Abstract
Plasma electrolytic oxidation (PEO) ceramic coating modified by carbon nanotubes (CNTs) was prepared on Mg–Gd–Y alloy. The microstructure, hydrophobicity and corrosion resistance of the coating were investigated by SEM, contact angle meter and electrochemical test system. Carbon nanotubes (CNTs) are staggered in the ceramic coating and partially filled with plasma discharge micropores. To some extent, CNTs can promote the plasma discharge and improve the film formation rate. With the increase of the content of CNTs, the content of carbon nanotubes in the ceramic coating increases. CNTs can effectively improve the hydrophobicity of ceramic coating. With the increase of the content of CNTs, the corrosion potential Ecoor and polarization resistance Rp increase, the corrosion current icoor decreases and the AC impedance |Z| increases, which leads to the decrease of corrosion rate.
1 Introduction
In recent years, there has been an increasing attention focused on corrosion protection of Mg alloys because the poor corrosion resistance has severely restricted their widespread application in the automotive, aerospace and electronics industries (Acheson et al. 2019; Gao et al. 2020; Jayaraja et al. 2019; Peng et al. 2020). Surface treatment is a very important method to improve the corrosion resistance of Mg alloys (Ma et al. 2017; Sun et al. 2020). The common surface treatment methods of magnesium alloys include chemical conversion, anodic oxidation, plasma electrolytic oxidation, organic coating, and vapor deposition, etc. Chemical conversion is brittle and thin, and is generally used as an intermediate process. Because magnesium alloy is easy to oxidize; anodic oxidation, vapor deposition, and other technologies generally need complicated pretreatment processes such as alkaline washing, pickling and activation. Therefore, the process is complex and the stability of coating quality is reduced. The organic coating has good corrosion resistance, but its adhesion to metal substrate is limited. Plasma electrolytic oxidation (PEO) has become the core technology of surface treatment of Mg alloys due to its excellent comprehensive properties, simple pretreatment process and industrial characteristics (Bai et al. 2020; Dou et al. 2017; Ly et al. 2019; Muhaffel et al. 2019).
However, most of the existing surface treatment methods for Mg alloys are still inadequate in wet and electrolyte environments. Shi et al. (2020) studied the corrosion resistance of selenium-containing coatings on WE43 magnesium alloy by plasma electrolytic oxidation, and found that the addition of selenium can effectively reduce the corrosion current.
However, the inherent microporosity of plasma discharge results in limited corrosion resistance. An effective approach to protect Mg alloys from corrosion is to create a super-hydrophobic surface with a large water contact angle, which can exhibit better resistance to corrosion compared with a hydrophilic surface (Jiang et al. 2018; Jin et al. 2019; Liu et al. 2018; Yao et al. 2020; Zhang et al. 2017). Cui et al. (2017) prepared the composite coating of plasma electrolytic oxidation and sodium stearate; the results show that the superhydrophobic composite coating is very helpful to improve the corrosion resistance. Luo et al. (2018) prepared a hydrophobic surface on an extruded Mg–5Sn–1Zn alloy by plasma electrolytic oxidation with a subsequent stearic acid (SA) modification. The results show that the maximum water contact angle of 122.5° can be obtained by the PEO with the SA modification, which can be attributed to the rough island-structures and volcano-like structures with the formation of the SA coating.
Plasma electrolytic oxidation can form a rough micro/nanostructured surface with lotus leaf like structure, which improves the hydrophobicity (Ma et al. 2012). However, there are few studies on the one-step method of plasma electrolytic oxidation to achieve hydrophobicity. Zheng et al. (2019) prepared superhydrophobic coating on AZ31 magnesium alloy by one-step electrodeposition, which significantly improved the preparation efficiency and reduced the corrosion current. The author has studied the PEO technology modified by ZrO2, SiO2 and new carbon nano materials. It is found that nanoparticles can adjust the size and distribution of micro pores in plasma discharge, which has an important impact on the hydrophobicity and corrosion resistance of the ceramic coating. The porous out-layer of PEO can offer a rough surface to create hydrophobic coating, meanwhile, the presence of a higher pore density on the surface of the PEO coating increases the effective surface area and thus the tendency of nanomaterials effectively entering into the discharge micropores, and then further regulates the micro/nano structure of PEO ceramic layer (Li et al. 2019). However, there are few reports on one-step preparation of hydrophobic coatings by plasma electrolytic oxidation modified by nano materials such as carbon nanotubes (CNTs) (Sharma et al. 2020; Yürektürk et al. 2015).
This work is focused on the structure, hydrophobicity, and corrosion resistance of the modified PEO coating by CNTs, obtaining a comparison of corrosion resistance of CNTs with different contents.
2 Materials and methods
Mg–9Gd–3Y–0.5Zr (wt%) alloy was used in the experiment. PEO ceramic coating was prepared by plasma electrolytic oxidation equipment. During the PEO process, the specimen was used as anode. The current density was 5 A/dm2, duty cycle was 10% and frequency was 800 Hz. The time of plasma electrolytic oxidation was 10 min. The experimental solution was Na2SiO4 (15 g/L), NaOH (1 g/L) and CNTs with concentrations between 0.15 and 0.5 g/L. After the CNTs were dispersed in ultrasonic nano dispersion apparatus for 30 min, they were added into the electrolyte, and the PEO reaction was carried out in continuous ultrasonic dispersion.
The microstructure and elemental analysis of the coatings were characterized by SEM and EDS (FEI Quanta 400). The hydrophobicity was tested by contact angle measurements, and polarization curves and AC impedance tests (PARSTAT 4000) were performed to evaluate corrosion resistance.
3 Results and discussion
3.1 The morphology of CNTs and its influence on arc discharge voltage
TEM micrograph of CNTs is shown in Figure 1. As seen in Figure 1, the length of CNTs was about 0.5–2 μm, the diameter was about 30–50 nm, and the ratio of length-diameter was 16:1 to 40:1.
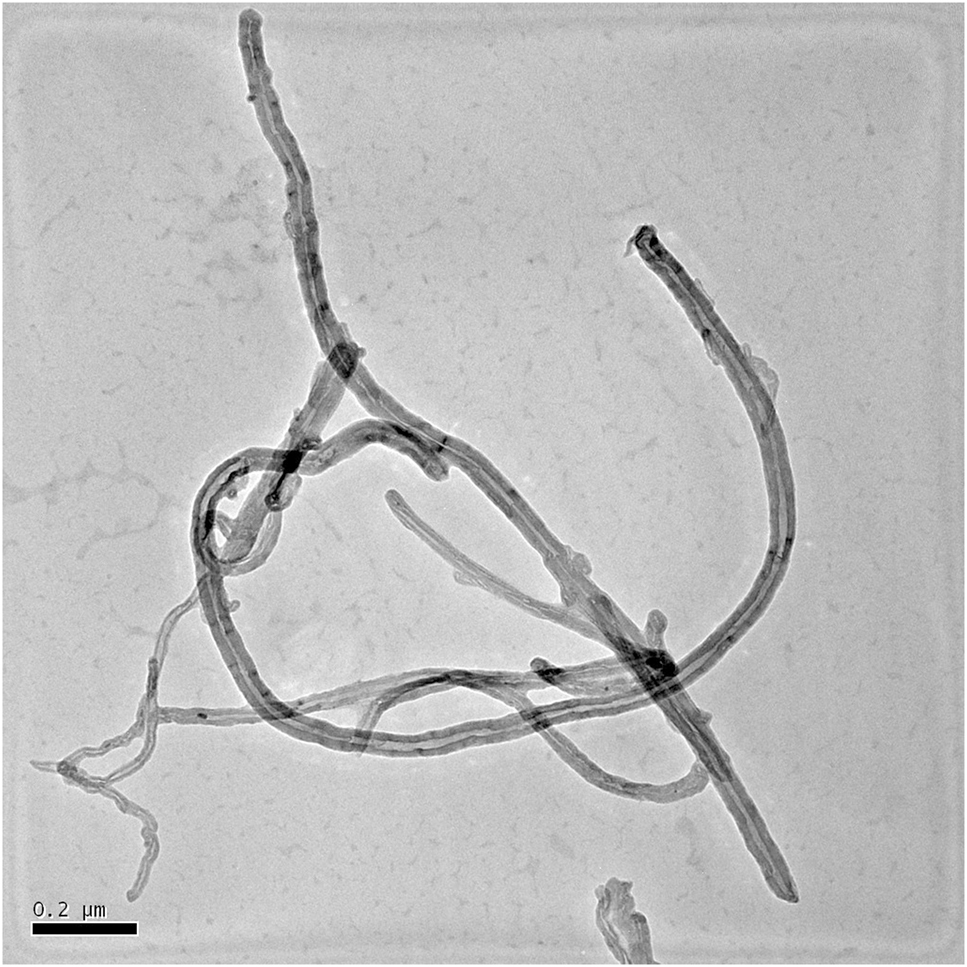
TEM micrograph of carbon nanotubes (CNTs).
The change of arc discharge voltage of the sample with the content of CNTs in the electrolyte during plasma electrolytic oxidation is shown in Figure 2. It can be seen that the CNTs can effectively reduce plasma discharge voltage. With the increase of the content of CNTs, the arc discharge voltage decreased gradually. Due to the good conductivity of CNTs, it can promote the cathode plasma discharge.
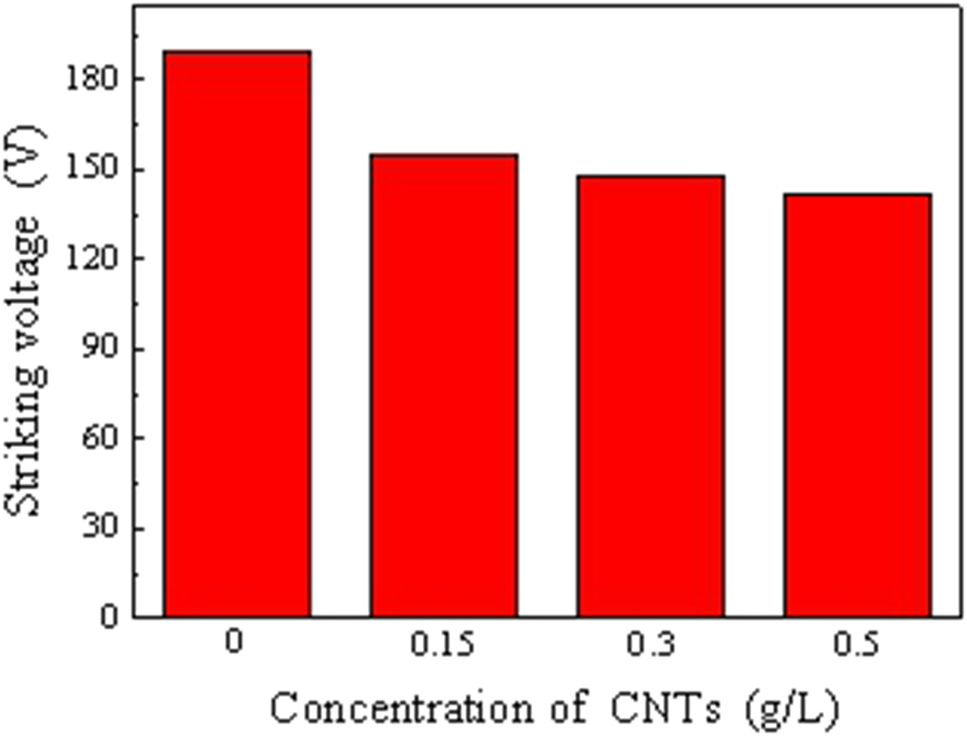
Arc discharge voltage with different content of carbon nanotubes (CNTs).
3.2 Effect of CNTs on the microstructure of PEO ceramic coating
The micro morphologies of the surface and cross section of the ceramic coating without CNTs are shown in Figure 3. As seen in Figure 3a, a lot of 1–4 μm discharge micropores were formed on the coating surface under plasma discharge, and some of them were connected. There were spray deposition oxidation products around the discharge micropores which was rapidly melted by discharge at high temperature and rapidly cooled and solidified by electrolyte. On the one hand, the gas phase produced by the reaction cannot overflow the discharge channel in time. On the other hand, the plasma discharge forms the channel. As seen in Figure 3b, PEO ceramic coating was crisscross and well combined. There were uniformly distributed plasma discharge micropores in the ceramic coating. The ceramic layer sintered at high temperature by plasma discharge has good thermal insulation and insulation characteristics.
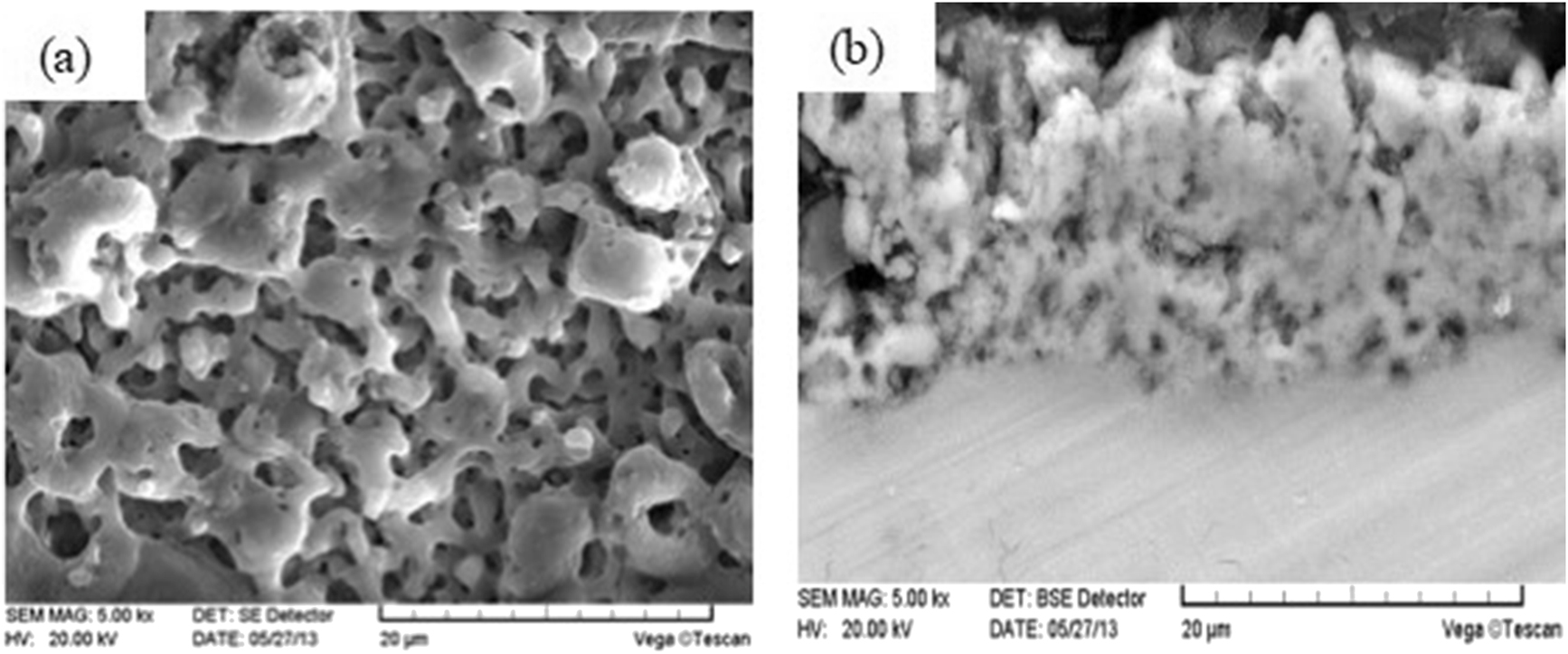
Morphology of plasma electrolytic oxidation (PEO) ceramic coatings: (a) surface morphology; (b) cross-section morphology.
The microstructure and element analysis of ceramic coatings with different content of CNTs are shown in Figure 4. In terms of surface morphology, the CNTs were staggered and deposited on the surface of PEO ceramic coating. Compared with the coating without CNTs, the plasma discharge micropores on the surface of PEO-CNTs composite coating were significantly reduced, because CNTs effectively filled the plasma discharge micropores. As seen in Figure 4b, d, and f, with the increase of the content of CNTs, the content of carbon nanotubes in the coating increased. The addition of CNTs has an effect on the micro and nano structure of the ceramic layer, and then affects the hydrophobicity and corrosion resistance of the ceramic layer, but has little effect on the conductivity and heat resistance of the insulating ceramic layer.
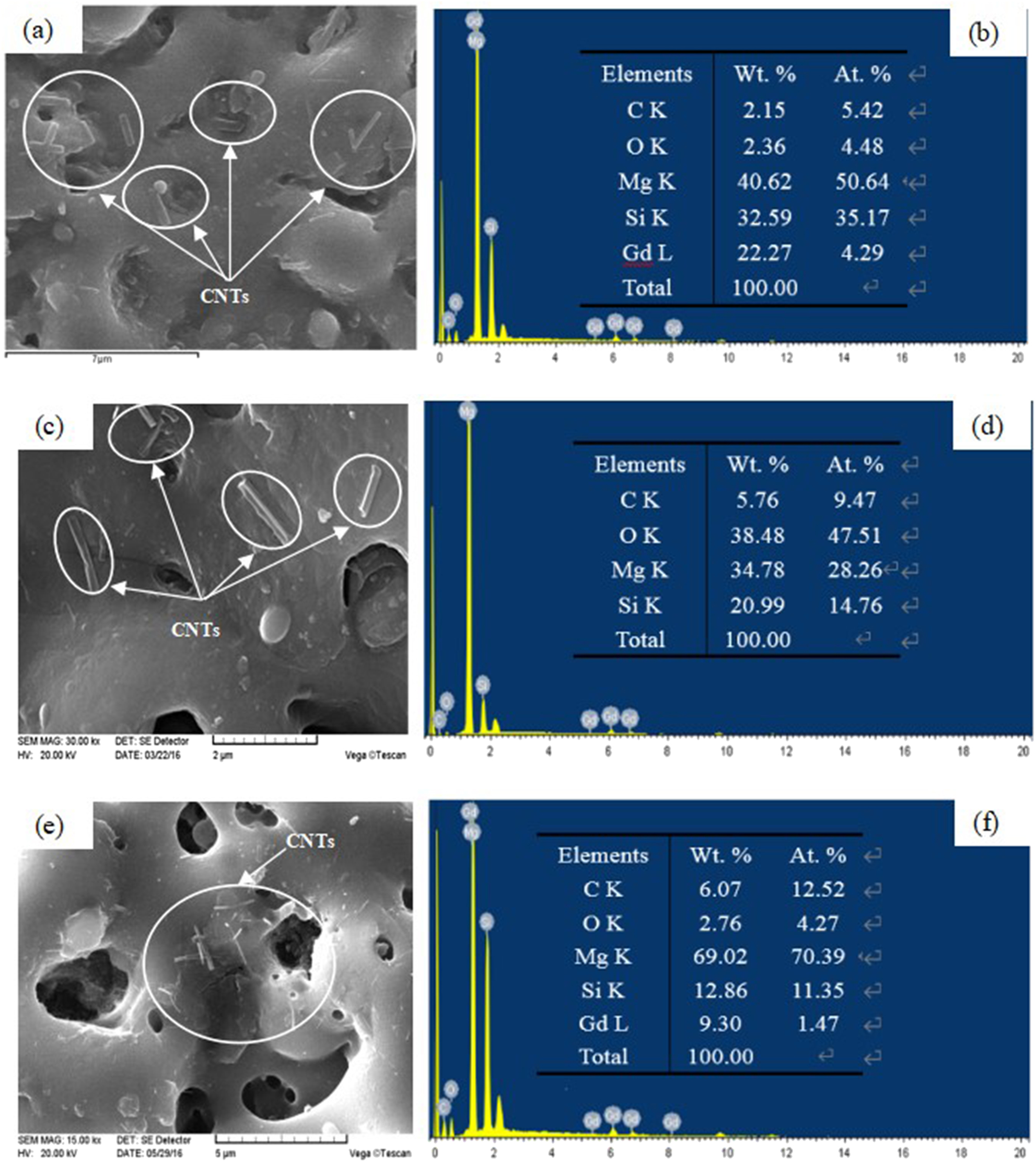
Morphology of plasma electrolytic oxidation (PEO) ceramic coatings with different concentrations of carbon nanotubes (CNTs) (a, b) 0.15 g/L; (c, d) 0.3 g/L; (e, f) 0.5 g/L.
The cross-sectional morphologies of PEO ceramic coatings with different CNTs contents are shown in Figure 5. It can be seen from the cross-section morphology that PEO coating and substrate were crisscross and well bonded. With the increase of CNTs content, the thickness of PEO ceramic coating gradually increased, which showed that CNTs could promote the plasma discharge to some extent. Due to the addition of CNTs, the surface density of ceramic coating was increased, which is quite different from the coating without CNTs. The outer layer of the coating without CNTs was a loose layer. Compared with the inner layer, there are more micropores, and even some discharge through-holes were formed due to the high voltage continuous breakdown at the later stage of discharge. With the CNTs addition, CNTs fill a part of discharge channel, which improves the density of the coating surface.
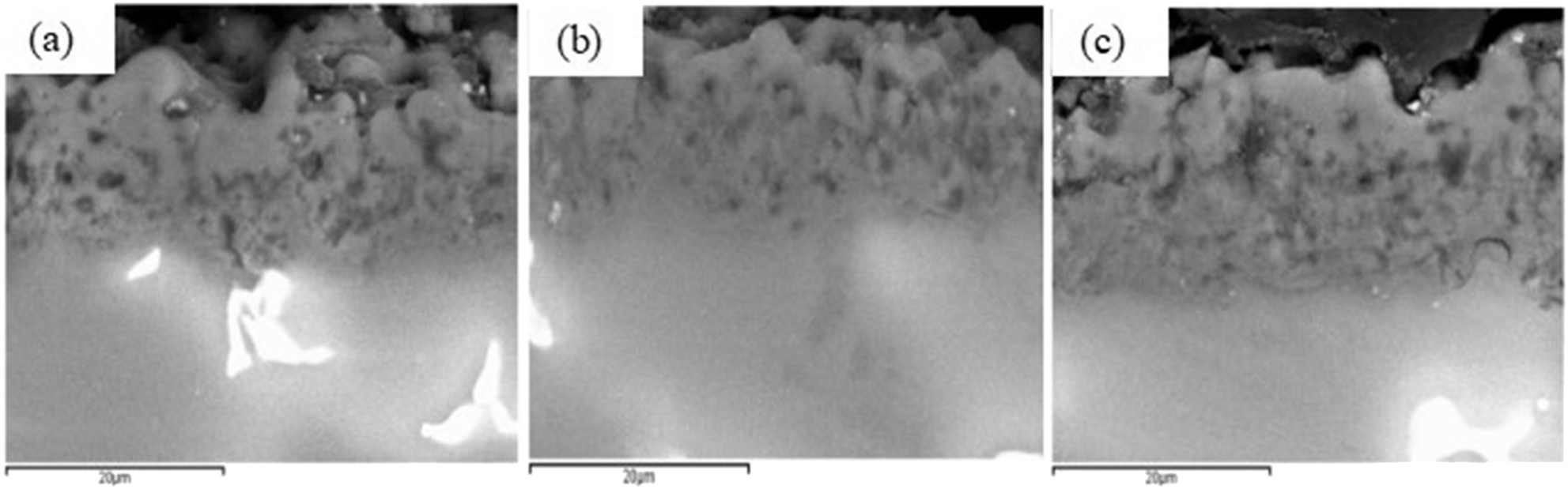
Cross section morphology of coatings with different concentrations of CNTs: (a) 0.15 g/L; (b) 0.3 g/L; (c) 0.5 g/L.
3.3 Hydrophobicity of PEO-CNTs composite coating
The contact angle measurement results of PEO-CNTs composite coating with different content of CNTs are shown in Figure 6. As seen in Figure 6a and b, both Mg–Gd–Y alloy and PEO ceramic coatings are hydrophilic. However, the contact angle of PEO ceramic coating is smaller than that of magnesium alloy. On the one hand, due to the uneven surface of PEO ceramic coating, it has a typical micro–nano porous structure, and there are relatively dense micropores on the surface. When contacting with distilled water, it has strong capillary adsorption and condensation, and there is strong van der Waals force, which leads to the strengthening of the effect of its surface and distilled water, and makes distilled water spread on its surface. On the other hand, due to the diversity and heterogeneity of the chemical composition of PEO layer, the surface of PEO film has higher surface free energy. According to the theory of similar dissolve mutually, PEO coating with high surface free energy and high polar component has strong compatibility to strong polar water molecules, which can induce distilled water to spread on its surface.
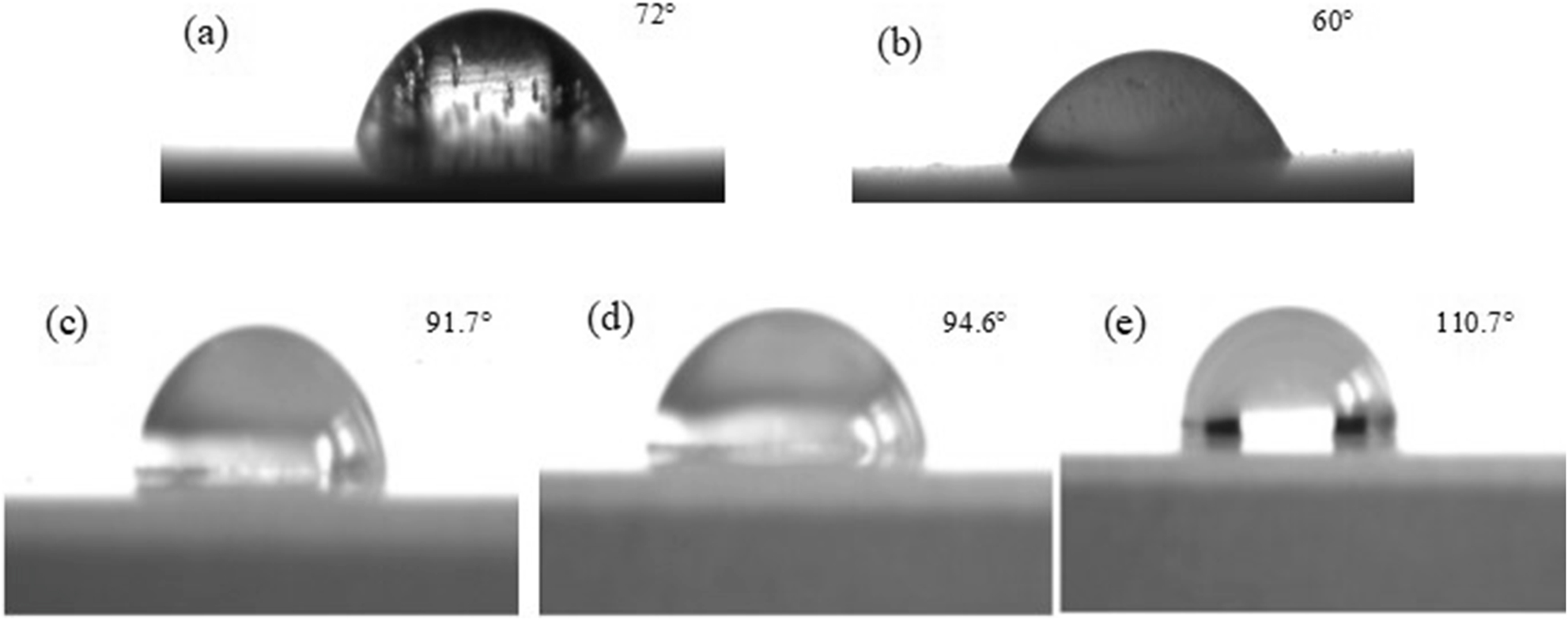
Hydrophobicity of PEO-CNTs coating with different contents of carbon nanotubes (CNTs): (a) Mg–Gd–Y alloy; (b) 0 g/L; (c) 0.15 g/L; (d) 0.3 g/L; (e) 0.5 g/L.
After the modification of CNTs, PEO ceramic layer shows hydrophobicity, and with the increase of CNTs content, the hydrophobicity increases. The wettability of the material surface is determined by the chemical composition and micro geometry of the surface, and the surface free energy and surface roughness play a key role in the wettability. After the modification of CNTs, the surface of the ceramic layer is relatively compact, but the microstructure of PEO coating is not changed. The composite film still has a micro–nano porous rough structure. Therefore, the main reason for the hydrophilic to hydrophobic transition of composite membranes depends on the change of chemical composition. Due to the strong hydrophobicity of CNTs, the surface of modified PEO layer has low surface free energy. According to the theory of similar dissolve mutually, when the composite membrane with low surface free energy contacts with distilled water, it has a certain repulsion to the strong polar distilled water molecules, which greatly reduces the van der Waals force and capillary adsorption force caused by the micro–nano porous rough structure. Therefore, the distilled water does not have enough surface tension and driving force to spread on the surface of the composite membrane. Finally, the surface modification from PEO film hydrophilic to composite film hydrophobic is realized, and the composite film modified by CNTs with hydrophobic characteristics is obtained.
3.4 Corrosion rate of PEO-CNTs composite coating
The corrosion rate of PEO-CNTs composite coating in 3.5% NaCl solution is shown in Figure 7. The addition of CNTs can significantly slow down the corrosion rate of PEO-CNTs composite coating, and the corrosion rate decreases with the increase of CNT concentration. On the one hand, the addition of CNTs can increase the growth thickness of the coating and slow down the diffusion of corrosion medium to the substrate. On the other hand, the unique long chain structure of CNTs can effectively fill the micropores and microcracks on the surface of the composite coating, so that the coating density is improved, which further prevents the diffusion of Cl− ions and improves the corrosion performance of the coating.
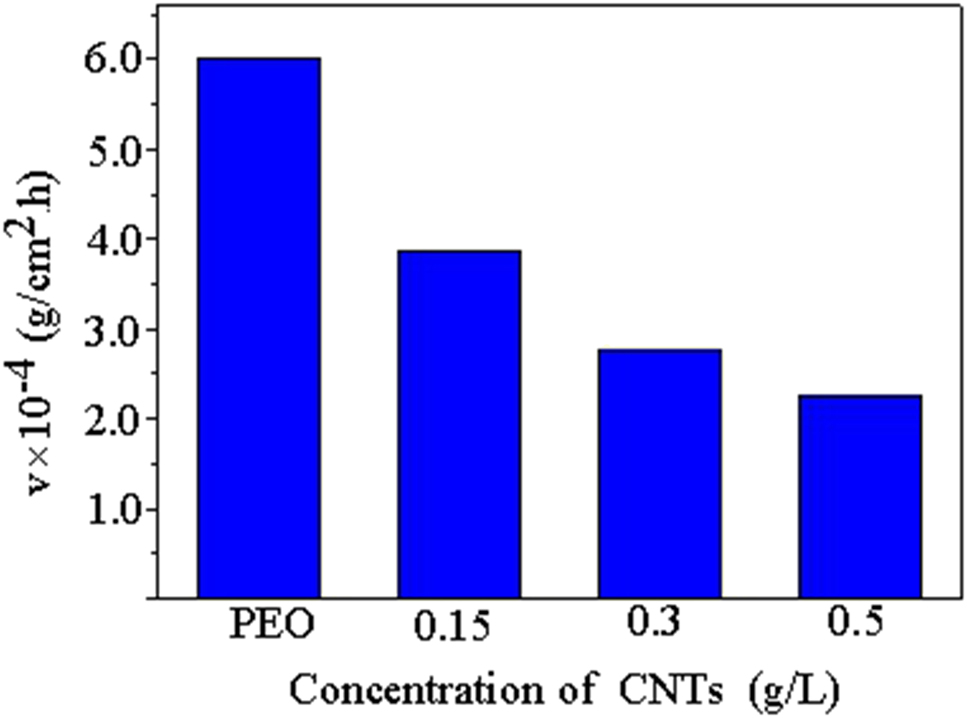
Corrosion rate of PEO-CNTs composite coating.
3.5 Polarization curve and corrosion morphology of PEO-CNTs coating
The polarization curve of PEO-CNTs composite coating in 3.5% NaCl solution at different concentrations of CNTs at room temperature is shown in Figure 8. The electrochemical parameters of PEO-CNTs composite coating at different concentrations of CNTs, such as Ecorr, icorr, bc, ba, and Rp, are shown in Table 1. As seen in Figure 8 and Table 1, after adding CNTs, the corrosion rate of ceramic coating decreased rapidly. With the increase of CNTs concentration, the self-corrosion potential Ecorr of the composite coating increased gradually, which indicated that the corrosion resistance tendency of the coating increased gradually, and the current density icorr decreased gradually and the corrosion rate decreased. In addition, the polarization resistance increased with the increase of CNT concentration, which indicated that the resistance of electrode reaction increased.
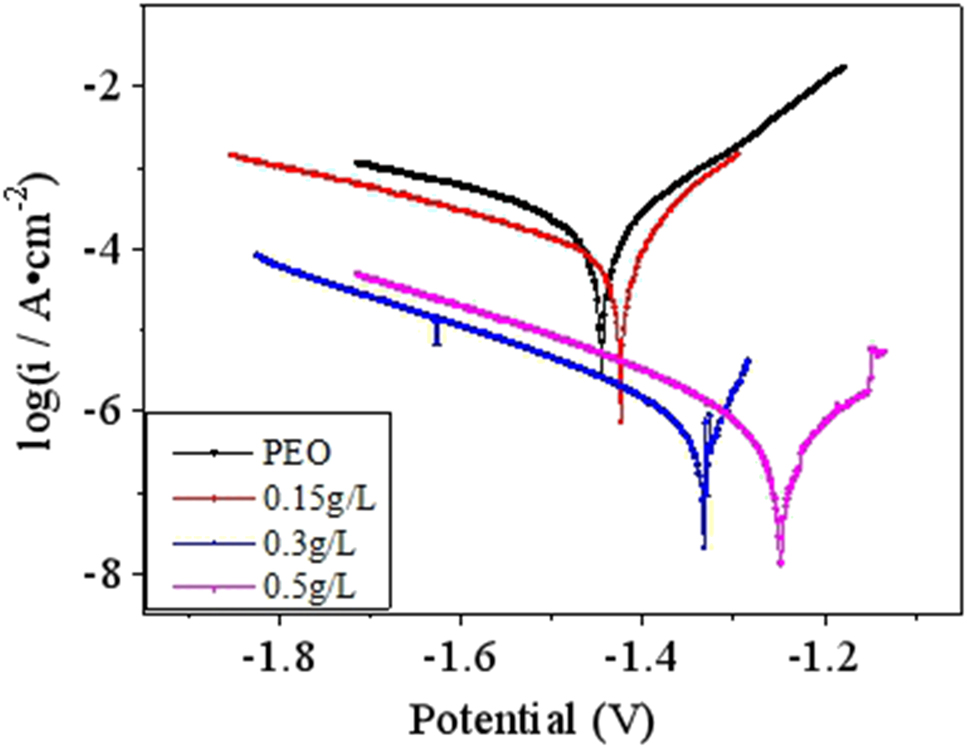
Polarization curve of PEO-CNTs composite coating.
Relevant electrochemical parameters fitted by Tafel extrapolation.
| Content of CNTs (g/L) | E corr/V | i corr/μA/cm2 | b a /mV | b c /mV | R p (Ω·cm2) |
|---|---|---|---|---|---|
| PEO | −1.45 | 12.28 | 189 | 433.5 | 4660 |
| 0.15 | −1.43 | 4.51 | 101.7 | 360.8 | 7645 |
| 0.3 | −1.33 | 0.54 | 57.3 | 164.7 | 34,225 |
| 0.5 | −1.25 | 0.11 | 228.3 | 214.1 | 425,080 |
The corrosion morphology of PEO coating is shown in Figure 9. After 24 h corrosion, it can be seen that PEO coating has obvious corrosion phenomenon, the surface of ceramic layer has been corroded and dissolved in a large area, and the microcracks along the PEO discharge hole are gradually corroded and deepened. After adding CNTs, the corrosion of ceramic layer is obviously weakened. Only the corrosion of plasma discharge micropores and microcracks is gradually deepened. Due to the chemical stability of CNTs and the hydrophobicity of ceramic layer modified by CNTs, it can prevent chloride, oxygen and water from participating in the reaction with the matrix. Moreover, CNTs can fill the plasma discharge micropores and microcracks. Therefore, the overall compactness of the film is improved, and the crack growth on the surface of the film is delayed, which further improves the corrosion resistance of the ceramic layer.
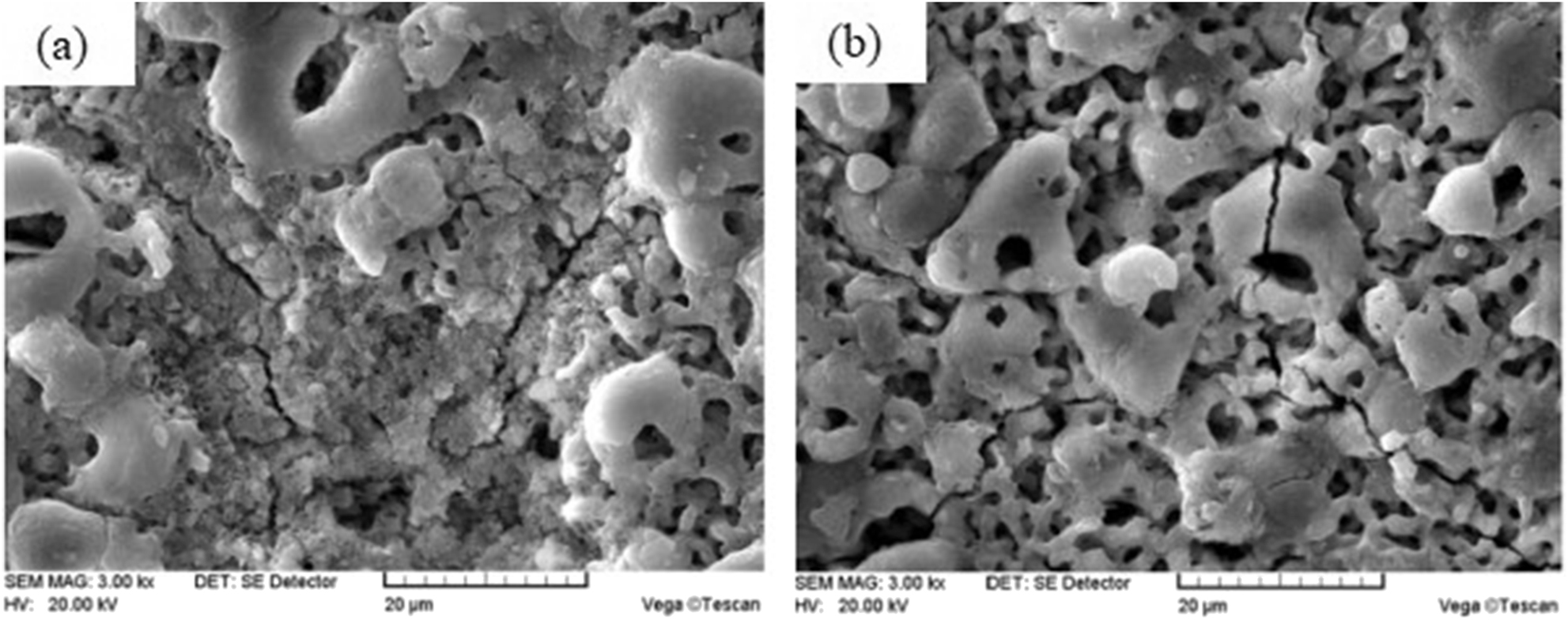
Corrosion morphology of plasma electrolytic oxidation (PEO) coating: (a) plasma electrolytic oxidation (PEO) coating without carbon nanotubes (CNTs); (b) 0.5 g/L CNTs.
Under the action of micro arc discharge, the substrate surface is in the state of high temperature and high pressure, and there is a great temperature difference between the inside and outside of the discharge channel. With the micropore as the center, the molten oxide rapidly cools and solidifies in the electrolyte, which leads to the formation of cracks due to the excessive stress on the surface of the film. These microcracks and discharge channels together constitute a place for energy exchange with the outside world in the process of plasma electrolytic oxidation, and they are also channels for gas discharge. When it is in NaCl solution, Cl− has strong penetrability, and it is concentrated in micropores and cracks. Crevice corrosion and galvanic cell with large cathode and small anode are formed at the crack, which leads to the crack tip extending continuously due to the stress concentration at the anode. CNTs can effectively fill plasma discharge micropores and microcracks, reduce the corrosion of Cl−, and the bridging toughening of CNTs can effectively reduce the crack growth rate.
3.6 Electrochemical impedance spectroscopy of PEO-CNTs coating
The AC impedance of PEO-CNTs composite coating in 3.5% NaCl solution at 0.15, 0.3 and 0.5 g/L concentrations of CNTs is shown in Figure 10. It can be seen that with the increase of CNTs content, the impedance value |Z| of ceramic layer increased, the resistance of electrode reaction increased and the corrosion rate decreased. Figure 11 and Table 2 are the equivalent circuits and fitting results of the AC impedance spectrum. In Figure 11, R1 is the solution resistance, Q is the constant phase angle element, R2 is the ceramic layer resistance. It can be seen from Table 2 that with the increase of CNTs content, the charge transfer resistance R2 of ceramic layer increases, which indicates that the resistance of electrode reaction increases. Charge transfer resistance (CTR) is the resistance of metal ions to transfer into solution through the electric double layer at the interface between metal and solution. Plasma discharge micropores are the areas where Cl− ions are easy to accumulate and corrode. CNTs can effectively fill the plasma discharge micropores, effectively inhibit the diffusion of Cl− ions and improve the interfacial charge transfer resistance. The increase of dispersion index n indicates that the density of ceramic layer increases, the diffusion rate of corrosive ions, such as Cl−, decreases, and the corrosion resistance of ceramic layer increases.
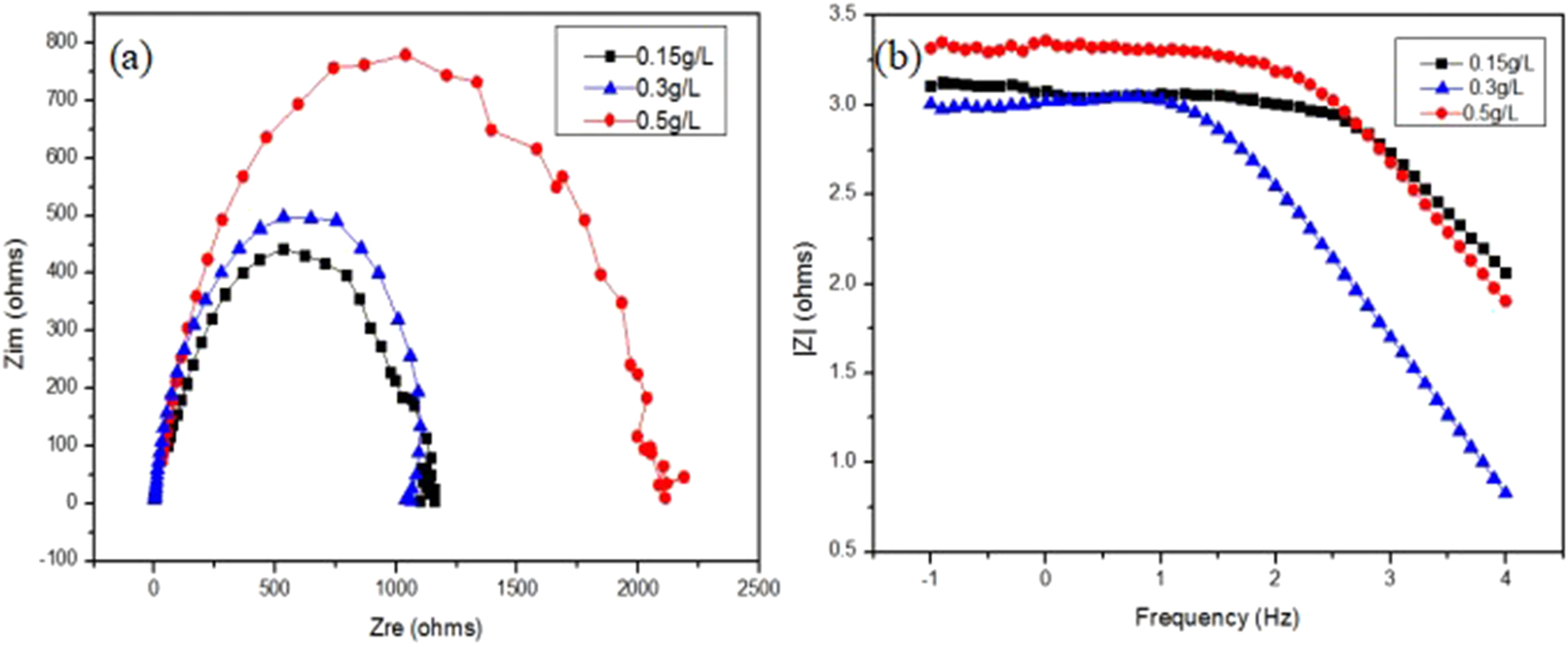
AC impedance of PEO-CNTs composite coating: (a) Nyquist diagram; (b) Bode diagram.
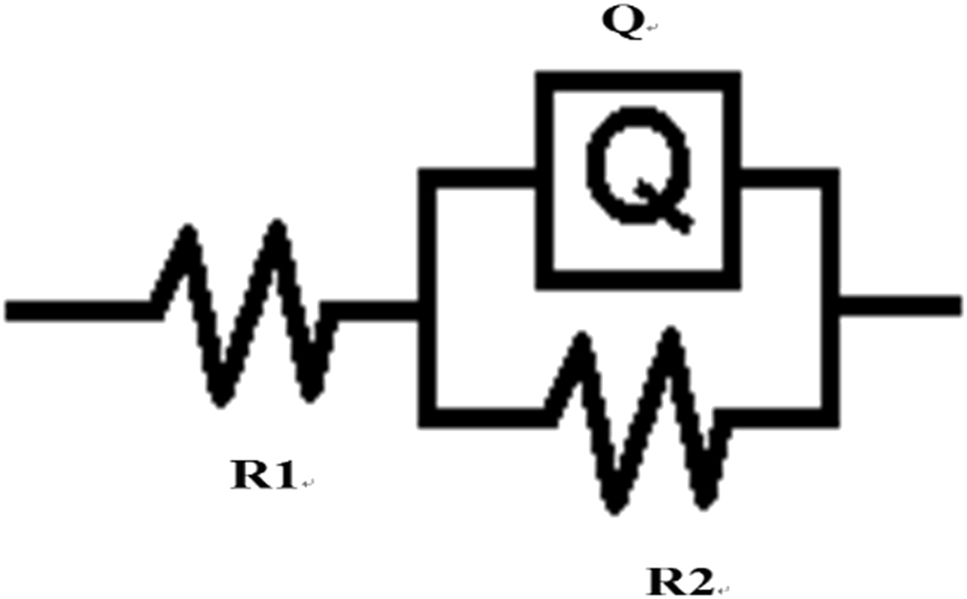
Equivalent electrical circuit of PEO-CNTs composite coating.
EIS data for PEO-CNTs composite coating.
| Content of CNTs (g/L) | R 1 (Ω·cm2) | Q (F·cm−2) | N | R 2 (Ω·cm2) |
|---|---|---|---|---|
| 0.15 | 2.09 | 3.5E−8 | 0.8 | 2.2E4 |
| 0.3 | 1.37 | 1.5E−7 | 0.8 | 2.4E4 |
| 0.5 | 3.40 | 6.8E−7 | 0.9 | 3.0E5 |
4 Conclusions
The microstructure, hydrophobicity, and corrosion resistance of PEO-CNTs composite coating were studied. CNTs with a diameter of about 40 nm and a length of about 1 μm are evenly staggered in the ceramic layer and partially filled with plasma discharge micropores. CNTs can effectively reduce arc discharge voltage and promote the growth of ceramic layer. With the increase of the content of CNTs, the content of carbon nanotubes in the ceramic coating increases, and the density of the ceramic layer increases. CNTs can effectively improve the hydrophobicity of ceramic coating. With the increase of the content of CNTs, the corrosion potential Ecoor and polarization resistance Rp increase, the corrosion current icoor decreases, the AC impedance |Z| and the charge transfer resistance R2 increase, which leads to the decrease of corrosion rate.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This research work is financially supported by the Youth Innovation Team of Shaanxi Universities: Metal corrosion protection and surface engineering technology, Key projects of Natural Science Basic Research in Shaanxi Province (2021JZ-54), Shaanxi Provincial Department of Education Industrialization Cultivation project (17JF009) and Yulin Science and Technology project (2018-2-30).
-
Conflicts of interest: The authors declare no conflicts of interest regarding this article.
References
Acheson, J.G., McKillop, S., Lemoine, P., Boyd, A.R., and Meenan, B.J. (2019). Control of magnesium alloy corrosion by bioactive calcium phosphate coating: implications for resorbable orthopaedic implants. Materialia 6: 100291, https://doi.org/10.1016/j.mtla.2019.100291.Search in Google Scholar
Bai, L.J., Kou, G., Zhao, K., Chen, G.T., and Yan, F.X. (2020). Effect of in-situ micro-arc oxidation coating on the galvanic corrosion of AZ31Mg coupled to aluminum alloys. J. Alloys Compd. 775: 1077–1085.10.1016/j.jallcom.2018.10.154Search in Google Scholar
Cui, L.Y., Liu, H.P., Zhang, W.L., Han, Z.Z., and Deng, M.X. (2017). Corrosion resistance of a superhydrophobic micro-arc oxidation coating on Mg–4Li–1Ca alloy. J. Mater. Sci. Technol. 33: 1263–1271, https://doi.org/10.1016/j.jmst.2017.10.010.Search in Google Scholar
Dou, J.H., Gu, G.C., and Chen, C.Z. (2017). Effects of calcium salts on microstructure and corrosion behavior of micro-arc oxidation coatings on Mg–2Zn–1Ca–0.8 Mn alloy. Mater. Lett. 196: 42–45, https://doi.org/10.1016/j.matlet.2017.03.028.Search in Google Scholar
Gao, F., Hu, Y.D., Li, G.C., Liu, S., and Pan, C.J. (2020). Layer-by-layer deposition of bioactive layers on magnesium alloy stent materials to improve corrosion resistance and biocompatibility. Bioactive Mater. 5: 611–623, https://doi.org/10.1016/j.bioactmat.2020.04.016.Search in Google Scholar PubMed PubMed Central
Jayaraja, R.K., Sabarib, S.S., and Tejab, K.P. (2019). Enhancing the corrosion resistance of stir zone of friction stir welded AZ31b magnesium alloy using micro arc oxidation coatings. Mater. Today 15: 68–75.10.1016/j.matpr.2019.05.026Search in Google Scholar
Jiang, D., Zhou, H., Wan, S., Cai, G.Y., and Dong, Z.H. (2018). Fabrication of superhydrophobic coating on magnesium alloy with improved corrosion resistance by combining micro-arc oxidation and cyclic assembly. Surf. Coating Technol. 339: 155–166, https://doi.org/10.1016/j.surfcoat.2018.02.001.Search in Google Scholar
Jin, Q., Tian, G.Y., Li, J.X., Zhao, Y., and Yan, H. (2019). The study on corrosion resistance of superhydrophobic magnesium hydroxide coating on AZ31B magnesium alloy. Colloid Surf. A 577: 8–16, https://doi.org/10.1016/j.colsurfa.2019.05.060.Search in Google Scholar
Li, Z.X., Yu, Q.L., Zhang, C.Y., Liu, Y.P., and Zhou, F. (2019). Synergistic effect of hydrophobic film and porous MAO membrane containing alkynol inhibitor for enhanced corrosion resistance of magnesium alloy. Surf. Coating Technol. 357: 515–525, https://doi.org/10.1016/j.surfcoat.2018.10.054.Search in Google Scholar
Liu, A.H. and Xu, J.L. (2018). Preparation and corrosion resistance of superhydrophobic coatings on AZ31 magnesium alloy. Trans. Nonferr. Metal. Soc. 28: 2287–2293, https://doi.org/10.1016/s1003-6326(18)64873-3.Search in Google Scholar
Luo, D., Liu, Y., Yin, X.M., Wang, H.Y., Han, Z.W., and Ren, L.Q. (2018). Corrosion inhibition of hydrophobic coatings fabricated by micro-arc oxidation on an extruded Mg–5Sn–1Zn alloy substrate. J. Alloys Compd. 731: 731–738, https://doi.org/10.1016/j.jallcom.2017.10.017.Search in Google Scholar
Ly, X.N. and Yang, S. (2019). Influence of current mode on microstructure and corrosion behavior of micro-arc oxidation (MAO) biodegradable Mg–Zn–Ca alloy in Hank’s solution. Surf. Coating. Technol. 358: 331–339, https://doi.org/10.1016/j.surfcoat.2018.11.040.Search in Google Scholar
Ma, C.F., Nagai, A., Yamazaki, Y., Toyama, T., and Yamashita, K. (2012). Electrically polarized micro-arc oxidized TiO2 coatings with enhanced surface hydrophilicity. Acta Biomater. 8: 860–865, https://doi.org/10.1016/j.actbio.2011.09.021.Search in Google Scholar PubMed
Ma, H.J., Gu, Y.H., Liu, S.J., Che, J.T., and Yang, D.W. (2017). Local corrosion behavior and model of micro-arc oxidation HA coating on AZ31 magnesium alloy. Surf. Coating Technol. 331: 179–188, https://doi.org/10.1016/j.surfcoat.2017.10.053.Search in Google Scholar
Muhaffel, F. and Cimenoglu, H. (2019). Development of corrosion and wear resistant micro-arc oxidation coating on a magnesium alloy. Surf. Coating Technol. 357: 822–832, https://doi.org/10.1016/j.surfcoat.2018.10.089.Search in Google Scholar
Peng, J.H., Zhang, Z., Long, C., Chen, H.H., and Wu, Y.C. (2020). Effect of crystal orientation and {101̅2} twins on the corrosion behaviour of AZ31 magnesium alloy. J. Alloys Compd. 827: 154096, https://doi.org/10.1016/j.jallcom.2020.154096.Search in Google Scholar
Sharma, V., Goyat, M.S., Hooda, A., and Bhargav, P.K. (2020). Recent progress in nano-oxides and CNTs based corrosion resistant superhydrophobic coatings: a critical review. Prog. Org. Coating 140: 105512, https://doi.org/10.1016/j.porgcoat.2019.105512.Search in Google Scholar
Shi, X.T., Zhu, Y.Y., Zhang, S.F., and Zhao, R.F. (2020). Characteristics of selenium-containing coatings on WE43 magnesium alloy by micro-arc oxidation. Mater. Lett. 261: 126944, https://doi.org/10.1016/j.matlet.2019.126944.Search in Google Scholar
Sun, J.Y., Cai, S., Wei, J.L., and Shen, K. (2020). Long-term corrosion resistance and fast mineralization behavior of micronano hydroxyapatite coated magnesium alloy in vitro. Ceram. Int. 46: 824–832, https://doi.org/10.1016/j.ceramint.2019.09.038.Search in Google Scholar
Yao, W.H., Wu, L., Huang, G.S., Jiang, B., Atrensc, A., and Pan, F.S. (2020). Superhydrophobic coatings for corrosion protection of magnesium alloys. J. Mater. Sci. Technol. 52: 100–118, https://doi.org/10.1016/j.jmst.2020.02.055.Search in Google Scholar
Yürektürk, Y., Muhaffel, F., and Baydoğan, M. (2015). Characterization of micro arc oxidized 6082 aluminum alloy in an electrolyte containing carbon nanotubes. Surf. Coating Technol. 269: 83–90.10.1016/j.surfcoat.2014.12.058Search in Google Scholar
Zhang, C.L., Zhang, F., Song, L., Zeng, R.C., and Han, E.H. (2017). Corrosion resistance of a superhydrophobic surface on micro-arc oxidation coated Mg–Li–Ca alloy. J. Alloys Compd. 728: 815–826, https://doi.org/10.1016/j.jallcom.2017.08.159.Search in Google Scholar
Zheng, T., Hu, Y., Pan, F., Zhang, Y., and Tang, A. (2019). Fabrication of corrosion resistant superhydrophobic coating on magnesium alloy by one step electrodeposition method. J. Magnes. Alloy 7: 193–202, https://doi.org/10.1016/j.jma.2019.05.006.Search in Google Scholar
© 2021 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Review
- A review on the corrosion resistance of electroless Ni-P based composite coatings and electrochemical corrosion testing methods
- Original Articles
- Water-droplet erosion behavior of high-velocity oxygen-fuel-sprayed coatings for steam turbine blades
- Corrosion characteristics of plasma spray, arc spray, high velocity oxygen fuel, and diamond jet coated 30MnB5 boron alloyed steel in 3.5 wt.% NaCl solution
- Corrosive-wear behavior of LSP/MAO treated magnesium alloys in physiological environment with three pH values
- Effect of carbon nanotubes on microstructure and corrosion resistance of PEO ceramic coating of magnesium alloy
- Investigating the efficacy of Curcuma longa against Desulfovibrio desulfuricans influenced corrosion in low-carbon steel
- Annual Reviewer Acknowledgement
- Reviewer acknowledgement Corrosion Reviews volume 39 (2021)
Articles in the same Issue
- Frontmatter
- Review
- A review on the corrosion resistance of electroless Ni-P based composite coatings and electrochemical corrosion testing methods
- Original Articles
- Water-droplet erosion behavior of high-velocity oxygen-fuel-sprayed coatings for steam turbine blades
- Corrosion characteristics of plasma spray, arc spray, high velocity oxygen fuel, and diamond jet coated 30MnB5 boron alloyed steel in 3.5 wt.% NaCl solution
- Corrosive-wear behavior of LSP/MAO treated magnesium alloys in physiological environment with three pH values
- Effect of carbon nanotubes on microstructure and corrosion resistance of PEO ceramic coating of magnesium alloy
- Investigating the efficacy of Curcuma longa against Desulfovibrio desulfuricans influenced corrosion in low-carbon steel
- Annual Reviewer Acknowledgement
- Reviewer acknowledgement Corrosion Reviews volume 39 (2021)

