyran] scaffold
- An efficient asymmetric approach to the R-enantiomer impurity of esomeprazole
- Synthesis, optical and electrochemical properties of 2-[(9H-fluoren-2-yl)aryl]-1H-benz[d]imidazole and 2,7-bis[(1H-benz[d]imidazol-2-yl)aryl]- 9H-fluorene derivatives
- Synthesis and fluorescence of pyrazolines substituted with pyrimidine and ferrocene subunits
- Design and synthesis of a novel rhodamine-based chemosensor and recognition study to Fe3+
- An efficient, one-pot three-component synthesis of 4H-thiazolo[3,2-a][1,3,5]triazin-6-one derivatives
- Microwave-assisted synthesis and antibacterial evaluation of new derivatives of 1,2-dihydro-3H-pyrazolo[3,4-d]pyrimidin-3-one
- Efficient assembly of quinoxaline derivatives from benzene-1,2-diamines, dialkyl acetylenedicarboxylates and ninhydrin
Articles in the same Issue
- Frontmatter
- Preliminary Communication
- Design, synthesis, and anticancer activity of novel aryl/heteroaryl chalcone derivatives
- Research Articles
- A simple and convenient method for the synthesis of 1,3,5-triazine-nitrolic acids. The first X-ray investigation of Z-isomeric nitrolic acid
- Pot, atom and step-economic (PASE) synthesis of medicinally relevant spiro[oxindole-3,4′-pyrano[4,3-b]pyran] scaffold
- An efficient asymmetric approach to the R-enantiomer impurity of esomeprazole
- Synthesis, optical and electrochemical properties of 2-[(9H-fluoren-2-yl)aryl]-1H-benz[d]imidazole and 2,7-bis[(1H-benz[d]imidazol-2-yl)aryl]- 9H-fluorene derivatives
- Synthesis and fluorescence of pyrazolines substituted with pyrimidine and ferrocene subunits
- Design and synthesis of a novel rhodamine-based chemosensor and recognition study to Fe3+
- An efficient, one-pot three-component synthesis of 4H-thiazolo[3,2-a][1,3,5]triazin-6-one derivatives
- Microwave-assisted synthesis and antibacterial evaluation of new derivatives of 1,2-dihydro-3H-pyrazolo[3,4-d]pyrimidin-3-one
- Efficient assembly of quinoxaline derivatives from benzene-1,2-diamines, dialkyl acetylenedicarboxylates and ninhydrin

