Abstract
Tungstic acid was used as a low-cost and readily available heterogeneous catalyst for the synthesis of 3,3-bis(1H-indol-3-yl)indolin-2-one derivatives from indoles. The reaction parameters, including catalyst quantity, solvents, temperature, and time were optimized. The present method has several advantages, such as mild conditions, simple work-up, elimination of anhydrous condition, easy recovery of catalyst and its recyclability as compared with existing methods.
Introduction
Among biologically active heterocyclic compounds [1–3], derivatives of oxindole [3,3-bis(1H-indol-3-yl) indolin-2-one, 3a in Scheme 1) attract much attention as anti-inflammatory [4], anti-HIV [5],andantitumor [6] agents, among other things [7, 8]. Oxindole is also an integral component of many natural products including convolutamydines [9], arundaphine [10], donaxaridine [11], paratunamide [12], and maremycins [13].
There are many reactions known for the synthesis of oxindole derivatives by condensation of isatins and indoles in the presence of various catalysts [14–30]. Although these methods work well, many of them involve harsh reaction conditions, long reaction time, or the use of corrosive acids. Most of the Lewis acid catalysts, being moisture sensitive, require usually more than stoichiometric amounts, the use of inert atmosphere, and easily undergo decomposition.
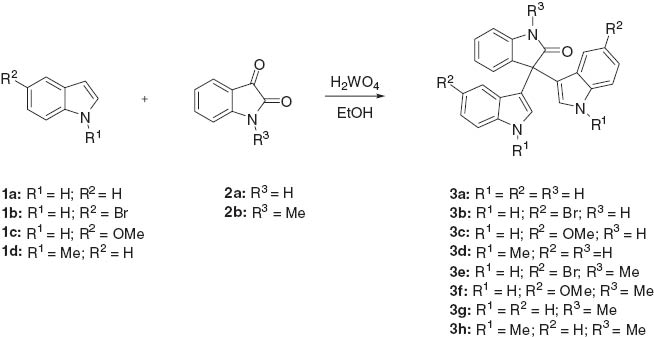
We have been involved in the study of the catalytic activity of tungstic acid in organic reactions. Many tungstic acid-catalyzed organic transformations are known [31–33]. Tungstic acid, a low-cost and readily available heterogeneous catalyst, exhibits high catalytic efficiency. We found that tungstic acid efficiently catalyzes condensation of indoles with isatins to form corresponding oxindole derivatives in high yields. The present method involves relatively mild conditions and easy work-up procedure (Scheme 1).
The reaction of indole 1a with isatin 2a in the presence of tungstic acid to form of 3,3-bis(indol-3-yl)indolin-2-one 3a was taken as model for the optimization study. To evaluate the catalyst quantity, a reaction of 1a and 2a was carried out using various amounts of catalyst (2.5, 5, 7.5, 10, 12.5, and 15 mol%). It was found that 10 mol% of catalyst gave a maximum yield in minimum time. Larger amounts of the catalyst loading neither increased the yield nor shortened the conversion time. For solvent optimization, various solvents such as toluene, chloroform, N,N-dimethylformamide, dichloromethane, methanol, t-butyl alcohol, and ethanol were used. Among the solvents studied, ethanol gave the best yield (92%) at 30°C. The use of methanol was avoided because of its toxicity. There was an increase in the yield of 3a up to 6 h; however, no substantial increase in the yield of 3a was noted when the reaction was continued beyond 6 h. A marginal increase in the yield was also recorded as temperature was increased from 30°C to refluxing in ethanol. The catalyst reusability study showed that tungstic acid can be reused up to three cycles without a substantial decrease in yield. Thus, the yield of 3a was 91% in the first cycle, 83% in the second cycle, and 71% for using the catalyst for the third time.
Conclusion
An efficient and expeditious method is reported for the synthesis of 3,3-bis(indol-3-yl)indolin-2-one derivatives 3a–h using tungstic acid as a heterogeneous catalyst. The method has the advantages of operational simplicity and mild conditions. The catalyst can be easily regenerated and reused up to three cycles without significant loss in the yield.
Experimental
1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were determined in DMSO-d6 on a Bruker-400 FT NMR spectrometer (Bruker India Scientific Pvt. Ltd., India). For regeneration and reusability, the catalyst was heated in dry ethanol at reflux temperature for half an hour, washed with dry ethanol, and dried at room temperature (~30°C).
General procedure
A mixture of indole 1a–d (1.59 g, 13.58 mmol), corresponding isatin 2a–b (1.0 g, 6.79 mmol), and tungstic acid (10 mol%) in ethanol (10 mL) was stirred for 6 h at room temperature. Then, the mixture was filtered through a celite pad to remove the suspended catalyst. Concentration on a rotary evaporator followed by silica gel chromatography eluting with mixtures of light petroleum and ethyl acetate furnished the 3,3-bis(1H-indol-3-yl)indolin-2-one 3a–h.
3,3-Bis(1H-indol-3-yl)indolin-2-one (3a) [34]
White solid, 92% (lit. 92%), mp >300°C; 1H NMR: δ 6.78 (t, 2H, J = 7 Hz), 6.81 (s, 2H), 6.95 (m, 4H), 7.22 (t, 4H, J = 8 Hz), 7.34 (d, 2H, J = 8 Hz), 10.61 (s, 1H), 10.97 (s, 2H).
3,3-Bis(5-bromo-1H-indol-3-yl)indolin-2-one (3b) [35]
White solid, 92% (lit. 70%), mp 264–266°C; 1H NMR: δ 6.92 (s, 2H), 7.00 (m, 2H), 7.16 (m, 3H), 7.27 (t, 1H, J = 7.6 Hz), 7.36 (m, 4H), 10.77 (s, 1H) 11.25 (s, 2H); 13C NMR: δ52.6, 110.3, 111.5, 114.2, 114.3, 122.3, 123.1, 124.1, 125.3, 126.4, 127.7, 128.7, 134.0, 136.1, 141.6, 178.9.
3,3-Bis(5-methoxy-1H-indol-3-yl) indolin-2-one (3c) [36]
White solid, 92% (lit. 95%), mp 240–244°C; 1H NMR: δ 3.52 (s, 6H, OCH3), 6.69 (m, 4H), 6.85 (s, 2H), 6.97 (m, 2H), 7.23 (m, 4H), 10.62 (s, 1H), 10.82 (s, 2H); 13C NMR: δ 52.9, 55.5, 103.8, 109.9, 110.9, 112.5, 114.0, 122.0, 125.4, 125.6, 126.5, 128.3, 132.6, 135.0, 141.8, 152.8, 179.2.
3,3-Bis(1-methyl-1H-indol-3-yl)indolin-2-one (3d) [37]
White solid, 89% (lit. 83%), mp >300°C; 1H NMR: δ 3.70 (s, 6H, CH3), 6.83 (m, 2H), 6.88 (s, 2H), 6.95 (m, 2H), 7.08 (m, 2H), 7.22 (m, 4H), 7.37 (d, 2H, J = 8.4 Hz), 10.63 (s, 1H); 13C NMR: δ 32.8, 52.8, 110.1, 110.2, 113.8, 118.9, 121.3, 121.5, 122.1, 125.3, 126.4, 128.4, 128.9, 134.9, 137.7, 141.6, 179.0.
3,3-Bis(5-bromo-1H-indol-3-yl)-1-methylindolin-2-one (3e)
White solid, 91%, mp 280–284°C; 1H NMR: δ 3.25 (s, 3H, CH3), 6.91 (s, 2H), 7.07 (m, 3H), 7.14 (m, 2H), 7.26 (m, 2H), 7.38 (m, 3H), 11.25 (s, 2H). 13C NMR: δ 26.8 (CH3), 52.2, 109.5, 111.5, 114.0, 114.3, 123.0, 124.1, 125.0, 126.4, 127.6, 129.0, 133.1, 136.1, 143.0, 177.2. Anal. Calcd for C25H17Br2N3O: C, 56.10; H, 3.20; N, 7.85. Found: C, 56.12; H, 3.24; N, 7.82.
3,3-Bis(5-methoxy-1H-indol-3-yl)-1-methylindolin-2-one (3f)
White solid, 88%, mp 236–240°C; 1H NMR: δ 3.27 (s, 3H, CH3), 3.51 (s, 6H, OCH3), 6.58 (s, 2H), 6.76 (m, 2H), 6.84 (d, 2H, J = 2.4 Hz), 7.03 (m, 2H), 7.27 (m, 4H), 10.84 (s, 2H); 13C NMR: δ 26.6, 52.5, 55.5, 103.3, 109.0, 111.0, 112.6, 113.8, 122.7, 125.1, 125.6, 126.4, 128.5, 132.6, 134.0, 143.3, 152.9, 177.4. Anal. Calcd for C27H23N3O3: C, 74.12; H, 5.30; N, 9.60. Found: C, 74.08; H, 5.32; N, 9.58.
3,3-Bis(1H-indol-3-yl)-1-methylindolin-2-one (3g) [37]
White solid, 90% (lit. 85%), mp >300°C; 1H NMR: δ 3.26 (s, 3H, CH3), 6.78 (m, 2H), 6.80 (s, 2H), 6.99 (m, 3H), 7.09 (m, 3H), 7.36 (m, 4H), 10.99 (s, 2H); 13C NMR: δ 26.7, 52.6, 109.2, 112.1, 114.5, 118.8, 121.1, 121.4, 122.8, 124.8, 126.0, 128.6, 134.2, 137.3, 143.3, 177.4.
1-Methyl-3,3-bis(1-methyl-1H-indol-3-yl)indolin-2-one (3h) [37]
White solid, 88% (lit. 80%), mp 230–232°C; 1H NMR: δ 3.25 (s, 3H, CH3), 3.70 (s, 6H, CH3), 6.81 (m, 2H), 6.87 (s, 2H), 7.00 (m, 1H), 7.04 (m, 2H), 7.08 (m, 3H), 7.17 (m, 1H), 7.30 (m, 3H); 13C NMR: δ 26.7,32.8, 52.4, 109.2, 110.3, 113.6, 119.0, 121.3, 121.6, 122.8, 125.0, 126.4, 128.5, 128.9, 134.0, 137.8, 143.1, 177.2.
G.M. Patel thanks UGC, New Delhi, India, for the award of a research fellowship under the RFSMS Scheme.
References
[1] Gilchrist, T. L. Heterocyclic chemistry. Academic Press: London, 1997, pp. 231.Search in Google Scholar
[2] Demirayak, S.; Kayagil, I.; Yurttas, L. Microwave supported synthesis of some novel 1,3-diarylpyrazino[1,2-a]benzimidazole derivatives and investigation of their anticancer activities Eur. J. Med. Chem. 2011, 46, 411–416.Search in Google Scholar
[3] Ito, H.; Sakakibara, J.; Ueda T. Antitumor effect of 6-phenyl-7(6H)-isoselenazolo[4,3-d]pyrimidone on the growth of Ehrlich ascites tumor. Cancer Lett. 1985, 28, 61–68.Search in Google Scholar
[4] Natarajan, A.; Fan, Y. H.; Chen, H.; Guo, Y.; Iyasere, J.; Harbinski, F.; Christ, W. J.; Aktas, H.; Halperin, J. A. 3,3-Diaryl-1,3-dihydroindol-2-ones as antiproliferatives mediated by translation initiation inhibition. J. Med. Chem. 2004, 47, 1882–1885.Search in Google Scholar
[5] Ratan Bal, T.; Anand, B.; Yogeeswari, P.; Sriram, D. Synthesis and evaluation of anti-HIV activity of isatin β-thiosemicarbazone derivatives Bioorg. Med. Chem. Lett. 2005, 15, 4451–4455.Search in Google Scholar
[6] Tripathy, R.; Reiboldt, A.; Messina, P. A.; Iqbal, M.; Singh, J.; Bacon, E. R.; Angeles, Th. S.; Yang, Sh. X.; Albom, M. S.; Robinson, C.; et al. Structure-guided identification of novel VEGFR-2 kinase inhibitors via solution phase parallel synthesis Bioorg. Med. Chem. Lett. 2006, 16, 2158–2162.Search in Google Scholar
[7] Amal Raj, A.; Raghunathan, R.; Sridevikumaria, M. R.; Raman, N. Synthesis, antimicrobial and antifungal activity of a new class of spiro pyrrolidines. Bioorg. Med. Chem. 2003, 11, 407–419.Search in Google Scholar
[8] Maskell, L.; Blanche, E. A.; Colucci, M. A.; Whatmore, J. L.; Moody, Ch. J. Synthesis and evaluation of prodrugs for anti-angiogenic pyrrolylmethylidenyl oxindoles. Bioorg. Med. Chem. Lett. 2007, 17, 1575–1578.Search in Google Scholar
[9] Zhang, H. P.; Kamano, Y.; Ichihara, Y.; Kizu, H.; Komiyama, K.; Itokawa, H.; Pettit, G. R. Isolation and structure of convolutamydines B–D from marine bryozoan Amathia convoluta. Tetrahedron 1995, 51, 5523–5528.Search in Google Scholar
[10] Khuzhaev, V. U.; Zhalolov, I.; Turguniv, K. K.; Tashkhodzhaev, B.; Levkovich, M. G.; Arpova, S. F.; Shashkov, A. S. Alkaloids from Arundo donax: XVII. Structure of the dimeric indole alkaloid arundaphine. Chem. Nat. Compd. 2004, 40, 269–272.Search in Google Scholar
[11] Rasmussen, H. B.; MacLeod, J. K. Total synthesis of donaxaridine. J. Nat. Prod. 1997, 60, 1152–1154.Search in Google Scholar
[12] Kagata, T.; Saito, S.; Shigemori, H.; Ohsaki, A.; Ishiyama, H.; Kubota, T.; Kobayashi, J. Paratunamides A–D, oxindole alkaloids from Cinnamodendron axillare. J. Nat. Prod. 2006, 69, 1517–1521.Search in Google Scholar
[13] Tang, Y. Q.; Sattler, I.; Thiericke, R.; Grabley, S.; Feng, X. Z. Maremycins C and D, new diketopiperazines, and maremycins E and F, novel polycyclic spiro-indole metabolites isolated from Streptomyces sp. Eur. J. Org. Chem. 2001, 66, 261–267.Search in Google Scholar
[14] Jursic, B. S.; Stevens, E. D. Preparation of dibarbiturates of oxindole by condensation of isatin and barbituric acid derivatives. Tetrahedron Lett. 2002, 43, 5681–5683.Search in Google Scholar
[15] Klumpp, D. A.; Yeung, K. Y.; Prakash, G. K. S.; Olah, G. A. Preparation of 3,3-diaryloxindoles by superacid-induced condensations of isatins and aromatics with a combinatorial approach. J. Org. Chem. 1998, 63, 4481–4484.Search in Google Scholar
[16] Marsden, S. P.; Watson, E. L.; Raw, S. A. Facile and general synthesis of quaternary 3-aminooxindoles. Org. Lett. 2008, 10, 2905–2908Search in Google Scholar
[17] Felpin, F. X.; Ibarguren, O.; Nassar Hardy, L.; Fouquet, E. Synthesis of oxindoles by tandem Heck-reduction-cyclization (HRC) from a single bifunctional, in situ generated Pd/C catalyst. J. Org. Chem. 2009, 74, 1349–1352Search in Google Scholar
[18] Malkov, A. V.; Kabeshov, M. A.; Bella, M.; Kysilka, O.; Malyshev, D. A.; Pluhackova, K.; Kocovsky, P. Vicinal amino alcohols as organocatalysts in asymmetric cross-aldol reaction of ketones: application in the synthesis of convolutamydine A. Org. Lett. 2007, 9, 5473–5476.Search in Google Scholar
[19] Azizian, J.; Mohammadi, A.; Karimi, N.; Mohammadizadeh, M.; Karimi, A. Silica sulfuric acid a novel and heterogeneous catalyst for the synthesis of some new oxindole derivatives. Catal. Commun. 2006, 7, 752–755.Search in Google Scholar
[20] Chatterjee, A.; Manna, S.; Benerji, J.; Prange, T.; Shoolery, J. Lewis-acid-induced electrophilic substitution in indoles with acetone Part 2. J. Chem. Soc. Perkin Trans. I 1980, 553–555.10.1039/P19800000553Search in Google Scholar
[21] Babu, G.; Sridhar, N.; Perumal, P. T. A convenient method of synthesis of bis-indolylmethanes: indium trichloride catalyzed reactions of indole with aldehydes and Schiff’s bases. Synth. Commun. 2000, 30, 1609–1614.Search in Google Scholar
[22] Nagarajan, R.; Perumal, P. InCl3 and In(OTf)3 catalyzed reactions: synthesis of 3-acetyl indoles, bis-indolylmethane and indolylquinoline derivatives. Tetrahedron 2002, 58, 1229–1232.10.1016/S0040-4020(01)01227-3Search in Google Scholar
[23] Chen, D. P.; Yu, L. B.; Wang, P.G. Lewis acid-catalyzed reactions in protic media: lanthanide-catalyzed reactions of indoles with aldehydes or ketones. Tetrahedron Lett. 1996, 37, 4467–4470.Search in Google Scholar
[24] Mi, X.; Lu, S.; He, J.; Cheng, J. P. Dy(OTf)3 in ionic liquid: an efficient catalytic system for reactions of indole with aldehydes/ketones or imines. Tetrahedron Lett. 2004, 45, 4567–4570.Search in Google Scholar
[25] Gibbs, T. J. K.; Tomkinson, N. C. O. Aminocatalytic preparation of bisindolylalkanes. Org. Biomol. Chem. 2005, 3, 4043–4045.Search in Google Scholar
[26] Yadav, J. S.; Reddy, B. V. S.; Sunitha, S. Efficient and eco-friendly process for the synthesis of bis(1H-indol-3-yl)methanes using ionic liquids. Adv. Synth. Catal. 2003, 345, 349–352.Search in Google Scholar
[27] Firouzabadi, H.; Iranpoor, N.; Jafari, A. A. Aluminumdodecatungstophosphate (AlPW12O40), a versatile and a highly water tolerant green Lewis acid catalyzes efficient preparation of indole derivatives. J. Mol. Catal. A Chem. 2006, 244, 168–172.Search in Google Scholar
[28] Ko, S.; Lim, C.; Tu, Z.; Wang, Y. F.; Wang, C. C.; Yao, C. F. CAN and iodine-catalyzed reaction of indole or 1-methylindole with α,β-unsaturated ketone or aldehyde. Tetrahedron Lett. 2006, 47, 487–492.Search in Google Scholar
[29] Yadav, J. S.; Reddy, B. V. S.; Murthy, C. V. S.; Kumar, G. M.; Madan, C. Lithium perchlorate catalyzed reactions of indoles: an expeditious synthesis of bis(indolyl)methanes. Synthesis 2001, 5, 783–788.Search in Google Scholar
[30] Bandgar, B. P.; Shaikh, K. A. Molecular iodine-catalyzed efficient and highly rapid synthesis of bis(indolyl)methanes under mild conditions. Tetrahedron Lett. 2003, 44, 1959–1961.Search in Google Scholar
[31] Singh, V. K.; Deota, P. T. Tungstic acid-hydrogen peroxide a simple reagent for oxidation of cycloocta-1,5-diene preparation of (Z) cyclooct-5-ene-trans-1,2-diol and 1,6-dicarbomethoxy (Z) hex-3-ene. Synth. Commun. 1988, 18, 617–624.Search in Google Scholar
[32] Deota, P. T.; Desai, R.; Valodkar, V. B., Reaction of tungstic acid-hydrogen peroxide with endo-dicyclopentadiene: an unusual observation. J. Chem. Res. (S) 1998, 9, 562–563.Search in Google Scholar
[33] Hui, C.; Wei-Lin, D.; Ruihua, G.; Yong, C.; Hexing, L.; Kangnian, F. New green catalytic manufacture of glutaric acid from the oxidation of cyclopentane-1,2-diol with aqueous hydrogen peroxide. Appl. Catal. A Gen. 2007, 328, 226–236.Search in Google Scholar
[34] Moghadam, K. R.; Kiasaraie, M. S.; Amlashi, H. T. Synthesis of symmetrical and unsymmetrical 3,3-di(indolyl)indolin-2-ones under controlled catalysis of ionic liquids. Tetrahedron 2010, 66, 2316–2321.Search in Google Scholar
[35] Tabatabaeian, K.; Mamaghani, M; Mahmoodi, N; Khorshidi, A. Ruthenium-catalyzed efficient routes to oxindole derivatives. Can. J. Chem. 2009, 87, 1213–1217.Search in Google Scholar
[36] Kamal, A.; Srikanth, Y. V. V.; Khan, N. A.; Shaik, T. B.; Ashraf, M. Synthesis of 3,3-diindolyl oxyindoles efficiently catalysed by FeCl3and their in vitro evaluation for anticancer activity. Bioorg. Med. Chem. Lett. 2010, 20, 5229–5231, and references cited therein.Search in Google Scholar
[37] Ji, S. J.; Wang, S. Y. Facile synthesis of 3,3-di(heteroaryl)indolin-2-one derivatives catalyzed by ceric ammonium nitrate (CAN) under ultrasound irradiation. Tetrahedron 2006, 62, 1527–1535.Search in Google Scholar
©2013 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Masthead
- Masthead
- Reviews
- Polyoxin and nikkomycin analogs: recent design and synthesis of novel peptidyl nucleosides
- Synthesis of fused heterocycles derived from 2H-1,4-benzoxazin-3(4H)-ones
- Research Articles
- Green synthesis of 1-monosubstituted 1,2,3-triazoles via ‘click chemistry’ in water
- Synthesis of a novel fused tricyclic heterocycle, pyrimido[5,4-e][1,4]thiazepine, and its derivatives
- Synthesis of 2-[(quinolin-8-yloxy)methyl]quinoline-3-carboxylic acid derivatives
- Pyrimidine-5-carbonitriles – part III: synthesis and antimicrobial activity of novel 6-(2-substituted propyl)-2,4-disubstituted pyrimidine-5-carbonitriles
- Tungstic acid-catalyzed synthesis of 3,3-bis (1H-indol-3-yl)indolin-2-one derivatives
- One-pot synthesis of dihydropyrano[c]chromene derivatives by using BF3•SiO2 as catalyst
Articles in the same Issue
- Masthead
- Masthead
- Reviews
- Polyoxin and nikkomycin analogs: recent design and synthesis of novel peptidyl nucleosides
- Synthesis of fused heterocycles derived from 2H-1,4-benzoxazin-3(4H)-ones
- Research Articles
- Green synthesis of 1-monosubstituted 1,2,3-triazoles via ‘click chemistry’ in water
- Synthesis of a novel fused tricyclic heterocycle, pyrimido[5,4-e][1,4]thiazepine, and its derivatives
- Synthesis of 2-[(quinolin-8-yloxy)methyl]quinoline-3-carboxylic acid derivatives
- Pyrimidine-5-carbonitriles – part III: synthesis and antimicrobial activity of novel 6-(2-substituted propyl)-2,4-disubstituted pyrimidine-5-carbonitriles
- Tungstic acid-catalyzed synthesis of 3,3-bis (1H-indol-3-yl)indolin-2-one derivatives
- One-pot synthesis of dihydropyrano[c]chromene derivatives by using BF3•SiO2 as catalyst

