Abstract
Objectives
This randomized controlled pilot study aimed to investigate the therapeutic effects of Baduanjin exercise – a traditional Chinese mind-body practice integrating gentle movements, diaphragmatic breathing, and mindfulness – on alleviating aromatase inhibitor-induced menopausal symptoms, reducing cancer-related fatigue, and modulating systemic inflammatory markers (IL-6, IL-1β, TNF-α) in breast cancer patients.
Methods
70 patients undergoing aromatase inhibitor therapy were recruited from Guangdong General Hospital and randomly assigned to either a Baduanjin exercise group (n=35) or a usual-care control group (n=35). The Baduanjin exercise group completed a supervised 12-week Baduanjin exercise regimen (three sessions weekly, 90 min/session), while controls maintained habitual activity. Menopausal symptoms were assessed using the Kupperman Index, fatigue severity via the Piper Fatigue Scale, and serum inflammatory markers (IL-6, IL-1β, TNF-α) through enzyme-linked immunosorbent assays at baseline and post-intervention.
Results
39 participants (55.7 %) completed the trial (intervention: n=24; control: n=15). The Baduanjin exercise group demonstrated significant reductions in arthralgia severity (p<0.05) and fatigue subdomains (affective: p<0.05; cognitive/mood: p<0.05) compared to controls. Notably, IL-6 levels increased significantly in the Baduanjin exercise group vs. the control group (p<0.05), while IL-1β and TNF-α showed no intergroup differences.
Conclusions
Baduanjin exercise may ameliorate aromatase inhibitor-induced menopausal symptoms and fatigue in breast cancer patients, with distinct modulations in inflammatory biomarkers. Its low-intensity, culturally tailored design supports feasibility as an adjunctive therapy, warranting further investigation into IL-6-mediated mechanisms.
Introduction
Breast cancer is the most prevalent malignancy among women globally and the leading cause of cancer-related morbidity in China [1]. Advances in multimodal therapies, including surgery, endocrine therapy, and targeted treatments, have significantly improved survival rates, particularly for hormone receptor-positive cases, which constitute over 50 % of diagnoses [2], [3], [4]. Aromatase inhibitors, as a core treatment modality for adjuvant endocrine therapy in postmenopausal hormone receptor-positive breast cancer patients, reduce the overall recurrence risk by 27 % compared with no extended treatment after the initial 5-year therapy, underscoring their long-term recurrence prevention value [5]. However, their clinical benefits are negated by a high discontinuation rate (36.9 % within five years) due to treatment-emergent adverse effects such as arthralgia, vasomotor symptoms, and fatigue, which impair quality of life and increase mortality risk among non-adherent patients [6], 7].
Non-pharmacological interventions, particularly exercise, are increasingly recognized for mitigating therapy-related toxicities. Aerobic and resistance training demonstrate efficacy in reducing symptoms, yet their high-intensity protocols often exclude patients with functional limitations or comorbidities [8], [9], [10], [11]. In contrast, Baduanjin exercise – a traditional Chinese mind-body exercise that integrates gentle, rhythmic movements, diaphragmatic breathing, and mindfulness meditation – offers a low-intensity, accessible alternative [12], [13], [14]. Preliminary evidence suggests its potential in alleviating cancer-related fatigue and modulating inflammatory pathways [15], [16], [17], but rigorous evaluation in aromatase inhibitor-treated populations remains lacking. This single-center randomized controlled pilot study focused on verifying intervention feasibility and preliminary efficacy.
Breast cancer patients treated with aromatase inhibitors frequently experience persistent menopausal-like symptoms (e.g., fatigue, hot flashes, arthralgia), which reduce treatment adherence and impair quality of life (QOL). For QOL assessment in this population, existing studies using standardized questionnaires have confirmed a significant burden: the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) shows patients have notably lower scores in physical function, emotional function, and global QOL, with physical discomfort scores negatively correlated with treatment adherence [18], [19], [20]; the Functional Assessment of Cancer Therapy-Endocrine Symptoms (FACT-ES) (designed for endocrine therapy populations) accurately captures the interference of aromatase inhibitor – specific symptoms (e.g., joint pain, hot flashes) on QOL, where the magnitude of score reduction is directly associated with symptom severity [21], [22], [23]; the Breast Cancer-Specific Module 23 (BR23) reveals disease-specific concerns (e.g., body image distress, fear of recurrence) further exacerbate QOL impairment [24], [25], [26]. Thus, based on the existing evidence, we suggest that future exercise intervention studies for this group consider including these three questionnaires pre- and post-intervention to allow for a comprehensive evaluation of QOL improvements. While exercise interventions are increasingly recommended to manage therapy-related side effects, evidence shows that regular physical activity across various forms effectively alleviates menopause-associated symptoms [27], 28]. Existing protocols, however, primarily focus on aerobic or resistance training, which may not adequately accommodate the functional limitations common in this population. The aim of this randomized controlled pilot study was to first verify the feasibility of a 12-week supervised Baduanjin program in this cohort, and second, to explore its preliminary efficacy in alleviating menopausal-like symptoms (assessed via the Kupperman Index and Piper Fatigue Scale) and modulating serum inflammatory biomarkers among patients treated with aromatase inhibitors. The summary of this article is presented in Figure 1. By integrating culturally rooted, low-intensity exercise with objective biomarker analysis, this study sought to address critical gaps in current supportive care strategies, particularly how mind-body exercises distinct from conventional aerobic or resistance training may regulate inflammation to improve symptom outcomes [29], 30]. Such insights could inform tailored interventions to enhance therapeutic adherence and survivorship experiences in breast cancer patients treated with aromatase inhibitors.

Graphical representation of this study. Key points: (1) breast cancer patients on aromatase inhibitors often experience menopausal symptoms (e.g., arthralgia) and cancer-related fatigue, which hinder treatment adherence, while culturally tailored mind-body interventions like Baduanjin are under-researched for these issues. (2) a randomized pilot study enrolled 39 breast cancer patients on aromatase inhibitors, with 24 in a 12-week supervised Baduanjin exercise group and 15 in control group, assessing symptoms via the Kupperman index/piper fatigue scale and serum inflammatory markers. (3) the Baduanjin group showed reduced arthralgia severity, improved fatigue dimensions, and altered serum inflammatory markers, supporting its feasibility as a culture-specific intervention with further investigation needed on inflammation-symptom links. Figure created with BioRender.
Materials and methods
Study design
This single-center randomized controlled pilot study was implemented at Guangdong Provincial People’s Hospital following Ethical Approval from the Institutional Review Board of Guangdong General Hospital (Approval No. GDREC2016424H-R1). The research protocol strictly complied with the ethical principles outlined in the Declaration of Helsinki. Prospective registration was completed at ClinicalTrials.gov (Identifier: NCT03162133; Registration Date: 12 May 2017). Written informed consent was obtained from all participants before commencement of the trial. As a pilot study, its primary objectives were to assess the feasibility of the 12-week Baduanjin intervention (including adherence rates and safety) and explore preliminary efficacy signals to inform future large-scale trials.
Participants
Participant recruitment was systematically conducted at the breast surgery clinic of Guangdong General Hospital between November 2017 and April 2018. Inclusion criteria included: 1) age ranging from 18 to 75 years; 2) histologically confirmed stage I to III breast cancer diagnosed between 0.5 and 8 years prior to enrollment; 3) continuous aromatase inhibitor therapy for more than six months; 4) menopausal syndrome severity confirmed by a modified Kupperman Index score of 6 or higher [31]; and 5) no prior Baduanjin exercise practice within the preceding six months. Exclusion criteria encompassed: i) severe comorbidities (e.g., uncontrolled cardiovascular disease, severe osteoporosis) or planned surgical interventions during the study period; and ii) participation in high-intensity physical activities exceeding 5 h per week. Diagnostic verification was performed through comprehensive medical record review. Baseline assessments were systematically collected to characterize participants, including: 1) demographic characteristics (age, marital status, employment status); 2) clinical profiles (breast cancer stage, time since initial diagnosis, duration of aromatase inhibitor therapy); 3) health status indicators (comorbidities, treatment-related complications); and 4) pre-interventional physical activity profiles. Participants were randomly assigned to the Baduanjin exercise or control group via a computer-generated simple randomization sequence (1:1 allocation) managed by an independent statistician not involved in participant recruitment or outcome assessment. Allocation concealment was ensured using sequentially numbered, opaque, sealed envelopes. Baseline characteristics and attrition rate are presented in Results (Table 1).
Baseline characteristics of patients.a
| Characteristics | Control group (n=35) | Exercise group (n=35) | p-Valueb |
|---|---|---|---|
| Age, years | 54.43 (8.68) | 54.31 (7.24) | 0.95 |
| Marital status, no. (%) | |||
| Married | 29 (82.9) | 25 (71.4) | 0.25 |
| Divorced/separated | 1 (2.9) | 0 (0) | |
| Single | 1 (2.9) | 3 (8.6) | |
| Unclear | 4 (11.3) | 7 (20) | |
| Employment status, no. (%) | |||
| Employed full or part-time | 17 (48.6) | 13 (37.1) | 0.32 |
| Unemployed | 3 (8.6) | 3 (8.6) | |
| Retired | 15 (42.8) | 19 (54.3) | |
| Unclear | 0 (0) | 0 (0) | |
| Time since diagnosis, years | 2.91 (1.7) | 3.17 (2.64) | 0.63 |
| Time since initiating AI, years | 2.42 (1.85) | 2.77 (2.37) | 0.50 |
| Overall stage, no. (%) | |||
| I | 6 (17.1) | 7 (20) | 0.57 |
| II | 20 (57.1) | 21 (60) | |
| III | 9 (25.8) | 7 (20) | |
| Missing/NA | 0 (0) | 0 (0) | |
| Attrition | 20 (57.1) | 11 (31.4) | 0.32 |
-
SD, standard deviation; AI, aromatase inhibitors. aData are presented as the mean (SD) for continuous variables and frequency (percentage) for categorical variables. bp-Value for difference between groups.
Study interventions
The intervention protocol for the Baduanjin exercise group consisted of a 12-week supervised regimen involving three structured sessions per week, each lasting 90 min. Each session was conducted in a group format at the Sports Medicine and Rehabilitation Center of Guangzhou Sport University, with on-site supervision by certified coaches to ensure the accuracy of movements. Each session was systematically divided into three phases: a 10-min preparatory warm-up, a 70-min standardized Baduanjin exercise regimen (comprising eight foundational postures executed eight times per session), and a 10-min cooldown period. Movement execution was synchronized with diaphragmatic breathing techniques (inhalation during postural extension phases, exhalation during relaxation phases). Instruction was provided by certified Qigong instructors with a minimum of five years of professional experience, adhering strictly to the 2017 National Fitness Qigong Baduanjin Standardized Guidelines [32]. The control cohort received routine clinical care without additional exercise interventions. Regarding blinding procedures, while it was not feasible to blind participants or Baduanjin instructors to the group allocation due to the physical nature of the intervention, blinding was rigorously implemented for both scale assessments (e.g., Kupperman Index, Piper Fatigue Scale) and blood sample analysis. Trained research assistants conducting scale evaluations and laboratory technicians responsible for cytokine detection (TNF-α, IL-6, IL-1β) were kept unaware of participant grouping to prevent subjective bias. Additionally, an independent statistician uninvolved in data collection performed all analyses using de-identified data, and all assessors underwent standardized training to ensure consistent operational procedures. These measures were collectively implemented to minimize potential measurement bias.
Outcome measurements
Menopausal symptom severity was quantified through standardized instruments (Kupperman Index and Piper Fatigue Scale) during baseline and post-intervention (48 h after the final exercise session) to avoid acute exercise-induced fluctuations in cytokines.
The Kupperman index
The modified Kupperman index, validated for evaluating menopausal symptoms in Chinese breast cancer survivors [31], comprises a cumulative score derived from 11 symptom-specific subscales: hot flashes, paresthesia, insomnia, nervousness, melancholia, vertigo, fatigue, joint and muscle pain, headaches, palpitations, and formication. Each subscale employs a scoring range from 0 to 3, where 0 indicates absence of symptoms, 1 represents mild symptoms, 2 corresponds to moderate symptoms, and 3 denotes severe symptoms. The total score is calculated by applying differential weighting coefficients: hot flashes are assigned a fourfold weighting, paresthesia, insomnia, and nervousness receive double weighting, while all remaining symptoms are assigned single weighting. This calculation method yields a maximum possible total score of 45.
The piper fatigue scale
Fatigue severity was quantified using the validated revised Piper Fatigue Scale [33], a widely used tool with established reliability in cancer populations [30], 34] which comprises 22 items systematically structured across four clinically recognized fatigue dimensions: behavioral manifestation/severity, affective interpretation, sensory perception, and cognitive-emotional impact. Each item utilized an 11-point Likert scale (0=no fatigue; 10=extreme fatigue), with domain-specific composite scores calculated as arithmetic means of constituent items. The global fatigue index was subsequently derived from averaging these four domain scores, providing a comprehensive fatigue assessment metric.
Serum inflammatory biomarkers assay
Fasting venous blood samples were collected under standardized morning conditions during both baseline and post-intervention phases (minimum 48-h interval after the final session). Serum separation was performed through centrifugation at 5,000 revolutions per minute for 10 min under controlled temperature conditions (4 °C). Quantitative measurement of interleukin-1β (IL-1β; R&D Systems catalog DLB50), interleukin-6 (IL-6; catalog D6050B), and tumor necrosis factor-α (TNF-α; catalog DTA00D) was conducted using validated enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN, USA) according to established protocols. All samples underwent duplicate testing with averaged results to ensure analytical precision, while laboratory analysts remained blinded to group assignments.
Statistical analysis
Statistical analyses were performed using SPSS Statistics version 25.0 (IBM Corp., Armonk, NY). Prior to analyses, normality of continuous variables was verified using the Shapiro–Wilk test, with all outcome measures meeting normality criteria (p>0.05). Baseline characteristics were presented as mean with standard deviation (SD) for continuous variables and frequency (percentage) for categorical measures. Intergroup comparisons of continuous parameters were analyzed via independent two-sample t-tests, whereas categorical variables were examined using Pearson’s chi-square tests. Longitudinal outcome measures were expressed as mean ± SD. Between-group differences across timepoints (baseline vs. post-intervention) were evaluated through independent t-tests, while within-group temporal changes were assessed using paired-sample t-tests. Statistical significance was defined as two-tailed p values <0.05. Given the pilot nature of this study, no correction for multiple comparisons was applied to prioritize detection of preliminary efficacy signals, with results interpreted cautiously.
Results
Subject baseline characteristics
The participants flow through all stages of the study is comprehensively depicted in Figure 2. Of the 70 participants initially enrolled and randomized, a total of 39 individuals (55.7 %) successfully completed the full 12 – week intervention protocol. The attrition rate was 31.4 % (11 out of 35 participants) in the Baduanjin exercise group and 57.1 % (20 out of 35 participants) in the control group. Statistical analysis revealed no significant difference in attrition rates between the two groups. For the control group, which began with 35 randomized participants, 9 participants were lost to follow-up due to a lack of sustained interest in the study procedures. Additionally, 11 participants chose to discontinue the intervention prematurely. As a result, 15 participants in the control group completed all aspects of the study. In the Baduanjin exercise group, starting with an equal initial cohort of 35 randomized participants, 3 participants were lost to follow-up because of diminished interest, and 8 participants discontinued the intervention. Ultimately, 24 participants in the Baduanjin group fulfilled the entire study protocol. Demographic and clinical characteristics of the included patients at baseline are presented in Table 1, revealing no statistically significant intergroup differences in baseline parameters including age distribution, marital status, employment status, disease duration (range: 0.5–8 years post-diagnosis), duration of aromatase inhibitor therapy, or tumor staging classification.
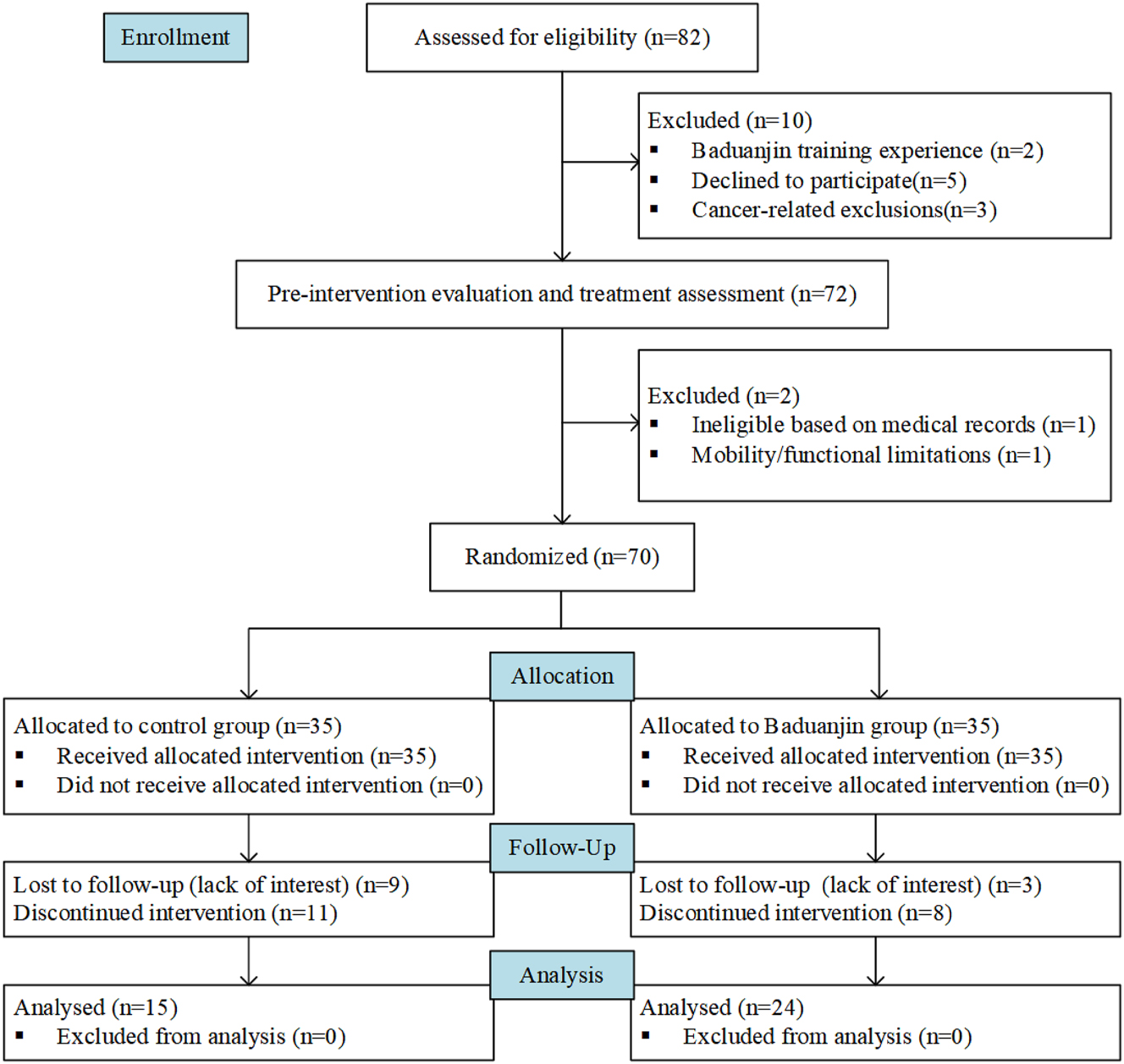
Flow diagram showing participant enrollment and grouping.
The figure depicts the participant flow throughout the study. Initially, 82 individuals were enrolled and screened for eligibility. Among them, 10 were excluded: 2 had prior Baduanjin training experience, 5 declined participation, and 3 met cancer-related exclusion criteria. The remaining 72 participants underwent pre-intervention evaluation, with 2 further excluded (1 due to ineligibility per medical records, 1 with mobility/functional limitations). A total of 70 participants were then randomized: 35 to the control group (maintaining their usual lifestyle without Baduanjin intervention) and 35 to the Baduanjin group (receiving the allocated intervention). During follow-up, 9 control participants were lost to follow-up (due to disinterest) and 11 discontinued the intervention; in the Baduanjin group, 3 were lost to follow-up (disinterest) and 8 discontinued the intervention. Finally, 15 participants from the control group and 24 from the Baduanjin group were included in the analysis.
Effects of Baduanjin exercise on the Kupperman index
Table 2 presents the longitudinal changes in Kupperman Index scores following the 12-week intervention. Baseline assessments demonstrated comparable scores between the Baduanjin exercise and control groups for all individual symptom domains and total composite scores. Post-intervention assessment revealed no significant between-group differences in total Kupperman Index scores. However, the Baduanjin exercise group exhibited a statistically significant reduction in arthralgia severity compared to controls (p<0.05). A clinically observable decrease in hot flash frequency was also documented in the Baduanjin exercise group, though this difference did not achieve statistical significance (p=0.059).
Effects of Baduanjin exercise on the Kupperman index.a
| Baseline | p-Valueb | Post-intervention | p-Valueb | Change | p-Valuec | ||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Exercise | Control | Exercise | Control | Exercise | ||||
| Hot flashes | 0.73 ± 0.59 | 0.96 ± 0.91 | 0.400 | 1.33 ± 0.62 | 1.08 ± 0.77 | 0.298 | 0.60 ± 0.63 | 0.13 ± 0.80 | 0.059 |
| Paresthesia | 1.00 ± 0.92 | 1.04 ± 0.62 | 0.867 | 1.00 ± 0.85 | 0.79 ± 0.66 | 0.394 | 0.00 ± 0.93 | −0.25 ± 0.79 | 0.375 |
| Insomnia | 0.93 ± 0.70 | 1.00 ± 0.72 | 0.779 | 0.93 ± 0.70 | 0.96 ± 0.75 | 0.918 | 0.00 ± 0.53 | −0.04 ± 0.91 | 0.873 |
| Nervousness | 0.87 ± 0.52 | 0.92 ± 0.58 | 0.787 | 0.67 ± 0.61 | 0.67 ± 0.64 | 0.657 | −0.20 ± 0.77 | −0.17 ± 0.70 | 0.890 |
| Melancholia | 0.47 ± 0.64 | 0.79 ± 0.83 | 0.205 | 0.60 ± 0.63 | 0.50 ± 0.66 | 0.643 | 0.13 ± 0.52 | −0.29 ± 0.95 | 0.122 |
| Vertigo | 0.80 ± 0.56 | 0.88 ± 0.80 | 0.752 | 0.67 ± 0.64 | 0.60 ± 0.63 | 1.000 | −0.13 ± 0.74 | −0.21 ± 0.88 | 0.786 |
| Fatigue | 1.13 ± 0.35 | 1.13 ± 0.67 | 0.965 | 1.07 ± 0.45 | 0.96 ± 0.36 | 0.415 | −0.07 ± 0.26 | −0.17 ± 0.82 | 0.649 |
| Arthralgia and myalgia | 1.87 ± 0.64 | 1.96 ± 0.69 | 0.681 | 1.80 ± 0.68 | 1.33 ± 0.48 | 0.467 | −0.07 ± 0.59 | −0.62 ± 0.82 | 0.029& |
| Headache | 0.67 ± 0.48 | 0.67 ± 0.70 | 1.000 | 0.87 ± 0.74 | 0.63 ± 0.58 | 0.262 | 0.20 ± 0.68 | −0.04 ± 0.62 | 0.262 |
| Palpitations | 0.93 ± 0.88 | 0.58 ± 0.77 | 0.183 | 0.53 ± 0.74 | 0.46 ± 0.66 | 0.744 | −0.40 ± 0.83 | −0.13 ± 0.54 | 0.215 |
| Formication | 0.47 ± 0.52 | 0.46 ± 0.51 | 0.961 | 0.40 ± 0.51 | 0.42 ± 0.65 | 0.933 | −0.07 ± 0.46 | −0.04 ± 0.69 | 0.902 |
| Total score | 14.9 ± 5.64 | 16.2 ± 6.26 | 0.504 | 16.47 ± 5.08 | 14.29 ± 6.58 | 0.282 | 1.60 ± 3.68 | −1.92 ± 6.99 | 0.081 |
-
SD, standard deviation. aData were presented as mean ± SD; control group (n=15); exercise group (n=24). p-Valueb for difference between groups at baseline or post-intervention. p-Valuec for change between groups from baseline to post-intervention.
Effects of Baduanjin exercise on the piper fatigue scale
Table 3 details the shifts in fatigue levels as indicated by the Piper Fatigue Scale. At the outset, equivalence was noted between the groups. Post-intervention, however, the Baduanjin exercise group exhibited a notably reduced level of affective meaning compared to the control group, achieving statistical significance (p<0.05). In comparing the control group, the Baduanjin exercise group showed significant decreases of both affective meaning and cognitive/mood (p<0.05).
Effects of Baduanjin exercise on the piper fatigue scale.a
| Baseline | p-Valueb | Post-intervention | p-Valueb | Change | p-Valuec | ||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Exercise | Control | Exercise | Control | Exercise | ||||
| Behavioral/severity | 2.79 ± 2.37 | 3.07 ± 2.41 | 0.727 | 2.86 ± 2.31 | 2.25 ± 1.89 | 0.378 | 0.07 ± 0.81 | −0.82 ± 1.84 | 0.088 |
| Affective meaning | 1.97 ± 1.83 | 3.00 ± 2.55 | 0.181 | 3.02 ± 2.51 | 1.75 ± 1.27 | 0.043 | 1.06 ± 1.83 | −1.24 ± 2.45 | 0.003 |
| Sensory | 4.29 ± 2.31 | 4.23 ± 2.46 | 0.934 | 4.15 ± 2.02 | 3.49 ± 1.52 | 0.255 | −0.15 ± 2.61 | −0.74 ± 2.19 | 0.453 |
| Cognitive/mood | 3.16 ± 1.88 | 4.01 ± 2.66 | 0.283 | 3.88 ± 2.12 | 3.24 ± 1.49 | 0.275 | 0.72 ± 1.34 | −0.78 ± 2.15 | 0.021 |
| Total score | 3.04 ± 0.1.79 | 3.57 ± 2.27 | 0.448 | 3.47 ± 1.74 | 2.69 ± 1.34 | 0.124 | 0.42 ± 0.74 | −0.89 ± 1.75 | 0.009 |
-
PFS, piper fatigue scale; SD, standard deviation. aData were presented as mean ± SD; control group (n=15); exercise group (n=24). p-Valueb for difference between groups at baseline or post-intervention. p-Valuec for change between groups from baseline to post-intervention.
Effects of Baduanjin exercise on serum inflammatory biomarkers
Table 4 presents the longitudinal changes in serum inflammatory biomarkers. Baseline and post-intervention comparisons showed no statistically significant between-group differences in IL-6 concentrations. However, the Baduanjin exercise group demonstrated a significant elevation in IL-6 levels compared to the control group when analyzing within-group changes across the intervention period (p<0.05).
Effects of Baduanjin exercise on serum inflammatory biomarkers.a
| Baseline | p-Valueb | Post-intervention | p-Valueb | Change | p-Valuec | ||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Exercise | Control | Exercise | Control | Exercise | ||||
| IL-1β, pg/mL | 67.41 ± 15.34 | 66.34 ± 15.82 | 0.835 | 80.49 ± 22.67 | 66.89 ± 15.27 | 0.031 | 13.07 ± 25.71 | 0.56 ± 16.64 | 0.072 |
| IL-6, pg/mL | 3.54 ± 0.42 | 3.60 ± 0.56 | 0.717 | 4.56 ± 0.72 | 6.15 ± 1.29 | 0.000 | 1.02 ± 0.47 | 2.54 ± 1.25 | 0.000 |
| TNF-α, pg/mL | 49.00 ± 9.46 | 47.53 ± 6.94 | 0.577 | 56.35 ± 6.57 | 49.80 ± 7.44 | 0.008 | 7.34 ± 10.60 | 2.27 ± 5.79 | 0.061 |
-
IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; SD, standard deviation. aData were presented as mean ± SD; control group (n=15); exercise group (n=24). p-Valueb for difference between groups at baseline or post-intervention. p-Valuec for change between groups from baseline to post-intervention.
Discussion
Contemporary advancements in oncological diagnostics and therapeutics have significantly enhanced prognostic outcomes for breast cancer [35]. However, treatment-induced complications – particularly among patients undergoing aromatase inhibitor therapy – persistently compromise quality-adjusted life years through menopausal symptom burden and cancer-related fatigue [36], 37]. This randomized controlled pilot study provides novel evidence that a 12-week Baduanjin exercise intervention protocol effectively attenuates menopausal symptomatology (particularly arthralgia severity) and mitigates multidimensional fatigue parameters in this clinical population. Mechanistically, the observed IL-6 modulation suggests potential anti-inflammatory pathways underlying these therapeutic effects, though further investigation is required to elucidate precise biological mechanisms.
Baduanjin exercise, a multimodal mind-body practice rooted in traditional Chinese medicine, combines physical postures with cognitive engagement and regulated breathing techniques, demonstrating therapeutic benefits for chronic conditions in female breast cancer patients [38]. This low-complexity intervention, comprising eight biomechanically optimized movements derived from historical wellness traditions, exhibits high accessibility across diverse demographics [17]. The efficacy of Baduanjin for improving psychosocial well-being in breast cancer patients is supported by accumulating evidence from systematic reviews and meta-analyses. These analyses have consistently confirmed its benefits in enhancing health-related quality of life and alleviating depressive symptoms [12], [13], [14, 32], 39]. The present findings provide supportive evidence regarding the potential of Baduanjin exercise in ameliorating vasomotor disturbances, with particular reference to reductions in hot flash frequency observed in aromatase inhibitor-treated patient cohorts [40]. These outcomes appear congruent with established clinical recommendations advocating mind-body interventions as therapeutic strategies for addressing treatment-induced menopausal symptoms [41]. Notably, we observed clinically meaningful reductions in arthralgia severity – a prevalent complication affecting distal joints that frequently compromises treatment adherence [42]. While previous meta-analyses report limited analgesic effects of Baduanjin exercise [13], our results suggest outcome variability may stem from differences in pain etiology (e.g., postoperative vs. treatment-induced), intervention protocols, and population-specific adaptations, underscoring the need for phenotype-stratified investigations.
Breast cancer survivors undergoing aromatase inhibitor therapy frequently report elevated fatigue severity concomitant with menopausal symptomatology. The fundamental mechanism behind fatigue is often linked to inflammation, a response typically triggered by standard cancer therapies, including chemotherapy, or by the cancer itself [43]. Our findings demonstrate Baduanjin exercise’s efficacy in attenuating multidimensional fatigue manifestations, particularly affective and cognitive/mood dimensions, corroborating existing evidence of its therapeutic potential against cancer-related fatigue [39]. Contemporary randomized controlled trial data substantiate these anti-fatigue effects across heterogeneous patient populations, though reduced effect consistency is noted in cohorts aged over 55 years [15]. Mechanistic investigations implicate aromatase inhibitors as potential contributors to fatigue pathophysiology through inflammatory pathways [44], 45], with circulating biomarkers like IL-6 exhibiting dual regulatory properties in inflammatory cascades [46]. IL-6 exhibits context-dependent dual roles in cancer, acting as either a suppressor or promoter of tumor development. During exercise, IL-6 is primarily released from skeletal muscles, whereas in inflammatory foci or the tumor microenvironment, it is secreted by leukocytes and stromal cells. Muscle-derived IL-6 exerts protective effects by enhancing insulin sensitivity in glycogen-storing tissues, stimulating anti-inflammatory cytokine production, mobilizing cytotoxic immune cells, and reducing DNA damage in cancer cells – biological actions that may inhibit cancer initiation and progression. Conversely, sustained IL-6 signaling within inflamed or tumor microenvironments drives chronic low-grade inflammation and activates pro-tumorigenic pathways, thereby promoting cancer advancement [29]. Additionally, IL-6 has been implicated as a critical mediator of pathological pain, including bone cancer pain, through mechanisms such as nociceptor sensitization and amplification of inflammatory signaling cascades [47].
Discrepancies between our findings and other Baduanjin studies likely stem from population-specific differences. For example, middle-aged women demonstrated reduced IL-6 after 12-week Baduanjin, likely reflecting systemic anti-inflammatory effects in metabolically intact populations [48]. Post-stroke patients with depression similarly exhibited decreased IL-6 following combined Baduanjin and psychotherapy, potentially via neuroendocrine-immune regulation of stress-driven inflammation [49]. Conversely, cognitively frail older adults showed non-significant IL-6 changes post-24-week Baduanjin practice, possibly due to age-related immunosenescence or baseline inflammatory lability [50]. Our cohort’s distinct characteristics – breast cancer survivors receiving aromatase inhibitors – introduce critical variables: these agents may disrupt muscle metabolic equilibrium, potentially augmenting myokine IL-6 secretion during rhythmic motor activity [42], 51]. This muscle-derived IL-6 could represent a transient adaptive response promoting glucose homeostasis and anti-inflammatory cytokine synthesis [29], distinguishable from pathogenic IL-6 signaling within tumor microenvironments. The observed elevation might therefore indicate beneficial “metabolic-inflammatory” uncoupling, wherein exercise-induced myokines mitigate therapy-related metabolic disturbances without potentiating pro-tumorigenic cascades. Notably, our observed IL-6 elevation post-Baduanjin exercise contrasts with conventional exercise-induced anti-inflammatory responses [30], suggesting modality-specific immunomodulatory mechanisms requiring further elucidation.
This study provides novel insights into Baduanjin exercise’s therapeutic effects on aromatase inhibitor-associated menopausal symptoms in breast cancer patients, while acknowledging methodological constraints. A key limitation is the small completed sample size (total n=39), which limits the feasibility of sensitivity analyses; multiple imputation or subgroup analyses in such small samples may introduce bias, so additional sensitivity tests were not performed [52]. This underscores the necessity for future studies with expanded sample sizes to verify the robustness of the findings. Other limitations include the notable attrition discrepancy (57.1 % in the control group vs. 31.4 % in the Baduanjin exercise group, p=0.03) – likely due to the lack of structured supervision in the control group – potentially biasing results toward participants with milder symptoms or higher adherence in the control cohort. From a patient management perspective, these findings highlight the importance of personalized support to enhance adherence: clinicians should proactively identify patients at risk of attrition (e.g., those showing early signs of disinterest or perceived burden), use regular follow-ups and tailored motivational counseling to improve retention, and integrate peer-support systems (where experienced participants share their journeys) to boost patients’ confidence and commitment to interventions like Baduanjin. Additionally, the heterogeneous age range (36–70 years), absence of neurocognitive assessments, and 12-week intervention duration (insufficient to address chronic inflammation from prolonged aromatase inhibitor use) further restrict the generalizability of conclusions. In summary, this pilot study suggests that Baduanjin exercise may serve as a feasible, low-intensity adjunctive therapy for ameliorating aromatase inhibitor-induced menopausal symptoms and fatigue, with distinct immunomodulatory effects on IL-6. Further research with larger samples, extended interventions, comprehensive mechanistic assessments (including exploration of IL-6’s dual roles), and refined methodologies (e.g., standardized retention strategies and age-stratified analyses) is needed to validate these findings and clarify the underlying mechanisms.
Conclusions
This pilot study provides preliminary evidence that 12-week Baduanjin exercise may alleviate specific aromatase inhibitor-induced menopausal symptoms (notably arthralgia) and reduce multidimensional fatigue in breast cancer patients, with concurrent modulation of inflammatory markers such as IL-6, tentatively supporting its potential as a low-intensity complementary intervention for managing treatment-related complications; however, due to limitations including a small sample size (n=39), notable attrition bias, and short intervention duration, these findings should be interpreted cautiously, and further well-powered trials with larger cohorts, extended follow-up, and mechanistic assessments – particularly regarding IL-6’s context-dependent role – are needed to validate its therapeutic value.
Funding source: This work was supported by the Guangdong Provincial Key Laboratory of Physical Activity and Health Promotion [grant numbers: 2021B1212040014] and the Guangdong Provincial Key Laboratory of Human Sports Performance Science [grant numbers: 2024B1212020009].
-
Research ethics: This study was performed under the rules of the Declaration of Helsinki, approved by the Ethics Committee of Guangdong General Hospital (GDREC2016424H (R1)).
-
Informed consent: Informed consent was obtained from all individuals included in this study, or their legal guardians or wards.
-
Author contributions: Jingwen Liao and Hao Lan analyzed the data, drew the graph, and drafted the tables and manuscript. Hao Lan conceived and designed the research, performed the research, analyzed the data, contributed to materials and analysis tools. Kun Wang provided clinical diagnosis information and other support for patients. Li Cai performed the research and conducted the Badaunjin exercise program. Min Hu and Xiaohui Hou designed the research, provided assistance, and reviewed the manuscript and tables. The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: We used the AI tool DeepSeek to assist with language polishing and improving the readability of the initial manuscript draft. All AI-generated content was thoroughly reviewed, revised, and approved by the authors, who take full responsibility for the entire content of the published work.
-
Conflict of interest: The authors declare no conflict of interest. Min Hu serves as an Editor-in-Chief for Translational Exercise Biomedicine, but was not involved in the handling, editorial review, or decision-making process for this manuscript. Xiaohui Hou serves as an In-house Managing-editor for Translational Exercise Biomedicine, but was not involved in the handling, editorial review, or decision-making process for this manuscript.
-
Research funding: This work was supported by the Guangdong Provincial Key Laboratory of Physical Activity and Health Promotion [grant numbers: 2021B1212040014] and the Guangdong Provincial Key Laboratory of Human Sports Performance Science [grant numbers: 2024B1212020009].
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Bray, F, Laversanne, M, Sung, H, Ferlay, J, Siegel, RL, Soerjomataram, I, et al.. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229–63. https://doi.org/10.3322/caac.21834.Suche in Google Scholar PubMed
2. Xiong, X, Zheng, LW, Ding, Y, Chen, YF, Cai, YW, Wang, LP, et al.. Breast cancer: pathogenesis and treatments. Signal Transduct Targeted Ther 2025;10:49. https://doi.org/10.1038/s41392-024-02108-4.Suche in Google Scholar PubMed PubMed Central
3. De La Cruz, L, Blankenship, SA, Chatterjee, A, Geha, R, Nocera, N, Czerniecki, BJ, et al.. Outcomes after oncoplastic breast-conserving surgery in breast cancer patients: a systematic literature review. Ann Surg Oncol 2016;23:3247–58. https://doi.org/10.1245/s10434-016-5313-1.Suche in Google Scholar PubMed
4. Kohler, BA, Sherman, RL, Howlader, N, Jemal, A, Ryerson, AB, Henry, KA, et al.. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst 2015;107:djv048. https://doi.org/10.1093/jnci/djv048.Suche in Google Scholar PubMed PubMed Central
5. Papakonstantinou, A, Villacampa, G, Navarro, V, Oliveira, M, Valachis, A, Pascual, T, et al.. Adjuvant endocrine treatment strategies for non-metastatic breast cancer: a network meta-analysis. eClinicalMedicine 2025;81:103116. https://doi.org/10.1016/j.eclinm.2025.103116.Suche in Google Scholar PubMed PubMed Central
6. Gradishar, WJ, Moran, MS, Abraham, J, Aft, R, Agnese, D, Allison, KH, et al.. NCCN Guidelines(R) insights: Breast cancer, Version 4.2021. J Natl Compr Cancer Netw 2021;19:484–93. https://doi.org/10.6004/jnccn.2021.0023.Suche in Google Scholar PubMed
7. Runowicz, CD, Leach, CR, Henry, NL, Henry, KS, Mackey, HT, Cowens-Alvarado, RL, et al.. American cancer society/American society of clinical oncology breast cancer survivorship care guideline. CA Cancer J Clin 2016;66:43–73. https://doi.org/10.3322/caac.21319.Suche in Google Scholar PubMed
8. Banning, M. Adherence to adjuvant therapy in post-menopausal breast cancer patients: a review. Eur J Cancer Care (Engl) 2012;21:10–9. https://doi.org/10.1111/j.1365-2354.2011.01295.x.Suche in Google Scholar PubMed
9. Kyvernitakis, I, Ziller, V, Hars, O, Bauer, M, Kalder, M, Hadji, P. Prevalence of menopausal symptoms and their influence on adherence in women with breast cancer. Climacteric 2014;17:252–9. https://doi.org/10.3109/13697137.2013.819327.Suche in Google Scholar PubMed
10. Peterson, LL, Ligibel, JA. Physical activity and breast cancer: an opportunity to improve outcomes. Curr Oncol Rep 2018;20:50. https://doi.org/10.1007/s11912-018-0702-1.Suche in Google Scholar PubMed
11. Bender, CM, Sereika, SM, Gentry, AL, Zhu, Y, Wagner, M, Cuglewski, C, et al.. Aerobic exercise and aromatase inhibitor-associated musculoskeletal symptoms: results of a randomized clinical trial. Support Care Cancer 2025;33:244. https://doi.org/10.1007/s00520-025-09257-4.Suche in Google Scholar PubMed
12. Gong, X, Rong, G, Wang, Z, Zhang, A, Li, X, Wang, L. Baduanjin exercise for patients with breast cancer: a systematic review and meta-analysis. Compl Ther Med 2022;71:102886. https://doi.org/10.1016/j.ctim.2022.102886.Suche in Google Scholar PubMed
13. Ye, XX, Ren, ZY, Vafaei, S, Zhang, JM, Song, Y, Wang, YX, et al.. Effectiveness of baduanjin exercise on quality of life and psychological health in postoperative patients with breast cancer: a systematic review and meta-analysis. Integr Cancer Ther 2022;21:15347354221104092. https://doi.org/10.1177/15347354221104092. .Suche in Google Scholar PubMed PubMed Central
14. Chen, Y, Zuo, X, Tang, Y, Zhou, Z. The effects of Tai chi and Baduanjin on breast cancer patients: systematic review and meta-analysis of randomized controlled trials. Front Oncol 2024;14:1434087. https://doi.org/10.3389/fonc.2024.1434087.Suche in Google Scholar PubMed PubMed Central
15. Liu, H, Liu, S, Xiong, L, Luo, B. Efficacy of Baduanjin for treatment of fatigue: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltim) 2023;102:e34707. https://doi.org/10.1097/md.0000000000034707.Suche in Google Scholar
16. Zou, L, SasaKi, JE, Wang, H, Xiao, Z, Fang, Q, Zhang, M. A systematic review and meta-analysis baduanjin qigong for health benefits: randomized controlled trials. Evid Based Complement Alternat Med 2017;2017:4548706. https://doi.org/10.1155/2017/4548706.Suche in Google Scholar PubMed PubMed Central
17. Koh, TC. Baduanjin – an ancient Chinese exercise. Am J Chin Med 1982;10:14–21. https://doi.org/10.1142/s0192415x8200004x.Suche in Google Scholar
18. Cocks, K, Wells, JR, Johnson, C, Schmidt, H, Koller, M, Oerlemans, S, et al.. Content validity of the EORTC quality of life questionnaire QLQ-C30 for use in cancer. Eur J Cancer 2023;178:128–38. https://doi.org/10.1016/j.ejca.2022.10.026.Suche in Google Scholar PubMed
19. Musoro, JZ, Coens, C, Sprangers, MAG, Brandberg, Y, Groenvold, M, Flechtner, HH, et al.. Minimally important differences for interpreting EORTC QLQ-C30 change scores over time: a synthesis across 21 clinical trials involving nine different cancer types. Eur J Cancer 2023;188:171–82. https://doi.org/10.1016/j.ejca.2023.04.027.Suche in Google Scholar PubMed
20. Hiensch, AE, Depenbusch, J, Schmidt, ME, Monninkhof, EM, Pelaez, M, Clauss, D, et al.. Supervised, structured and individualized exercise in metastatic breast cancer: a randomized controlled trial. Nat Med 2024;30:2957–66. https://doi.org/10.1038/s41591-024-03143-y.Suche in Google Scholar PubMed PubMed Central
21. Fallowfield, LJ, Leaity, SK, Howell, A, Benson, S, Cella, D. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat 1999;55:189–99. https://doi.org/10.1023/a:1006263818115.10.1023/A:1006263818115Suche in Google Scholar PubMed
22. Tolaney, SM, Guarneri, V, Seo, JH, Cruz, J, Abreu, MH, Takahashi, M, et al.. Long-term patient-reported outcomes from monarchE: abemaciclib plus endocrine therapy as adjuvant therapy for HR+, HER2-node-positive, high-risk, early breast cancer. Eur J Cancer 2024;199:113555. https://doi.org/10.1016/j.ejca.2024.113555.Suche in Google Scholar PubMed
23. Lustberg, M, Fan-Havard, P, Wong, FL, Hill, K, Phelps, MA, Herrera, KW, et al.. Randomized placebo-controlled, double-blind clinical trial of nanoemulsion curcumin in women with aromatase inhibitor-induced arthropathy: an Alliance/NCORP pilot trial. Breast Cancer Res Treat 2024;205:61–73. https://doi.org/10.1007/s10549-023-07223-4.Suche in Google Scholar PubMed PubMed Central
24. Chie, WC, Chang, KJ, Huang, CS, Kuo, WH. Quality of life of breast cancer patients in Taiwan: validation of the Taiwan Chinese version of the EORTC QLQ-C30 and EORTC QLQ-BR23. Psychooncology 2003;12:729–35. https://doi.org/10.1002/pon.727.Suche in Google Scholar PubMed
25. Paramanandam, VS, Dylke, E, Clark, GM, Daptardar, AA, Kulkarni, AM, Nair, NS, et al.. Prophylactic use of compression sleeves reduces the incidence of arm swelling in women at high risk of breast cancer-related lymphedema: a randomized controlled trial. J Clin Oncol 2022;40:2004–12. https://doi.org/10.1200/jco.21.02567.Suche in Google Scholar PubMed
26. Dent, R, Cortés, J, Pusztai, L, McArthur, H, Kümmel, S, Bergh, J, et al.. Neoadjuvant pembrolizumab plus chemotherapy/adjuvant pembrolizumab for early-stage triple-negative breast cancer: Quality-of-Life results from the randomized KEYNOTE-522 study. J Natl Cancer Inst 2024;116:1654–63. https://doi.org/10.1093/jnci/djae129.Suche in Google Scholar PubMed PubMed Central
27. Wu, S, Shi, Y, Zhao, Q, Men, K. The relationship between physical activity and the severity of menopausal symptoms: a cross-sectional study. BMC Womens Health 2023;23:212. https://doi.org/10.1186/s12905-023-02347-7.Suche in Google Scholar PubMed PubMed Central
28. Kim, MJ, Cho, J, Ahn, Y, Yim, G, Park, HY. Association between physical activity and menopausal symptoms in perimenopausal women. BMC Womens Health 2014;14:122. https://doi.org/10.1186/1472-6874-14-122.Suche in Google Scholar PubMed PubMed Central
29. Orange, ST, Leslie, J, Ross, M, Mann, DA, Wackerhage, H. The exercise IL-6 enigma in cancer. Trends Endocrinol Metabol 2023;34:749–63. https://doi.org/10.1016/j.tem.2023.08.001.Suche in Google Scholar PubMed
30. Hiensch, AE, Mijwel, S, Bargiela, D, Wengström, Y, May, AM, Rundqvist, H. Inflammation mediates exercise effects on fatigue in patients with breast cancer. Med Sci Sports Exerc 2021;53:496–504. https://doi.org/10.1249/mss.0000000000002490.Suche in Google Scholar
31. Tao, M, Shao, H, Li, C, Teng, Y. Correlation between the modified Kupperman index and the menopause rating scale in Chinese women. Patient Prefer Adherence 2013;7:223–9. https://doi.org/10.2147/ppa.s42852.Suche in Google Scholar PubMed PubMed Central
32. Liao, J, Chen, Y, Cai, L, Wang, K, Wu, S, Wu, L, et al.. Baduanjin’s impact on quality of life and sleep quality in breast cancer survivors receiving aromatase inhibitor therapy: a randomized controlled trial. Front Oncol 2022;12:807531. https://doi.org/10.3389/fonc.2022.807531.Suche in Google Scholar PubMed PubMed Central
33. Piper, BF, Dibble, SL, Dodd, MJ, Weiss, MC, Slaughter, RE, Paul, SM, editors. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum 1998;25:677–8410.1037/t18854-000Suche in Google Scholar
34. Hsiao, CP, Wang, D, Kaushal, A, Saligan, L. Mitochondria-related gene expression changes are associated with fatigue in patients with nonmetastatic prostate cancer receiving external beam radiation therapy. Cancer Nurs 2013;36:189–97. https://doi.org/10.1097/ncc.0b013e318263f514.Suche in Google Scholar
35. Burstein, HJ, Lacchetti, C, Anderson, H, Buchholz, TA, Davidson, NE, Gelmon, KA, et al.. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol 2019;37:423–38. https://doi.org/10.1200/jco.18.01160.Suche in Google Scholar PubMed
36. Khosrow-Khavar, F, Filion, KB, Bouganim, N, Suissa, S, Azoulay, L. Aromatase inhibitors and the risk of cardiovascular outcomes in women with breast cancer: a population-based cohort study. Circulation 2020;141:549–59. https://doi.org/10.1161/circulationaha.119.044750.Suche in Google Scholar
37. Henry, NL, Azzouz, F, Desta, Z, Li, L, Nguyen, AT, Lemler, S, et al.. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol 2012;30:936–42. https://doi.org/10.1200/jco.2011.38.0261.Suche in Google Scholar
38. Leung, KW, Yang, YJ, Hui, SS, Woo, J. Mind-body health benefits of traditional Chinese qigong on women: a systematic review of randomized controlled trials. Evid Based Complement Alternat Med 2021;2021:7443498. https://doi.org/10.1155/2021/7443498.Suche in Google Scholar PubMed PubMed Central
39. Kuo, CC, Wang, CC, Chang, WL, Liao, TC, Chen, PE, Tung, TH. Clinical effects of baduanjin qigong exercise on cancer patients: a systematic review and meta-analysis on randomized controlled trials. Evid Based Complement Alternat Med 2021;2021:6651238. https://doi.org/10.1155/2021/6651238.Suche in Google Scholar PubMed PubMed Central
40. Chlebowski, RT, Mortimer, JE, Crandall, CJ, Pan, K, Manson, JE, Nelson, R, et al.. Persistent vasomotor symptoms and breast cancer in the Women’s health initiative. Menopause 2018;26:578–87. https://doi.org/10.1097/gme.0000000000001283.Suche in Google Scholar
41. Liu, J, Nie, G, Li, Y, Wen, Z, Lu, L, Xie, L, et al.. Nonhormonal hot flash management for breast cancer survivors: a systematic review and network meta-analysis. Evid Based Complement Alternat Med 2020;2020:4243175. https://doi.org/10.1155/2020/4243175.Suche in Google Scholar PubMed PubMed Central
42. Hyder, T, Marino, CC, Ahmad, S, Nasrazadani, A, Brufsky, AM. Aromatase inhibitor-associated musculoskeletal syndrome: understanding mechanisms and management. Front Endocrinol 2021;12:713700. https://doi.org/10.3389/fendo.2021.713700.Suche in Google Scholar PubMed PubMed Central
43. Bower, JE, Lamkin, DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun 2013;30:S48–57. https://doi.org/10.1016/j.bbi.2012.06.011.Suche in Google Scholar PubMed PubMed Central
44. Bauml, J, Chen, L, Chen, J, Boyer, J, Kalos, M, Li, SQ, et al.. Arthralgia among women taking aromatase inhibitors: is there a shared inflammatory mechanism with co-morbid fatigue and insomnia? Breast Cancer Res 2015;17:89. https://doi.org/10.1186/s13058-015-0599-7.Suche in Google Scholar PubMed PubMed Central
45. Mao, JJ, Farrar, JT, Bruner, D, Zee, J, Bowman, M, Seluzicki, C, et al.. Electroacupuncture for fatigue, sleep, and psychological distress in breast cancer patients with aromatase inhibitor-related arthralgia: a randomized trial. Cancer 2014;120:3744–51. https://doi.org/10.1002/cncr.28917.Suche in Google Scholar PubMed PubMed Central
46. Fuster, JJ, Walsh, K. The good, the bad, and the ugly of interleukin-6 signaling. EMBO J 2014;33:1425–7. https://doi.org/10.15252/embj.201488856.Suche in Google Scholar PubMed PubMed Central
47. Zhou, YQ, Liu, Z, Liu, ZH, Chen, SP, Li, M, Shahveranov, A, et al.. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation 2016;13:141. https://doi.org/10.1186/s12974-016-0607-6.Suche in Google Scholar PubMed PubMed Central
48. Chen, HH, Yeh, ML, Lee, FY. The effects of baduanjin qigong in the prevention of bone loss for middle-aged women. Am J Chin Med 2006;34:741–7. https://doi.org/10.1142/s0192415x06004259.Suche in Google Scholar
49. Liu, Y, Chen, C, Du, H, Xue, M, Zhu, N. Impact of Baduanjin exercise combined with rational emotive behavior therapy on sleep and mood in patients with poststroke depression: a randomized controlled trial. Medicine (Baltim) 2024;103:e38180. https://doi.org/10.1097/md.0000000000038180.Suche in Google Scholar PubMed PubMed Central
50. Ye, Y, Wan, M, Lin, H, Xia, R, He, J, Qiu, P, et al.. Effects of Baduanjin exercise on cognitive frailty, oxidative stress, and chronic inflammation in older adults with cognitive frailty: a randomized controlled trial. Front Public Health 2024;12:1385542. https://doi.org/10.3389/fpubh.2024.1385542.Suche in Google Scholar PubMed PubMed Central
51. Gupta, A, Henry, NL, Loprinzi, CL. Management of aromatase inhibitor-induced musculoskeletal symptoms. JCO Oncol Pract 2020;16:733–9. https://doi.org/10.1200/op.20.00113.Suche in Google Scholar
52. Sterne, JA, White, IR, Carlin, JB, Spratt, M, Royston, P, Kenward, MG, et al.. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. https://doi.org/10.1136/bmj.b2393.Suche in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter on behalf of Shangai Jiao Tong University and Guangzhou Sport University
This work is licensed under the Creative Commons Attribution 4.0 International License.


