Abstract
Objective
Investigation of the anticarcinogenic effects of natural products with low toxicity is very important in the development of new therapeutic strategies against cancer. Ginnalin A (GA) is one of the most important phenolic compounds of Acer genus and its anticancer effect has been shown that in various cancer cell lines. SB203580, a p38 MAPK inhibitor, can inhibit cell proliferation independently of p38 MAPK. The objective of this study was to investigate combination effect of GA and SB203580 on Hep-3B cell line.
Material and methods
Cell viability was determined by using XTT method after the treatment with GA, SB203580 and combination of both. Anticarcinogenic effects of GA and SB203580 both in single and in combination have been analyzed with Caspase-3 activity assay and expression levels of important genes involved in cell cycle and apoptosis were evaluated by qPCR.
Results
GA and SB203580 have shown additive effect on Hep-3B cells in the combination inhibited 50% of cell viability. And, SB203580 increased the effect of GA on activation of Caspase-3 and expressions of genes important in apoptosis and cell cycle.
Conclusion
This study indicates that GA and SB203580 can be an effective for development of new therapeutic strategies in hepatocellular carcinoma.
Öz
Amaç
Düşük toksisiteli doğal ürünlerin antikarsinojenik etkilerinin araştırılması, kansere karşı yeni tedavi stratejilerinin geliştirilmesinde oldukça önemlidir. Ginnalin A (GA), Acer cinsinin en önemli fenolik bileşiklerinden biridir ve çeşitli kanser hücre hatlarında antikanser etkisi gösterilmiştir. Bir p38 MAPK inhibitörü olan SB203580, p38 MAPK’den bağımsız olarak hücre proliferasyonunu inhibe edebilmektedir. Bu çalışmanın amacı, GA ve SB203580’in Hep-3B hücre hattında kombinasyon etkisinin araştırılmasıdır.
Yöntem
GA, SB203580 ve her ikisinin kombinasyonu ile muameleden sonra hücre canlılığı XTT yöntemi kullanılarak belirlenmiştir. GA ve SB203580’in hem tek başına hem de kombine olarak antikarsinojenik etkileri Kaspaz-3 aktivite testi ile analiz edilmiş ve hücre döngüsü ile apoptozda önemli genlerin ekspresyon seviyeleri qPCR ile değerlendirilmiştir.
Bulgular
GA ve SB203580, Hep-3B hücreleri üzerinde hücre canlılığının %50’sini inhibe ettikleri kombinasyonda additif etki göstermiştir. Ayrıca, SB203580 GA’nın Kaspaz-3 aktivasyonu ve apoptoz ile hücre döngüsünde önemli olan genlerin ifadeleri üzerindeki etkisini arttırmıştır.
Sonuç
Bu çalışma, GA ve SB203580’in hepatosellüler karsinomada yeni terapötik stratejilerin geliştirilmesinde etkili olabileceğini göstermektedir.
Introduction
Hepatocellular carcinoma (HCC), the third leading cause of cancer-related death worldwide, is the most common type of primary liver cancer with a rate of 80–90%. It is the fifth most frequent cancer type in men, while is ranked seventh in women [1], [2]. However, the incidence of HCC varies in different geographical regions depending on the risk factors. The most important risk factors for HCC are chronic infection with Hepatitis B, C and D viruses, alcohol intake and aflatoxin exposure. Accordingly, the incidence of HCC is high in the East, Southeast and Central Asia and the sub-Saharan Africa where the prevalence of hepatitis infections is high. In a similar manner, HCC cases are frequently encountered in South China and sub-Saharan Africa where foods are contaminating with aflatoxin [3], [4], [5]. Also, non-alcoholic steatohepatitis, obesity, metabolic diseases such as diabetes and genetic susceptibility can be associated with development of HCC [6].
There are various treatment approaches for HCC: liver transplantation, surgical resection, percutaneous ablation, trans-arterial chemoembolization, radioembolization and chemotherapy [7], [8]. Especially, liver transplantation, liver resection and percutaneous ablation are the most effective treatment options, but these are effective for HCC only in case of early stage [9]. In addition, because of the late diagnosis of HCC due to the slow development of the disease and lack of biological markers that can detect the onset of the disease, HCC patients are diagnosed at advanced stage [10], [11]. Thereby, chemotherapeutic approaches are applied in treatment for advanced HCC [12]. Especially, sorafenib, a multiple kinase inhibitor, is used as an antiangiogenic agent in advanced HCC patients and can increase the survival time [13]. However, drug resistance is observed in patients who initially responded to sorafenib [14]. Therefore, novel therapeutic strategies are needed for the HCC.
One of the therapeutic strategies developed against cancer is to take advantage of potential anticancer activities of natural products. It has been reported that phenolic compounds, found in natural products such as plants, fruits and vegetables, have antibacterial, antiviral, antiatherosclerotic, anti-inflammatory and anticarcinogenic effects as well as antioxidant properties [15], [16]. Phenolic compounds, secondary metabolites with one or more aromatic rings possessing one or more hydroxyl groups, are classified as phenolic acids and analogs, flavonoids, tannins, stilbenes, curcuminoids, coumarins, lignans, quinines and others [17]. Gallotannins are the hydrolyzable tannins and Chinese tannin, Turkish tannin, Tara tanin, Acer tanin and Hamamelis tanin are the important examples of gallotannins. Acer tannin extracted from Acer genus commonly known as maple [18], [19]. Ginnalins are found in several members of the Acer genus and it has been shown that Ginnalin A (GA), one of the most abundant phenolic compound in Acer genus, has various pharmacological properties such as antioxidant, antibacterial, a-glucosidase enzyme inhibition [20], [21]. In studies with human breast cancer and human colorectal cancer cell lines it has been shown that GA has anticarcinogenic effects on these cells and is the more effective than Ginnalin B and C [22], [23]. One of the most important strategies in the treatment of cancer is the targeting of signaling pathways, particularly those associated with cancer development. For this purpose, various inhibitor molecules are used to determine the role of proteins involved in signaling pathways. SB203580, a pyridinyl imidazole, is used to elucidate the role of p38 mitogen-activated protein kinase (MAPK) in various biological processes [24]. p38 MAPK participates in regulation of apoptosis, growth inhibition, differentiation, cell cycle arrest, and acts as a tumor suppressor by virtue of these roles [25], [26]. Although SB203580 is a p38 MAPK inhibitor, it has been reported that SB203580 suppresses cell proliferation independently of p38 MAPK. This antiproliferative activity of SB203580 has been described by inhibiting the phosphorylation of protein kinase B (PKB), a protooncogen [27]. In a study with hepatocellular carcinoma cells, it has been indicated that SB203580 induces autophagy which may be responsible for the antiproliferative effect of SB203580 [24].
There is no study investigating the anticarcinogenic effect of GA on HCC cells. Also, as a natural product, the effectiveness of GA in the combination strategies is unknown. On the other hand, little is known about the antiproliferative activity of SB203580 on HCC cells. Therapeutic agents developed against cancer affect not only the cancer cells but also non-malignant cells. The use of these agents with high doses also causes side effects. Therefore, combined application of them can reduce side effects, may also cause similar effects with lower doses depending on the synergistic or additive effect. In this study, we aimed to investigate the possible synergistic or additive effect of GA and SB203580 on Hep-3B human HCC cells. For this, primarily the combination activity of GA and SB203580 on the cell viability was assessed, then, expression levels of genes, important in apoptosis and cell cycle, were determined and Caspase-3 activity assay was performed to show anticarcinogenic effect.
Materials and methods
Cell culture
Hep-3B (ATCC® HB-8064™) human HCC cell line obtained from the ATCC (Manassass, VA, USA). The cells were cultured in EMEM medium containing 2 mM L-glutamine supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C in a humidified atmosphere of 5% CO2-95% air. GA (Carbosynth, Cat # FG65656) and SB203580 (Cayman Chemical, Cat # 152121-47-6) were purchased commercially and stock solutions were prepared with 0.1% DMSO in PBS.
Cytotoxicity assay
The XTT method was used to assess the cytotoxic effects of GA, SB203580 and combination of them in Hep-3B cells and each experiment was carried out in triplicate. The cells (5×103 cells/well) were seeded into 96-well plates and incubated for 24 h. After the incubation, the cells were treated with 0–500 μM GA and 0–100 μM SB203580 for 24, 48 and 72 h. Then, XTT solution was added to the each well and absorbance values of the working groups were read at 450 nm (reference wavelength 630 nM) in a microplate reader after the 4 h. The concentrations of GA and SB203580 inhibited 50% of cell viability (IC50) was determined with CompuSyn Version 1.0 software and the concentrations of combination applied to the cells were determined considering the IC50 values of GA and SB203580. Accordingly, the cells were treated with GA (0–300 μM) and SB203580 (0–60 μM) in a ratio of 5:1 for 72 h followed by XTT analysis.
The effect sizes of combination treatment of GA and SB203580 on the Hep-3B cells were determined by calculating the combination index (CI) with the CompuSyn Version 1.0 software by Ting-Chao Chou and Nick Martin. This program is based on the median-effect analysis according to the Chou-Talalay method and synergy, additivity, and antagonism are defined as CI<1, CI=1, and CI>1, respectively [28], [29]. According to the obtained results, GA and SB203580 were applied to the cells at IC50 or in combination that shown additive effect.
Analysis of Caspase-3 activity
The activity of Caspase-3 was measured using the Caspase 3 Assay Kit (BioVision;Cat # K106-25) according to the manufacturer’s instructions. Briefly, cytosolic extracts of cells, treated with GA, SB203580 and combination of them, were obtained with cell lysis buffer and the protein concentrations were measured using the Bradford reagent. Analysis was performed with 100 μg protein sample for each group. Caspase-3 activity was determined by detection of chromophore p-nitroaniline (p-NA) after cleavage from the labeled substrate DEVD-p-NA by active Caspase-3 in a microplate reader at a wavelength of 400 nm. Each experiment was carried out in triplicate.
RNA isolation and qPCR analysis
For the evaluation of gene expression levels, total RNAs were extracted from control and treatment groups using TRIzol Reagent and reverse transcription was performed using iScript™ cDNA Synthesis Kit (Bio-Rad) according to the manufacturer’s instructions.
The effects of GA, SB203580 and combination of them on CASP3, CASP7, CASP8, CASP9, BCL2, BAX, FAS, CYCS, P53, TNF, TNFR1, TRADD, P21, CCND1, CCND2, CCND3, CDK4 and CDK6 gene expressions were evaluated by qPCR (Biorad CFX Connect) analysis. The qPCR reaction was performed using EvaGreen qPCR Mastermix (Mastermix-S) and qPCR protocol was applied as denaturation at 95°C for 10 min, followed by 35 cycles consisting of denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. The primers suitable for the target genes were designed using IDT PrimerQuest (https://eu.idtdna.com/site) and primer sequences were shown in Table 1. ACTB and CYPA were used in the normalization of the results as housekeeping genes.
Primers sequences used in qPCR analysis.
| Gene | Primer sequence |
|---|---|
| ACTB | F:5-TGAACGGGAAGCTCACTGG-3 R:5-TCCACCACCCTGTTGCTGTA-3 |
| CYPA | F:5-TATCTGCACTGCCAAGACTGAGTG-3 R:5-CTTCTTGCTGGTCTTGCCATTCC-3 |
| CASP3 | F:5-GAGCCATGGTGAAGAAGGAATA-3 R:5-TCAATGCCACAGTCCAGTTC-3 |
| CASP7 | F:5-CGAAACGGAACAGACAAAGATG-3 R:5-TTAAGAGGATGCAGGCGAAG-3 |
| CASP8 | F:5-GCCCAAACTTCACAGCATTAG-3 R:5-GTGGTCCATGAGTTGGTAGATT-3 |
| CASP9 | F:5-CGACCTGACTGCCAAGAAA-3 R:5-CATCCATCTGTGCCGTAGAC-3 |
| BCL2 | F:5-GTGGATGACTGAGTACCTGAAC-3 R:5-GAGACAGCCAGGAGAAATCAA-3 |
| BAX | F:5-GGAGCTGCAGAGGATGATTG-3 R:5-GGCCTTGAGCACCAGTTT-3 |
| FAS | F:5-GTGATGAAGGACATGGCTTAGA-3 R:5-GCCCAAACTTCACAGCATTAG-3 |
| CYCS | F:5-GGAGAGGATACACTGATGGAGTA-3 R:5-GTCTGCCCTTTCTTCCTTCTT-3 |
| P53 | F:5-GAGATGTTCCGAGAGCTGAATG-3 R:5-TTTATGGCGGGAGGTAGACT-3 |
| TNF | F:5- CCTCCTCTCTGCCATCAAG-3 R:5- CCAGATAGATGGGCTCATACC-3 |
| TNFR1 | F:5-CGAGATCGATCGGCTGGAG-3 R:5-GTCCATGTCGCGGAGCA-3 |
| TRADD | F:5-TTTCTGTTCCAGGGTCAGC-3 R:5-GCCATTTGAGACCCACAGA-3 |
| P21 | F:5-TGGACCTGTCACTGTCTTGTA-3 R:5-AGAAATCTGTCATGCTGGTCTG-3 |
| CCND1 | F:5-GTTCGTGGCCTCTAAGATGAA -3 R:5-AGGTTCCACTTGAGCTTGTT-3 |
| CCND2 | F:5-GAAGGACATCCAACCCTACAT-3 R:5-AGAAGTGCGAAGAAGAGGTC-3 |
| CCND3 | F:5-GACCTGGCTGCTGTGATT-3 R:5-AAGGTCTGGGCATGCTTT-3 |
| CDK4 | F:5-ATTGGTGTCGGTGCCTATG-3 R:5-AACTGTGCTGATGGGAAGG-3 |
| CDK6 | F:5-ATGCCGCTCTCCACCATC-3 R:5-GTGACACTGTGCACACATCAAA-3 |
Statistical analysis
Data of Caspase-3 activity were analyzed statistically using IBM SPSS 21.0 (SPSS Inc., Chicago, IL, USA) and differences between the groups were analyzed by one-way ANOVA test. The gene expressions analysis were performed by using the 2−ΔΔCT method with RT2 Profiles™ PCR Array Data Analysis. p-value <0.05 was considered to be statistically significant.
Results
Effect of GA, SB203580 and combination of them on cell viability
The cytotoxic effect of GA, SB203580 and combination of them was determined using the XTT assay. GA and SB203580 inhibited the cell viability of Hep-3B cells in a concentration and time manner as shown in Figures 1 and 2. The IC50 of GA and SB203580 were found to be 155 μM and 32.5 μM in of Hep-3B cells for 72 h, respectively.

Concentration and time dependent manner inhibitory effects of GA on cell viability in Hep-3B cells.
The cells were treated with GA at different concentrations and time intervals. The cytotoxic effect was assessed by XTT assay. Values represent the mean±SD at three independent experiments (Cont: control; untreated cells).
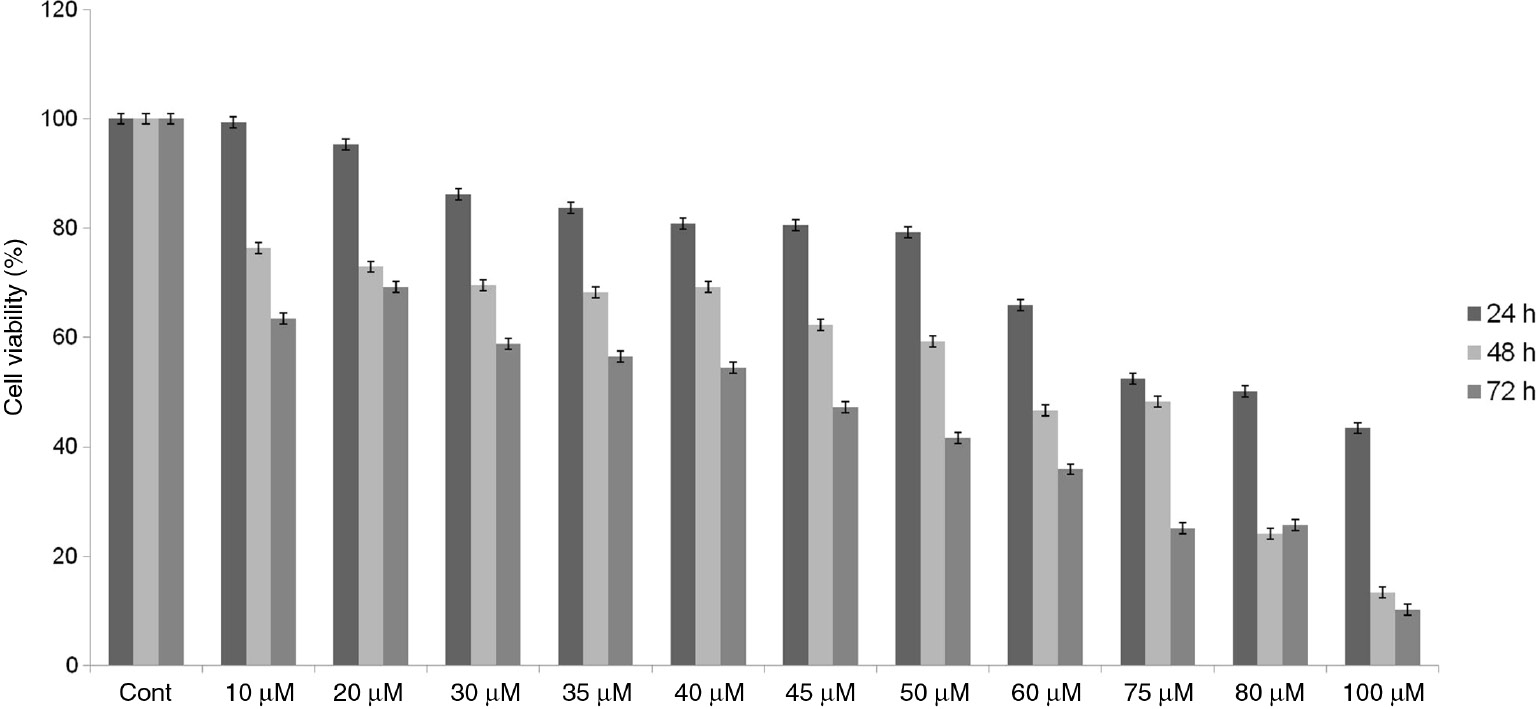
Effect of SB203580 on the cell viability in Hep-3B cells.
The cells were treated with SB203580 at different concentrations and time intervals. The cytotoxic effect was assessed by XTT assay. Values represent the mean±SD at three independent experiments (Cont: control; untreated cells).
After the determitation of IC50 values, GA and SB203580 applied to the cells in a ratio of 5:1 for 72 h. According to the results of CI, GA and SB203580 have shown additive effect on Hep-3B cells at concentrations of combination which inhibited 50% of cell viability (CI=1). These concentrations were 86.22 μM and 17.24 μM for GA and SB203580, respectively. However, it has been found that the combination of GA and SB203580 exerts an antagonistic effect when the cell inhibition exceeds 50% (Figure 3).

Isobologram for combination of GA and SB203580. Isobologram was generated using CompuSyn Version 1.0 software and CI values were calculated.
The percent of cell growth inhibition was presented as the fraction affected (Fa) cells in this isobologram. According to this, Fa=0.5, Fa=0.75 and Fa=0.90 shows 50%, 75% and 90% inhibition, respectively. The data point, below the line, shows synergistic effect, above the line shows antagonistic effect and on the line shows additive effect. Data are the average results of three independent experiments.
Considering these results, the cells were treated with GA and SB203580 at the concentrations which they showed additive effect on Hep-3B cells for 72 h as well as IC50 of GA or SB203580 for subsequent experiments.
Effect of GA, SB203580 and combination of them on Caspase-3 activity
After the treatment with SB203580 and combined with GA, the Caspase-3 activity of cells increased to 7.8 and 8.2 folds, respectively, compared to the control group (p<0.05). These increases were also found to be significant compared to the group which GA was treated with IC50 (p<0.05). On the other hand, there was no significant difference in Caspase-3 activity between the group in which the SB203580 was applied with IC50 and the combination group (p>0.05) (Figure 4).

Caspase-3 activity in Hep-3B cells.
The cells were untreated (control) or treated with GA, SB203580 and combination of them. Data were presented as the mean±standard deviation of three replicate experiments (Cont: control; untreated cells) *p<0.05.
Effects of GA, SB203580 and combination of them on expressions of genes associated with apoptosis and cell cycle
The effects of GA, SB203580 and combination of them on expressions of genes are important in apoptosis and cell cycle were determined using qPCR analysis, after total RNA isolation and cDNA synthesis from control and treatment groups cells were performed.
According to the qPCR results, after the treatment with IC50 of GA, the expression of CASP3, CASP8, CASP9, CYCS and P53 genes significantly increased to 12.09, 10.14, 3.37, 16.15 and 4.15 folds, respectively, compared with the control group. On the other hand, the expression of CCND1 and CDK6 genes was significantly decreased to 4.16 and 6.78 folds. When the cells were treated with IC50 of SB203580 the expression of CASP3, CASP7, CASP8 and BAX genes significantly increased to 15.7, 4, 12.14 and 4.2 folds, respectively, and the expression of CCND1 and CDK4 genes was significantly decreased to 6.75 and 3.15 folds, respectively. After the combined treatment, a significant increase was observed in the expression of CASP3, CASP9, BAX and CYCS genes as 18.38, 15.37, 14 and 13.82 folds, respectively. A significant decrease was observed in the expression of BCL2, CCND1, CDK4 and CDK6 genes as 2.19, 8.96, 9.78 and 7.65 folds, respectively, compared with the control group (p<0.05) (Figure 5). No significant change was observed in the other genes.

Effect of GA, SB203580 and combination of them on expression of important genes in apoptosis and cell cycle for 72 h.
Values represent the mean±SD at three independent experiments. *p<0.05.
Discussion
Hepatocellular carcinoma (HCC), is a large proportion of the total liver cancer burden in the world, has an ever-increasing incidence [30], [31]. Despite the increasing incidence, limited efficacy of current treatment options requires the development of new treatment strategies [32]. Recent studies have shown that natural products can modulate different signaling mechanisms involved in the carcinogenesis process [33]. Accordingly, the investigation of anticarcinogenic activities of natural products in various cancer cells contributes to the development of new therapeutic strategies against cancer.
GA is a phenolic compound found in Acer sp., especially Acer ginnala. It has been reported that the extracts of Acer sp. have antiproliferative effect on colon cancer cells and arrest the cell cycle in S phase [22]. In another study, González-Sarrías reported that GA arrested the cell cycle in the S- and G2/M-phases and decreased cyclins A and D1 protein levels in breast and colon cancer cells [23]. SB203580, investigated for its combination effect with GA on HCC cells in present study, is a p38 MAPK inhibitor and it is frequently used to clarify the role of p38 MAPK. On the other hand, several studies have shown that SB203580 inhibits cell proliferation independent of p38 MAPK. Lali et al. reported that SB203580 can inhibit phosphorylation of retinoblastoma protein, a cell cycle regulatory molecule, in interleukin-2-stimulated T cells. Researchers have shown that SB203580 suppresses PI 3-kinase/protein kinase B (PKB) which is involved in the regulation of retinoblastoma phosphorylation [27]. It is known that retinoblastoma is a tumor suppressor and it inhibits the activity of E2F transcription factors in hypophosphorylated state, thus, cell cycle progression is suppressed. The phosphorylation of retinoblastoma via cyclin-dependent kinases leads to the release of E2F which causes the progression of the cell cycle [34].
In another study, it has reported that SB203580 induces autophagy via activating adenosine monophosphate-activated protein kinase (AMPK) and death-associated protein kinase (DAPK) in HCC cells. The researchers have suggested that it can be associated with antiproliferative effect of SB203580 [24].
In this study, the combination effect of GA, has anticarcinogenic effect in various cancer cell lines, and SB203580 which can suppress the proliferation of cancer cells has been investigated in the Hep-3B human HCC cell line. Firstly, the effect of GA and SB203580 on cell viability in HCC cells was analyzed and IC50 values for 72 h were found to be 155 μM and 32.5 μM, respectively. Then, GA and SB203580 were treated to the cells in a ratio of 5:1 for 72 h.
The results of CI analysis showed that GA and SB203580 have additive effect with low concentrations (Fa≥0.5) on HCC cells. For further analysis, GA and SB203580 were treated to the cells with IC50 of them and in combination (86.22 μM GA and 17.24 μM SB203580) that shown additive effect with leading to 50% inhibition (CI=1).
The one of the most important features of cancer cells is evasion from apoptosis. For this reason, the induction of apoptosis is the most important step for maintenance of the number of healthy cells and clearance oftumor [35]. It is known that the cell death pathways have an important place in therapeutic strategies. One of the best indicators in evaluating the efficacy of a new therapeutic agent is the induction of apoptosis in cancer cells. For this purpose, in this study, apoptotic effect of GA, SB203580 and combination of both was evaluated with Caspase-3 activity assay and gene expression analysis.
Apoptosis is induced through two major pathways; intrinsic and extrinsic. Activation of Caspase-3 which occurs through proteolytic cleavage of procaspase-3 is one of the most important steps for the induction of apoptosis in both pathways [36]. In this study, SB203580 and combined with GA increased the Caspase-3 activity in HCC cells. However, GA did not cause a significant increase in Caspase-3 activity. Although they were applied to the cells with lower concentrations in combination, it has been observed that a significant increase in Caspase-3 activity as 8.2 fold. It is thought that this result confirms the additive effect observed in cytotoxicity analysis. After the combined treatment, a significant increase was observed in the expression of CASP3, CASP9, BAX and CYCS genes and a significant decrease was observed in the expression of BCL2 gene. The release of cytochrome c into cytosol from mitochondria is mediated by the apoptotic protein Bax, while the antiapoptotic protein Bcl-2 blocks the release of cytochrome c in intrinsic pathway of apoptosis. In addition, activation of Caspase-9 and Caspase-3 occurs with release of cytochrome c 37]. Caspase-3, encoded by the CASP3 gene, is the most important effector proteinase in the caspase cascade because when it is activated, apoptosis process begins irreversibly [38]. According to the qPCR results, a significant increase was determined in expression of CYCS gene which encodes cytochrome c protein. It is thought that this result correlates with the reduction in the BCL2 gene and increase in the BAX gene. Also, when the increase in CASP3 and CASP9 genes expression is considered, it can be suggested that GA and SB induce intrinsic pathway of apoptosis. However, more comprehensive and advanced analyzes are needed for this. Furthermore, when GA and SB203580 were applied alone to HCC cells, expression of important genes, responsible for apoptosis induction, was also affected. However, it is observed that the combination of GA and SB203580 has a greater effect on the expression of genes important in apoptosis.
On the other hand, expressions of genes encoding key proteins of cell cycle were also analyzed in this study. A significant decrease was observed in the expression of CCND1, CDK4 and CDK6 genes in combination group. Furthermore, when GA and SB203580 were applied alone to HCC cells, expression of these genes was also affected.
CCND1,2,3/CDK4,6 complex is responsible for the progression of the G1 phase in the cell cycle [39]. The reductions in expression of CCND1, CDK4 and CDK6 genes which are encode these members of the complex can considered an important data indicating that GA and SB203580 can block the cell cycle in the G1 phase.
The use of therapeutic agents with high doses causes side effects in cancer treatment. Therefore, synergistic or additive effects of possible chemotherapeutic agents in combination treatments are very important because they are effective together with lower doses. In this study, it has been shown that GA and SB203580 have an additive activity in HCC cells. Our findings indicated that GA and SB203580 can be an effective for development of new therapeutic strategies in HCC.
Acknowledgements
This study was produced from the MSc thesis of Pınar Özden and supported by N.E.U. Scientific Research Projects (BAP #171318001).
Conflict of interest statement: There are no conflicts of interest.
References
1. Tam K. The roles of doxorubicin in hepatocellular carcinoma. ADMET & DMPK 2003;1:29–44.10.5599/admet.1.3.7Suche in Google Scholar
2. Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of hepatocellular carcinoma in the Asia-Pacific Region. Gut Liver 2016;10:332–9.10.5009/gnl15257Suche in Google Scholar PubMed PubMed Central
3. Gao J, Xie L, Yang WS, Zhang W, Gao S, Wang J, et al. Risk factors of hepatocellular carcinoma—current status and perspectives. Asian Pac J Cancer Prev 2012;13:743–52.10.7314/APJCP.2012.13.3.743Suche in Google Scholar PubMed
4. Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog 2017;16:1.10.4103/jcar.JCar_9_16Suche in Google Scholar PubMed PubMed Central
5. Chen C. Global elimination of viral hepatitis and hepatocellular carcinoma: opportunities and challenges. Gut 2018;67:595–8.10.1136/gutjnl-2017-315407Suche in Google Scholar PubMed
6. Massarweh NN, El-Serag HB. Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control 2017;24:1073274817729245.10.1177/1073274817729245Suche in Google Scholar PubMed PubMed Central
7. Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol 2014;20:4115–27.10.3748/wjg.v20.i15.4115Suche in Google Scholar PubMed PubMed Central
8. Golabi P, Fazel S, Otgonsuren M, Sayiner M, Locklear CT, Younossi ZM. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine (Baltimore) 2017;96:e5904.10.1097/MD.0000000000005904Suche in Google Scholar PubMed PubMed Central
9. Gwiasda J, Schulte A, Kaltenborn A, Ramackers W, Kleine M, Beetz O, et al. Identification of the resection severity index as a significant independent prognostic factor for early mortality and observed survival >5 and >10 years after liver resection for hepatocellular carcinoma. Surg Oncol 2017;26:178–87.10.1016/j.suronc.2017.03.004Suche in Google Scholar PubMed
10. Deng G, Zeng S, Ma J, Zhang Y, Qu Y, Han Y, et al. The anti-tumor activities of Neferine on cell invasion and oxaliplatin sensitivity regulated by EMT via Snail signaling in hepatocellular carcinoma. Sci Rep 2017;7:41616.10.1038/srep41616Suche in Google Scholar PubMed PubMed Central
11. Le Grazie M, Biagini MR, Tarocchi M, Polvani S, Galli A. Chemotherapy for hepatocellular carcinoma: the present and the future. World J Hepatol 2017;9:907–20.10.4254/wjh.v9.i21.907Suche in Google Scholar PubMed PubMed Central
12. Ikeda M, Morizane C, Ueno M, Okusaka T, Ishii H, Furuse J. Chemotherapy for hepatocellular carcinoma: current status and future perspectives. Jpn J Clin Oncol 2018;48:103–14.10.1093/jjco/hyx180Suche in Google Scholar PubMed
13. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–90.10.1056/NEJMoa0708857Suche in Google Scholar PubMed
14. Xu Y, Huang J, Ma L, Shan J, Shen J, Yang Z, et al. MicroRNA-122 confers sorafenib resistance to hepatocellular carcinoma cells by targeting IGF-1R to regulate RAS/RAF/ERK signaling pathways. Cancer Lett 2016;371:171–81.10.1016/j.canlet.2015.11.034Suche in Google Scholar PubMed
15. Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 2004;74:2157–84.10.1016/j.lfs.2003.09.047Suche in Google Scholar PubMed PubMed Central
16. Galati G, O’Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med 2004;37:287–303.10.1016/j.freeradbiomed.2004.04.034Suche in Google Scholar PubMed
17. Huang WY, Cai YZ, Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr Cancer 2010;62:1–20.10.1080/01635580903191585Suche in Google Scholar PubMed
18. Chung KT, Wong TY, Wei CI, Huang YW, Lin Y. Tannins and human health: a review. Crit Rev Food Sci Nutr 1998;38:421–64.10.1080/10408699891274273Suche in Google Scholar PubMed
19. Park KH, Yoon KH, Yin J, Le TT, Ahn HS, Yoon SH, et al. Antioxidative and anti-inflammatory activities of galloyl derivatives and antidiabetic activities of Acer ginnala. Evid Based Complement Alternat Med 2017;2017:6945912.10.1155/2017/6945912Suche in Google Scholar PubMed PubMed Central
20. Han SS, Lo SC, Choi YW, Kim JH, Baek SH. Antioxidant activity ofcrude extract and pure compounds of Acer ginnala Max. Bull Korean Chem Soc 2004;25:389–91.10.5012/bkcs.2004.25.3.389Suche in Google Scholar
21. Honma A, Koyama T, Yazawa K. Anti-hyperglycemic of sugar maple Acer saccharum and its constituent acertannin. Food Chem 2010;123:390–4.10.1016/j.foodchem.2010.04.052Suche in Google Scholar
22. González-Sarrías A, Li L, Seeram NP. Effects of maple (Acer) plant part extracts on proliferation, apoptosis and cell cycle arrest of human tumorigenic and non-tumorigenic colon cells. Phytother Res 2012;26:995–1002.10.1002/ptr.3677Suche in Google Scholar
23. González-Sarrías A, Ma H, Edmonds ME, Seeram NP. Maple polyphenols, ginnalins A-C, induce S- and G2/M-cell cycle arrest in colon and breast cancer cells mediated by decreasing cyclins A and D1 levels. Food Chem 2013;136:636–42.10.1016/j.foodchem.2012.08.023Suche in Google Scholar
24. Zhang H, Chen GG, Zhang Z, Chun S, Leung BC, Lai PB. Induction of autophagy in hepatocellular carcinoma cells by SB203580 requires activation of AMPK and DAPK but not p38 MAPK. Apoptosis 2012;17:325–34.10.1007/s10495-011-0685-ySuche in Google Scholar
25. Deacon K, Mistry P, Chernoff J, Blank JL, Patel R. p38 Mitogen-activated protein kinase mediates cell death and p21-activated kinase mediates cell survival during chemotherapeutic drug-induced mitotic arrest. Mol Biol Cell 2003;14:2071–87.10.1091/mbc.e02-10-0653Suche in Google Scholar
26. Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J 2010;429:403–17.10.1042/BJ20100323Suche in Google Scholar
27. Lali FV, Hunt AE, Turner SJ, Foxwell BM. The pyridinyl imidazole inhibitor SB203580 blocks phosphoinositide-dependent protein kinase activity, protein kinase B phosphorylation, and retinoblastoma hyperphosphorylation in interleukin-2-stimulated T cells independently of p38 mitogen-activated protein kinase. J Biol Chem 2000;275:7395–402.10.1074/jbc.275.10.7395Suche in Google Scholar
28. Chou TC, Talalay P. Analysis of combined drug effects: a new look at a very old problem. Trends Pharmacol Sci 1983;4:450–4.10.1016/0165-6147(83)90490-XSuche in Google Scholar
29. Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984;22:27–55.10.1016/0065-2571(84)90007-4Suche in Google Scholar
30. Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol 2005;40:225–35.10.1007/s00535-005-1566-3Suche in Google Scholar PubMed
31. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90.10.3322/caac.20107Suche in Google Scholar PubMed
32. Alnajjar AM, Elsiesy HA. Natural products and hepatocellular carcinoma: a review. Hepatoma Res 2015;1:119–24.10.4103/2394-5079.167379Suche in Google Scholar
33. Anantharaju PG, Gowda PC, Vimalambike MG, Madhunapantula SV. An overview on the role of dietary phenolics for the treatment of cancers. Nutr J 2016;15:99.10.1186/s12937-016-0217-2Suche in Google Scholar PubMed PubMed Central
34. Stone JG, Siedlak SL, Tabaton M, Hirano A, Castellani RJ, Santocanale C, et al. The cell cycle regulator phosphorylated retinoblastoma protein is associated with tau pathology in several tauopathies. J Neuropathol Exp Neurol 2011; 70:578–87.10.1097/NEN.0b013e3182204414Suche in Google Scholar PubMed PubMed Central
35. Baig S, Seevasant I, Mohamad J, Mukheem A, Huri HZ, Kamarul T. Potential of apoptotic pathway-targeted cancer therapeutic research: Where do we stand? Cell Death Dis 2016;7:e2058.10.1038/cddis.2015.275Suche in Google Scholar PubMed PubMed Central
36. Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature 2001;411:342–8.10.1038/35077213Suche in Google Scholar PubMed
37. Nakamura H, Kumei Y, Morita S, Shimokawa H, Ohya K, Shinomiya K. Antagonism between apoptotic (Bax/Bcl-2) and anti-apoptotic (IAP) signals in human osteoblastic cells under vector-averaged gravity condition. Ann N Y Acad Sci 2003;1010:143–7.10.1196/annals.1299.023Suche in Google Scholar PubMed
38. Li F, Cao B, Ge JF, Zhao J, Jiang D, Zheng SY. Expression of caspase-3 gene in gastric adenocarcinoma cell line SGC-7901 via Ad-FasL. Int J Clin Exp Pathol 2016;9:357–62.Suche in Google Scholar
39. Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet 2008;9:115–28.10.1038/nrg2269Suche in Google Scholar PubMed
©2019 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Research Article
- National External Quality Assessment follow-up: 2010–2017 Turkish experience
- Review Article
- Cofilin-1 as a potential biomarker to evaluate acute kidney injury
- Research Articles
- Can SCUBE1 be used to predict the early diagnosis, lesion volume and prognosis of acute ischemic stroke?
- Evaluation of the effects of different treatment modalities on angiogenesis in heart failure patients with preserved ejection fraction via VEGF and sVEGFR-1
- Effects of platelet rich plasma on the gastric serosal surface neomucosa formation: an experimental rodent model
- Short Communication
- IVSII-74 T>G: As harmless as we thought?
- Research Articles
- Cyclosporine-A induces apoptosis in human prostate cancer cells PC3 and DU145 via downregulation of COX-2 and upregulation of TGFβ
- New insights into the interaction between mammalian butyrylcholinesterase and amitriptyline: a combined experimental and computational approach
- Targeting epidermal growth factor receptor pathway with irreversible tyrosine kinase inhibitor
- SAHA modulates cell proliferation, colony forming and epithelial-mesenchymal transition in CCA cells
- Ginnalin A and SB203580 show additive effect on Hep-3B hepatocellular carcinoma cell line
- Gene expression data analysis for characterizing shared and type specific mechanisms of HCC and B-CLL
- The analysis of surface saccharide profiles through fluorescein-labelled lectins in a rat pancreatic tissue with established metabolic syndrome model
- Case Reports
- Galactorrhea and hyperprolactinemia during vortioxetine use: case report
- Alkaline phosphatase ınterference in an unconjugated estriol assay causing a false positive Down syndrome screening result
- Research Article
- The effect of Paracetamol exposure on hepatic and renal tissues during statin usage
Artikel in diesem Heft
- Frontmatter
- Research Article
- National External Quality Assessment follow-up: 2010–2017 Turkish experience
- Review Article
- Cofilin-1 as a potential biomarker to evaluate acute kidney injury
- Research Articles
- Can SCUBE1 be used to predict the early diagnosis, lesion volume and prognosis of acute ischemic stroke?
- Evaluation of the effects of different treatment modalities on angiogenesis in heart failure patients with preserved ejection fraction via VEGF and sVEGFR-1
- Effects of platelet rich plasma on the gastric serosal surface neomucosa formation: an experimental rodent model
- Short Communication
- IVSII-74 T>G: As harmless as we thought?
- Research Articles
- Cyclosporine-A induces apoptosis in human prostate cancer cells PC3 and DU145 via downregulation of COX-2 and upregulation of TGFβ
- New insights into the interaction between mammalian butyrylcholinesterase and amitriptyline: a combined experimental and computational approach
- Targeting epidermal growth factor receptor pathway with irreversible tyrosine kinase inhibitor
- SAHA modulates cell proliferation, colony forming and epithelial-mesenchymal transition in CCA cells
- Ginnalin A and SB203580 show additive effect on Hep-3B hepatocellular carcinoma cell line
- Gene expression data analysis for characterizing shared and type specific mechanisms of HCC and B-CLL
- The analysis of surface saccharide profiles through fluorescein-labelled lectins in a rat pancreatic tissue with established metabolic syndrome model
- Case Reports
- Galactorrhea and hyperprolactinemia during vortioxetine use: case report
- Alkaline phosphatase ınterference in an unconjugated estriol assay causing a false positive Down syndrome screening result
- Research Article
- The effect of Paracetamol exposure on hepatic and renal tissues during statin usage

