Abstract
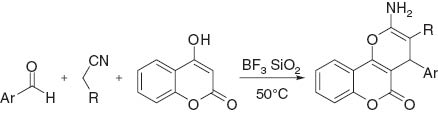
Silica-supported boron trifluoride (BF3•SiO2) is an efficient, readily available, and reusable catalyst for the synthesis of 2-amino-5-oxo-4-aryl-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile or carboxylic acid ethyl ester derivatives by condensation of 4-hydroxycoumarin, an aldehyde, and an alkylnitrile.
Introduction
Silica-supported boron trifluoride, BF3•SiO2, is easy to prepare, shows unusually high Brønsted acidity that can be controlled by activation temperature, and exhibits considerable catalytic activity [1]. BF3•SiO2 is a solid super acid [2]. It is used as a catalyst in several organic transformations, such as Claisen-Schmidt condensations [3], synthesis of 14-substituted 14H-dibenzo[a,j]xanthenes [4] and 1,2,4,5-tetrasubstituted imidazoles [5], polymerization of styrene [6], and the preparation of polyfunctionalized piperidin-4-ones [7], α-amino phosphonates [8], quinoxalines [9], and 3,4-dihydropyrimidin-2(1H)-ones [10].
Pyrans constitute one of the major classes of naturally occurring compounds [11–15]. Pyran derivatives exhibit biological activities, can be photochromic [16–20], and can be used as intermediates for the synthesis of various compounds, including pyranopyridines [21], polyazanaphthalenes [22], pyrano[2]pyrimidines [23], and pyridin-2-ones [24]. Recently, several methods have been reported for the synthesis of 2-amino-5-oxo-dihydropyrano[3,2-c]chromene derivatives through a three-component condensation of 4-hydroxycoumarin with aldehydes and alkyl nitriles. This reaction can be catalyzed by a variety of catalysts [25–37].
Results and discussion
We report that BF3•SiO2 is an efficient and reusable catalyst for the synthesis of 2-amino-5-oxo-4-aryl-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile or carboxylic acid ethyl ester derivatives (Table 1). The reaction of 4-hydroxycoumarin (2 mmol) with 4-nitrobenzaldehyde (2.1 mmol) and malononitrile (2.1 mmol) was investigated for optimization of the reaction conditions (Table 1, entry 3). The optimized conditions are given in Experimental section. It should be noted that the best results were obtained in the absence of any solvent. The reusability of the BF3•SiO2 catalyst was also examined. After each run, the reaction mixture was cooled to room temperature, and the catalyst was separated from the organic product by treatment with chloroform. It was shown that the catalyst could be reused many times, although a gradual decline in activity was observed.
Synthesis of 2-amino-5-oxo-4-aryl-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile or carboxylic acid ethyl ester derivatives in the presence of BF3•SiO2.
| Entry | Ar | R | Yield (%)/mp (°C); this work | Yield (%)/mp (°C); reported [ref.] |
|---|---|---|---|---|
| 1 | 4-F-C6H4 | CN | 93/260–261 | 96/260–262 [26] |
| 2 | 2,3-Cl2-C6H3 | CN | 92/281–282 | 90/280–282 [25] |
| 3 | 4-O2N-C6H4 | CN | 95/177–178 | 85/177–178 [35] |
| 4 | 3-O2N-C6H4 | CN | 95/257–258 | 84/257–258 [34] |
| 5 | 3-Cl-C6H4 | CN | 94/247–248 | 86/246–248 [26] |
| 6 | 4-MeO-C6H4 | CN | 93/220–222 | 78/220–222 [34] |
| 7 | 4-Cl-C6H4 | CN | 92/257–259 | 88/256–258 [30] |
| 8 | 4-Br-C6H4 | CN | 93/247–248 | 80/247–249 [26] |
| 9 | 2-Cl-C6H4 | CN | 90/263–264 | 80/262–264 [35] |
| 10 | 2-Me-C6H4 | CN | 88/264–265 | 87/264–266 [26] |
| 11 | 2,4-Cl2-C6H3 | CN | 90/257–258 | 86/255–257 [25] |
| 12 | 4-Me-C6H4 | CN | 89/219–220 | 80/219–220 [35] |
| 13 | 2-Me-C6H4 | CN | 88/264–265 | 87/264–266 [26] |
| 14 | C6H5 | CN | 90/253–254 | 10/253–255 [34] |
| 15 | 4-Cl-C6H4 | COOEt | 90/192–194 | 89/192–194 [27] |
| 16 | 3-O2N-C6H4 | COOEt | 91/248–250 | 90/247–250 [25] |
| 17 | 4-O2N-C6H4 | COOEt | 91/240–242 | 91/241–243 [27] |
See Experimental for conditions.
Conclusions
The preparation of 2-amino-5-oxo-4-aryl-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile or carboxylic acid ethyl ester derivatives by the reaction of 4-hydroxycoumarin, an aldehyde, and an alkyl nitrile is efficiently catalyzed by BF3•SiO2. In contrast to many other acidic catalysts, this reagent does not need special precautions for handling or storage.
Experimental
General
The catalyst BF3•SiO2 was prepared as previously reported [1, 2]. Products were characterized by IR and 1H NMR spectroscopy and by comparison of their melting points with the literature values. Melting points were determined on a Buchi melting point B-540 B.V.CHI apparatus.
General procedure for the synthesis of compounds shown in Table 1
A mixture of 4-hydroxycoumarin (2 mmol), an aldehyde (2.1 mmol), an alkyl nitrile (2.1 mmol), and BF3•SiO2 (0.6 g, 25 mol%) was heated at 50°C. The progress of the reaction was monitored by TLC. After completion of the reaction, 15–25 min, the mixture was extracted with chloroform and filtered to recover the catalyst. The chloroform extract was concentrated and the residue was crystallized from isopropanol to afford the 2-amino-5-oxo-4-aryl-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile or carboxylic acid ethyl ester derivative.
Financial support for this work by the Research Council of University of Jiroft is gratefully acknowledged.
References
[1] Wilson, K.; Clark, J. H. Synthesis of a novel supported solid acid BF3 catalyst. Chem. Commun. 1998, 2135–2136.10.1039/a806060fSearch in Google Scholar
[2] Klapotke, T. M.; Mc Monagle, F.; Spence, R. R.; Winfield, J. M. γ-Alumina-supported boron trifluoride: catalysis, radiotracer studies and computations. J. Fluorine Chem. 2006, 127, 1446–1453.Search in Google Scholar
[3] Sadegi, B.; Mirjalili, B. F.; Hashemi, M. M. BF3•SiO2: an efficient reagent system for the one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles. Tetrahedron Lett. 2008, 49, 2575–2577.Search in Google Scholar
[4] Mirjalili, B. F.; Bamoniri, A.; Akbari, A. BF3·SiO2: an efficient alternative for the synthesis of 14-aryl or alkyl-14H-dibenzo[a,j]xanthenes. Tetrahedron Lett. 2008, 49, 6454–6456.Search in Google Scholar
[5] Sadegi, B.; Mirjalili, B. F.; Hashememi, M. M. BF3•SiO2: an efficient heterogeneous alternative for regio-chemo and stereoselective Claisen-Schmidt condensation. J. Iran. Chem. Soc. 2008, 5, 694–698.Search in Google Scholar
[6] Boodhoo, K. V. K.; Dunk, W. A. E.; Vicevic, M.; Jachuck, R. J.; Sage, V.; Macquarrie, D. J.; Clark, J. H. Classical cationic polymerisation of styrene in a spinning disc reactor using silica supported BF3 catalyst. J. Appl. Polym. Sci. 2006, 101, 8–19.Search in Google Scholar
[7] Dindulkar, S. D.; Parthiban P.; Jeong, Y. T. BF3•SiO2 is a simple and efficient Lewis acid catalyst for the one-pot synthesis of polyfunctionalized piperidin-4-ones. Monatsh Chem. 2012, 143, 113–118.Search in Google Scholar
[8] Reddy, M. V.; Dindulkar, S. D.; Jeong, Y. T. BF3•SiO2-catalyzed one-pot synthesis of α-aminophosphonates in ionic liquid and neat conditions. Tetrahedron Lett. 2011, 52, 4764–4767.Search in Google Scholar
[9] Mirjalili, B. F.; Bamoniri, A.; Akbari, A. Nano-BF3•SiO2: a reusable and eco-friendly catalyst for synthesis of quinoxalines. Chem. Heterocycl. Comp. 2011, 47, 487–491.Search in Google Scholar
[10] Mirjalili, B. F.; Bamoniri, A.; Akbari, A. One-pot synthesis of 3,4-dihydropyrimidin-2(1 H)-ones (thiones) promoted by nano-BF3•SiO2. J. Iran. Chem. Soc. 2011, 8, 135–140.Search in Google Scholar
[11] Tang, Y.; Oppenheimer, J.; Song, Z.; You, L.; Zhang, X.; Hsung, R. P. Strategies and approaches for constructing 1-oxadecalins. Tetrahedron 2006, 62, 10785–10813.Search in Google Scholar
[12] McKee, T. C.; Fuller, R. W.; Covington, C. D.; Cardellina, J. H., II; Gulakowski, R. J.; Krepps, B. L.; McMahon, J. B.; Boyd, M. R. New pyranocoumarins isolated from calophyllum lanigerum and calophyllum teysmannii. J. Nat. Prod. 1996, 59, 754–758.Search in Google Scholar
[13] McKee, T. C.; Covington, C. D.; Fuller, R. W.; Bokesch, H. R.; Young, S.; Cardellina, J. H., II; Kadushin, M. R.; Soejarto, D. D.; Stevens, P. F.; Cragg, G. M.; et al. Pyranocoumarins from tropical species of the genus Calophyllum: a chemotaxonomic study of extracts in the national cancer institute collection. J. Nat. Prod. 1998, 61, 1252–1256.Search in Google Scholar
[14] Wu, S.-J.; Chen, I.-S. Alkaloids from Zanthoxylum simulans. Phytochemistry 1993, 34, 1659–1661.Search in Google Scholar
[15] Jung, E. J.; Park, B. H.; Lee, Y. R. Environmentally benign, one-pot synthesis of pyrans by domino Knoevenagel/6π-electrocyclization in water and application to natural products. Green Chem. 2010, 12, 2003–2011.Search in Google Scholar
[16] Kumar, S.; Hernandez, D.; Hoa, B.; Lee, Y.; Yang, J. S.; McCurdy, A. Synthesis, photochromic properties, and light-controlled metal complexation of a naphthopyran derivative. Org. Lett. 2008, 10, 3761–3764.Search in Google Scholar
[17] Rawat, M.; Prutyanov, V.; Wulff, W. D. Chromene chromium carbene complexes in the syntheses of naphthopyran and naphthopyrandione units present in photochromic materials and biologically active natural products. J. Am. Chem. Soc. 2006, 128, 11044–11053.Search in Google Scholar
[18] Fedorova, O. A.; Maure, F.; Chebunkova, A. V.; Strokach, Y. P.; Valova, T. M.; Kuzmina, L. G.; Howard, J. A. K.; Wenzel, M.; Gloe, K.; Lokshin, V.; et al. Investigation of cation complexation behavior of azacrown ether substituted benzochromene. J. Phys. Org. Chem. 2007, 20, 469–483.Search in Google Scholar
[19] Hepworth, J. D.; Heron, B. M. Chapter 2 Synthesis and photochromic properties of naphthopyrans. Prog. Heterocycl. Chem. 2005, 17, 33–62.Search in Google Scholar
[20] Delbaer, S.; Micheau, J.-C.; Vermeersch, G. NMR kinetic investigations of the photochemical and thermal reactions of a photochromic chromene. J. Org. Chem. 2003, 68, 8968–8973.Search in Google Scholar
[21] Ren, Q.; Siau, W-Y.; Du, Z.; Zhang, K.; Wang, J. Expeditious assembly of a 2-amino-4H-chromene skeleton by using an enantioselective Mannich intramolecular ring cyclization-tautomerization cascade sequence. Chem. Eur. J. 2011, 17, 7781–7785.Search in Google Scholar
[22] Adbel-Fattah, A. H.; Hesien, A. M.; Metwally, S. A.; Elnagdi, M. H. The reaction of ethyl 6-amino-5-cyano-4-aryl-2-methyl-4H-pyran-3- carboxylate with nucleophilic reagents. Liebigs Ann. Chem. 1989, 585–588.10.1002/jlac.1989198901102Search in Google Scholar
[23] Quintela, J. M.; Peinador, C.; Moreira, M. J. A novel synthesis of pyrano[2,3-d]pyrimidine derivatives. Tetrahedron 1995, 51, 5901–5912.Search in Google Scholar
[24] Srivastava, S.; Batra, S.; Bhaduri, A. P. A facile acid-catalyzed ring transformation of 4H-pyrans to 1,2,3,4-tetrahydropyridin-2-ones and 3,4-dihydronaphtho[1,2-b]pyran-2(H)-ones. Indian J. Chem. Sect. B 1996, 35B, 602–604.Search in Google Scholar
[25] Heravi, M. M.; Alimadadi Jani, B.; Derikvand, F.; Bamoharram, F. F.; Oskooie, H. A. Three component, one-pot synthesis of dihydropyrano[3,2-c]chromene derivatives in the presence of H6P2W18O62•18H2O as a green and recyclable catalyst. Catal. Commun. 2008, 10, 272–275.Search in Google Scholar
[26] Mehrabi, H.; Abusaidi. H. Synthesis of biscoumarin and 3,4-dihydropyrano[c]chromene derivatives catalysed by sodium dodecyl sulfate (SDS) in neat water. J. Iran. Chem. Soc. 2010, 7, 890–894.Search in Google Scholar
[27] Khurana, J. M.; Nand, B.; Saluja, P. DBU: a highly efficient catalyst for one-pot synthesis of substituted 3,4-dihydropyrano[3,2-c]chromenes, dihydropyrano[4,3-b]pyranes, 2-amino-4H-benzo[H]chromenes and 2-amino-4H benzo[g]chromenes in aqueous medium. Tetrahedron 2010, 66, 5637–5641.Search in Google Scholar
[28] Khurana, J. M.; Kumar, S. Tetrabutylammonium bromide (TBAB): a neutral and efficient catalyst for the synthesis of biscoumarin and 3,4-dihydropyrano[c]chromene derivatives in water and solvent-free conditions. Tetrahedron Lett. 2009, 50, 4125–4127.Search in Google Scholar
[29] Shaterian, H. R.; Oveisi, A. R. A simple green approach to the synthesis of 2-amino-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile derivatives catalyzed by 3-hydroxypropanaminium acetate (HPAA) as a new ionic liquid. J. Iran. Chem. Soc. 2011, 8, 545–552.Search in Google Scholar
[30] Mohammadi Ziarani, G.; Badiei, A.; Azizi, M.; Zarabadi, P. Synthesis of 3,4-dihydropyrano[c]chromene derivatives using sulfonic acid functionalized silica (SiO2PrSO3H). Iran. J. Chem. Chem. Eng. 2011, 30, 59–65.Search in Google Scholar
[31] Ghorbani-Vaghei, R.; Toghraei-Semiromi, Z.; Karimi-Nami, R. One-pot synthesis of 4H-chromene and dihydropyrano[3,2-c]chromene derivatives in hydroalcoholic media. J. Braz. Chem. Soc. 2011, 5, 905–909.Search in Google Scholar
[32] Zheng, J.; Li, Y-Q. One-pot synthesis of tetrahydrobenzo[b]pyran and dihydropyrano[c]chromene derivatives in aqueous media by using trisodium citrate as a green catalyst. Arch. Appl. Sci. Res. 2011, 3, 381–388.Search in Google Scholar
[33] Alizadeh, A.; Khodaei, M. M.; Beygzadeh, M.; Kordestani, D.; Feyzi, M. Biguanide-functionalized Fe3O4/SiO2 magnetic nanoparticles: an efficient heterogeneous organosuperbase catalyst for various organic transformations in aqueous media, Bull. Korean Chem. Soc. 2012, 33, 2547–2580.Search in Google Scholar
[34] Khoobi, M.; Ma’mani, L.; Rezazadeh, F.; Zareie, Z.; Foroumadi, A.; Ramazani, A.; Shafiee, A. One-pot synthesis of 4H-benzo[b]pyrans and dihydropyrano[c]chromenes using inorganic-organic hybrid magnetic nanocatalyst in water. J. Mol. Catal. A: Chem. 2012, 359, 74–80.Search in Google Scholar
[35] Tabatabaeian, K.; Heidari, H.; Mamaghani M.; Mahmoodi, N. O. Ru(II) complexes bearing tertiary phosphine ligands: a novel and efficient homogeneous catalyst for one-pot synthesis of dihydropyrano[3,2-c]chromene and tetrahydrobenzo[b]pyran derivatives. Appl. Organomet. Chem. 2012, 26, 56–61.Search in Google Scholar
[36] Niknam, K.; Jamali, A. Silica-bonded n-propylpiperazine sodium n-propionate as recyclable basic catalyst for synthesis of 3,4-dihydropyrano[c]chromene derivatives and biscoumarins. Chin. J. Catal. 2012, 33, 1840–1849.Search in Google Scholar
[37] Shaterian, H. R.; Arman, M.; Rigi, F. Domino Knoevenagel condensation, Michael addition, and cyclization using ionic liquid, 2-hydroxyethylammonium formate, as a recoverable catalyst. J. Mol. Liq. 2011, 158, 145–150.Search in Google Scholar
©2013 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Masthead
- Masthead
- Reviews
- Polyoxin and nikkomycin analogs: recent design and synthesis of novel peptidyl nucleosides
- Synthesis of fused heterocycles derived from 2H-1,4-benzoxazin-3(4H)-ones
- Research Articles
- Green synthesis of 1-monosubstituted 1,2,3-triazoles via ‘click chemistry’ in water
- Synthesis of a novel fused tricyclic heterocycle, pyrimido[5,4-e][1,4]thiazepine, and its derivatives
- Synthesis of 2-[(quinolin-8-yloxy)methyl]quinoline-3-carboxylic acid derivatives
- Pyrimidine-5-carbonitriles – part III: synthesis and antimicrobial activity of novel 6-(2-substituted propyl)-2,4-disubstituted pyrimidine-5-carbonitriles
- Tungstic acid-catalyzed synthesis of 3,3-bis (1H-indol-3-yl)indolin-2-one derivatives
- One-pot synthesis of dihydropyrano[c]chromene derivatives by using BF3•SiO2 as catalyst
Articles in the same Issue
- Masthead
- Masthead
- Reviews
- Polyoxin and nikkomycin analogs: recent design and synthesis of novel peptidyl nucleosides
- Synthesis of fused heterocycles derived from 2H-1,4-benzoxazin-3(4H)-ones
- Research Articles
- Green synthesis of 1-monosubstituted 1,2,3-triazoles via ‘click chemistry’ in water
- Synthesis of a novel fused tricyclic heterocycle, pyrimido[5,4-e][1,4]thiazepine, and its derivatives
- Synthesis of 2-[(quinolin-8-yloxy)methyl]quinoline-3-carboxylic acid derivatives
- Pyrimidine-5-carbonitriles – part III: synthesis and antimicrobial activity of novel 6-(2-substituted propyl)-2,4-disubstituted pyrimidine-5-carbonitriles
- Tungstic acid-catalyzed synthesis of 3,3-bis (1H-indol-3-yl)indolin-2-one derivatives
- One-pot synthesis of dihydropyrano[c]chromene derivatives by using BF3•SiO2 as catalyst

