Abstract
An efficient ligand- and catalyst-free intramolecular S-arylation leading to the direct synthesis of indalothiochromen-4-ones from simple dithioesters under mild conditions has been developed. This method is particularly noteworthy given its experimental simplicity, high generality, and good functional group toleration.
The indole subunits have always fascinated the researchers for their recurrent presence in most of the biologically active natural products [1–5]. The polycyclic annulated indole compounds have drawn the interest of chemists to design their synthesis and study their interaction with enzymes in biological systems [6–10]. For example, polycyclic indole alkaloids such as ajmaline [11], ervincidine [12], alistonitrine [13], MGM-16, and mitragynine [14] are important in both biology and medicinal chemistry.
A wide range of sulfur-heterocyclic scaffolds such as thiopyrans and fused-thiopyran derivatives, including benzothiopyrans, have been reported for their biological activity [15–19]. Annulation of the indole ring to the thiopyran makes it even more interesting in the synthetic and biological perspective [9, 20, 21]. On the basis of these considerations and our interest in developing new synthetic strategies for the synthesis of biologically viable heterocycles [22–28], we herein report an improved method for the synthesis of indalothiochromen-4-ones.
The literature reports reveal only a few synthetic methods for the construction of indalothiochromen-4-ones. The earliest report on the synthesis of such compounds is the reaction of propiolic acid and 3-mercaptoindole followed by treatment with phosphoric acid [29], but this method has not attracted much attention. Indoline-2-thione was exploited more as a starting material than any other reactants [21, 30–33]. The thio-Claisen rearrangement of 2-(4′-aryloxybut-2′-ynylthio)-1-methylindole derived from indoline-2-thione and 1-aryloxy-4-chlorobut-2-yne has also been reported [34]. On the other hand, the use of dithioesters as a sulfur source to afford indalothiochromen-4-ones has not been explored yet to the best of our knowledge. Herein, we report the new and efficient synthesis of indalothiochromen-4-ones by the reaction of simple 1-(2-chloro-1H-indol-3-yl)ethanone and dithioesters under basic conditions at room temperature.
The starting materials N-alkyl/aryl indoles 1a-c were synthesized according to the reported method [35]. Initially, the Vilsmeier-Haack reaction of oxindoles (A) yielded 3-acetyl 2-chloro indoles (B). These products were next, either benzylated or methylated using benzyl bromide and methyl iodide, respectively (Scheme 1). The starting materials dithioesters were also synthesized by the known method with slight modifications. The aryl halides were converted into Grignard reagents, which were further allowed to react with carbon disulfide to form a thiocarbonyl-thio salt. Subsequently, treatment of the thiocarbonyl-thio salt with an alkyl halide yielded the desired dithioesters 2a-j[36]. As can be seen from Scheme 1, 1-(2-chloro-1H-indol-3-yl)ethanone (1a) was treated with dithioester 2a in DMF in the presence of sodium hydride at room temperature to furnish 2-phenyl-thiopyrano[2,3-b]indol-4-(9H)-one thiochromone (3a) in good yield. The mildest conditions possible under which the reaction of compound 1a with dithioester 2a would proceed with a synthetically useful rate were investigated. The reaction conducted under reflux in DMF in the presence of NaH (1.5 Eq) required 24 h to reach completion and provided the desired product 3a in 40% yield. The use of other bases than NaH, including K2CO3, TEA, KOH,t-BuOK, and MeONa, gave rise to significantly lower yields or no product 3a at all.
![Scheme 1 Synthesis route of 2-aryl/heteroaryl-thiopyrano[2,3-b]indol-4-(9H)–ones.](/document/doi/10.1515/hc-2014-0206/asset/graphic/j_hc-2014-0206_scheme_001.jpg)
Synthesis route of 2-aryl/heteroaryl-thiopyrano[2,3-b]indol-4-(9H)–ones.
Therefore, the reaction was optimized by varying the amount of the base NaH, solvent, and temperature. The reaction was found to proceed efficiently at room temperature with the highest yield of 60% of 3aobtained in the presence of 2.5 Eq of NaH. A decrease in the amount of NaH to 2.0 Eq had negative effect on the yield of 3a (55%), whereas the use of 3.0 Eq did not lead to any significant increase in the yield compared to the application of 2.5 Eq of NaH. Screening of various solvents, including toluene, THF, diethyl ether and DMF, showed that DMF is the most suitable medium. The reaction of 1a with 2a showed no improvement upon raising the temperature. Subsequently, the reactions of N-alkyl/aryl indole derivatives 1a-c with wide range of dithioesters 2a-jwere evaluated under the optimized conditions. In all cases, the products were obtained in moderate to good yield at room temperature.
Interestingly, the LCMS analysis of crude product 3h suggested a contamination with a trace amount of 9-benzyl-2-(4-(methylthio)phenyl)thiopyrano[2,3-b]indol-4(9H)-one [28, 37] (not shown). The suggested SNAr substitution reaction of the halogen atom by the methylthio group did not take place with 4-chlorophenyl and 4-bromophenyl substituted indalothiochromen-4-ones 3f,g. The heterocyclic dithioesters of benzothiophene 2i and thiophene 2j underwent the reaction with 1aand 1c, efficiently furnishing the corresponding indalothiochromen-4-ones 3i, 3j,and 3min 63%, 56%, and 63% yields, respectively. 6-Bromo substituted indalothiochromen-4-ones 3nand 3owere obtained in the respective yields of 52% and 48%. The structures of all these products were characterized and confirmed by spectral analyses.
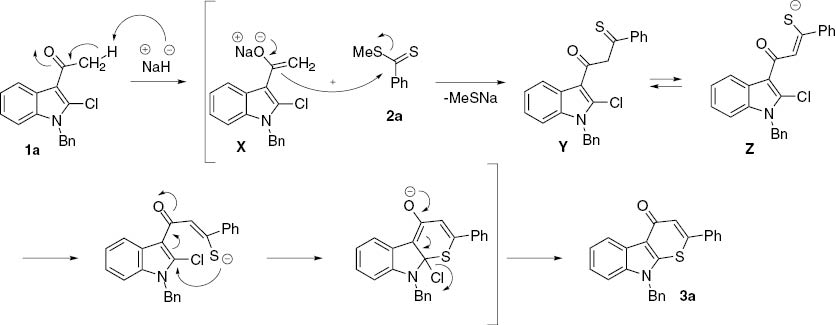
We propose that the reaction proceeds according to the mechanism shown in Scheme 2, exemplified by the particular reaction of 1a with 2a. The carbanion intermediate X undergoes a reaction with dithioester 2a to afford 1,3-thioketone Y, which then undergoes keto-enol tautomerization to generate another intermediate product Z. Finally, an intramolecular addition of mercapto group in Z followed by elimination of halide ion led to the formation of the observed product 3a.
Experimental details
All materials were purchased from known commercial sources and used without further purification. Thin layer chromatography (TLC) analysis was performed with silica gel 60F254 aluminum sheets (Merck). Mixtures of hexanes and ethyl acetate in different ratios were used in TLC analysis. Melting points were determined in open capillaries and are uncorrected. Infrared (IR) spectra were recorded on FT-IR spectrometer in KBr pellets. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were collected at NMR Facility, IOE, University of Mysore.
One-pot synthesis of indalothiochromen-4-ones 3a-o
A solution of N,N-dimethylformamide (DMF, 10.5 mL, 3 Eq) in dichloromethane (20 mL) was treated dropwise at 0°C with a solution of phosphorus oxychloride (11 mL, 3 Eq) in dichloromethane (20 mL). The mixture was stirred for 15 min, then treated slowly with a solution of 2-oxindole (A, 5 g, 1 Eq) in chloroform (20 mL), and stirred under reflux for 5 h. After the addition of crushed ice and stirring for an additional 20 min, the separation of two layers was observed. The aqueous layer was adjusted to pH 7 with sodium acetate. The mixture was left at room temperature overnight, and then the resultant precipitate of product B was collected by filtration, washed with water, and dried [35].
Next, a mixture of compound B (2.5 g) and potassium hydroxide (1.25 g, 1.5 Eq) in dry DMF (10 mL) was stirred for 10 min before treatment with benzyl bromide (1.4 mL, 1.1 Eq). The mixture was stirred at room temperature until substrate B was completely consumed, as monitored by TLC analysis. The mixture was then poured into water and extracted with ethyl acetate. The organic extract was dried over sodium sulfate and concentrated. The residue of product 1awas crystallized from diethyl ether.
A solution of compound 1 (1.0 mmol, 1.0 Eq) in DMF (2 mL) was added at 0°C to a 60% suspension of NaH in mineral oil (2.5 mmol, 2.5 Eq), and the mixture was stirred for 15 min at room temperature. Then a solution of dithioester 2 (1.0 mmol, 1.0 Eq) in DMF (2 mL) was added over a period of 10 min at 0°C, and the mixture was stirred at room temperature for an additional 7 h. The progress of the reaction was monitored by TLC. The mixture was poured into water and extracted with ethyl acetate (2×25 mL). The combined organic layers were washed with brine (25 mL) and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure, and the crude product was passed through a small plug of silica eluting with hexane/ethyl acetate (8:2) to afford indalothiochromen-4-ones 3a-o. Representative products are characterized as follows. Characterization of all compounds is given in Supplementary Information.
9-Benzyl-2-phenylthiopyrano[2,3-b]indol-4(9H)-one (3a)
Compound 3a was obtained by the reaction of 1a and 2a in 60% yield as a pale brown solid; mp 164–166°C; 1H NMR (CDCl3): δ 8.65 (d, J = 4.8 Hz, 1H), 7.60 (t, J = 3.6 Hz, 2H), 7.46–7.45 (m, 3H), 7.38–7.36 (m, 3H), 7.29–7.25 (m, 4H), 7.14 (d, J = 6.4 Hz, 2H), 5.43 (s, 2H); 13C NMR (CDCl3): δ 178.6, 143.9, 142.9, 137.9, 136.5, 134.6, 130.3, 129.0, 127.2, 127.0, 126.6, 124.7, 122.7, 122.4, 114.2, 108.9, 48.2; IR: 1597, 1481, 1474, 1419, 1334, 873, 750, 743, 717, 612 cm−1; MS (ESI): m/z = 368.1 (M +). Anal. Calcd for C24H17NOS: C, 78.45; H, 4.66; N, 3.81. Found: C, 78.43; H, 4.64; N, 3.79.
9-Benzyl-2-(3,4,5-trimethoxyphenyl)thiopyrano[2,3-b]indol-4(9H)-one (3e)
Compound 3e was obtained by the reaction of 1a and 2e in 76% yield as pale yellow solid; mp 169–171°C; 1H NMR (DMSO-d6): δ 8.43 (d, J = 7.7 Hz, 1H), 7.76 (d, J = 8.1 Hz, 1H), 7.42 (t, J = 7. 2 Hz, 1H), 7.44–7.23 (m, 7H), 7.02 (s, 2H), 5.73 (s, 2H), 3.89 (s, 6H), 3.73 (s, 3H); 13C NMR (DMSO-d6): δ 177.8, 153.8, 144.1, 142.7, 138.1, 136.0, 131.8, 129.3, 128.4, 127. 5, 126.2, 125.0, 124.4, 122.6, 121.8, 110.7, 105.1, 60.6, 56.6, 48.0; IR: 1599, 1505, 1479, 1420, 1332, 1249, 1126, 1008, 829, 758, 667 cm−1; MS (ESI): m/z = 458.7 (M +). Anal. Calcd for C24H16ClNOS: C, 70.88; H, 5.07; N, 3.06. Found: C, 70.85; H, 5.05; N, 3.05.
9-Benzyl-2-(4-fluorophenyl)thiopyrano[2,3-b]indol-4(9H)- one (3h)
Compound 3h was obtained by the reaction of 1a and 2h in 53% yield as creamy solid; mp 155–157°C; 1H NMR (CDCl3): δ 8.59 (d, J = 4.8 Hz, 1H), 7.61–7.57 (m, 2H), 7.42–7.25 (m, 7H), 7.20–7.13 (m, 4H), 5.47 (s, 2H); 13C NMR (CDCl3): δ 165.3, 162.8, 138.0, 134.3, 132.3, 129.2, 128.3, 126.1, 125.1, 124.3, 122.7, 116.4, 109.0, 48.4; IR: 1607, 1486, 1452, 1230, 1162, 1107, 1334, 833, 746, 559 cm−1; MS (ESI): m/z = 386.6 (M +). Anal. Calcd for C24H16ClNOS: C, 74.78; H, 4.18; N, 3.63. Found: C, 74.76; H, 4.16; N, 3.62.
2-(Benzo[b]thiophen-3-yl)-9-benzylthiopyrano[2,3-b]indol-4(9H)-one (3j)
Compound 3j was obtained by the reaction of 1a and 2j in 56% yield as amber solid; mp 145–149°C; 1H NMR (CDCl3): δ 8.47 (d, J = 7.2 Hz, 1H), 8.01 (d, J = 8.0 Hz, 2H), 7.85–7.75 (m, 4H), 7.42–7.31 (m, 5H), 7.25–7.18 (m, 2H), 7.12 (d, J = 7.6 Hz, 1H), 5.51 (s, 2H); 13C NMR (CDCl3): δ 182.1, 147.8, 141.1, 137.5, 135.9, 132.1, 129.3, 128.8, 128.6, 127.6, 126.6, 125.1, 124.6, 122.5, 122.2, 121.4, 119.8, 112.5, 110.9, 109.9, 60.7; IR: 1586, 1480, 1424, 1419, 1266, 1117, 755, 752, 733, 401 cm−1; MS (ESI): m/z = 423.8 (M +). Anal. Calcd for C26H17NOS2: C, 73.73; H, 4.05; N, 3.31. Found: C, 73.72; H, 4.03; N, 3.30.
6-Bromo-9-methyl-2-(p-tolyl)thiopyrano[2,3-b]indol-4(9H)- one (3n)
Compound 3n was obtained by the reaction of 1c and 2b in 52% yield as pale brown solid; mp 168–170 °C; 1H NMR (CDCl3): δ 8.73 (s, 2H), 8.59 (d, J = 6.8 Hz, 1H), 7.41 (dd, J = 6.6, 1.2 Hz, 2H), 7.34–7.29 (m, 2H), 6.94 (d, J = 8.8 Hz, 1H), 3.81 (s, 3H), 2.12 (s, 3H); 13C NMR (CDCl3): δ 176.8, 147.9, 138.0, 136.4, 134.8, 129.0, 128.8, 128.7, 126.3, 124.7, 123.5, 121.1, 113.8, 111.8, 110.2, 36.6, 21.3; IR: 1594, 1480, 1424, 1363,1234, 1194, 1052, 1016, 851, 810, 732, 705 cm−1; MS (ESI): m/z = 322.12 (M +). Anal. Calcd for C19H14BrNOS: C, 59.38; H, 3.67; N, 3.64. Found: C, 59.36; H, 3.66; N, 3.63.
Supplementary material
Experimental procedures for 1a-c and 2a-j and characterization of the remaining products 3 are available as supplementary material on the journal’s website.
Acknowledgments
The Ministry of Human Resource Development (MHRD) and the University Grant Commission (UGC), New Delhi, India, are acknowledged for recognizing the University of Mysore as the Institute of Excellence and for the financial assistance. We also thank NMR Facility, IOE, University of Mysore, Manasagangotri, Mysore, India, for spectral data.
References
[1] Negi, A.; Singla, R.; Singh, V. Indole based alkaloid in cancer: an overview. PharmaTutor 2014, 2, 76–82.Search in Google Scholar
[2] Aygun, A.; Pindur, U. Chemistry and biology of new marine alkaloids from the indole and annelated indole series. Curr. Med. Chem. 2003, 10, 1113–1127.10.2174/0929867033457511Search in Google Scholar
[3] Srivastava, A.; Pandeya, S. N. Indole a versitile nucleus in pharmaceutical field. Int. J. Current Pharm. Rev. Res. 2011, 1, 1–17.Search in Google Scholar
[4] Lounasmaa, M.; Tolvanen, A. Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Nat. Prod. Rep. 2000, 17, 175–191.10.1039/a809402kSearch in Google Scholar
[5] Vine, K. L.; Matesic, L.; Locke, J. M.; Ranson, M.; Skropeta, D. Cytotoxic and anticancer activities of isatin and its derivatives: a comprehensive review. Anticancer. Agents Med. Chem. 2009, 9, 397–414.10.2174/1871520610909040397Search in Google Scholar
[6] Takada, S.; Ishizuka, N.; Sasatani, T.; Makisumi, Y,; Jyoyama, H.; Hatakeyama, H.; Asanuma F.; Hirose, K. Studies on fused indoles. II. Structural modifications and analgesic activity of 4-aminomethyltetrahydrothiopyrano [2, 3-b] indoles. Chem. Pharm. Bull. (Tokyo) 1984, 32, 877–886.10.1248/cpb.32.877Search in Google Scholar
[7] El-Agrody, A. M.; Fouda, A. M.; Al-Dies, A.-A. M. Studies on the synthesis, in vitro antitumor activity of 4H-benzo[h]chromene, 7H-benzo[h]chromene[2,3-d]pyrimidine derivatives and structure-activity relationships of the 2-,3- and 2,3-positions. Med. Chem. Res. 2014, 23, 3187–3199.10.1007/s00044-013-0904-xSearch in Google Scholar
[8] Parmar, N. J.; Labana, B. M.; Barad, H. A.; Kant, R.; Gupta, V. K. An efficient domino Knoevenagel/hetero-Diels-Alder route to some novel thiochromenoquinoline-fused polyheterocycles. Monatshefte für Chemie 2014, 145, 1179–1189.10.1007/s00706-014-1187-8Search in Google Scholar
[9] Takada, S.; Makisumi, Y. Studies on fused indoles. I. Novel synthesis of 4-aminomethyltetrahydrothiopyrano [2, 3-b] indoles through a thio-claisen rearrangement. Chem. Pharm. Bull. (Tokyo) 1984, 32, 872–876.10.1248/cpb.32.872Search in Google Scholar
[10] Chu, X.-Q.; Zi, Y.; Lu, X.-M.; Wang, S.-Y.; Ji, S.-J. Intramolecular Pd-catalyzed C–H functionalization: construction of fused tetracyclic bis-indole alkaloid analogues. Tetrahedron 2014, 70, 232–238.10.1016/j.tet.2013.11.079Search in Google Scholar
[11] Mullish, B. H.; Fofaria, R. K.; Smith, B. C.; Lloyd, K.; Lloyd, J.; Goldin, R. D.; Dhar, A. Severe cholestatic jaundice after a single administration of ajmaline; a case report and review of the literature. BMC Gastroenterol. 2014, 14, 60–70.10.1186/1471-230X-14-60Search in Google Scholar
[12] Rallapalli, S. K.; Namjoshi, O. A.; Tiruveedhula, V. V. N. P. B.; Deschamps, J. R.; Cook, J. M. Stereospecific total synthesis of the indole alkaloid ervincidine. Establishment of the C-6 hydroxyl stereochemistry. J. Org. Chem. 2014, 79, 3776–3780.10.1021/jo402692uSearch in Google Scholar
[13] Zhu, G.-Y.; Yao, X.-J.; Liu, L.; Bai, L.-P.; Jiang, Z.-H. Alistonitrine A, a caged monoterpene indole alkaloid from Alstonia scholaris. Org. Lett. 2014, 16, 1080–1083.10.1021/ol403625gSearch in Google Scholar
[14] Matsumoto, K.; Narita, M.; Muramatsu, N.; Nakayama, T.; Misawa, K.; Kitajima, M.; Tashima, K.; Devi, L. A.; Suzuki, T.; Takayama, H.; et al. Orally active opioid μ/δ dual agonist MGM-16, a derivative of the indole alkaloid mitragynine, exhibits potent antiallodynic effect on neuropathic pain in mice. J. Pharmacol. Exp. Ther. 2014, 348, 383–392.10.1124/jpet.113.208108Search in Google Scholar
[15] Van Vliet, L. A.; Rodenhuis, N.; Dijkstra, D.; Wikström, H.; Pugsley, T. A.; Serpa, K. A.; Meltzer, L. T.; Heffner, T. G.; Wise, L. D.; Lajiness, M. E.; et al. Synthesis and pharmacological evaluation of thiopyran analogues of the dopamine D3 receptor-selective agonist (4aR,10bR)-(+)-trans-3,4,4a,10b-tetrahydro-4-n-propyl-2H,5H [1b]enzopyrano[4,3-b]-1,4-oxazin-9-ol (PD 128907). J. Med. Chem. 2000, 43, 2871–2882.10.1021/jm0000113Search in Google Scholar
[16] Brown, M. J.; Carter, P. S.; Fenwick, A. S.; Fosberry, A. P.; Hamprecht, D. W.; Hibbs, M. J.; Jarvest, R. L.; Mensah, L.; Milner, P. H.; O’Hanlon, P. J.; et al. The antimicrobial natural product chuangxinmycin and some synthetic analogues are potent and selective inhibitors of bacterial tryptophanyl tRNA synthetase. Bioorg. Med. Chem. Lett. 2002, 12, 3171–3174.10.1016/S0960-894X(02)00604-2Search in Google Scholar
[17] Sugita, Y.; Hosoya, H.; Terasawa, K.; Yokoe, I.; Fujisawa, S.; Sakagami, H. Cytotoxic activity of benzothiepins against human oral tumor cell lines. Anticancer Res. 2001, 21, 2629–2632.Search in Google Scholar
[18] Quaglia, W.; Pigini, M.; Piergentili, A.; Giannella, M.; Gentili, F.; Marucci, G.; Carrieri, A.; Carotti, A.; Poggesi, E.; Leonardi, A.; et al. Structure-activity relationships in 1,4-benzodioxan-related compounds. 7. Selectivity of 4-phenylchroman analogues for α(1)-adrenoreceptor subtypes. J. Med. Chem. 2002, 45, 1633–1643.10.1021/jm011066nSearch in Google Scholar
[19] Carter, J. S.; Rogier, J. D. J.; Talley, J. J. Dihydrobenzopyrans, dihydrobenzothiopyrans, and tetrahydroquinolines for the treatment of cox-2-mediated disorders. J. Med. Chem. 2001,44,211-220.Search in Google Scholar
[20] Voskressensky, L. G.; Festa, A. A.; Varlamov, A. V. Domino reactions based on Knoevenagel condensation in the synthesis of heterocyclic compounds. Recent advances. Tetrahedron 2014, 70, 551–572.10.1016/j.tet.2013.11.011Search in Google Scholar
[21] Majumdar, K. C.; Ponra, S.; Ghosh, T. Green approach to highly functionalized thiopyrano derivatives via domino multi-component reaction in water. RSC Adv. 2012, 2, 1144–1150.10.1039/C1RA00655JSearch in Google Scholar
[22] Raghavendra, G. M.; Ramesha, A. B.; Revanna, C. N.; Nandeesh, K. N.; Mantelingu, K.; Rangappa, K. S. One-pot tandem approach for the synthesis of benzimidazoles and benzothiazoles from alcohols. Tetrahedron Lett. 2011, 52, 5571–5574.10.1016/j.tetlet.2011.08.037Search in Google Scholar
[23] Vinayaka, A. C.; Sadashiva, M. P.; Wu, X.; Biryukov, S. S.; Stoute, J. A.; Rangappa, K. S.; Gowda, D. C. Facile synthesis of antimalarial 1,2-disubstituted 4-quinolones from 1,3-bisaryl-monothio-1,3-diketones. Org. Biomol. Chem. 2014, 12, 8555–8561.10.1039/C4OB01455CSearch in Google Scholar
[24] Ramesha, A. B.; Raghavendra, G. M.; Nandeesh, K. N.; Rangappa, K. S.; Mantelingu, K. Tandem approach for the synthesis of imidazo[1,2-a]pyridines from alcohols. Tetrahedron Lett. 2013, 54, 95–100.10.1016/j.tetlet.2012.10.112Search in Google Scholar
[25] Kumar, S. V.; Yadav, S. K.; Raghava, B.; Saraiah, B.; Ila, H.; Rangappa, K. S.; Hazra A. Cyclocondensation of arylhydrazines with 1,3-bis(het)arylmonothio-1,3-diketones and 1,3-bis(het)aryl-3-(methylthio)-2-propenones: synthesis of 1-aryl-3,5-bis(het)arylpyrazoles with complementary regioselectivity. J. Org. Chem. 2013, 78, 4960–4973.10.1021/jo400599eSearch in Google Scholar
[26] Revanna, C. N.; Raghavendra, G. M.; Jenifer Vijay, T. A.; Rangappa, K. S.; Badregowda, D. G.; Mantelingu, K. Propylphosphonic anhydride-catalyzed tandem approach for Biginelli reaction starting from alcohols. Chem. Lett. 2014, 43, 178–180.10.1246/cl.130732Search in Google Scholar
[27] Nandeesh, K. N.; Raghavendra, G. M.; Revanna, C. N.; Jenifer Vijay, T. a.; Rangappa, K. S.; Mantelingu, K. Recyclable, graphite-catalyzed, four-component synthesis of functionalized pyrroles. Synth. Commun. 2014, 44, 1103–1110.10.1080/00397911.2013.848368Search in Google Scholar
[28] Jenifer Vijay, T. A.; Nandeesh, K. N.; Raghavendra, G. M.; Rangappa, K. S.; Mantelingu, K. Transition metal free intramolecular S-arylation: one-pot synthesis of thiochromen-4-ones. Tetrahedron Lett. 2013, 54, 6533–6537.10.1016/j.tetlet.2013.09.094Search in Google Scholar
[29] Brown, E. R. Indolothiopyrones. US 4032537 A 19770628, 1977.Search in Google Scholar
[30] Majumdar, K. C.; Ponra, S.; Nandi, R. K. One-pot efficient green synthesis of spirooxindole-annulated thiopyran derivatives via Knoevenagel condensation followed by Michael addition. Tetrahedron Lett. 2012, 53, 1732–1737.10.1016/j.tetlet.2012.01.099Search in Google Scholar
[31] Suzuki, T.; Matsuhisa, A.; Miyata, K.; Yanagisawa, I.; Ohta, M. Novel 5-hydroxytryptamine (5-HT3) receptor antagonists. Synthesis and structure-activity relationships of 9-methyl-2, 3, 4, 9-tetrahydrothiopyrano[2, 3-b]indol-4-one derivatives. Chem. Pharm. Bull. (Tokyo) 1997, 45, 101–106.10.1248/cpb.45.101Search in Google Scholar
[32] Kopecky, J.; Smejkal, J. The synthesis of 1,4-dihydroxy- and 1,4-dihalogenobicyclo[2.2.2]octanes. Tetrahedron Lett. 1967, 8, 3889–3891.10.1016/S0040-4039(01)89746-XSearch in Google Scholar
[33] Majumdar, K. C.; Taher, A.; Ray, K. Domino-Knoevenagel-hetero-Diels-Alder reactions: an efficient one-step synthesis of indole-annulated thiopyranobenzopyran derivatives. Tetrahedron Lett. 2009, 50, 3889–3891.10.1016/j.tetlet.2009.04.054Search in Google Scholar
[34] Majumdar, K. C.; Alam, S. Regioselective unusual formation of spirocyclic 4-{2′-benzo(2′,3′-dihydro)furo}-9-methyl-2,3,9-trihydrothiopyrano[2,3-b]indole by 4-exo-trig aryl radical cyclization and rearrangement. Org. Lett. 2006, 8, 4059–4062.10.1021/ol061531gSearch in Google Scholar
[35] Monge, A.; Palop, J.; Ramirez, C.; Font, M.; Fernandez-Alvarez, E. New 5H-1,2,4-triazino[5,6-b]indole and aminoindole derivatives. Synthesis and studies as inhibitors of blood platelet aggregation, anti-hypertensive agents and thromboxane synthetase inhibitors. Eur. J. Med. Chem. 1991, 26, 179–188.10.1016/0223-5234(91)90027-KSearch in Google Scholar
[36] Perrier, S.; Takolpuckdee, P. Macromolecular design via reversible addition-fragmentation chain transfer (RAFT)/xanthates (MADIX) polymerization. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5347–5393.10.1002/pola.20986Search in Google Scholar
[37] Li, M.; Cao, H.; Wang, Y.; Lv, X.-L.; Wen, L. One-pot multicomponent cascade reaction of N,S-ketene acetal: solvent-free synthesis of imidazo[1,2-a]thiochromeno[3,2-e]pyridines. Org. Lett. 2012, 14, 3470–3473.10.1021/ol301441vSearch in Google Scholar
Supplemental Material
The online version of this article (DOI: 10.1515/hc-2014-0206) offers supplementary material, available to authorized users.
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Reviews
- Synthesis of quinazolines and quinazolinones via palladium-mediated approach
- Media with photoinduced irreversible fluorescence
- Synthesis of quinolines and acridines by the reaction of 2-(perfluoroalkyl)anilines with lithium and Grignard reagents
- Preliminary Communications
- Synthesis of tricyclic indolizidines from ethyl isocyanoacetate
- Ligand- and catalyst-free intramolecular C-S bond formation: direct access to indalothiochromen- 4-ones
- Research Articles
- Preparation of optically active 4-substituted γ-lactones by lipase-catalyzed optical resolution
- One-pot synthesis of 4-alkyl-2-amino-4H-chromene derivatives
- Ring transformation and antimicrobial activity of indolyl-substituted 2(3H)-furanones
Articles in the same Issue
- Frontmatter
- Reviews
- Synthesis of quinazolines and quinazolinones via palladium-mediated approach
- Media with photoinduced irreversible fluorescence
- Synthesis of quinolines and acridines by the reaction of 2-(perfluoroalkyl)anilines with lithium and Grignard reagents
- Preliminary Communications
- Synthesis of tricyclic indolizidines from ethyl isocyanoacetate
- Ligand- and catalyst-free intramolecular C-S bond formation: direct access to indalothiochromen- 4-ones
- Research Articles
- Preparation of optically active 4-substituted γ-lactones by lipase-catalyzed optical resolution
- One-pot synthesis of 4-alkyl-2-amino-4H-chromene derivatives
- Ring transformation and antimicrobial activity of indolyl-substituted 2(3H)-furanones

