Abstract
The development of light-sensitive media based on organic, mostly heterocyclic compounds that have no fluorescence in their initial form but provide fluorescent photoproducts formation is considered in this review. Materials with photoinduced irreversible fluorescence appear to be the most promising in the design of recording media for 3D archive optical memory. Photoactivatable fluorophores are also of interest for use in cell biology.
Introduction
Materials possessing functionality that can be activated by light irradiation are of great importance because of their potential use in optical data storage, printing, drug delivery systems, and biological function imaging, among other things.
Among the type of functionality, fluorescence switching is one of the most important functions for volumetric optical data storage [1]. The access to the stored data is achieved by measuring the fluorescence emitted by the fluorescing photoproduct [2–5]. Therefore, light-sensitive organic recording media based on photochemical transformations of organic compounds appear to be the most promising in the design of write once read many (WORM) recording media for 3D bitwise archive optical memory. These media have, in principle, a higher resolution than the currently used temperature-sensitive materials.
Another type of WORM material recording media is composed of two components: a photoacid generator (PAG) and a dye precursor (DP). The DP molecules are colorless and stable in neutral media; however, they become strongly colored and fluorescing in the presence of an acid produced by the light-sensitive PAG molecules when they are exposed to light. For example, lactone forms of some dyes have no fluorescence in neutral media, but their open forms are strongly fluorescent in the presence of acid. Different compounds were tested as photosensitive acid generators. They are differed in the two-photon absorption cross section at the writing wavelength, photoreaction efficiency, and thermal stability.
Here, we review the heterocyclic compounds that are of interest to diminish subside processes of destruction along irradiation, and by doing so, they increase the resolution and information capacity of the WORM materials. Several publications have also described the use of photoactivatable fluorophores in cell biology [6].
Media based on fluorophores activated by photo generators
The process of information recording using fluorophores activated by PAGs is shown in Scheme 1 [2]. A light-sensitive PAG produces acid molecules after absorbing one UV photon (one-photon process) or two visible photons (two-photon process). The DP molecules become strongly colored and fluorescing in the presence of generated acid.

In earlier experiments, o-nitrobenzaldehyde has been used as an acid generator, which upon excitation with UV light [7] undergoes phototransformation into o-nitrosobenzoic acid. Rhodamine В base 1 has been used as a DP, which was found to react with the o-nitrosobenzoic acid to form the colored rhodamine В dye 2 (Scheme 2). After excitation with 355 nm light, the solution develops a strong pink color, and a bright red fluorescence is observed from this form when the solution is illuminated with 532 nm light. An identical color change and fluorescence are observed after 355 nm irradiation when the same two components are dispersed in a solid poly(methyl methacrylate) (PMMA) matrix. In the case of solid matrices, both unexposed and colored areas and unwritten and written areas of the polymer film or block do not show any spectral changes or degradation at room temperature, when they are stored in the dark.

Because o-nitrobenzaldehyde has a very weak absorption band at 355 nm, two-photon writing with 1064- and 532-nm beams has low efficiency. To increase the efficiency of the writing process, a new memory material has been developed. 1-Nitro-2-naphthaldehyde (NNA) is used as the acid photogenerating component instead of o-nitrobenzaldehyde. Because of the additional benzene ring in the molecular structure, the absorption spectrum of NNA is red shifted compared to that of o-nitrobenzaldehyde, and consequently, the absorption band at 355 nm is very intense. The nitrosonaphthoic acid, after excitation of with 355 nm light, undergoes a reaction with base 1 transforming this colorless DP into a deep-colored fluorescing dye 2.
A variety of DPs and acid generators suitable for this type of memory material exist [8–11]. However, to be suitable for use with two-photon 3D memory devices, these molecules must possess the following characteristics:
The photoprocesses that generate the acid must have a high quantum efficiency.
Both the write and the read forms of the 3D material should have a long-term (years) stability at room temperature.
The written form should be a light stable, strongly fluorescing dye that can sustain its fluorescence efficiency at least 106 reading cycles without degradation.
The material should be highly soluble in monomers and the corresponding polymer hosts.
The absorption spectrum of the acid generator should have high cross-sectional absorption in the region of 355 nm or other easily accessible two-photon wavelength to perform two-photon excitation, for example, the 1064- and the 532-nm (SHG) pulses from Nd:YAG laser, that are currently used for 3D volume writing.
To date, triarylsulfonium salts have been found to be effective PAGs.
A norbornene-derived protected quinizarin 3 as a precursor dye has been prepared for fluorescence imaging by illumination [12]. The t-butoxycarbonyl-protected (t-Boc) precursor is readily prepared from leuco quinizarin. The t-Boc protecting groups of the precursor are easily removed under illumination conditions, thus regenerating original properties of quinizarin 4(Scheme 3).

Accordingly, a thin film containing poly(methyl methacrylate) (PMMA), DP 3 (48 wt%), and PAG triphenylsulfonium triflate (TPSOTf, 5 wt%) has been prepared by spin casting a dioxane solution on a quartz plate. The irradiation of the polymer film generates strong protonic acid, which catalyzes the deprotection of the acid labile t-Boc groups and regenerates the original quinizarin moieties in the polymer film. The t-Boc-protected norbornenyl monomer has also been copolymerized with a hexylnorbornene to give a copolymer containing quinizarin DP. The t-Boc groups of quinizarin moieties in the copolymer are effectively removed by acids generated by the photoinduced decomposition of the PAG. The copolymer can potentially be used as a fluorescence imaging material in a polymer film.
A polymerizable quinizarin (Qz) DP 5 having both methacrylate and tert-butoxycarbonyl (t-Boc) groups has been prepared and radically copolymerized to obtain mono-t-Boc-protected quinizarin polymers as fluorescent imaging materials (Scheme 4) [13]. Compound 5 is a unique monomer having an acid-labile t-Boc blocking group along with a polymerizable methacrylate group. The polymers obtained by copolymerization of compound 5 with methyl methacrylate are readily modified to regenerate phenol groups by the deprotection of t-Boc groups in the quinizarin moieties by photochemical treatment in the presence of a PAG. The polymers 6 possess color and fluorescent imaging properties based on a photolithographic method: fluorescent images obtained without wet development and fluorescent relief patterns after wet development followed by flood exposure.

The generation of functional images by the selective immobilization of organic dyes in polymer films has been reported. The selective removal of labile acidic protecting groups by photoinduced chemical transformation followed by the chemisorption of organic dyes from the solution into the patterned polymer film affords effective functional images. Recently, finely resolved patterned fluorescent images with these transiently protected precursor molecules have been described. As part of efforts to produce functional images in polymer films, Kim and coworkers [14] have reported the synthesis of the novel copolymer 7 with pendant pyridylbenzoxazole groups, and its use in the generation of fluorescent images by photolithographic methods. The benzoxazole chromophore of 7 has an electron-donating amino group at one end and a pyridyl group at the other. The strongly fluorescent nature of 7 is expected to be affected when the electronic state of the benzoxazole chromophore is disturbed by interaction with an acid, as in 8 (Scheme 5). If the acid-induced fluorescence quenching is significant and occurs only in selected areas, then patterned fluorescent images will be obtained. The preparation of the pyridylbenzoxazole monomer has been described [14].

A novel nonfluorescent form of rhodamine 700 as a DP 9 has been developed by Walker and coworkers [15]. Its structure is shown in Scheme 6. The DP molecules have been synthesized by the titration of methanol solution of rhodamine 700 with a solution of potassium hydroxide in methanol. The DP molecules 9 are colorless and stable in neutral media; however, in the presence of acid, they are transformed to rhodamine 700 dye 10, which is a colored and strongly fluorescing molecule.

Two major components – DP and PAG – have been uniformly dispersed in the poly(methyl methacrylate) (PMMA) host to obtain a WORM medium. For writing information in a multilayer volumetric format, two 532-nm photon absorption generated by a picosecond Nd:YAG laser has been suggested. The photoinduced acid undergoes a reaction with 9 to generate the rhodamine 700 colored fluorescing molecules that form spots at the focus of the laser pulses (bits) inside the volume of the memory disk. The written information may be accessed by illumination of the written bits using a low power CW diode laser that emits at the 650-nm absorption maximum of the written form 10. The diode laser light induces the stored bits to fluorescence, and measuring the emitted fluorescence by means of a photomultiplier coupled to high NA optics accesses the stored information. The dye molecules 10 are very stable, and the written information may be stored for years without noticeable decay.
Despite several studies, the use of DP-PAG compositions for optical information recording has still unsolved problems. New PAGs are of interest to diminish subside processes of dye destruction upon irradiation and, therefore, to increase resolution and information capacity of the WORM materials. One possible solution is the photodehydrogenation of aryl(hetaryl)pyrazolines under illumination in carbon tetrachloride [16–18]. The following mechanism of the phototransformation of aryl(hetaryl)pyrazolines has been proposed (Scheme 7) [18].

The photodehydrogenation of pyrazolines is accompanied by an increase in the acidity of the medium [18], which can be successfully used for the fluorescence activation of the lactone forms of rhodamine dyes.
The irradiation of a solution that contains the lactone form of rhodamine B and pyrazoline 11 at the absorption maximum of the pyrazoline results in pink coloration both in carbon tetrachloride and in toluene containing CCl4 or C2Cl6 (2–5%). The changes in the electron absorption spectra upon the irradiation of the rhodamine B lactone form + pyrazoline 11composition in toluene in the presence of C2Cl6 are shown in Figure 1.

Electronic absorption spectra of pyrazoline 11(c=48 μm) and rhodamine B lactone form (c=50 μm) in CCl4 before (1) and after (2–11) irradiation at 420 nm.
It is important to note the ability of aryl(hetaryl)pyrazolines to photogenerate acidity and thus to activate the rhodamine dye fluorescence in polymer films as well [17]. The changes in the electronic absorption and emission spectra during the irradiation of a poly(methyl methacrylate) film (PMMA film) containing rhodamine B in lactone form and pyrazoline 11are shown in Figure 2.
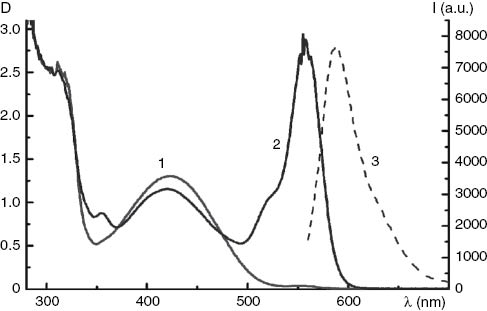
Electronic absorption (1, 2) and emission spectra (3) of PMMA film with dissolved pyrazoline 11, rhodamine B lactone form, and C2Cl6 before (1) and after (2, 3) irradiation at 420 nm.
Two subsequent organic reactions that initiate the coloring of a PMMA film, namely, the photodehydrogenation of pyrazoline and rhodamine B lactone ring opening (Scheme 2), occur in a polymer film. We have shown previously that the use of activating irradiation in the near UV region (at 300 nm) can lead to rhodamine lactone form opening and the decrease of the recording quality of the film [19]. However, in the presence of aryl(hetaryl)pyrazolines 11 and 12, the wavelength of activating irradiation is shifted to the visible region (near 400 nm) of the electronic absorption spectrum. It completely excludes the possible photodestruction of the starting rhodamine lactone form and provides good perspectives for using aryl(hetaryl)pyrazolines as effective PAGs in the design of the recording media for 3D archive optical memory [20]. It is also important to note that other dihydrohetarenes can also give rise to acidity under their illumination in the near UV region. The photoaromatization of dihydropyridines to pyridines has also been reported. The process takes place with CCl4 destruction, which is followed by proton generation [21].
Media based on photochemically activated fluorophores
Protection and deprotection reactions are essential in the synthesis of multifunctional compounds. In many cases, these reactions are conducted under acidic or basic conditions or require highly reactive reagents, leading to the limitation of use of protecting groups in the synthetic processes. From these viewpoints, much attention has been paid to photolabile protecting groups, which allow deprotection without additional reagents and under neutral conditions.
Chemically modified fluorophores, in which fluorescence is blocked by the presence of a photocleavable protecting group, have been introduced by Ware and coworkers [22]. Many photosensitive organic compounds as potential fluorophores have then been synthesized for various sensor applications. N-Acetyl or N-benzoylazine derivatives 13 shown in Scheme 8, which are known as leuco dyes, are used in many applications, including pressure-sensitive carbonless paper [23] and thermographic and photothermographic imaging [24]. Akiba and coworkers [1] have used these photosensitive derivatives to provide fluorescence activation under illumination with the formation of fluorescent oxazine dye 14 (Scheme 8).

Among various photocleavable protecting groups reported, the 6-nitroveratroyl-oxycarbonyl (NVOC) group has gained much attention because of its efficient removal upon UV irradiation. The preparation of NVOC-protected quinizarin 15 is shown in Scheme 9. The first application of the NVOC protecting group to the direct generation of patterned fluorescent images has been reported by Min and coworkers [25].

If the protecting group in nonfluorescing dye 15 is removed under photoinduced chemical transformation, the fluorescence can be regenerated, allowing patterned fluorescent images in the polymer film by selective removal of the protecting group in the exposed areas. The protecting group investigated earlier is the t-butyloxycarbonyl (t-Boc) group, which requires a PAG to cleave the protecting group in the exposed areas. The NVOC group, however, does not require acidic conditions for deprotection. To circumvent the use of PAG, photodegradation-induced fluorescence imaging that uses poly(silylene-p-phenylene)s in the absence of PAG has recently been reported. UV irradiation induces oxidation of the exposed area and results in fine fluorescent patterns [26].
To avoid using of PAG, a new polybenzoxazole 16 substituted with a hydroxyphenyl group has been synthesized by using a Suzuki coupling reaction accompanied by a simultaneous elimination reaction of acetyl protecting group of hydroxyl group (Scheme 10) [27]. The polymer in solution as well as in the film exhibits a strong emission (520 nm) with a large Stokes shift (approximately 200 nm) via excited-state intramolecular proton-transfer mechanism. The new conjugated polymer containing 2-(2′-hydroxyphenyl)benzoxazole exhibits a unique fluorescence quenching property upon UV irradiation, allowing convenient fluorescence imaging.

Thus, Lee and coworkers [27, 28] have reported a simple yet effective fluorescence imaging on the films of the new polymer by means of UV irradiation without the aid of any PAG and any additional processing such as baking or etching. The resulting fluorescence image is stable over 100 days of storage under ambient conditions.
For the long-term stability of a patterned image, appropriate control of recording reactivity, such as a limited basicity of the fluorophore, is necessary to achieve photochemically gated protonation that occurs only under the selective recording light (the photoacid-abundant condition). On the basis of this consideration, a novel quinoline-based fluorophore with controlled basicity 17has been designed (Scheme 11) [29]. Its structure is characterized by intramolecular hydrogen bonding that is introduced to reduce basicity of the nitrogen atom of quinoline. Kim and Park have reported the H-bond-induced gated protonation and the stable fluorescence imaging of 17 in terms of the comparison with an H-bond-free analogue 18 that belongs to a class of conventional basic fluorophores.

Chromone, quinolone, and thiochromone derivatives have attracted attention for their unique photochemical properties, including photoabsorption, photoreaction, and photochromism. However, there are no reports of using them as photolabile protecting groups. Kitani and coworkers [30] have prepared novel photolabile thiochromone S,S-dioxide 19 (Scheme 12) as a protecting group for various alcohols, amines, and carboxylic acids. The photodeprotection proceeds smoothly to release alcohols, amines, or carboxylic acids almost quantitatively under irradiation with 313 nm light. As the reaction proceeds, the absorption at near 365 nm increases, and a new fluorescent emission at 440 nm is observed because of the formation of the tetracyclic compound 20. The photoproduct 20 has 100 times stronger fluorescent intensity than the starting thiochromone S,S-dioxide 19. The applications of thiochromone type protecting groups to other functional groups as well as the elucidation of the reaction mechanism are under way.

A large series of photoactive chromone derivatives have been synthesized by Krayushkin and coworkers [31]. Derivatives of 2-furyl-3-acetylchromones are of interest as photosensitive organic systems designed for use in various photocontrolled photonic devices. 2-Furyl-3-acetylchromones undergo irreversible changes under UV irradiation to form fluorescent products 21 providing optical information reading (Scheme 13).

Photochemical transformations of the synthesized compounds in polymeric matrices to develop photoluminescent recording media have also been studied. Unlike their behavior in solution, the excitation of fluorescence by light at the maximum of the photoproduct’s absorption band leads to a very high fluorescence intensity. It has been shown that among the studied polymeric bindings, the use of poly(methyl methacrylate) provides the highest fluorescence intensity. Judging from kinetic data, the fluorescence intensity of the photoproducts increases sharply on going from solution to a polymeric matrix at comparably equal changes of the photoinduced absorbance [31–35].
Several publications have described the use of photoactivatable fluorophores in cell biology [6, 36–41]. The blocked fluorophore is used to label a cellular protein in vitro. The labeled protein is then microinjected into a living cell and allowed to equilibrate with the endogenous unlabeled protein. Subsequent photolysis using near-UV light over a small region of the cell unmasks the fluorophore. The movement of the protein bearing the fluorescent tag can be observed. Corriе and Trentham have described two photoactivatable fluorescein derivatives for labeling cysteine residues [6]. One of the derivatives bears a lipophilic anchor group. The etherification of both phenolic hydroxyl groups of fluorescein locks the molecule into its nonfluorescent lactone form (Scheme 14). Fluorescein has been unsymmetrically substituted with a 2-nitrobenzyl ether and one of three variously functionalized alkyl ethers to give compounds 22, 23, and 24.

The 2-nitrobenzyl group can be selectively removed by photolysis with near-UV light to regenerate a fluorescent species 25, whereas the second ether group contains one of a range of functions, maleimido or iodoacetyl for ligation to proteins, or a long alkyl chain to promote association with lipid membranes.
A new, bright photoactivatable organic fluorophore that can be imaged at the single-molecule level in living cells has been reported [42]. The DCDHF class of single-molecule cellular labels [43–45], in which an amine donor is connected to a dicyanomethylenedihydrofuran acceptor via a conjugated п-bonded network, has been used. Lord and coworkers [42] have reengineered a red-emitting DCDHF to produce the fluorogen 26, a molecule that is dark until photoactivated with a short burst of low-intensity violet light. Photoactivation of 26 leads to conversion of the azide moiety to an amine 27, which shifts the absorption to long wavelengths and creates a bright, red emitter that is photostable enough to be imaged on the single-molecule level in living cells (Scheme 15). Thus, photoactivation of an azide-based DCDHF fluorogen provides a new class of labels that can be useful for superresolution imaging schemes that require active control of single molecules in the chemically and optically complex medium of the cell.

The photoactivatable DCDHF single-molecule fluorogen [42] is an example of a larger class based on replacing a donor group in a push-pull chromophore with a photoactivatable azide group. Unlike the other photoswitching systems, photoactivating the azido-DCDHF does not require other additives (i.e. oxygen scavengers and exogenous thiol) [46–48] and thus may find greater ease of use in living systems. The next step with use of these photoactivatable DCDHF systems is to apply specific targeting schemes to direct the label to desired locations. These molecules may also be used for fluorogenic photoaffinity labeling [49]; assuming a binding pocket is engineered for the fluorogen, a flash of blue light can simultaneously turn on fluorescence and induce a covalent bond formation between the DCDHF and the biomolecule.
A novel type of photoprotecting group for carbonyl compounds has been described. Efforts toward developing photolabile protecting groups for carbonyl compounds date back three decades, and progress has been made since [50–61]. Nevertheless, apparent drawbacks hinder the use of those approaches in organic synthesis and biomedical research. For example, protecting groups based on a well-established o-nitrobenzyl photochemistry have the inherent limitations of being sensitive to reactive organometallic reagents and reducing reagents, which restricts the scope of their application in organic synthesis. It also has been pointed out that their photochemical properties are not ideal for biological research [62–64]. The protecting diol is readily accessed in one step from a commercially available material [65]. The installation of the protecting group upon the carbonyl compounds is achieved in excellent yields (Scheme 16). The carbonyl compounds in their protected form 28 are remarkably stable under various conditions and can be released photochemically with high efficiency.

Conclusion
Heterocyclic compounds that possess irreversible photoinduced fluorescent changes are effective candidates for the design of the recording media in 3D archive optical memory. The transition of these compounds from a nonfluorescent form to highly fluorescent one can undergo directly upon illumination or with intermediate photogeneration of acid that then provides effective fluorophore activation. The application of heterocyclic compounds in these media is rather perspective because it provides a possibility to shift the wavelength of activating irradiation to the visible region (near 400 nm) of the electronic absorption spectrum and excludes completely the possible photodestruction of the starting fluorophore passive form. The photoactivation may also provide a new class of labels that would be useful in biochemical studies for superresolution imaging schemes that require active control of single molecules in the chemically and optically complex medium of the cell.
References
[1] Akiba, M.; Dvornikov, A. S.; Rentzepis, P. M. Formation of oxazine dye by photochemical reaction of N-acyl oxazine derivatives. J. Photochem. Photobiol. A. Chem. 2007, 190, 69–76.10.1016/j.jphotochem.2007.03.014Search in Google Scholar
[2] Dvornikov, A. S.; Rentzepis, P. M. Novel organic ROM materials for optical 3D memory devices. Opt. Commun. 1997, 136, 1–6.10.1016/S0030-4018(96)00645-1Search in Google Scholar
[3] Liang, Y. C.; Dvornikov, A. S.; Rentzepis, P. M. Fluorescent photochromic fulgides. Res. Chem. Intermed. 1998, 24, 905–914.10.1163/156856798X00609Search in Google Scholar
[4] Pudavar, H. E.; Joshi, M. P.; Prasad, P. N.; Reinhardt, B. A. High-density three-dimensional optical data storage in a stacked compact disk format with two-photon writing and single photon readout. Appl. Phys. Lett. 1999, 74, 1338–1340.10.1063/1.123543Search in Google Scholar
[5] Liang, Y.; Dvornikov, A. S.; Rentzepis, P. M. Synthesis and photochemistry of photochromicfluorescing indol-2-ylfulgimides. J. Mater. Chem. 2000, 10, 2477–2482.10.1039/b002374oSearch in Google Scholar
[6] Corriе, J. E. T.; Trentham, D. R. Synthesis of photoactivatable fluorescein derivatives bearing side chains with varying properties. J. Chem. Soc. Perkin Trans. 11995, 16, 1993–2000.10.1039/p19950001993Search in Google Scholar
[7] George, M. V.; Scaiano, J. C. Photochemistry of o-nitrobenzaldehyde and related studies. J. Phys. Chem. 1980, 84, 492–495.10.1021/j100442a007Search in Google Scholar
[8] Pappas, S. P. Photogeneration of acid: Part 6. A review of basic principles for resist imaging applications. J. Imaging Technol. 1985, 11, 146–157.Search in Google Scholar
[9] McKean, D. R.; Schaedeli, U.; MacDonald, S. A. Acid photogeneration from sulfonium salts in solid polymer matrices. J. Polym. Sci. Part A1989, 27, 3927–3935.10.1002/pola.1989.080271205Search in Google Scholar
[10] Scaiano, J. C.; Barra, M.; Calabrese, G.; Sinta, R. Photochemistry of 1,2-dibromoethane in solution. A model for the generation of hydrogen bromide. J. Chem. Soс. Chem. Commun. 1992, 19, 1418–1419.10.1039/c39920001418Search in Google Scholar
[11] Shirai, M.; Tsunooka, M. Photoacid and photobase generators: chemistry and applications to polymeric materials. Prog. Polym. Sci. 1996, 21, 1–45.10.1016/0079-6700(95)00014-3Search in Google Scholar
[12] Ahn, K.-D.; Lee, J.-H.; Cho, I.; Park, K. H.; Kang, J.-H.; Han, D. K.; Kim, J.-M. Norbornene-derived quinizarin dye molecules for photoimaging in polymer films based on chemical amplification. J. Photopolym. Sci. Technol. 2000, 13, 493–496.10.2494/photopolymer.13.493Search in Google Scholar
[13] Lee, C.-W.; Yuan, Z.; Ahn, K.-D.; Lee, S.-H. Color and fluorescent imaging of t-BOC-protected quinizarin methacrylate polymers. Chem. Mater. 2002, 14, 4572–4575.10.1021/cm020016jSearch in Google Scholar
[14] Kim, J.-M.; Chang, T.-E.; Kang, J.-H.; Park, K. H.; Han, D.-K.; Ahn, K.-D. Photoacid-induced fluorescence quenching: a new strategy for fluorescent imaging in polymer films. Angew. Chem., Int. Ed. 2000, 39, 1780–1782.10.1002/(SICI)1521-3773(20000515)39:10<1780::AID-ANIE1780>3.0.CO;2-HSearch in Google Scholar
[15] Walker, E.; Dvornikov, A.; Coblentz, K.; Rentzepis, P. Terabyte recorded in two-photon 3D disk. Appl. Opt. 2008, 47, 4133–4139.10.1364/AO.47.004133Search in Google Scholar
[16] Traven, V. F.; Ivanov, I. V.; Pavlov, A. S.; Manaev, A. V.; Voevodina, I. V.; Barachevskii, V. A. Quantitative photooxidation of 4-hydroxy-3-pyrazolinylcoumarins to pyrazolyl derivatives. Mendeleev Commun. 2007, 17, 345–346.10.1016/j.mencom.2007.11.016Search in Google Scholar
[17] Traven, V. F.; Ivanov, I. V.; Dolotov, S. M.; Kobeleva, O. I.; Valova, T. M.; Barachevsky, V. A. Aryl(hetaryl)pyrazolines as new photoacid generators for optical information recording. J. Photochem. Photobiol. A. Chem. 2014, 295, 34–39.10.1016/j.jphotochem.2014.08.016Search in Google Scholar
[18] Traven, V. F.; Ivanov, I. V. New reaction of photoaromatization of aryl- and hetarylpyrazolines. Russ. Chem. Bull. Int. Ed. 2008, 57, 1063–1069.10.1007/s11172-008-0135-3Search in Google Scholar
[19] Traven, V. F.; Dolotov, S. M.; Ivanov, I. V.; Barachevsky, V. A.; Kobeleva, O. I.; Valova, T. M.; Platonova, I. V.; Ajt, A.O. Light-sensitive polymer material having fluorescent information reading. Pat. RU 2478116 C2, March 17, 2011.Search in Google Scholar
[20] Ivanov, I. V.; Dolotov, S. M.; Kobeleva, O. I.; Valova, T. M.; Barachevsky, V. A.; Traven, V. F. Photoactivation of fluorescence of rhodamine dyes in the presence of haloalkanes. Russ. Chem. Bull. Int. Ed. 2013, 62, 1195–1200.10.1007/s11172-013-0163-5Search in Google Scholar
[21] Jin, M.-Z.; Yang, L.; Wu, L.-M.; Liu, Y.-C.; Liu, Z.-L. Novel photoinduced aromatization of Hantzsch 1,4-dihydropyridines. Chem. Commun. 1998, 22, 2451–2452.10.1039/a807093hSearch in Google Scholar
[22] Ware, B. R.; Brvenik, L. J.; Cummings, R. T.; Furukawa, R. H.; Krafft, G. A. Fluorescence Photoactivation and Dissipation (FPD). In Applications of Fluorescence in the Biomedical Sciences. Taylor, D. L.; Waggoner, A. S.; Murphy, R. F.; Lanni, F.; Birge, R. R., Eds. Alan R. Liss: New York, 1986; pp 141–157.Search in Google Scholar
[23] Potts, H. A.; Wood, A. H.; Cook, C. C. The decomposition of N-benzoylleucomethylene blue during the pressure-sensitive copying process. I. Identification of reaction products. J. Appl. Chem. Biotechnol. 1972, 22, 651–657.10.1002/jctb.5020220602Search in Google Scholar
[24] Thien, T. V. Thiazine, Oxazine, and Phenazine Leuco Dyes. In Chemistry and Application of Leuco Dyes. Muthyala, R., Ed. Plenum Press: New York, 1997; pp 67–95.Search in Google Scholar
[25] Min, S.-J.; Ahn, K.-D.; Kim, J.-M. Patterned fluorescence images using a photocleavable NVOC-protected quinizarin. Bull. Korean Chem. Soc. 2005, 26, 1437–1439.10.5012/bkcs.2005.26.9.1437Search in Google Scholar
[26] Ohshita, J.; Uemura, T.; Kim, D.-H.; Kunai, A.; Kunugi, Y.; Kakimoto, M. Preparation of poly(silylene-p-phenylene)s containing a pendant fluorophore and their applications to PL imaging. Macromolecules2005, 38, 730–735.10.1021/ma040108pSearch in Google Scholar
[27] Lee, J. K.; Kim, H.-J.; Kim, T. H.; Lee, C.-H.; Park, W. H.; Kim, J.; Lee, T. S. A new synthetic approach for polybenzoxazole and light-induced fluorescent patterning on its film. Macromolecules2005, 38, 9427–9433.10.1021/ma051112jSearch in Google Scholar
[28] Park, S.; Kim, S.; Seo, J.; Park, S. Y. Strongly fluorescent and thermally stable functional polybenzoxazole film: excited-state intramolecular proton transfer and chemically amplified photopatterning. Macromolecules2005, 38, 4557–4559.10.1021/ma050009rSearch in Google Scholar
[29] Kim, S.; Park, S. Y. Photochemically gated protonation effected by intramolecular hydrogen bonding: towards stable fluorescence imaging in polymer films. Adv. Mater. 2003, 15, 1341–1344.10.1002/adma.200305050Search in Google Scholar
[30] Kitani, S.; Sugawara, K.; Tsutsumi, K.; Morimoto, T.; Kakiuchi, K. Synthesis and characterization of thiochromone S,S-dioxides as new photolabile protecting groups. Chem. Commun. 2008, 18, 2103–2105.10.1039/b801860jSearch in Google Scholar
[31] Krayushkin, M. M.; Levchenko, K. S.; Yarovenko, V. N.; Zavarzin, I. V.; Barachevsky, V. A.; Puankov, Yu. A.; Valova, T. M.; Kobeleva, O. I. Synthesis and study of photosensitive chromone derivatives for recording media of archival three-dimensional optical memory. ARKIVOC2009, 9, 269–283.10.3998/ark.5550190.0010.916Search in Google Scholar
[32] Barachevsky, V. A.; Kobeleva, O. I.; Valova, T. M.; Ait, A. O.; Dunaev, A. A.; Gorelik, A. M.; Krayushkin, M. M.; Levchenko, K. S.; Yarovenko, V. N.; Kiyko, V. V.; et al. Photochromic and irreversible photofluorescent organic materials for 3D bitwise optical memory. Opt. Mem. Neural Netw. 2010, 19, 187–195.10.3103/S1060992X10020104Search in Google Scholar
[33] Barachevsky, V. A.; Strokach, Yu. P.; Puankov, Yu. A.; Kobeleva, O. I.; Valova, T. M.; Levchenko, K. S.; Yarovenko, V. N.; Krayushkin, M. M. Light-sensitive heterocyclic compounds for information nanotechnologies. ARKIVOC2009, 9, 70–95.10.3998/ark.5550190.0010.906Search in Google Scholar
[34] Krayushkin, M. M.; Levchenko, K. S.; Yarovenko, V. N.; Christoforova, L. V.; Barachevsky, V. A.; Puankov, Yu. A.; Valova, T. M.; Kobeleva, O. I.; Lyssenko, K. Synthesis and reactivity of 1-aryl-9H-thieno[3,4-b]chromon-9-ones. New J. Chem. 2009, 33, 2267–2277.10.1039/b9nj00237eSearch in Google Scholar
[35] Barachevsky, V. A.; Kobeleva, O. I.; Ait, A. O.; Gorelik, A. M.; Valova, T. M.; Krayushkin, M. M.; Yarovenko, V. N.; Levchenko, K. S.; Kiyko, V. V.; Vasilyuk, G. T. Optical polymer materials with photocontrolled fluorescence. Opt. Mater. 2013, 35, 1805–1809.10.1016/j.optmat.2013.03.005Search in Google Scholar
[36] Mitchison, T. J. Polewards microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J. Cell. Biol. 1989, 109, 637–652.10.1083/jcb.109.2.637Search in Google Scholar
[37] Reinsch, S. S.; Mitchison, T. J.; Kirschner, M. Microtubule polymer assembly and transport during axonal elongation. J. Cell. Biol. 1991, 115, 365–379.10.1083/jcb.115.2.365Search in Google Scholar
[38] Sawin, K. E.; Mitchison, T. J. Poleward microtubule flux mitotic spindles assembled in vitro. J. Cell. Biol. 1991, 112, 941–954.10.1083/jcb.112.5.941Search in Google Scholar
[39] Theriot, J. A.; Mitchison, T. J. Actin microfilament dynamics in locomoting cells. Nature1991, 352, 126–131.10.1038/352126a0Search in Google Scholar
[40] Theriot, J. A.; Mitchison, T. J.; Tilney, L. G.; Portnoy, D. A. The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature1992, 357, 257–260.10.1038/357257a0Search in Google Scholar
[41] Okabe, S.; Hirokawa, N. Differential behavior of photoactivated microtubules in growing axons of mouse and frog neurons. J. Cell. Biol. 1992, 117, 105–120.10.1083/jcb.117.1.105Search in Google Scholar
[42] Lord, S. J.; Conley, N. R.; Lee, H. D.; Samuel, R.; Liu, N.; Twieg, R. J.; Moerner, W. E. A photoactivatable push−pull fluorophore for single-molecule imaging in live cells. J. Am. Chem. Soc. 2008, 130, 9204–9205.10.1021/ja802883kSearch in Google Scholar
[43] Willets, K. A.; Nishimura, S. Y.; Schuck, P. J.; Twieg, R. J.; Moerner, W. E. Nonlinear optical chromophores as nanoscale emitters for single-molecule spectroscopy. Acc. Chem. Res. 2005, 38, 549–556.10.1021/ar0401294Search in Google Scholar
[44] Nishimura, S. Y.; Lord, S. J.; Klein, L. O.; Willets, K. A.; He, M.; Lu, Z.; Twieg, R. J.; Moerner, W. E. Diffusion of lipid-like single-molecule fluorophores in the cell membrane. J. Phys. Chem. B2006, 110, 8151–8157.10.1021/jp0574145Search in Google Scholar
[45] Lord, S. J.; Lu, Z.; Wang, H.; Willets, K. A.; Schuck, P. J.; Lee, H. D.; Nishimura, S. Y.; Twieg, R. J.; Moerner, W. E. Photophysical properties of acene DCDHF fluorophores: long-wavelength single-molecule emitters designed for cellular imaging. J. Phys. Chem. A2007, 111, 8934–8941.10.1021/jp0712598Search in Google Scholar
[46] Bates, M.; Blosser, T. R.; Zhuang, X. Short-range spectroscopic ruler based on a single-molecule optical switch. Phys. Rev. Lett. 2005, 94, 108101-1–108101-4.10.1103/PhysRevLett.94.108101Search in Google Scholar
[47] Heilemann, M.; Margeat, E.; Kasper, R.; Sauer, M.; Tinnefeld, P. Carbocyanine dyes as efficient reversible single-molecule optical switch. J. Am. Chem. Soc. 2005, 127, 3801–3806.10.1021/ja044686xSearch in Google Scholar
[48] Rasnik, I.; McKinney, S. A.; Ha, T. Nonblinking and long-lasting single-molecule fluorescence imaging. Nat. Methods2006, 3, 891–893.10.1038/nmeth934Search in Google Scholar
[49] Kotzyba-Hibert, F.; Kapfer, I.; Goeldner, M. Recent trends in photoaffinity labeling. Angew. Chem. Int. Ed. 1995, 34, 1296–1312.10.1002/anie.199512961Search in Google Scholar
[50] Hebert, J.; Gravel, D. o-Nitrophenylethylene glycol: a photosensitive protecting group for aldehydes and ketones. Can. J. Chem. 1974, 52, 187–189.10.1139/v74-030Search in Google Scholar
[51] Gravel, D.; Hebert, J.; Thoraval, D. o-Nitrophenylethylene glycol as photoremovable protective group for aldehydes and ketones: syntheses, scope, and limitations. Can. J. Chem. 1983, 61, 400–410.10.1139/v83-072Search in Google Scholar
[52] Gravel, D.; Murray, S.; Ladouceur, G. o-Nitrobenzyl alcohol, a simple and efficient reagent for the photoreversible protection of aldehydes and ketones. J. Chem. Soc., Chem. Commun. 1985, 24, 1828–1829.10.1039/c39850001828Search in Google Scholar
[53] Friedrich, E.; Lutz, W.; Eichenauer, H.; Enders, D. Mild cleavage of N,N-dimethylhydrazones to carbonyl compounds with singlet oxygen. Synthesis1977, 12, 893–894.10.1055/s-1977-24626Search in Google Scholar
[54] Hoshino, O.; Sawaki, S.; Umezawa, B. Organic photochemistry. V. Dethioacetalization by photolysis in the presence of molecular oxygen. Chem. Pharm. Bull. 1979, 27, 538–540.10.1248/cpb.27.538Search in Google Scholar
[55] Aurell, M. J.; Boix, C.; Ceita, M. L.; Llopis, C.; Tortajada, A.; Mestres, R. Polymer-supported o-nitrophenylethylene glycols for photoremovable protection of aldehydes. J. Chem. Res. (S)1995, 12, 452–453.Search in Google Scholar
[56] Ceita, L.; Maiti, A. K.; Mestres, R.; Tortajada, A. o-Nitroaryldioxolanes for protection of pheromones. Study of the photodelivery of carbonyl compounds. J. Chem. Res. (S)2001, 10, 403–404.10.3184/030823401103168433Search in Google Scholar
[57] McHale, W. A.; Kutateladze, A. G. An efficient photo-SET-induced cleavage of dithiane-carbonyl adducts and its relevance to the development of photoremovable protecting groups for ketones and aldehydes. J. Org. Chem. 1998, 63, 9924–9931.10.1021/jo981697ySearch in Google Scholar
[58] Lin, W.; Lawrence, D. S. A strategy for the construction of caged diols using a photolabile protecting group. J. Org. Chem. 2002, 67, 2723–2726.10.1021/jo0163851Search in Google Scholar
[59] Lu, M.; Fedoryak, O. D.; Moister, B. R.; Dore, T. M. Bhc-diol as a photolabile protecting group for aldehydes and ketones. Org. Lett. 2003, 5, 2119–2122.10.1021/ol034536bSearch in Google Scholar
[60] Blanc, A.; Bochet, C. G. Bis(o-nitrophenyl)ethanediol: a practical photolabile protecting group for ketones and aldehydes. J. Org. Chem. 2003, 68, 1138–1141.10.1021/jo026347xSearch in Google Scholar
[61] Kantevari, S.; Narasimhaji, C. V.; Mereyala, H. B. Bis(4,5-dimethoxy-2-nitrophenyl)ethylene glycol: a new and efficient photolabile protecting group for aldehydes and ketones. Tetrahedron2005, 61, 5849–5854.10.1016/j.tet.2005.04.007Search in Google Scholar
[62] Park, C.-H.; Givens, R. S. New Photoactivated protecting groups. 6. p-Hydroxyphenacyl: A phototrigger for chemical and biochemical probes1,2. J. Am. Chem. Soc. 1997, 119, 2453–2463.10.1021/ja9635589Search in Google Scholar
[63] Corrie, J. E. T.; Barth, A.; Munasinghe, V. R. N.; Trentham, D. R.; Hutter, M. C. Photolytic cleavage of 1-(2-nitrophenyl)ethyl ethers involves two parallel pathways and product release is rate-limited by decomposition of a common hemiacetal intermediate. J. Am. Chem. Soc. 2003, 125, 8546–8554.10.1021/ja034354cSearch in Google Scholar
[64] Il’ichev, Y. V.; Schwörer, M. A.; Wirz, J. Photochemical reaction mechanisms of 2-nitrobenzyl compounds: methyl ethers and caged ATP. J. Am. Chem. Soc. 2004, 126, 4581–4595.10.1021/ja039071zSearch in Google Scholar
[65] Wang, P.; Hu, H.; Wang, Y. Novel photolabile protecting group for carbonyl compounds. Org. Lett. 2007, 9, 1533–1535.10.1021/ol070346fSearch in Google Scholar
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Reviews
- Synthesis of quinazolines and quinazolinones via palladium-mediated approach
- Media with photoinduced irreversible fluorescence
- Synthesis of quinolines and acridines by the reaction of 2-(perfluoroalkyl)anilines with lithium and Grignard reagents
- Preliminary Communications
- Synthesis of tricyclic indolizidines from ethyl isocyanoacetate
- Ligand- and catalyst-free intramolecular C-S bond formation: direct access to indalothiochromen- 4-ones
- Research Articles
- Preparation of optically active 4-substituted γ-lactones by lipase-catalyzed optical resolution
- One-pot synthesis of 4-alkyl-2-amino-4H-chromene derivatives
- Ring transformation and antimicrobial activity of indolyl-substituted 2(3H)-furanones
Articles in the same Issue
- Frontmatter
- Reviews
- Synthesis of quinazolines and quinazolinones via palladium-mediated approach
- Media with photoinduced irreversible fluorescence
- Synthesis of quinolines and acridines by the reaction of 2-(perfluoroalkyl)anilines with lithium and Grignard reagents
- Preliminary Communications
- Synthesis of tricyclic indolizidines from ethyl isocyanoacetate
- Ligand- and catalyst-free intramolecular C-S bond formation: direct access to indalothiochromen- 4-ones
- Research Articles
- Preparation of optically active 4-substituted γ-lactones by lipase-catalyzed optical resolution
- One-pot synthesis of 4-alkyl-2-amino-4H-chromene derivatives
- Ring transformation and antimicrobial activity of indolyl-substituted 2(3H)-furanones


