Abstract
Objectives
Exercise training induces several skeletal muscle adaptations. Beta-guanidinopropionic acid (β-GPA) is a creatine analog that simulates the effect of exercise to induce mitochondrial biogenesis. However, the effects of β-GPA on resistance training adaptation, such as muscle hypertrophy and mitochondrial biogenesis, are unclear. Therefore, using a resistance exercise model in rats, the present study was designed to investigate the effects of β-GPA administration on resistance training adaptations.
Methods
This study was approved by the Ethics Committee for Animal Experiments at Ritsumeikan University (approval number: BKC2022-009). Male Sprague Dawley rats were randomly divided into placebo or β-GPA groups. β-GPA (1000 mg/kg) was orally administered once daily, starting seven days before the initiation of electromyostimulation as a model for resistance exercise, and continued throughout the training period. Electromyostimulation was applied to the right gastrocnemius muscle via electrical stimulation every other day for a total of 12 sessions
Results
Peroxisome proliferators-activated receptor-γ co-activator-1α, a regulator of mitochondrial biogenesis, was significantly increased by the combination of training and β-GPA compared to the training leg (p<0.05). Protein expression of Total OXPHOS, a marker of mitochondrial content, was significantly increased by the combination of training and β-GPA compared to the training leg (p<0.05). β-GPA intake reduced muscle mass (main effect of β-GPA, p<0.05) and was associated with muscle protein breakdown-related Fbx32 and LC3-II protein expression levels but did not counteract the increase in muscle mass caused by resistance training.
Conclusions
Administration of exogenous β-GPA enhanced resistance training-induced mitochondrial biogenesis. Moreover, β-GPA still permitted resistance electromyostimulation-induced muscle mass gains, but that effect was attenuated as compared to placebo.
Introduction
Skeletal muscle is a massive, plastic tissue carrying 40 % of body mass [1]. Inactivity associated with aging, injury, or disease results in reduced skeletal muscle mass [2], reduced oxidative capacity, mitochondrial function [3], and insulin resistance [4], which can lead to multiple health problems. Exercise is known to be an effective intervention and is widely implemented to address these issues. Resistance and endurance exercises induce several adaptations in skeletal muscle, including muscle hypertrophy and mitochondrial adaptation (e.g., mitochondrial biogenesis), which may increase muscle strength and endurance performance. These adaptations induced by different exercise modes vary widely, with resistance exercise causing muscle hypertrophy [5] and endurance exercise causing more pronounced mitochondrial adaptations [6, 7]. From the point of view of mitochondria, resistance training had no impact on mitochondrial fractional synthesis rate and citrate synthase activity, while endurance training increased both parameters [8]. On the other hand, some studies reported that resistance exercise and training has been shown to enhance mitochondrial function, mitochondrial biogenesis, and mitochondrial fractional synthesis rate [9, 10]. These findings indicate that resistance exercise is a mode of exercise that induces significant muscle hypertrophy and a slight increase in mitochondrial biogenesis.
Creatine is a metabolite in energy supply, with 90–95 % of body’s total creatine stored in skeletal muscle [11]. Creatine and creatine phosphate also regulate the activity of intracellular energy sensor AMP-activated protein kinase (AMPK) [12]. AMPK is an upstream factor of peroxisome proliferators-activated receptor-γ co-activator-1α (PGC-1α), a regulator of mitochondrial biogenesis [13]; creatine depletion and the consequent decrease in creatine phosphate may be one trigger for mitochondrial biogenesis. Indeed, it has been shown that mitochondrial enzyme activity and AMPK phosphorylation are promoted in skeletal muscle pharmacologically and genetically engineered for creatine depletion within skeletal muscle [14, 15]. Beta-guanidinopropionic acid (β-GPA) has a similar substance structure to creatine. Therefore, β-GPA is thought to act by competitive inhibition against creatine, which reduces creatine and creatine phosphate in skeletal muscle by inhibiting creatine transport into the cell [15, 16]. β-GPA is also an exercise mimetic that can achieve the same muscle adaptations as endurance training. β-GPA decreased creatine and creatine phosphate in skeletal muscle and increased PGC-1α protein expression [13, 15]. β-GPA may, therefore, trigger the activation of mitochondrial biogenesis via inhibition of creatine transport into the cell and reduction of intracellular creatine. On the other hand, β-GPA increases muscle proteolysis, resulting in reduced muscle mass [17]. Thus, β-GPA induces various adaptations in skeletal muscle, including mitochondrial biogenesis and muscle mass loss.
Considering these findings, resistance training with β-GPA may gain both training adaptations in skeletal muscle. However, the effects of β-GPA on resistance training adaptations are unclear. Therefore, this study sought to determine the effects of β-GPA on resistance training adaptations, especially muscle hypertrophy and mitochondrial biogenesis using electromyostimulation. We hypothesized that administration of exogenous β-GPA would increase mitochondrial biogenesis concomitant with the electromyostimulation-induced muscle hypertrophy. The summary of this article is presented in Figure 1.
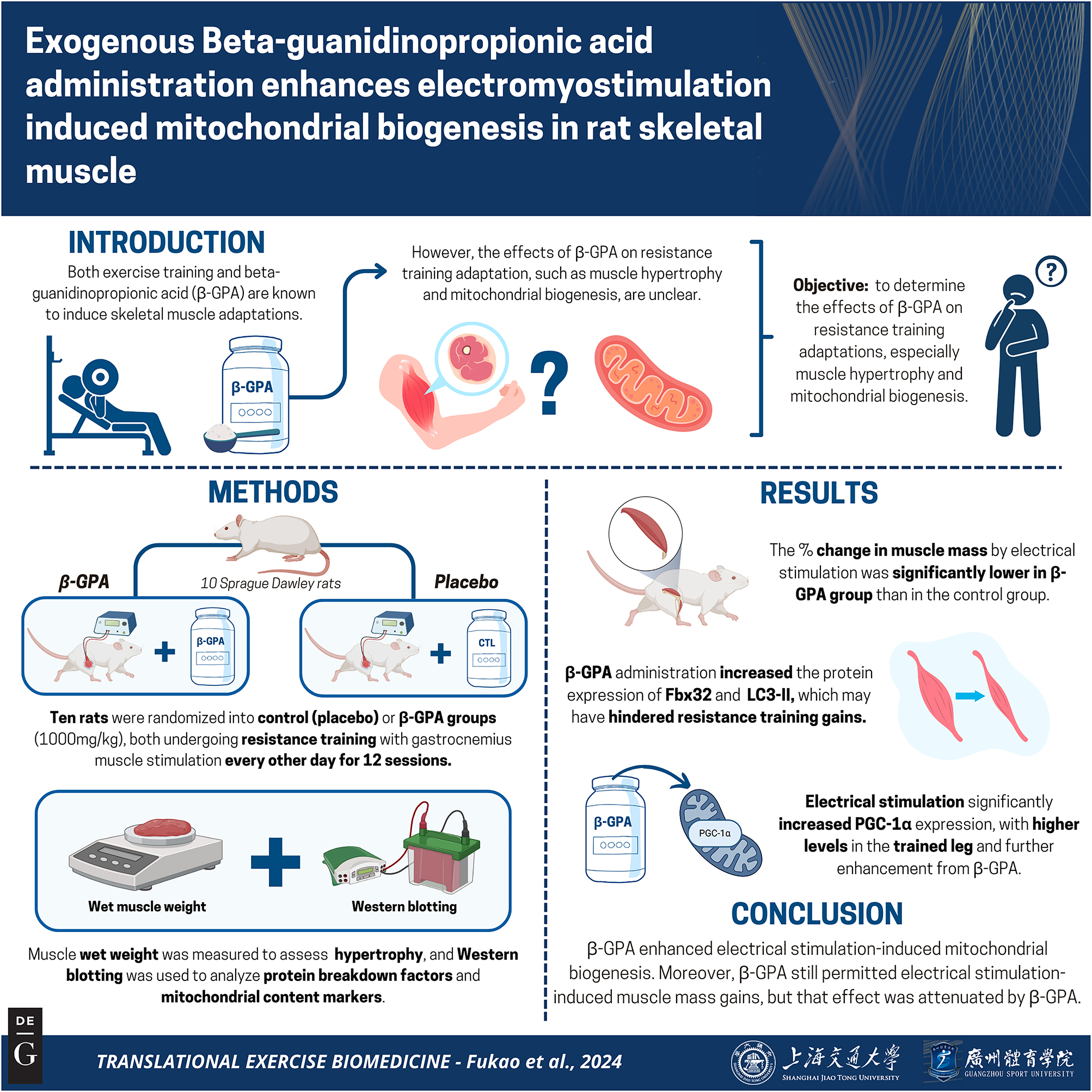
Graphical representation of the study. Key points: (1) The study found that combining β-GPA with electromyostimulation significantly increased mitochondrial biogenesis markers, such as PGC-1α and OXPHOS protein expression, in the trained muscle. (2) Despite promoting mitochondrial adaptations, β-GPA intake resulted in a reduction in muscle mass, as indicated by increased protein breakdown markers like Fbx32 and LC3-II. (3) While β-GPA reduced muscle mass overall, it did not completely counteract the muscle mass gains induced by electromyostimulation. Figure created with BioRender.
Methods
Animals and experimental design
This study was approved by Ethics Committee for Animal Experiments at Ritsumeikan University (approval number: BKC2022-009) and conducted following the Declaration of Helsinki. Ten seven-week-old male Sprague Dawley rats were obtained from Shimizu Laboratory Materials. All rats were maintained in an environment of 23 ± 1 °C with a 12-hourly light/dark cycle (light: 8:00–20:00, dark: 20:00–8:00) and provided water and food ad libitum. After a one-week acclimatization period, rats were randomly assigned to the Control or β-GPA groups. For one week before resistance exercise, rats in the β-GPA group received β-GPA (1,000 mg/kg, Combi-blocks, San Diego, CA, USA). From day 8, resistance exercise was performed on each rat’s right leg every two days for 12 sessions. During the training period, β-GPA or placebo was continued to be administered once daily. Forty-eight hours following the end of the last resistance exercise, the rats’ right and left gastrocnemius muscles were removed after a 12-h fasting. The muscles were stored at −80 °C until they were analyzed. An overview of the study is shown in Figure 2.
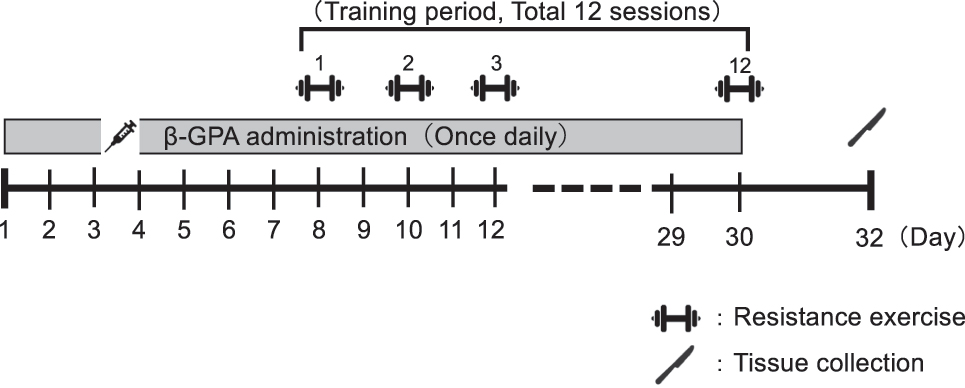
An overview of the study.
Administration of β-GPA
β-GPA (1,000 mg/kg) was dissolved in 1.5 mL distilled water, and forced oral administration was performed using a sonde. The administration was implemented after the resistance exercise. In the Control group, an equivalent volume of distilled water was administered.
Resistance exercise protocol
Resistance exercise by electromyostimulation was carried out as described in a previous study [18, 19]. In brief, under isoflurane inhalation anesthesia, the right leg was shaved, and exposed skin was cleaned with ethanol. The rats were then positioned supine, and the right leg was placed on a footplate and secured at a 90° ankle joint angle. The gastrocnemius muscle of the right leg was (the Trained leg) stimulated percutaneously by electrodes (Vitrode V, Ag/AgCl: Nihon Kohden, Tokyo, Japan) connected to an electrical stimulator (SEN-0823: Nihon Kohden, Tokyo, Japan) and an isolator (SS-104j; Nihon Kohden, Tokyo, Japan). The electrodes were put over the triceps surae muscle. The frequency during electrical stimulation was set at 100 Hz, with the voltage (−30 V) adjusted to exert maximum isometric contraction (pulse width: 4 ms, shape: square wave). Five sets of electrical stimulation were performed, each consisting of 10 cycles of 3 s electrical stimulation with a seven-second interval between each set. There was a three-minute rest between each set. Resistance exercise was performed once every two days for 12 sessions. The left gastrocnemius was treated as the Untrained leg. A previous study showed that 12 resistance exercise sessions using this protocol resulted in significant muscle hypertrophy in the gastrocnemius muscle [19].
Western blotting
Western blotting was conducted as previously described [18]. In short, frozen muscle samples were powdered and homogenized by RIPA buffer (Cell Signaling Technology, Danvers, MA, USA) with cOmplete Mini protease inhibitor cocktail (11836153001, Sigma-Aldrich, St. Louis, MO, USA) and PhosSTOP phosphatase inhibitor cocktail (4906845001, Sigma-Aldrich, St. Louis, MO, USA). After centrifugation of the homogenate (10,000 g, 10 min, 4 °C), the protein concentration of the collected supernatant was determined (Protein Assay Rapid Kit Wako II 295–78401, FUJIFILM Wako Pure Chemical, Osaka, Japan). Then, 3 × Blue Loading Buffer, DTT (Blue Loading Buffer Pack, #7722 Cell Signaling Technology, Danvers, MA, USA), supernatant, and distilled water were mixed, and the mixture was then boiled at 95 °C for 5 min. Only samples for OXPHOS were not boiled. The samples were stored at −80 °C until they were processed for western blotting. Each sample was separated by electrophoresis (150 V, 80 min) on a 10 % TGX gel (#1610173, Bio-Rad, Hercules, CA, USA) with 5 µg or 10 µg of protein and transferred (30 V, 90 min) onto PVDF membranes (#1620177, Bio-Rad, Hercules, CA, USA). The membrane was washed with Tris-Buffered saline containing 0.1 % Tween 20 (TBS-T) for 5 min and blocked by TBS-T containing 5 % skim milk for 60 min at room temperature. After blocking, the membrane was washed three times for 5 min in TBS-T and incubated overnight (4 °C) with primary antibody. The following antibodies were used: PGC-1α (#516–577, Millipore, CA, USA), Total OXPHOS Rodent WB Antibody Cocktail (#ab110413, Abcam, Cambridge, UK), total-p38 MAPK (#9212, Cell Signaling Technology, Danvers, MA, USA), phospho-p38 MAPK Thr180/Tyr182 (#9211, Cell Signaling Technology, Danvers, MA, USA), total-AMPKα (#2532, Cell Signaling Technology, Danvers, MA, USA), phospho-AMPKα Thr172 (#2531, Cell Signaling Technology, Danvers, MA, USA), total-CaMKII (#4436, Cell Signaling Technology, Danvers, MA, USA), total-CaMKII (#4436, Cell Signaling Technology, Danvers, MA, USA), phospho-CaMKII Thr286 (#12716, Cell Signaling Technology, Danvers, MA, USA), Calcineurin (#2614, Cell Signaling Technology, Danvers, MA, USA), DSCR1 (#sc-377507, Santa Cruz Biotechnology, Dallas, TX, USA), LC3B (#2775, Cell Signaling Technology, Danvers, MA, USA), Fbx32 (#168372 Abcam, Cambridge, UK), MuRF1 (#sc-398608 Santa Cruz Biotechnology, Dallas, TX, USA). The following day, the membrane was washed by TBS-T for 5 min x three, and the secondary antibody (Anti-rabbit IgG, HRP-linked Antibody, #7074, Cell Signaling Technology, Danvers, MA, USA) (Anti-mouse IgG, HRP-linked Antibody, #7076, Cell Signaling Technology Danvers, MA, USA) was added to TBS-T and incubated at room temperature for 1 h. The membrane was rewashed in TBS-T for 5 min x three, and the bands were detected using Luminata Forte Western HRP Substrate (WBLUF0500, Millipore, CA, USA) with FUSION Chemiluminescence Imaging System (M&S Instruments, Osaka, Japan). Band intensities were calculated by standardizing the quantified value of the amount of protein applied to each lane by Ponceau S staining using Image J software version 1.53 k (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All values were expressed as mean ± SE. IBM SPSS Statistics ver. 28 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Data were analyzed using a two-way analysis of variance (resistance training × β-GPA), and multiple comparisons by Bonferroni were performed only when a significant interaction was found. The % change in muscle mass was analyzed by unpaired t-test. The significance level was set at p<0.05.
Results
Skeletal muscle mass
Muscle wet weight was calculated to assess the impact of β-GPA on resistance training-induced muscle hypertrophy. The results showed resistance training increased muscle mass (training effect: p<0.01), but β-GPA decreased respectively (β-GPA effect: p<0.001, Figure 3). The % change in muscle mass by resistance training was significantly lower in the β-GPA group than in the Control group (Control: 9.92 ± 1.70 %, β-GPA: 3.92 ± 1.63 %, p<0.05).
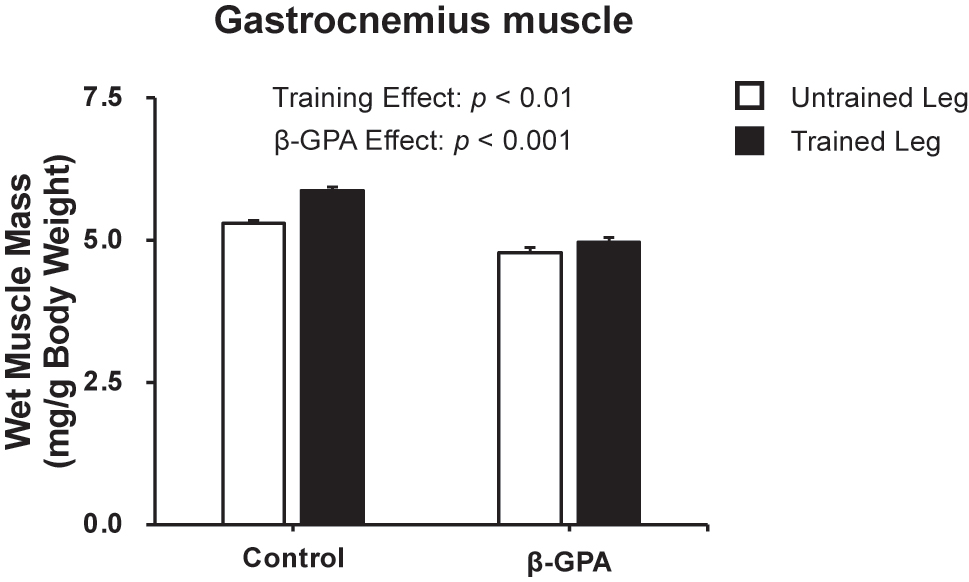
Gastrocnemius muscle wet weight after chronic resistance training and β-GPA ingestion in rat skeletal muscle (n=5 in each group). Data was expressed as mean ± SE.
Muscle protein breakdown-related factors
The protein expression levels of muscle proteolysis are shown in Figure 4. LC3B-I protein expression was increased by resistance training and β-GPA respectively (training effect: p<0.05, β-GPA effect: p<0.001, Figure 4A). A main effect of β-GPA was confirmed in the protein expression levels of LC3-II (β-GPA effect: p<0.05, Figure 4B), which is involved in autophagosome formation. However, any interventions did not change the LC3B-II/LC3B-I ratio (Figure 4C). A main effect of β-GPA was also detected in the protein expression levels of F-box protein 32 (Fbx32), a ubiquitin ligase (β-GPA effect: p<0.001, Figure 4D). Despite that, the protein expression level of MuRF1 was not changed by any interventions (Figure 4E).
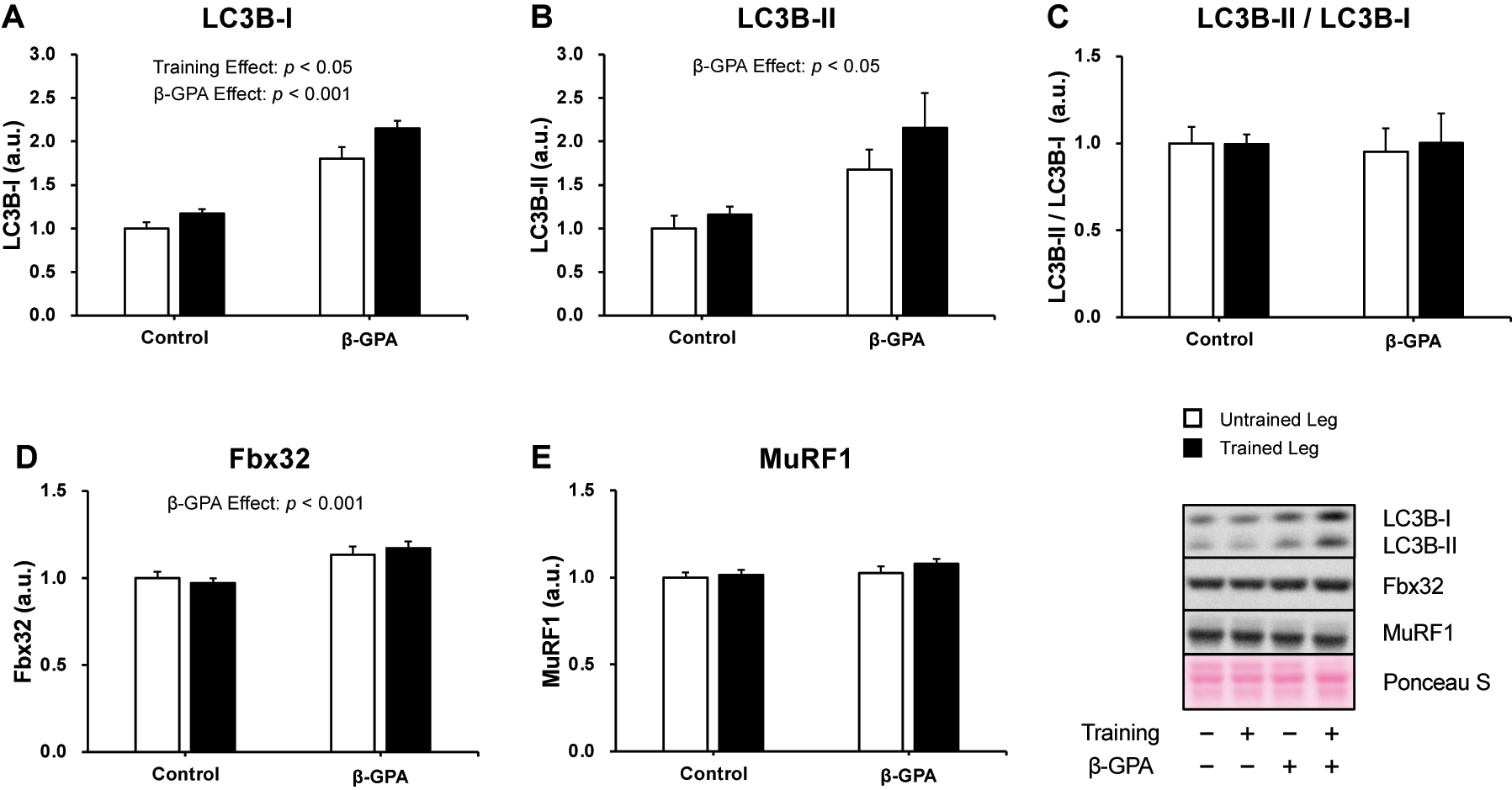
Muscle protein breakdown-related proteins were expressed after chronic resistance training and β-GPA ingestion in rat skeletal muscle (n=5 in each group). A: LC3B-I. B: LC3B-II. C: LC3B-II/LC3B-I. D: Fbx32. E: MuRF1. Data was expressed as mean ± SE.
Mitochondrial content markers
To estimate mitochondrial content, the protein expression levels of Complex I, II, III, IV, and V were evaluated (Figure 5). There is a significant interaction in Complex I protein expression levels (p<0.05, Partial η2 = 0.257, Figure 5A). Multiple comparisons showed that Complex I in the Trained leg was significantly higher than in the Untrained leg (p<0.05, Figure 5A). Protein expression of Complex I in the β-GPA + Trained leg was also significantly higher compared to the Trained leg and β-GPA leg (p<0.05, Figure 5A). In addition, a main effect of β-GPA was observed in the protein expression levels of Complex II (β-GPA effect: p<0.005, Figure 5B), and a trend was observed in Complex III and V (β-GPA effect: Complex III: p=0.059, β-GPA effect: Complex V: p=0.051, Figure 5C–E), respectively. Furthermore, a significant interaction between resistance training and β-GPA was observed for protein expression in Total OXPHOS (p<0.05, Partial η2 = 0.246, Figure 5F). Multiple comparisons showed that Total OXPHOS protein expression was significantly higher in the Trained leg than in the Untrained leg (p<0.05, Figure 5F). The protein expression level of Total OXPHOS was also significantly higher in the β-GPA + Trained leg than in the β-GPA + Untrained leg (p<0.05, Figure 5F). Furthermore, protein expression levels of Total OXPHOS in the β-GPA + Trained leg were significantly higher than in the Trained leg (p<0.05, Figure 5F).
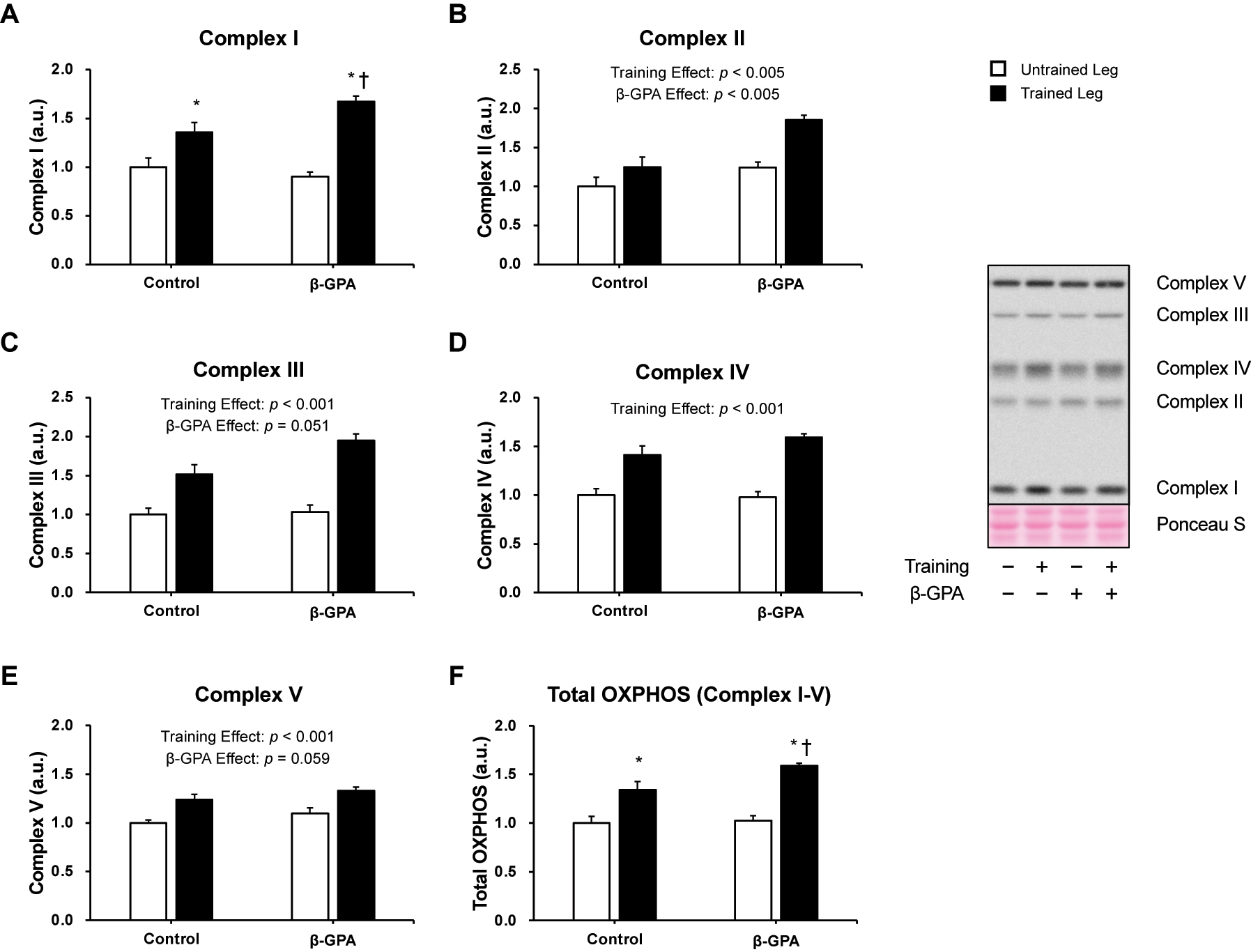
Markers of mitochondrial content protein expression in rat skeletal muscle (n=5 in each group). Data was expressed as mean ± SE. A: Complex I (NDUFB8). B: Complex II (SDHB). C: Complex III (UQCRC2). D: Complex IV (MTCO1). E: Complex V (ATP5A). F: Total OXPHOS (complexes I, II, III, IV, and V). *p<0.05 (vs. Untrained leg in the same group). †p<0.05 (vs. Trained leg in control group).
PGC-1α and upstream proteins
Figure 6 shows the protein levels of PGC-1α, a central regulator of mitochondrial biogenesis. The results showed a significant interaction between resistance training and β-GPA in PGC-1α protein expression (p<0.05, Partial η2 = 0.330, Figure 6). Multiple comparisons showed that the Trained leg had significantly higher protein expression of PGC-1α than the Untrained leg (p<0.05, Figure 6). β-GPA + Trained leg protein expression of PGC-1α was considerably higher than β-GPA leg and Trained leg (p<0.05, Figure 6). Protein expression and phosphorylation of factors regulating PGC-1α are shown in Figures 7 and 8. Protein expression of AMPKα was increased by (β-GPA effect: p<0.05, Figure 7A). A training effect was observed in the protein expression of MAPK (β-GPA effect: p<0.05 Figure 7C). On the other hand, AMPKα Thr172 phosphorylation was not changed by interventions (Figure 7B), and a main effect of β-GPA was observed for p38 MAPK phosphorylation (β-GPA effect: p<0.01, Figure 7D). Ca2+/calmodulin-dependent protein kinase II (CaMKII) and calcineurin protein expression levels were increased by resistance training (CaMKII: training effect: p<0.01, Calcineurin: training effect: p<0.05, Figure 8A–C). A trend by β-GPA (β-GPA effect: p=0.075) was observed for the phosphorylation of CaMKII (Figure 8B). A main effect of training and β-GPA was also observed for protein expression levels of Down Syndrome Critical Region 1 (DSCR1), an activation marker of calcineurin (training effect: p<0.05, β-GPA effect: p<0.05, Figure 8D).
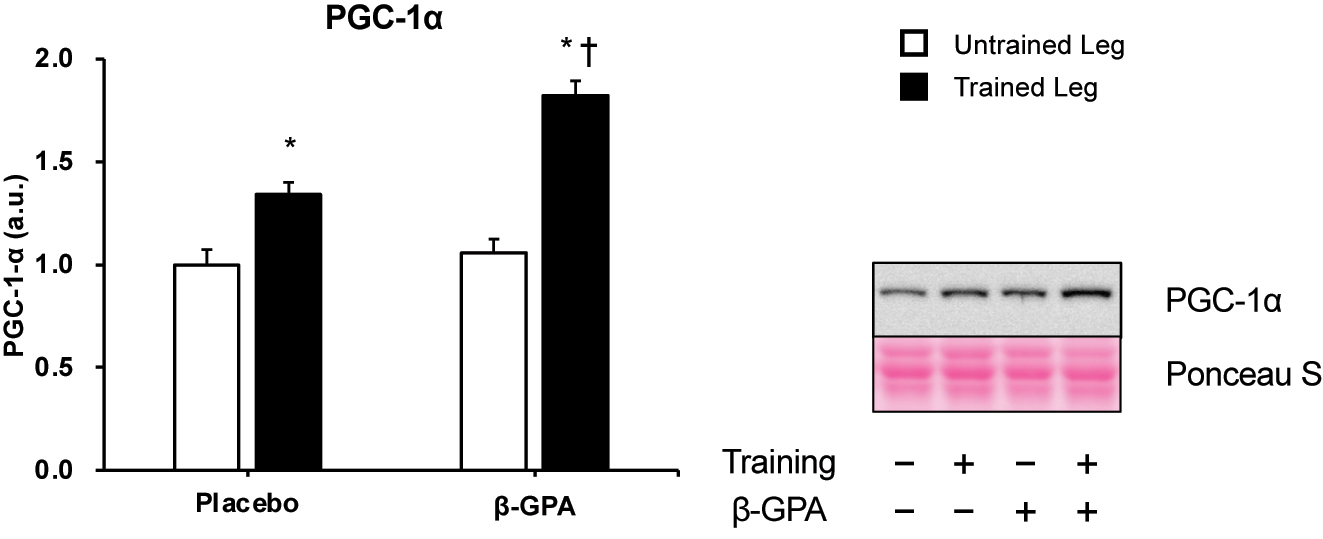
PGC-1α protein expression in rat skeletal muscle (n=5 in each group). Data was expressed as mean ± SE. *p<0.05 (vs. Untrained leg in the same group). †p<0.05 (vs. Trained leg in control group)
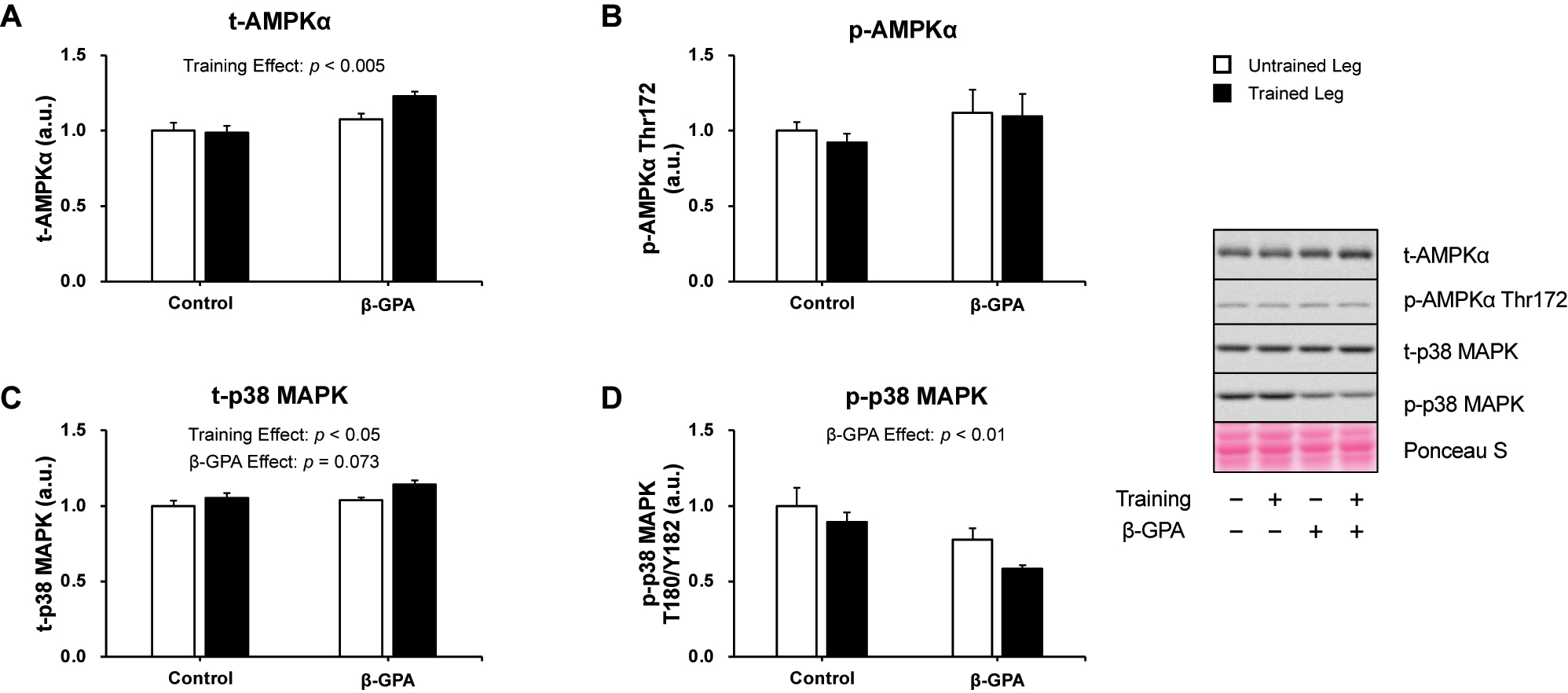
The expression of AMPK and p38 MAPK after chronic resistance training and β-GPA ingestion in rat skeletal muscle (n=5 in each group). A: AMPKα. B: phosphorylated AMPKα Thr172. C: p38 MAPK. D: phosphorylated p38 MAPK Thr180/Tyr182. Data was expressed as mean ± SE.
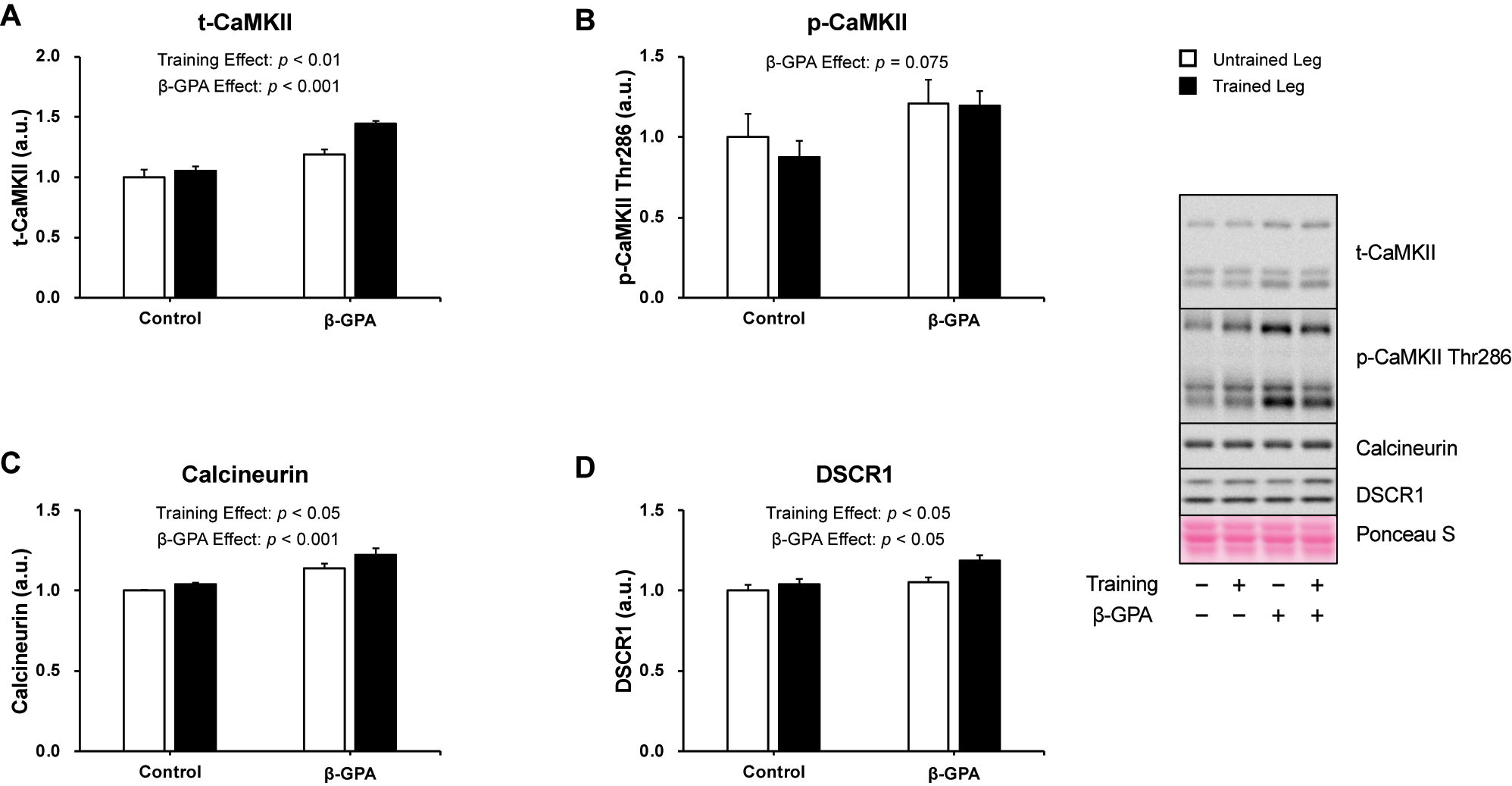
The expression of Ca2+-related proteins after chronic resistance training and β-GPA ingestion in rat skeletal muscle (n=5 in each group). A: CaMKII. B: phosphorylated CaMKII Thr286. C: Calcineurin. D: DSCR1. Data was expressed as mean ± SE.
Discussion
This study aimed to investigate the effects of β-GPA administration on resistance training-induced mitochondrial adaptation and muscle hypertrophic effects in rat skeletal muscle. The study’s main findings were [1]: β-GPA increased proteins involved in autophagy and the ubiquitin-proteasome. Moreover, muscle mass was reduced with β-GPA. Still, a significant main effect of resistance training indicated that the training response was intact [2]; β-GPA attenuated muscle mass gain through resistance training [3]; the integration of β-GPA with resistance training compared to resistance training alone resulted in higher mitochondrial protein content [4]; the integration of β-GPA with resistance training induced significantly higher increase in PGC-1α protein expression compared to resistance training alone [5]; the integration of β-GPA with resistance training additively increased protein expression of calcineurin and DSCR1. These findings suggest that β-GPA enhances mitochondrial biogenesis and still maintains the muscle mass gain through resistance training in rat skeletal muscle, but the training effect was significantly reduced compared to placebo.
Acute resistance exercise stimulates muscle protein synthesis, while long-term training leads to muscle hypertrophy [5, 20]. On the other hand, chronic β-GPA intake has been reported to activate muscle proteolysis and cause muscle atrophy [17, 21]. Similarly, in the results of the present study, muscle mass was increased by resistance training in both groups. The present study suggests for the first time that the combination of β-GPA and resistance training does not counteract the increase in muscle mass induced by resistance training in rat skeletal muscle, but the muscle hypertrophic response by resistance training was attenuated in the β-GPA group. It is believed that skeletal muscle mass is regulated by a net balance between muscle protein synthesis and degradation [22]. The autophagy-lysosomal and ubiquitin-proteasome systems are involved in muscle protein degradation [22]. It has been suggested that the enhancement of muscle proteolysis by β-GPA may be due to signaling pathways independent of AMPK [17]. Previous in vivo and in vitro studies reported that β-GPA promotes an increase in the density of autophagosomes and protein expression of Fbx32 [17, 23]. The current study showed that administration of β-GPA also increased the protein expression of Fbx32 and LC3-II. These findings suggest that β-GPA administration may have reduced skeletal muscle mass and attenuated muscle mass gain by resistance training due to the activation of the autophagy-lysosome system and ubiquitin-proteasome system in skeletal muscle. Chronic resistance training in mice does not increase Fbx32 mRNA or LC3-II protein expression [24]. Similarly, in the present study, resistance training did not enhance the protein expression levels of LC3-II and Fbx32. Our results indicated that the administration of β-GPA during the resistance training period did not induce protein degradation beyond the effect of β-GPA alone. The present study only evaluated marker proteins involved in muscle protein degradation, not those involved in muscle protein synthesis. The resistance exercise model used in the present research activates the mammalian target of rapamycin complex 1 (mTORC1) signaling involved in muscle protein synthesis immediately after acute resistance exercise until 24 h [25]. Since we collected muscle tissue 48 h after the final exercise session, it is likely that any transient activation of mTORC1 signaling as well as muscle protein synthesis would have already returned to baseline. Consequently, we did not include these markers in our current analysis. However, in the future, the effects of β-GPA administration on the muscle protein synthesis in response to acute resistance exercise should also be explored to offer a comprehensive assessment of its impact on muscle protein metabolism.
PGC-1α is a master regulator of mitochondrial biogenesis, and the activation of mitochondrial biogenesis increases mitochondrial content [26, 27]. The protein expression level of OXPHOS is also widely used to indicate mitochondrial content [24, 28, 29]. In the present study, β-GPA augmented the protein expression of Total OXPHOS and PGC-1α induced by resistance training. This suggests that β-GPA augments resistance training-induced mitochondrial biogenesis, resulting in a more significant increase in mitochondrial content. The expression of PGC-1α is regulated by signaling proteins such as AMPK, p38 MAPK, CaMKII, and calcineurin [27]. Acute resistance exercise phosphorylates these proteins except for calcineurin [18, 30], and training increases the protein expression of PGC-1α [20]. The creatine analog β-GPA also decreases intracellular creatine and creatine phosphate by inhibiting creatine transport into the muscle cell [15]. β-GPA has been reported to phosphorylate AMPK [15, 23] and subsequently promote mitochondrial biogenesis [15]. In contrast, another study found that two weeks of β-GPA intake did not promote AMPK phosphorylation [31]; therefore, it is unclear whether β-GPA activates AMPK in skeletal muscle. Our result supported the report by Nichenko [31]. In our experiment, oral administration of β-GPA was also not performed 48 h before tissue collection, which may have already eliminated β-GPA-induced AMPK activation. p38 MAPK is considered one of the upstream proteins of PGC-1α [32]. A previous study reported β-GPA caused p38 MAPK phosphorylation in vivo [15]. However, we did not observe p38 MAPK phosphorylation in the β-GPA group. This result suggested β-GPA may not affect p38 MAPK phosphorylation in rat skeletal muscle. Further research will be required to confirm the impacts of β-GPA on p38 MAPK and whether β-GPA induced-p38 MAPK activation is necessary for mitochondrial biogenesis using a transgenic model. In summary, in this study, AMPK and p38 MAPK may not be engaged with enhanced PGC-1α protein expression by resistance training and β-GPA.
Calcineurin and CaMKII are activated by increased intracellular Ca2+ concentrations [33, 34]. Intracellular Ca2+ concentrations are thought to play an essential role in mitochondrial biogenesis, regulating the expression level and activation of PGC-1α [27, 33]. Protein expression levels of calcineurin and DSCR1 used as an indicator of calcineurin activity [34], were enhanced by resistance training and β-GPA in the present study. These results suggested that β-GPA intake, in combination with resistance training, additively enhanced calcineurin activation. Skeletal muscle contraction may activate calcineurin because it transiently increases intracellular Ca2+ concentrations in the skeletal muscle [35]. In a previous study, DSCR1 mRNA levels were increased by an eight-week resistance training in young men [36]. β-GPA also increased protein expression levels of calcineurin and DSCR1. Furthermore, β-GPA enhanced CaMKII protein expression, and phosphorylation of CaMKII showed an increasing trend by β-GPA. Therefore, we suggest that the activation of resistance training-induced mitochondrial biogenesis by β-GPA was partially due to the activation of Ca2+-related signaling in this study. To our knowledge, no study reports the relationship between β-GPA and intracellular Ca2+. Although Gallo et al. implied that β-GPA might affect intracellular Ca2+ concentration because β-GPA led to fiber type transition from fast type to slow type [37, 38], the specific mechanism of how β-GPA activates calcineurin and CaMKII was not investigated in this study, and further investigations are required to address these questions.
Limitations
A limitation of this study is that mitochondrial content was assessed solely on the expression levels of the mitochondrial proteins Complex I through Complex V. The gold standard for evaluating mitochondrial content is electron microscopy [39]. Because mitochondrial content and function in skeletal muscle are altered by resistance training and β-GPA [23, 40], accurate assessments of mitochondrial content and function can provide essential insights into quantitative and qualitative changes of mitochondria.
Conclusions
In conclusion, administration of β-GPA stimulated the increase in PGC-1α induced by resistance training and activated mitochondrial biogenesis in rat skeletal muscle. Moreover, β-GPA still permitted resistance training-induced muscle mass gains, but that gain was attenuated compared to untreated animals, supporting the role of β-GPA in promoting proteolysis.
Funding source: Japan Society for the Promotion of Science
Award Identifier / Grant number: JP21KK0177
-
Research ethics: This study was approved by the Ethics Committee for Animal Experiments at Ritsumeikan University (BKC2022-009) and all experiments were performed according to the Declaration of Helsinki (revised in 2013).
-
Informed consent: Not applicable.
-
Author contributions: NF, JT, SF designed this study. NF, KO, RT conducted the study. NF performed the analysis. NF and SF wrote the manuscript. All authors approved the final version. The authors have accepted responsibility for the content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors states no conflict of interest.
-
Research funding: This research was supported by JSPS KAKENHI Grant Number JP21KK0177 to SF.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Frontera, WR, Ochala, J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int 2015;96:183–95. https://doi.org/10.1007/s00223-014-9915-y.Suche in Google Scholar PubMed
2. Wall, BT, Dirks, ML, Snijders, T, Senden, JM, Dolmans, J, van Loon, LJ. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol 2014;210:600–11. https://doi.org/10.1111/apha.12190.Suche in Google Scholar PubMed
3. Kenny, HC, Rudwill, F, Breen, L, Salanova, M, Blottner, D, Heise, T, et al.. Bed rest and resistive vibration exercise unveil novel links between skeletal muscle mitochondrial function and insulin resistance. Diabetologia 2017;60:1491–501. https://doi.org/10.1007/s00125-017-4298-z.Suche in Google Scholar PubMed
4. Bilet, L, Phielix, E, van de Weijer, T, Gemmink, A, Bosma, M, Moonen-Kornips, E, et al.. One-leg inactivity induces a reduction in mitochondrial oxidative capacity, intramyocellular lipid accumulation and reduced insulin signalling upon lipid infusion: a human study with unilateral limb suspension. Diabetologia 2020;63:1211–22. https://doi.org/10.1007/s00125-020-05128-1.Suche in Google Scholar PubMed PubMed Central
5. Mero, AA, Hulmi, JJ, Salmijarvi, H, Katajavuori, M, Haverinen, M, Holviala, J, et al.. Resistance training induced increase in muscle fiber size in young and older men. Eur J Appl Physiol 2013;113:641–50. https://doi.org/10.1007/s00421-012-2466-x.Suche in Google Scholar PubMed
6. Holloszy, JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 1967;242:2278–82. https://doi.org/10.1016/s0021-9258(18)96046-1.Suche in Google Scholar
7. Baldwin, KM, Klinkerfuss, GH, Terjung, RL, Molé, PA, Holloszy, JO. Respiratory capacity of white, red, and intermediate muscle: adaptative response to exercise. Am J Physiol 1972;222:373–8. https://doi.org/10.1152/ajplegacy.1972.222.2.373.Suche in Google Scholar PubMed
8. Wilkinson, SB, Phillips, SM, Atherton, PJ, Patel, R, Yarasheski, KE, Tarnopolsky, MA, et al.. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 2008;586:3701–17. https://doi.org/10.1113/jphysiol.2008.153916.Suche in Google Scholar PubMed PubMed Central
9. Porter, C, Reidy, PT, Bhattarai, N, Sidossis, LS, Rasmussen, BB. Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc 2015;47:1922–31. https://doi.org/10.1249/mss.0000000000000605.Suche in Google Scholar
10. Groennebaek, T, Jespersen, NR, Jakobsgaard, JE, Sieljacks, P, Wang, J, Rindom, E, et al.. Skeletal muscle mitochondrial protein synthesis and respiration increase with low-load blood flow restricted as well as high-load resistance training. Front Physiol 2018;9:1796. https://doi.org/10.3389/fphys.2018.01796.Suche in Google Scholar PubMed PubMed Central
11. Paddon-Jones, D, Børsheim, E, Wolfe, RR. Potential ergogenic effects of arginine and creatine supplementation. J Nutr 2004;134:2888S–94S. discussion 95S. https://doi.org/10.1093/jn/134.10.2888s.Suche in Google Scholar PubMed
12. Ponticos, M, Lu, QL, Morgan, JE, Hardie, DG, Partridge, TA, Carling, D. Dual regulation of the AMP-activated protein kinase provides a novel mechanism for the control of creatine kinase in skeletal muscle. EMBO J 1998;17:1688–99. https://doi.org/10.1093/emboj/17.6.1688.Suche in Google Scholar PubMed PubMed Central
13. Zong, H, Ren, JM, Young, LH, Pypaert, M, Mu, J, Birnbaum, MJ, et al.. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A 2002;99:15983–7. https://doi.org/10.1073/pnas.252625599.Suche in Google Scholar PubMed PubMed Central
14. Stockebrand, M, Sasani, A, Das, D, Hornig, S, Hermans-Borgmeyer, I, Lake, HA, et al.. A mouse model of creatine transporter deficiency reveals impaired motor function and muscle energy metabolism. Front Physiol 2018;9:773. https://doi.org/10.3389/fphys.2018.00773.Suche in Google Scholar PubMed PubMed Central
15. Williams, DB, Sutherland, LN, Bomhof, MR, Basaraba, SA, Thrush, AB, Dyck, DJ, et al.. Muscle-specific differences in the response of mitochondrial proteins to beta-GPA feeding: an evaluation of potential mechanisms. Am J Physiol Endocrinol Metab 2009;296:E1400–8. https://doi.org/10.1152/ajpendo.90913.2008.Suche in Google Scholar PubMed
16. Wyss, M, Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol Rev 2000;80:1107–213. https://doi.org/10.1152/physrev.2000.80.3.1107.Suche in Google Scholar PubMed
17. Baumgarner, BL, Nagle, AM, Quinn, MR, Farmer, AE, Kinsey, ST. Dietary supplementation of beta-guanidinopropionic acid (betaGPA) reduces whole-body and skeletal muscle growth in young CD-1 mice. Mol Cell Biochem 2015;403:277–85. https://doi.org/10.1007/s11010-015-2357-7.Suche in Google Scholar PubMed PubMed Central
18. Takegaki, J, Sase, K, Fujita, S. Repeated bouts of resistance exercise attenuate mitogen-activated protein-kinase signal responses in rat skeletal muscle. Biochem Biophys Res Commun 2019;520:73–8. https://doi.org/10.1016/j.bbrc.2019.09.050.Suche in Google Scholar PubMed
19. Ogasawara, R, Kobayashi, K, Tsutaki, A, Lee, K, Abe, T, Fujita, S, et al.. mTOR signaling response to resistance exercise is altered by chronic resistance training and detraining in skeletal muscle. J Appl Physiol 2013;114:934–40. https://doi.org/10.1152/japplphysiol.01161.2012.Suche in Google Scholar PubMed
20. Kitaoka, Y, Nakazato, K, Ogasawara, R. Combined effects of resistance training and calorie restriction on mitochondrial fusion and fission proteins in rat skeletal muscle. J Appl Physiol 2016;121:806–10. https://doi.org/10.1152/japplphysiol.00465.2016.Suche in Google Scholar PubMed
21. Ross, TT, Overton, JD, Houmard, KF, Kinsey, ST. beta-GPA treatment leads to elevated basal metabolic rate and enhanced hypoxic exercise tolerance in mice. Phys Rep 2017;5. https://doi.org/10.14814/phy2.13192.Suche in Google Scholar PubMed PubMed Central
22. Stokes, T, Hector, AJ, Morton, RW, McGlory, C, Phillips, SM. Recent perspectives regarding the role of dietary protein for the promotion of muscle hypertrophy with resistance exercise training. Nutrients 2018;10. https://doi.org/10.3390/nu10020180.Suche in Google Scholar PubMed PubMed Central
23. Crocker, CL, Baumgarner, BL, Kinsey, ST. β-guanidinopropionic acid and metformin differentially impact autophagy, mitochondria and cellular morphology in developing C2C12 muscle cells. J Muscle Res Cell Motil 2020;41:221–37. https://doi.org/10.1007/s10974-019-09568-0.Suche in Google Scholar PubMed PubMed Central
24. Takegaki, J, Ogasawara, R, Kotani, T, Tamura, Y, Takagi, R, Nakazato, K, et al.. Influence of shortened recovery between resistance exercise sessions on muscle-hypertrophic effect in rat skeletal muscle. Phys Rep 2019;7:e14155. https://doi.org/10.14814/phy2.14155.Suche in Google Scholar PubMed PubMed Central
25. Ogasawara, R, Fujita, S, Hornberger, TA, Kitaoka, Y, Makanae, Y, Nakazato, K, et al.. The role of mTOR signalling in the regulation of skeletal muscle mass in a rodent model of resistance exercise. Sci Rep 2016;6:31142. https://doi.org/10.1038/srep31142.Suche in Google Scholar PubMed PubMed Central
26. Hood, DA, Tryon, LD, Carter, HN, Kim, Y, Chen, CC. Unravelling the mechanisms regulating muscle mitochondrial biogenesis. Biochem J 2016;473:2295–314. https://doi.org/10.1042/bcj20160009.Suche in Google Scholar PubMed
27. Jornayvaz, FR, Shulman, GI. Regulation of mitochondrial biogenesis. Essays Biochem 2010;47:69–84. https://doi.org/10.1042/bse0470069.Suche in Google Scholar PubMed PubMed Central
28. Marshall, RN, Smeuninx, B, Seabright, AP, Morgan, PT, Atherton, PJ, Philp, A, et al.. No effect of five days of bed rest or short-term resistance exercise prehabilitation on markers of skeletal muscle mitochondrial content and dynamics in older adults. Phys Rep 2022;10:e15345. https://doi.org/10.14814/phy2.15345.Suche in Google Scholar PubMed PubMed Central
29. Mesquita, PHC, Lamb, DA, Parry, HA, Moore, JH, Smith, MA, Vann, CG, et al.. Acute and chronic effects of resistance training on skeletal muscle markers of mitochondrial remodeling in older adults. Phys Rep 2020;8:e14526. https://doi.org/10.14814/phy2.14526.Suche in Google Scholar PubMed PubMed Central
30. Kido, K, Ato, S, Yokokawa, T, Makanae, Y, Sato, K, Fujita, S. Acute resistance exercise-induced IGF1 expression and subsequent GLUT4 translocation. Phys Rep 2016;4. https://doi.org/10.14814/phy2.12907.Suche in Google Scholar PubMed PubMed Central
31. Nichenko, AS, Southern, WM, Atuan, M, Luan, J, Peissig, KB, Foltz, SJ, et al.. Mitochondrial maintenance via autophagy contributes to functional skeletal muscle regeneration and remodeling. Am J Physiol Cell Physiol 2016;311:C190–200. https://doi.org/10.1152/ajpcell.00066.2016.Suche in Google Scholar PubMed
32. Akimoto, T, Pohnert, SC, Li, P, Zhang, M, Gumbs, C, Rosenberg, PB, et al.. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem 2005;280:19587–93. https://doi.org/10.1074/jbc.m408862200.Suche in Google Scholar
33. Ojuka, EO, Jones, TE, Han, DH, Chen, M, Holloszy, JO. Raising Ca2+ in L6 myotubes mimics effects of exercise on mitochondrial biogenesis in muscle. Faseb J 2003;17:675–81. https://doi.org/10.1096/fj.02-0951com.Suche in Google Scholar PubMed
34. Yang, J, Rothermel, B, Vega, RB, Frey, N, McKinsey, TA, Olson, EN, et al.. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res 2000;87:E61–8. https://doi.org/10.1161/01.res.87.12.e61.Suche in Google Scholar PubMed
35. Kuo, IY, Ehrlich, BE. Signaling in muscle contraction. Cold Spring Harbor Perspect Biol 2015;7:a006023. https://doi.org/10.1101/cshperspect.a006023.Suche in Google Scholar PubMed PubMed Central
36. Lamas, L, Aoki, MS, Ugrinowitsch, C, Campos, GE, Regazzini, M, Moriscot, AS, et al.. Expression of genes related to muscle plasticity after strength and power training regimens. Scand J Med Sci Sports 2010;20:216–25. https://doi.org/10.1111/j.1600-0838.2009.00905.x.Suche in Google Scholar PubMed
37. Gallo, M, MacLean, I, Tyreman, N, Martins, KJ, Syrotuik, D, Gordon, T, et al.. Adaptive responses to creatine loading and exercise in fast-twitch rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol 2008;294:R1319–28. https://doi.org/10.1152/ajpregu.00631.2007.Suche in Google Scholar PubMed
38. Ecochard, L, Roussel, D, Sempore, B, Favier, R. Stimulation of HSP72 expression following ATP depletion and short-term exercise training in fast-twitch muscle. Acta Physiol Scand 2004;180:71–8. https://doi.org/10.1046/j.0001-6772.2003.01184.x.Suche in Google Scholar PubMed
39. Larsen, S, Nielsen, J, Hansen, CN, Nielsen, LB, Wibrand, F, Stride, N, et al.. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 2012;590:3349–60. https://doi.org/10.1113/jphysiol.2012.230185.Suche in Google Scholar PubMed PubMed Central
40. MacDougall, JD, Sale, DG, Elder, GC, Sutton, JR. Muscle ultrastructural characteristics of elite powerlifters and bodybuilders. Eur J Appl Physiol Occup Physiol 1982;48:117–26. https://doi.org/10.1007/bf00421171.Suche in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter on behalf of Shangai Jiao Tong University and Guangzhou Sport University
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Issue 3: Skeletal muscle, exercise, aging and chronic disease
- Section: Integrated exercise physiology, biology, and pathophysiology in health and disease
- Impact of exercise and fasting on mitochondrial regulators in human muscle
- Effectiveness of aerobic exercise interventions on balance, gait, functional mobility and quality of life in Parkinson’s disease: an umbrella review
- Creatine and strength training in older adults: an update
- Creatine supplementation strategies aimed at acutely increasing and maintaining skeletal muscle total creatine content in healthy, young volunteers
- Section: Physical activity/inactivity and health across the lifespan
- Independent mobility and physical activity among children residing in an ultra-dense metropolis
- Physical activity and cardiometabolic risk factors in sprint and jump-trained masters athletes, young athletes and non-physically active men
- Cross-sectional analysis of blood leukocyte responsiveness to interleukin-10 and interleukin-6 across age and physical activity level
- Section: Exercise and E-health, M-health, AI and technology
- Assessing core body temperature in a cool marathon using two pill ingestion strategies
- Issue 4: Preclinical and clinical approaches to translational exercise biomedicine
- Section: Integrated exercise physiology, biology, and pathophysiology in health and disease
- Nicotinic acid improves mitochondrial function and associated transcriptional pathways in older inactive males
- Exogenous Beta-guanidinopropionic acid administration enhances electromyostimulation-induced mitochondrial biogenesis in rat skeletal muscle
- How exercise shapes the anti-inflammatory environment in multiple sclerosis – a conceptual framework focusing on tryptophan-derived molecules in T cell differentiation
- Section: Personalized and advanced exercise prescription for health and chronic diseases
- Acute effects of high-intensity interval training on microvascular circulation: a case control study in uveal melanoma
- Discrepancies in walking speed measurements post-bed-rest: a comparative analysis of real-world vs. laboratory assessments
- Section: Sports medicine and movement science
- Lower-body strength, power and sprint front crawl performance
- Section: Letter to the editor
- Comment on: “A unique pseudo-eligibility analysis of longitudinal laboratory performance data from a transgender female competitive cyclist”
- Author’s response to “letter to the editor comment on: ‘A unique pseudo-eligibility analysis of longitudinal laboratory performance Data from a transgender female competitive cyclist’” by Lundberg, O’Connor, Kirk, Pollock, and Brown
Artikel in diesem Heft
- Frontmatter
- Issue 3: Skeletal muscle, exercise, aging and chronic disease
- Section: Integrated exercise physiology, biology, and pathophysiology in health and disease
- Impact of exercise and fasting on mitochondrial regulators in human muscle
- Effectiveness of aerobic exercise interventions on balance, gait, functional mobility and quality of life in Parkinson’s disease: an umbrella review
- Creatine and strength training in older adults: an update
- Creatine supplementation strategies aimed at acutely increasing and maintaining skeletal muscle total creatine content in healthy, young volunteers
- Section: Physical activity/inactivity and health across the lifespan
- Independent mobility and physical activity among children residing in an ultra-dense metropolis
- Physical activity and cardiometabolic risk factors in sprint and jump-trained masters athletes, young athletes and non-physically active men
- Cross-sectional analysis of blood leukocyte responsiveness to interleukin-10 and interleukin-6 across age and physical activity level
- Section: Exercise and E-health, M-health, AI and technology
- Assessing core body temperature in a cool marathon using two pill ingestion strategies
- Issue 4: Preclinical and clinical approaches to translational exercise biomedicine
- Section: Integrated exercise physiology, biology, and pathophysiology in health and disease
- Nicotinic acid improves mitochondrial function and associated transcriptional pathways in older inactive males
- Exogenous Beta-guanidinopropionic acid administration enhances electromyostimulation-induced mitochondrial biogenesis in rat skeletal muscle
- How exercise shapes the anti-inflammatory environment in multiple sclerosis – a conceptual framework focusing on tryptophan-derived molecules in T cell differentiation
- Section: Personalized and advanced exercise prescription for health and chronic diseases
- Acute effects of high-intensity interval training on microvascular circulation: a case control study in uveal melanoma
- Discrepancies in walking speed measurements post-bed-rest: a comparative analysis of real-world vs. laboratory assessments
- Section: Sports medicine and movement science
- Lower-body strength, power and sprint front crawl performance
- Section: Letter to the editor
- Comment on: “A unique pseudo-eligibility analysis of longitudinal laboratory performance data from a transgender female competitive cyclist”
- Author’s response to “letter to the editor comment on: ‘A unique pseudo-eligibility analysis of longitudinal laboratory performance Data from a transgender female competitive cyclist’” by Lundberg, O’Connor, Kirk, Pollock, and Brown


