Pressurized IntraThoracic Aerosol Chemotherapy (PITAC) directed therapy of patients with malignant pleural effusion and pleural metastasis
-
Pernille Schjødt Hansen
, Martin Graversen
, Sönke Detlefsen
, Alan Patrick Ainsworth
und Michael Bau Mortensen
Abstract
Objectives
Pressurized IntraThoracic Aerosol Chemotherapy (PITAC) has been suggested as a new therapy for patients with malignant pleural effusion (MPE) and/or pleural metastasis (PLM). The patients have a poor prognosis with a median survival of 3 to 12 months. We present feasibility, patient safety, and cytological/histological response assessment in PITAC-treated patients with MPE and/or PLM.
Methods
Patients eligible for PITAC and treated at Odense PIPAC Center were included. PITAC was performed in lateral decubitus or prone position under double-lumen endotracheal tube ventilation to allow exclusion of the lung if necessary. After positioning of the ultrasound-guided trocar, the second trocar is inserted by video-assisted thoracoscopy. MPE was evacuated and measured. Pleural lavage was performed if no or small amounts of MPE were present. MPE or pleural lavage fluid was analyzed cytologically. Visible PLM was biopsied and sent for histology assessment using a four-tiered Thoracic Regression Grading Score (TRGS). After a walkthrough of the safety checklist, the chemotherapy was nebulized followed by 30 min of passive diffusion. The chemotherapy and chemotherapy-saturated air was evacuated through a closed bag and ventilation system.
Results
We report data on 11 intended PITACs in five patients. Nine PITACs were completed and two PITACs were discontinued due to intraoperative complications or technical reasons. Response evaluation was available in three patients: one showed complete response (TRGS 1) and another stable disease (TRGS 2). Cytology was available from two patients: one showed conversion from malignant to benign. The 30-day mortality was zero.
Conclusions
PITAC appears to be safe and feasible.
Introduction
Malignant pleural effusion (MPE) is caused by malignant cells altering the equilibrium in parietal and visceral pleura resulting in fluid build-up [1]. Depending on the primary malignancy, median survival ranges from 3 to 12 months indicating advanced disease [2, 3]. Treatment options including talcum pleurodesis, indwelling pleural catheters, and serial ultrasound-guided pleurocentesis are all palliative and focus on relief of symptoms [4, 5].
Fifteen percent of Danish cancer patients suffer from MPE, and MPE accounts for 20 % of the total number of pleural effusions registered in Denmark 5], [6], [7. Pleural metastasis (PLM) develops due to the spread of malignant cells through the bloodstream, lymphatic vessels, shedding, or by direct invasion [8]. PLM is often associated with MPE and is the most frequently recurring metastatic malignancy involving the pleural membranes [9, 10]. Furthermore, PLM is also the second most frequent cause of pleural effusion in adults [8].
Pressurized IntraThoracic Aerosol Chemotherapy (PITAC) was first introduced in 2012 in Herne, Germany. During standard thoracoscopy, antineoplastic agents are nebulized under pressure into the pleural cavity through a CE-certified nebulizer [11, 12]. The PITAC procedure was conceptualized in parallel with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC), which is now used globally in patients with peritoneal metastasis (PM). Preclinical PIPAC studies have shown an increased local drug bioavailability compared to lavage-based treatments [13].
In a recent review, five retrospective case series on PITAC-directed therapy in patients with MPE and/or PLM were identified [14]. It concluded that study populations were heterogeneous with missing data on indication, procedure, patient and personnel health safety, postoperative complications, response assessment, and follow-up.
This study investigates feasibility, safety, and cytological–histological response in patients with MPE and/or PLM treated with PITAC.
Methods
This is a retrospective analysis of consecutive patients with PLM and/or MPE treated with PITAC at Odense PIPAC Center (OPC) from February 2018 to September 2021. Patients with an Eastern Cooperative Oncology Group (ECOG) performance status ≤2 were included by case-by-case assessment and discussed at the multidisciplinary PIPAC conference. They were informed regarding the experimental nature of PITAC including its potential effects, complications, and adverse events. Additionally, patients were informed about the off-label use of standard chemotherapeutic agents used for PIPAC at OPC since 2015.
Procedure
With the patient in either the lateral decubitus or prone position, the procedure was performed in general anesthesia using a double lumen endotracheal tube allowing exclusion of the ipsilateral lung if necessary (Figure 1).
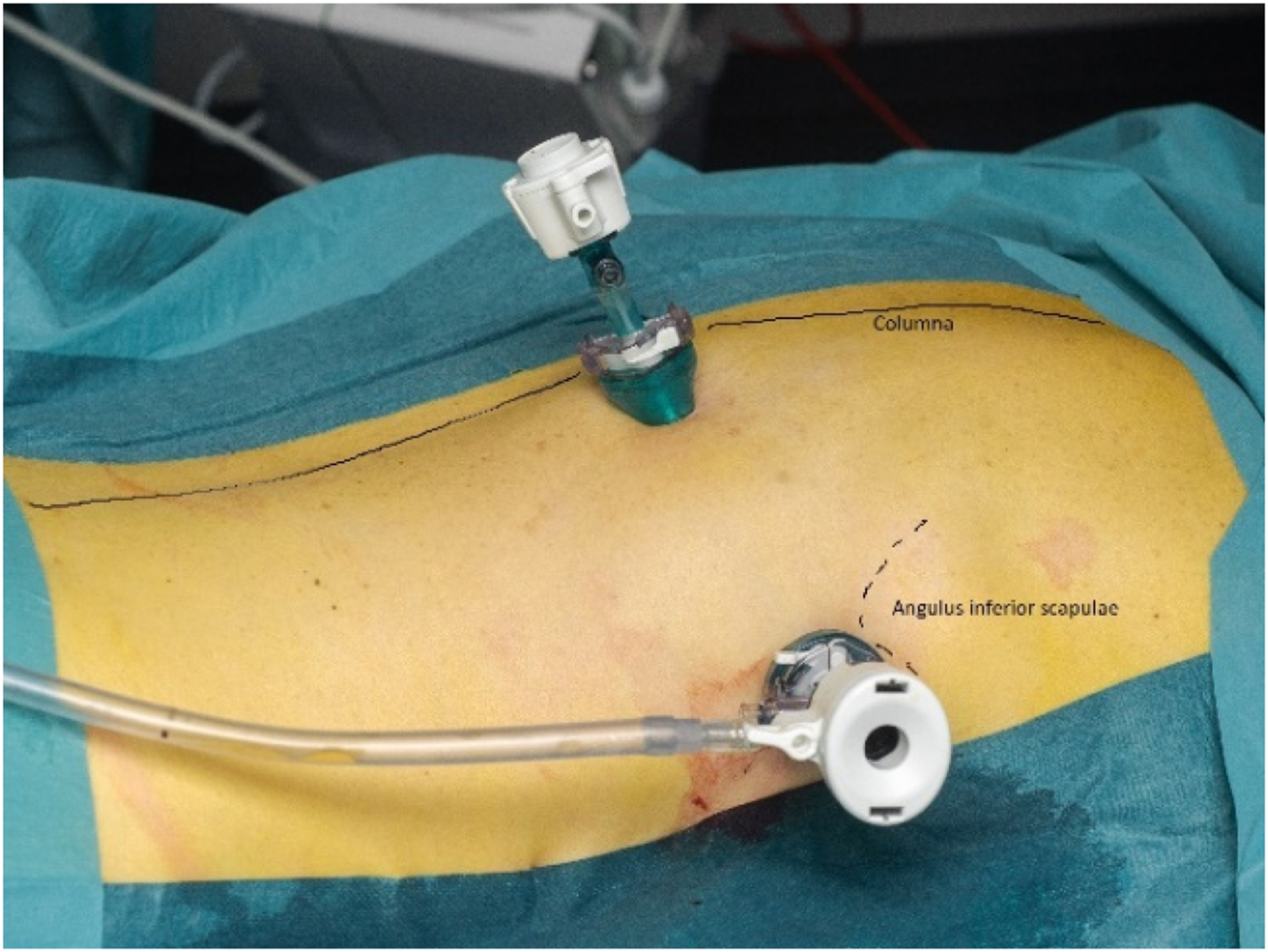
Pressurized IntraThoracic Aerosol Chemotherapy in prone position.
A 12 mm balloon trocar placed in the posterior axillary line just inferior to the inferior angle of scapula, and a 5 mm balloon trocar placed near the spine.
Pleural access was obtained ultrasound-guided along the anterior axillary line (AAL), posterior axillary line (PAL), or midaxillary line (MAL). Intrathoracic pressure was maintained throughout the procedure with normothermic CO2 at 12 mmHg.
After access to the pleural cavity, MPE was evacuated. The pleural cavity was flushed with saline (pleural lavage fluid, PLF) if less than 150 mL of MPE was present. MPE or PLF was analyzed cytologically. If present, parietal PLMs were biopsied and marked by clips, and a CE-certified nebulizer was inserted through the 12 mm trocar (Figures 2 and 3).
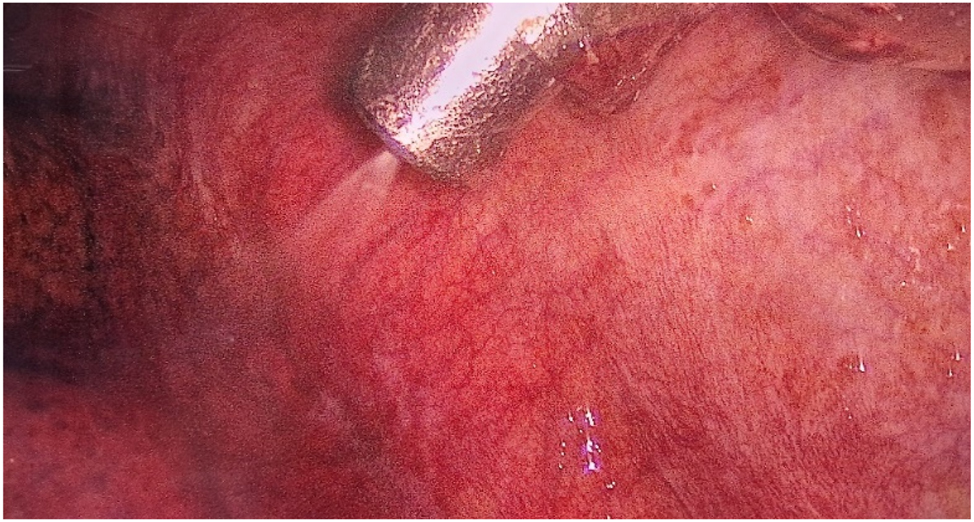
View of the left pleural cavity during nebulization of chemotherapy.
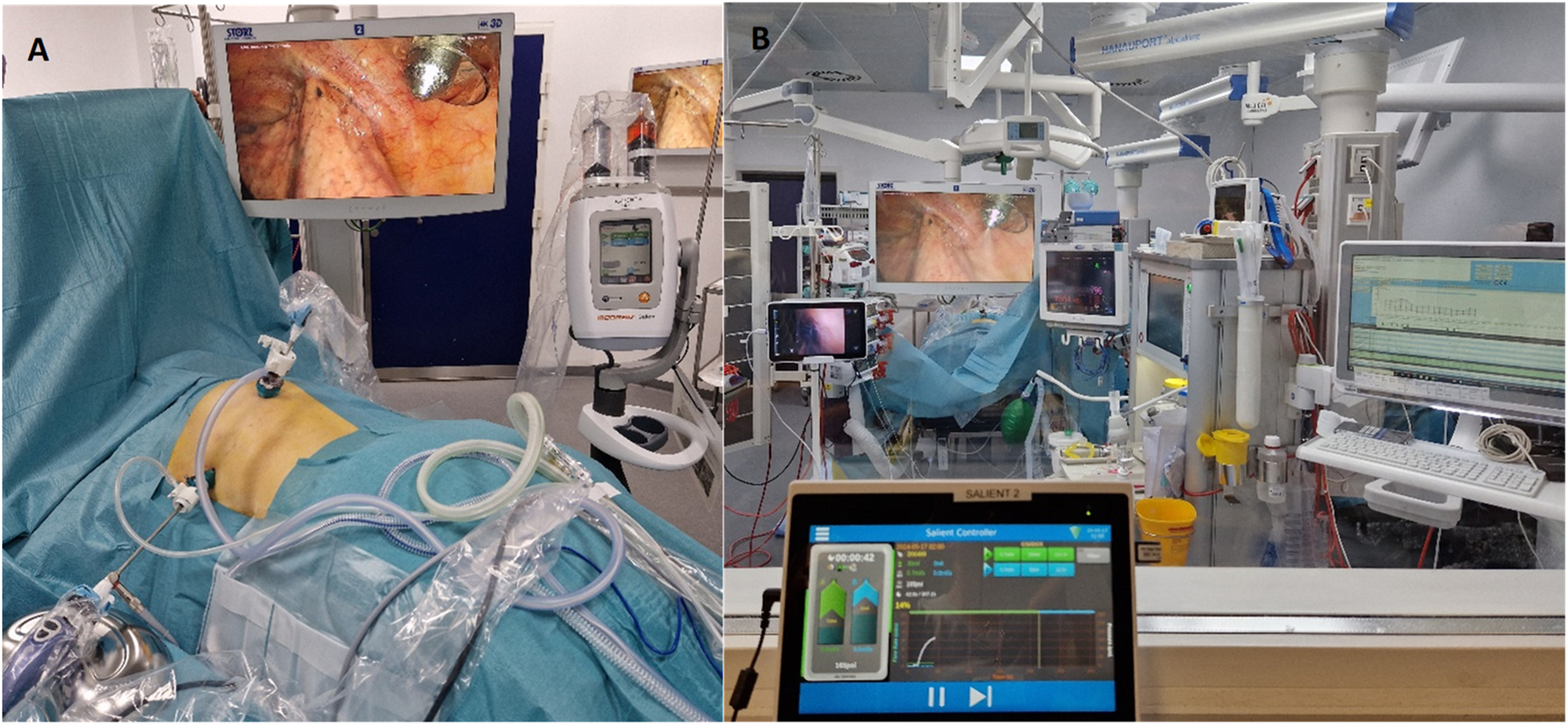
The arrangement during Pressurized IntraThoracic Aerosol Chemotherapy (PITAC) from inside and outside the operating room. (A) The arrangement from inside the operating room with the patient in prone position. Two trocars: 12 mm balloon trocar just next to the spine and a 5 mm trocar below the left scapulae. The left pleural cavity visible with the CE-certified nebulizer in the 12 mm balloon trocar. Microinjector loaded with cisplatin and doxorubicin connected to the CE-certified nebulizer covered by plastic bag. (B) The arrangement from outside the operating room. The following monitors are visual from the left: video-assisted double-lumen endotracheal tube with a view of the tracheal bifurcation, left pleural cavity with the active CE-certified nebulizer, and three monitors related to the anesthesiology. In the front, the remote-control tablet for the microinjector with increasing pressure during nebulization.
A safety checklist, adapted from the OPC standard PIPAC checklist (see Supplemental Material, Appendix 1), was completed before operating room (OR) staff left the OR [15]. The nebulization process was controlled from outside the room with a view of the patient and observation monitors (Figure 3). PITAC was performed using a standard pressure of maximum 300 Psi and a flow rate of 0.5–0.7 mL/s. After 30 min of passive diffusion, the chemotherapy and chemotherapy-contaminated carbon dioxide was evacuated through a closed bag and ventilation system. No chest tubes were inserted. The lung was reventilated under video-guidance when relevant, and the trocar sites closed according to standard thoracoscopy guidelines. The patients received a single prophylactic dose of cefuroxime (3 g) and metronidazole (1.5 g).
Based on chemotherapy applied during PIPAC, patients with colorectal (incl. appendix) cancer received oxaliplatin 92 mg/m2. Remaining patients received a combination of cisplatin 7.5 mg/m2 and doxorubicin 1.5 mg/m2 [16].
Histology and cytology
MPE cytology, PLF cytology, and pleural biopsies were assessed by the same pathologist (SD) who had experience with evaluation of the Peritoneal Regression Grading Score (PRGS) used in PIPAC-directed therapy [17, 18].
Three step sections were cut from the paraffin embedded tissue blocks and stained with hematoxylin–eosin (H&E), followed by a section immunostained for epithelial cell adhesion molecule (Ep-CAM) or, in case of malignant mesothelioma, Hector Battifora and MEsothelioma 1 (HBME1) [19]. A final series of three step sections were stained with H&E. In analogy with the PRGS, a four-tiered Thoracic Regression Grading Score (TRGS) was assessed: TRGS one equaled complete response, TRGS two equaled major response, TRGS three equaled minor response, and TRGS four equaled no response. If more than one pleural biopsy was obtained, the mean TRGS was also reported.
MPE and/or PLF were analyzed as described previously [20]. A minimum of 150 mL of MPE and/or PLF was centrifuged, and smears of the sediment were analyzed by conventional cytology (Papanicolaou and May-Giemsa Grünwald staining). Leftovers of the sediment were embedded in paraffin wax of which one section was stained with H&E. If necessary, further sections were analyzed with immunocytochemical markers such as calretinin, cluster of differentiation (CD) 56, carcinoembryonic antigen (CEA), cytokeratin (CK) 7, CK20, Ep-CAM, HBME-1, protein ΔNp63 (P40), synaptophysin, or thyroid transcription factor 1 (TTF1), as part of the diagnostic routine. The cytological specimens were diagnosed using a five-tiered system: malignant cells, cells suspicious of malignancy, atypical cells, no malignant cells, or other (including specimens unsuitable for diagnosis for technical reasons).
Assessment of complications
Patients were evaluated by PITAC surgeons before discharge. A hotline phone number was provided at discharge, and the patients were contacted after 2 weeks.
Surgery-related complications were graded according to the Clavien–Dindo Classification (grade I–V) [21], and PITAC-related adverse events according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [22].
Results
Procedures
Five patients were found eligible for PITAC-directed therapy (Figure 4). Indications for PITAC were MPE (n=2), PLM (n=1), or synchronous MPE and PLM (n=2) from malignant mesothelioma or metastasis from carcinoma of different origin (rectum, breast, ovary, or stomach) (Table 1).
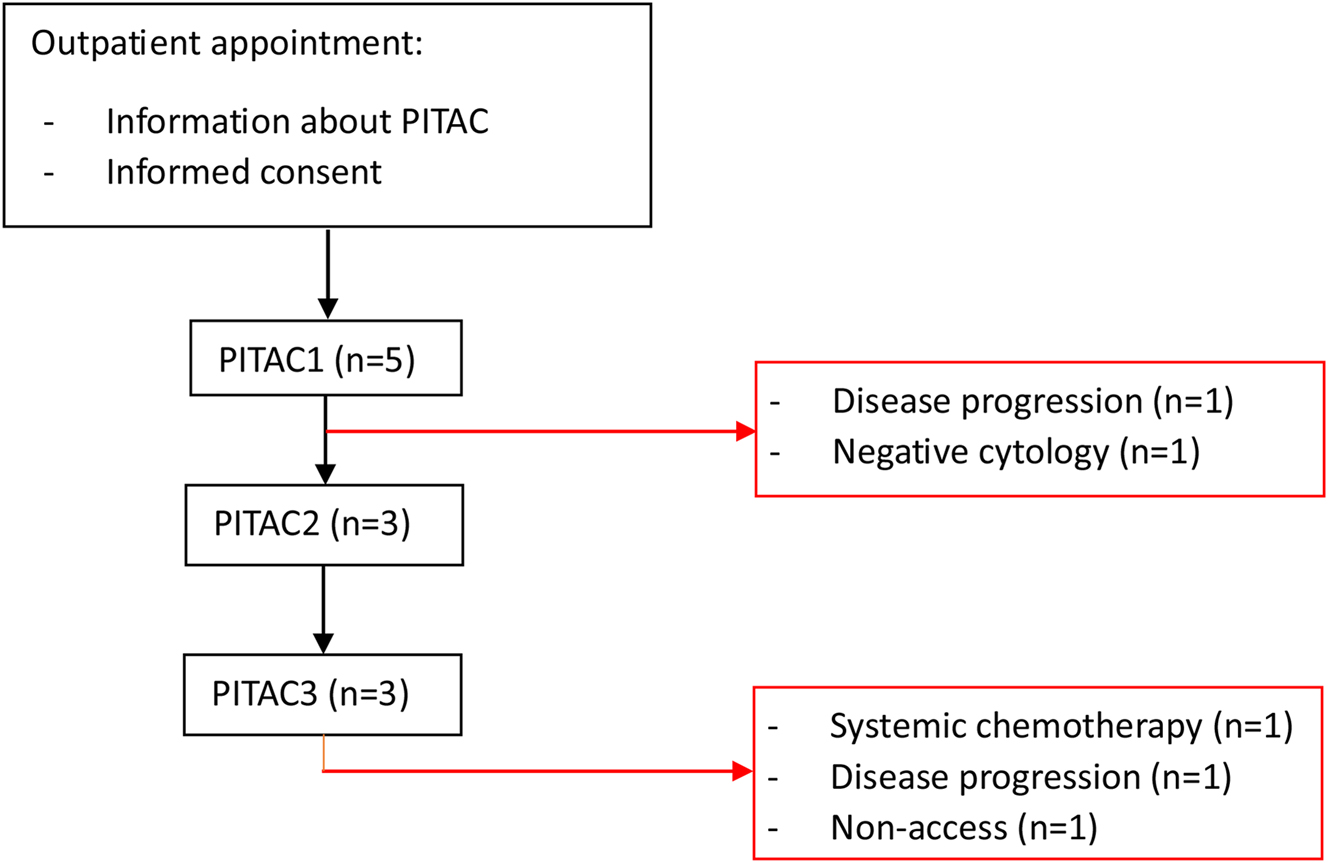
Patient flowchart.
Patient characteristics.
| Patient (primary) | 1 (Rectum) | 2 (Breast) | 3 (Ovary) | 4 (Peritoneum) | 5 (Stomach) |
|---|---|---|---|---|---|
| Gender, F/M | M | F | F | M | M |
| Age, years | 53 | 56 | 67 | 61 | 64 |
| ECOG performance status | 0 | 1 | 0 | 0 | 2 |
| Histology | Adenocarcinoma | HER2+ ductal carcinoma | High-grade serous adenocarcinoma | Malignant epithelioid mesothelioma | Poorly cohesive (signet ring cell) carcinoma |
| Other metastatic sites | No | No | No | No | No |
| Prior treatment | Systemic chemotherapy Lung resection SBRT |
Systemic chemotherapy Left-sided mastectomy Immunotherapy Herceptin treatment |
Systemic chemotherapy HIPEC Immunotherapy |
Systemic chemotherapy | Gastrectomy Systemic chemotherapy |
| Bidirectional treatment | Systemic chemotherapy | Immunotherapy | None | None | None |
| Indication for PITAC | Left-sided PLM | Bilateral MPE and PLM | Left-sided MPEa | Right-sided MPE and PLM | Left-sided MPE |
| Days between MPE/PLM diagnosis and PITAC1 | 843 | 108 | 873 | N/A | 40 |
-
aIndication was malignant pleural effusion (MPE), but both MPE and pleural metastasis (PLM) were found at PITAC1. ECOG, Eastern Cooperative Oncology Group; HER2+, human epidermal growth factor receptor two positive; SBRT, stereotactic body radiotherapy; HIPEC, heated intraperitoneal chemotherapy; N/A, not available.
Patients 1–3 received two or more PITACs and patient 4 received one PITAC (Table 2). For patient 5, two PITACs were planned but terminated due to an intraoperative complication during PITAC1 and later disease progression.
Procedures and outcome.
| Patient (primary) | 1 (Rectum) | 2 (Breast) | 3 (Ovary) | 4 (Peritoneum) | 5 (Stomach) |
|---|---|---|---|---|---|
| Positioning | Prone | PITAC1+2: Lateral PITAC3: Prone |
Lateral | Prone | Prone |
| Balloon trocar positioning | N/A | PAL IC7 and caudal AAL | MAL IC5-6 and caudal PAL/MAL IC-7-8 | PAL below the scapula and just right to the spine | PAL below the scapula and just right to the spine |
| No. of PITACs | 3 | 3 | 3 | 1 | 1 |
| MPE volume, mL | PITAC1: 50 PITAC2: 50 PITAC3: Non-access |
PITAC1: 1,250 PITAC2: 1,250 PITAC3: 1,100 |
PITAC1 (lhs): 30 PITAC2 (lhs): 0 PITAC3 (rhs): 700 |
PITAC1: 400 | PITAC1: N/A |
| Pleural lavage | PITAC1: Yes PITAC2: Yes PITAC3: Non-access |
PITAC1: No PITAC2: No PITAC3: No |
PITAC1 (lhs): Yes PITAC2 (lhs): Yes PITAC3 (rhs): No |
PITAC1: No | PITAC1: No |
| Cytology | PITAC1: Malignant cells PITAC2: Not suited for diagnosis PITAC3: NA |
PITAC1: Malignant cells PITAC2: No malignant cells PITAC3: No malignant cells |
PITAC1 (lhs): Malignant cells PITAC2 (lhs): Malignant cells PITAC3 (rhs): Malignant cells |
PITAC1: Atypical cells | PITAC1: NA |
| Histology, TRGSa | PITAC1: 3.0 (2 biopsies) PITAC2: 1 (1 biopsy) PITAC3: NA |
PITAC 1: NA PITAC 2: NA PITAC 3: NA |
PITAC1 (lhs): NA PITAC2 (lhs): 2 (1 biopsy) PITAC3 (rhs): 2 (1 biopsy) |
PITAC1: 1 (1 biopsy) | PITAC1: NA |
| Concurrent surgeries | No | PIPAC with all PITACs | One PIPAC at PITAC3 | One PIPAC at PITAC1 | No |
| Intraoperative complications (Clavien-Dindo) | Intercostal arterial bleed during biopsy (IIIb) | None | None | None | Lung lesion during access |
| Postoperative complications (CTCAE grade) | PITAC1: Chest pain (2) PITAC2: Vomiting (2), chest pain (2) PITAC3: None |
PITAC1: Chest pain (2) and urinary retention (1) PITAC2: Chest pain (2), subcutaneous emphysema (1), and urinary retention (1) PITAC3: Chest pain (2) |
None | PITAC1: Chest pain (2) and urinary retention (2) | PITAC1: Subcutaneous emphysema (1) |
| Length of stay, days | PITAC1: N/A PITAC2: 1 PITAC3: 0 |
PITAC1: 1 PITAC2: 1 PITAC3: 1 |
PITAC1 (lhs): 1 PITAC2 (lhs): 0 PITAC3 (rhs): 0 |
PITAC1: 1 | PITAC1: 1 |
-
aIn case of several biopsies taken prior to the same PITAC, mean Thoracic Regression Grading Score (TRGS) is given. bControl X-ray due to insufficient reventilation of the right lung due to PLM., AAL, anterior axillary line; CTCAE, Common Terminology Criteria for Adverse Events; lhs, left-hand side; MAL, midaxillary line; NA, not available; PAL, posterior axillary line; rhs, right-hand side.
Response assessment
Cytology
Pleural cytology was available in patients 1–4 (Table 2). For patient 1, malignant cells were found in the specimen taken prior to PITAC1, while the specimen from PITAC2 was inconclusive for technical reasons. Patient 2 underwent three PITACs, and malignant cells were detected in the specimen obtained prior to PITAC1 but no longer detectable prior to PITAC2 and -3. For patient 3, malignant cells were found in three consecutive specimens taken prior to PITAC1, -2, and -3. No comparison was possible for patient 4 since only one conclusive cytology specimen was available, showing atypical cells, why no additional PITACs were performed.
Histology
Biopsies were available from patient 1, 3, and 4 (Table 2). In patient 1, the mean baseline TRGS, prior to PITAC1, was 3.0 (Figure 5a–d). Prior to PITAC2, the biopsy showed complete regression (TRGS 1) (Figure 5e and f). Patient 3 had no biopsies taken at baseline, while biopsies taken prior to PITAC2 (left pleural cavity) and prior to PITAC3 (right pleural cavity) showed TRGS 2. Patient 4 showed TRGS one at baseline, prior to PITAC1, while no consecutive biopsies were available.
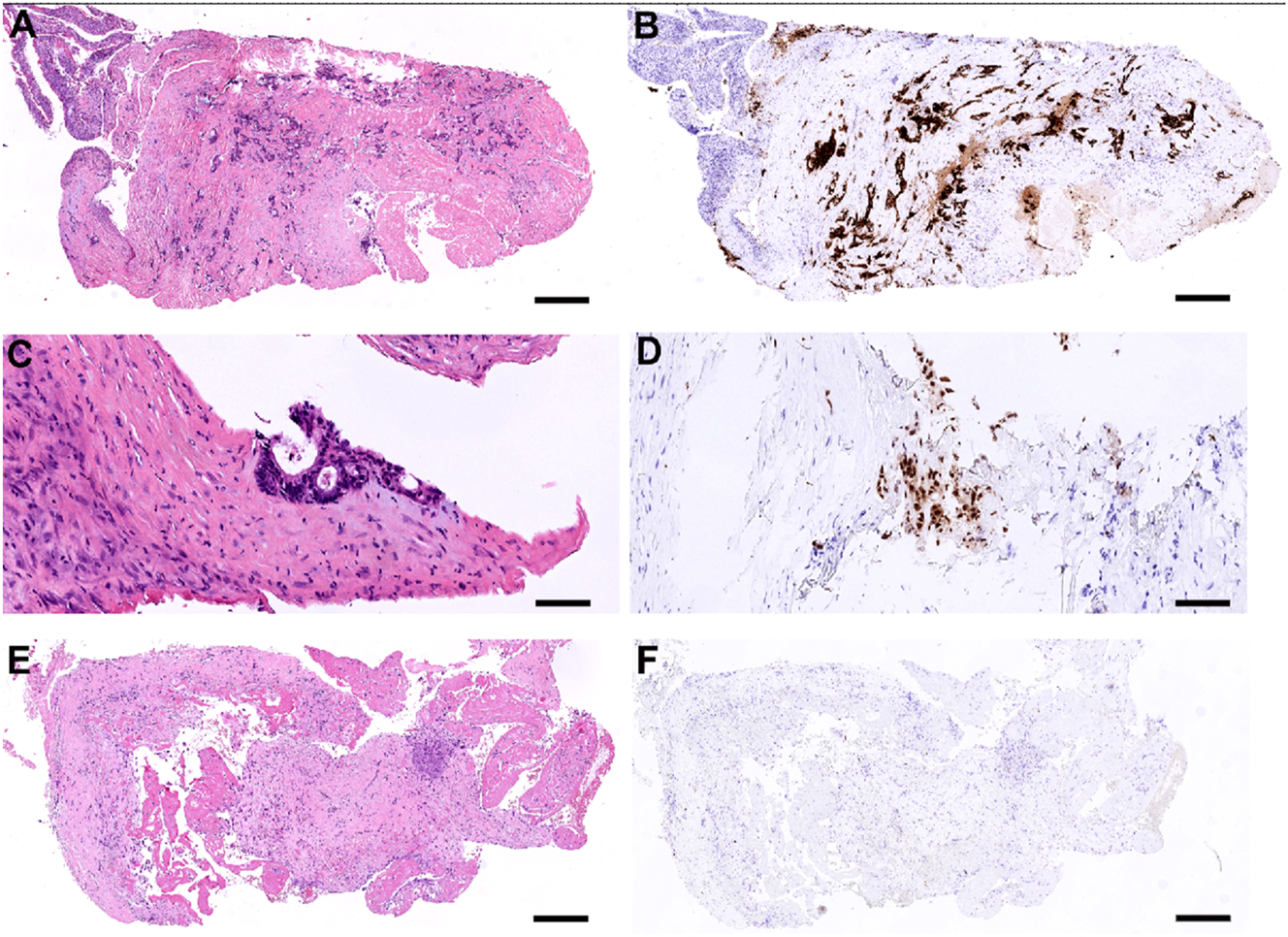
Histological features of pleural metastasis from rectal cancer before and after PITAC (patient 1). (A and B) Biopsy from the upper left-sided pleura at baseline, showing metastatic rectal adenocarcinoma, TRGS 4 (a, H&E. B, CDX2-immunostaining). Scale bar: 500 μm. (C and D) Higher magnification of biopsy from the lower left-sided pleura at baseline, showing a small focus of metastatic rectal adenocarcinoma, TRGS 2 (C, H&E. D, CDX2-immunostaining). Scale bar: 100 μm. (E and F) Biopsy from the left pleura taken prior to PITAC2, showing complete response (TRGS 1). Regressive fibrosis with mesothelial proliferation and fibrin is shown. No metastatic tumor cells are found at this location (E, H&E. F, CDX2-immunostaining). Scale bar: 350 μm.
Intra- and postoperative complications
Patient 5 scheduled for PITAC did not receive PITAC because of a lung lesion during access (uneventful postoperative recovery). Patient 1 had an intraoperative hemorrhage caused by the pleural biopsy at PITAC2, but this did not influence treatment. No intraoperative events that may have posed a risk to the occupational health safety were registered.
Mild to moderate (CTCAE≤2) medical AEs were reported by three patients (Table 2). Patient 1 had vomiting and chest pain after PITAC1 and -2. Patient 2, who received simultaneous PIPAC-directed therapy, had chest pain and urinary retention after PITAC1 and -2. The same patient had right-sided self-limiting subcutaneous emphysema. Patient 4 had mild chest pain and urinary retention after PITAC1.
No severe or life-threatening (CTCAE>2) postoperative medical AEs were reported. All patients were either discharged the same day or on the first postoperative day.
Follow-up
Based on CT scans, patient 1 showed stable disease for 11 months. Patient 2 had CT-verified disease progression within 6 months. Patient 3 showed no recurring MPE on the left-hand side during a lung ultrasound 4 weeks after PITAC2. Six weeks after PITAC2, a CT-scan showed small amounts of MPE bilaterally. Patient 4 had no evidence of malignant disease after PITAC1.
Discussion
In this retrospective analysis of patients with MPE and/or PLM treated with PITAC from 2018 to 2021, five patients were included on a case-by-case assessment, resulting in 11 intended PITAC treatments of which nine were completed. The included patients were heterogeneous in characteristics, including primary tumors and performance status. The use of PITAC has previously been reported in patients with malignant mesothelioma, ovarian, and gastric cancer [12, 23, 24]. To our knowledge, this is the first report of pleural metastasis from rectal and breast cancer treated with PITAC.
Two intraoperative complications were registered: one lung lesion during port insertion in a patient with left-sided MPE from gastric cancer and one intercostal arterial bleed during a biopsy in a patient with left-sided MPE from rectal cancer. In the first patient, it was assessed that it would not be safe to proceed with PITAC, but in the second case, PITAC was administered after hemostasis. Previous studies on PITAC have not reported intraoperative complications [12, 23], [24], [25], [26.
We identified two cases of CTCAE≤2. One study found no CTCAE>2 related to PITAC; however, CTCAE<2 was published as a mixture of PIPAC and PITAC data [24]. Another study found prolonged air-leakage in a patient with simultaneous lung wedge resections. Other possible complications such as toxic reactions or wound heling problems could not be determined, as data were a mixture of PIPAC and PITAC [25]. Thus, results on intra- and postoperative complications are lacking.
The most useful histological response data available from this study was from patient 1 with PLM from rectal adenocarcinoma. The patient was planned to receive three PITACs – two were completed and the third was with primary non-access. The biopsies showed complete regression from mean TRGS 3.0 prior to PITAC1 to TRGS 1 prior to PITAC2, indicating a local histological response. From patient 3 (PLM from ovarian cancer), pleural biopsies taken prior to PITAC2 and -3 both showed TRGS 2 (major histological response). Pre- and post-treatment biopsies for patients 2, 4, and 5 were unfortunately not available.
The use of the TRGS, in analogy to the PRGS in PM, is supported by recent data showing that the PRGS holds significant prognostic value in patients treated with PIPAC-directed therapy [16]. The present data suggest that PITAC may induce histological regression in PLM based on the TRGS, but at present, no other data are available. Further prospective data are needed to establish the utility of TRGS in the setting of PITAC further, and particularly its potential clinical impact.
Cytology for response evaluation of PITAC was not used previously, according to the published literature [12, 23], [24], [25], [26. This retrospective study found conversion in one patient with right-sided MPE from HER2+ ductal breast cancer. Before PITAC1, malignant cells were detectable in the MPE. At PITAC2 and -3, no malignant cells were detectable indicating that PITAC might have a positive effect on MPE in breast cancer patients. However, as breast cancer has never before been treated with PITAC and only one patient was included in this study, further studies are needed to support these results.
There are no data comparing PITAC to other available treatment options in patients with MPE such as talcum pleurodesis, indwelling pleural catheters, and repeated ultrasound-guided pleurocentesis. PITAC is performed under general anesthesia demanding that patients should be in good performance and this might limit some patients from PITAC compared to the conventional treatment options for MPE. Still, one very important feature with PITAC is the use of chemotherapy. The current treatment options focus on symptomatic relief only by removing the MPE and/or gluing the pleura together with talcum, but by applying chemotherapy to the pleural cavity, local treatment of malignant cells and/or visible PLM is also provided.
Whether PITAC is more or less safe and effective than the current treatment options cannot be determined based on the present study. However, it is interesting to note that PITAC-directed therapy seems technically feasible and may induce local response when evaluated in the same way as patients having PIPAC [12, 23], [24], [25], [26.
The advancement of PITAC since 2012 has been slow-paced, as merely 21 published patients have received a total of 38 PITACs [12, 23], [24], [25], [26. Five retrospective case series (six including the present) classify PITAC to phase-I of the IDEAL framework [27]. In May 2023, the first prospective phase-I study was approved at Odense PIPAC Center, Denmark, and patients are currently being recruited. The primary goal is to evaluate feasibility and safety of PITAC-directed therapy in patients with MPE, while secondary outcomes include MPE volume reduction and histological/cytological response [28].
Conclusions
PITAC seems technically feasible, safe, and able to induce histological and cytological response in some patients with MPE and/or PLM. However, prospective phase-I data are needed to investigate standard operating procedures, indication, patients and occupational health safety, optimal response assessment, and subsequently relevant short- and long-term outcomes.
-
Research ethics: The PITAC-OPC5 protocol has been approved by the Danish Medicines Agency and Ethical Committee (EU CT number 2023-503297-20-00).
-
Informed consent: Oral and written informed consent is obtained from all individuals included in this study.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission. PSH made substantial contribution to the conception of the work and drafted the manuscript. MG, SD, and APA revised the manuscript critically for important intellectual content. MBM made substantial contribution to the conception of the work and revised it critically for important intellectual content. The final manuscript was approved by all authors, and they all agree to be accountable for all aspects of the work.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: Authors state no conflict of interest.
-
Research funding: None declared.
References
1. Semaan, R, Feller-Kopman, D, Slatore, C, Sockrider, M. Malignant pleural effusions. Am J Respir Crit Care Med 2016;194:P11–2. https://doi.org/10.1164/rccm.1946P11. Epub 2016/09/16PubMed PMID: 27628085.Suche in Google Scholar PubMed
2. Asciak, R, Rahman, NM. Malignant pleural effusion: from diagnostics to therapeutics. Clin Chest Med 2018;39:181–93. https://doi.org/10.1016/j.ccm.2017.11.004. Epub 2018/02/13PubMed PMID: 29433714.Suche in Google Scholar PubMed
3. Egan, AM, McPhillips, D, Sarkar, S, Breen, DP. Malignant pleural effusion. QJM 2014;107:179–84. https://doi.org/10.1093/qjmed/hct245. Epub 2013/12/26PubMed PMID: 24368856.Suche in Google Scholar PubMed
4. Bashour, SI, Mankidy, BJ, Lazarus, DR. Update on the diagnosis and management of malignant pleural effusions. Respir Med 2022;196:106802. https://doi.org/10.1016/j.rmed.2022.106802. Epub 2022/03/15PubMed PMID: 35287006.Suche in Google Scholar PubMed
5. Fjællegaard, K, Petersen, JK, Armbuster, K, Jensen, HK, Skaarup, SH, Laursen, CB, et al.. Malign pleuraeffusion. Ugeskr Laeger 2021;183.Suche in Google Scholar
6. Clive, AO, Jones, HE, Bhatnagar, R, Preston, NJ, Maskell, N. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev 2016;2016:Cd010529. https://doi.org/10.1002/14651858.CD010529.pub2. Epub 2016/05/09PubMed PMID: 27155783; PubMed Central PMCID: PMCPMC6450218.Suche in Google Scholar PubMed PubMed Central
7. Ferreiro, L, Suárez-Antelo, J, Álvarez-Dobaño, JM, Toubes, ME, Riveiro, V, Valdés, L. Malignant pleural effusion: diagnosis and management. Cancer Res J 2020;2020:2950751. https://doi.org/10.1155/2020/2950751. Epub 2020/12/05PubMed PMID: 33273991; PubMed Central PMCID: PMCPMC7695997.Suche in Google Scholar PubMed PubMed Central
8. Hussein-Jelen, T, Bankier, AA, Eisenberg, RL. Solid pleural lesions. AJR Am J Roentgenol 2012;198:W512–20. https://doi.org/10.2214/ajr.11.7626. Epub 2012/05/25PubMed PMID: 22623565.Suche in Google Scholar
9. Desimpel, J, Vanhoenacker, FM, Carp, L, Snoeckx, A. Tumor and tumorlike conditions of the pleura and juxtapleural region: review of imaging findings. Insights into Imaging 2021;12:97. https://doi.org/10.1186/s13244-021-01038-x.Suche in Google Scholar PubMed PubMed Central
10. Shalahuddin, E, Hayati, F. Pleural metastasis of pulmonary adenocarcinoma mimicking diffuse mesothelioma: a case report and literature study. Radiol Case Rep 2023;18:818–23. https://doi.org/10.1016/j.radcr.2022.11.049. Epub 2022/12/31PubMed PMID: 36582758; PubMed Central PMCID: PMCPMC9793177.Suche in Google Scholar PubMed PubMed Central
11. Lurvink, RJ, Van der Speeten, K, Rovers, KP, de Hingh, I. The emergence of pressurized intraperitoneal aerosol chemotherapy as a palliative treatment option for patients with diffuse peritoneal metastases: a narrative review. J Gastrointest Oncol 2021;12:S259–s70. https://doi.org/10.21037/jgo-20-497. Epub 2021/05/11PubMed PMID: 33968442; PubMed Central PMCID: PMCPMC8100694.Suche in Google Scholar PubMed PubMed Central
12. Jonscher, N, Hummels, M, Giger-Pabst, U, Karljalainen, E, Zieren, J, Büchner, N, et al.. Chapter 18 pressurized IntraThoracic aerosol chemotherapy (PITAC). In: Raymond, MA, editor. The book of PIPAC – cancer under pressure. De Gruyter; 2014.10.1515/9783110366617.183Suche in Google Scholar
13. Solass, W, Kerb, R, Mürdter, T, Giger-Pabst, U, Strumberg, D, Tempfer, C, et al.. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol 2014;21:553–9. https://doi.org/10.1245/s10434-013-3213-1. Epub 2013/09/06PubMed PMID: 24006094; PubMed Central PMCID: PMCPMC3929768.Suche in Google Scholar PubMed PubMed Central
14. Hansen, PS, Graversen, M, Detlefsen, S, Mortensen, MB. Review on treatment of pleural metastasis and malignant pleural effusion with Pressurized IntraThoracic Aerosol Chemotherapy (PITAC). Pleura Peritoneum 2024;9:47–53. https://doi.org/10.1515/pp-2023-0048.Suche in Google Scholar PubMed PubMed Central
15. Graversen, M, Pedersen, PB, Mortensen, MB. Environmental safety during the administration of pressurized IntraPeritoneal aerosol chemotherapy (PIPAC). Pleura Peritoneum 2016;1:203–8. https://doi.org/10.1515/pp-2016-0019. Epub 2016/12/01PubMed PMID: 30911624; PubMed Central PMCID: PMCPMC6386395.Suche in Google Scholar PubMed PubMed Central
16. Graversen, M, Detlefsen, S, Ainsworth, AP, Fristrup, CW, Knudsen, AO, Pfeiffer, P, et al.. Treatment of peritoneal metastasis with pressurized intraperitoneal aerosol chemotherapy: results from the prospective PIPAC-OPC2 study. Ann Surg Oncol 2023;30:2634–44. https://doi.org/10.1245/s10434-022-13010-0. Epub 2023/01/06PubMed PMID: 36602663.Suche in Google Scholar PubMed
17. Solass, W, Sempoux, C, Detlefsen, S, Carr, NJ, Bibeau, F. Peritoneal sampling and histological assessment of therapeutic response in peritoneal metastasis: proposal of the Peritoneal Regression Grading Score (PRGS). Pleura Peritoneum 2016;1:99–107. https://doi.org/10.1515/pp-2016-0011. Epub 2016/06/01PubMed PMID: 30911613; PubMed Central PMCID: PMCPMC6328069.Suche in Google Scholar PubMed PubMed Central
18. Solass, W, Sempoux, C, Carr, NJ, Bibeau, F, Neureiter, D, Jäger, T, et al.. Reproducibility of the peritoneal regression grading score for assessment of response to therapy in peritoneal metastasis. Histopathology 2019;74:1014–24. https://doi.org/10.1111/his.13829. Epub 2019/01/29PubMed PMID: 30687944.Suche in Google Scholar PubMed
19. Detlefsen, S, Windedal, T, Bibeau, F, Bruhn, LV, Carr, N, Graversen, M, et al.. Role of immunohistochemistry for interobserver agreement of Peritoneal Regression Grading Score in peritoneal metastasis. Hum Pathol 2022;120:77–87. https://doi.org/10.1016/j.humpath.2021.12.005. Epub 2021/12/27PubMed PMID: 34954136.Suche in Google Scholar PubMed
20. Roensholdt, S, Detlefsen, S, Mortensen, MB, Graversen, M. Response evaluation in patients with peritoneal metastasis treated with pressurized IntraPeritoneal aerosol chemotherapy (PIPAC). J Clin Med 2023;12:1289. https://doi.org/10.3390/jcm12041289.Suche in Google Scholar PubMed PubMed Central
21. Clavien, PA, Barkun, J, de Oliveira, ML, Vauthey, JN, Dindo, D, Schulick, RD, et al.. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–96. https://doi.org/10.1097/SLA.0b013e3181b13ca2. Epub 2009/07/30PubMed PMID: 19638912.Suche in Google Scholar PubMed
22. Common Terminology Criteria for adverse events (CTCAE): U.S. Department of health and human services. National Institutes of Health. National Cancer Institute; 2017 [20-04-2023]. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.Suche in Google Scholar
23. Drevet, G, Maury, JM, Bakrin, N, Tronc, F. Technique of pressurized intrathoracic aerosol chemotherapy (PITAC) for malignant pleural effusion. Pleura Peritoneum 2020;5:20200129. https://doi.org/10.1515/pp-2020-0129. Epub 2021/02/13PubMed PMID: 33575461; PubMed Central PMCID: PMCPMC7823156.Suche in Google Scholar PubMed PubMed Central
24. Giger-Pabst, U, Demtröder, C, Falkenstein, TA, Ouaissi, M, Götze, TO, Rezniczek, GA, et al.. Pressurized IntraPeritoneal aerosol chemotherapy (PIPAC) for the treatment of malignant mesothelioma. BMC Cancer 2018;18:442. https://doi.org/10.1186/s12885-018-4363-0. Epub 2018/04/20PubMed PMID: 29669524; PubMed Central PMCID: PMCPMC5907219.Suche in Google Scholar PubMed PubMed Central
25. Kuchen, NCT, Hailemariam, S, Schoeb, O. Safety and efficacy of pressurized intraperitoneal/intrathoracic aerosol chemotherapy (PIPAC/PITAC) in patients with peritoneal and/or pleural carcinomatosis: a preliminary experience. J Med Ther 2018;2:1–6. 10.15761/JMT.1000127Suche in Google Scholar
26. Robella, M, Vaira, M, Borsano, A, Mossetti, C, M, DES. Low-dose pressurized intrathoracic aerosol chemotherapy (PITAC) as an alternative therapy for pleuropulmonary involvement in pseudomyxoma peritonei. Anticancer Res 2018;38:929–32. https://doi.org/10.21873/anticanres.12305. Epub 2018/01/29PubMed PMID: 29374723.Suche in Google Scholar PubMed
27. Baggaley, AE, Lafaurie, G, Tate, SJ, Boshier, PR, Case, A, Prosser, S, et al.. Pressurized intraperitoneal aerosol chemotherapy (PIPAC): updated systematic review using the IDEAL framework. Br J Surg 2022;110:10–8. https://doi.org/10.1093/bjs/znac284. Epub 2022/09/04PubMed PMID: 36056893; PubMed Central PMCID: PMCPMC10364525.Suche in Google Scholar PubMed PubMed Central
28. Hansen, PS, Graversen, M, Detlefsen, S, Ainsworth, AP, Fristrup, C, Eckhoff, L, et al.. Implementation and evaluation of Pressurized IntraThoracic Aerosol Chemotherapy (PITAC) for the treatment of patients with malignant pleural effusion - a Danish phase I study. Study protocol for the PITAC-OPC5 trial. Pleura Peritoneum 2024.10.1515/pp-2024-0014Suche in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/pp-2024-0008).
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Pressurized IntraThoracic Aerosol Chemotherapy (PITAC) directed therapy of patients with malignant pleural effusion and pleural metastasis
- Implementation and evaluation of Pressurized IntraThoracic Aerosol Chemotherapy (PITAC) for the treatment of patients with malignant pleural effusion: study protocol for the Danish phase-I PITAC-OPC5 study
- Extent of peritoneal metastases from colorectal cancer is not associated with changes in thrombin generation or fibrinolysis
- Letters to the Editor
- Questioning the role of HIPEC in patients with granulosa cell ovarian tumours
- Reply to: Questioning the role of HIPEC in patients with granulosa cell ovarian tumours
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Pressurized IntraThoracic Aerosol Chemotherapy (PITAC) directed therapy of patients with malignant pleural effusion and pleural metastasis
- Implementation and evaluation of Pressurized IntraThoracic Aerosol Chemotherapy (PITAC) for the treatment of patients with malignant pleural effusion: study protocol for the Danish phase-I PITAC-OPC5 study
- Extent of peritoneal metastases from colorectal cancer is not associated with changes in thrombin generation or fibrinolysis
- Letters to the Editor
- Questioning the role of HIPEC in patients with granulosa cell ovarian tumours
- Reply to: Questioning the role of HIPEC in patients with granulosa cell ovarian tumours


