Body composition and immunonutritional status in patients treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC) for gastrointestinal peritoneal metastases: a prospective single-center analysis
-
Stefano Rotolo
, Emanuele Rinninella
Abstract
Objectives
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a novel drug administration method with promising efficacy for the treatment of peritoneal metastases (PM). This study aimed to evaluate the prognostic value of an immunonutritional assessment on the feasibility, safety, and survival in this setting.
Methods
Data of PM patients undergoing PIPAC between September 2018 and May 2020 were prospectively recorded. A CT scan-derived body composition assessment was performed for each patient.
Results
Fifty-one patients were enrolled, of which 30 (58%) underwent multiple PIPAC cycles, with a pathological response rate of 55%. Prognostic nutritional index (PNI) and neutrophil-to-lymphocytes predicted completion of more than one PIPAC cycle, with a cut off of 36.5 and 4.8 respectively. Muscle attenuation and body fat tissues were associated with pathological response. At multivariate Cox regression analysis, only the presence of a low PNI (HR 2.41, 95% CI 1.08–5.46) was significantly associated with a worse OS.
Conclusions
A pretreatment immunonutritional assessment may provide valuable information for PIPAC patients’ selection and survival, while body composition parameters are able to predict pathological response. Further larger studies are needed to validate the role of these biomarkers in tailoring the treatment and monitoring PM patients undergoing PIPAC.
Introduction
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a novel locoregional chemotherapy recently proposed for patients affected by peritoneal dissemination from gastrointestinal and gynecological cancers. Several phase-I and phase-II studies reported reassuring safety data and high antitumoral efficacy of PIPAC alone or in combination with systemic chemotherapy [1], [2], [3]. Based on laparoscopy, PIPAC may be repeated several times, enhancing the chance to hit active neoplastic cells. Despite defined PIPAC schedules being lacking, it has been proposed that multiple administrations should be carried out to exert the best antiblastic efficacy [4], [5], [6]. Unfortunately, the reported rate of completing a three-cycle course hardly reaches 50% in published cohorts. This may not be surprising, given that PIPAC has been mostly administered in late-stage diseases in a palliative setting [7], [8], [9], [10], [11]. The selection of patients undergoing PIPAC relies on several factors and it is decided on a case-by-case basis in most studies, as precise PIPAC indications still need to be accurately defined [12].
A large body of evidence disclosed the role of the immunonutritional status in oncological patients, which entails their capacity to cope with surgical and antiblastic treatments. Several scores based on blood test, including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and prognostic nutritional index (PNI), have been reported to correlate with postoperative complications and survival in patients with gastrointestinal cancer [13]. NLR and PLR, as markers of systemic inflammatory response, are closely associated with cancer development, progression, and metastasis and have been used as prognostic indicators in many solid tumors [14], [15], [16]. Moreover, in the oncological setting, the computed tomography (CT) scan-derived body composition analysis has grown in interest in the last decade, due both to the high availability of CT scans in neoplastic patients [17, 18], and to the association of CT-derived parameters with an increased risk of chemotherapy toxicity and poor survival [19].
Nutritional status is a major determinant of surgical and oncological outcomes. In 2016, the Global Leadership Initiative on Malnutrition (GLIM), through the collaboration of the leading nutrition societies, defined a standardised approach for the diagnosis of malnutrition. The evaluation starts with the analysis of the “risk” status using any of the already validated screening tools. The second step is the assessment of malnutrition and its severity. To do so, different criteria have been identified and classified into phenotypic and etiological. Phenotypic criteria include involuntary weight loss, reduced BMI and/or reduced FFM, measured through validated procedures, such as bioelectrical impedance analysis and fat free mass index (FFMI). Etiological criteria are reduced food intake or assimilation and inflammation or disease burden. To diagnose malnutrition at least one phenotypic criterion and one etiologic criterion should be present. Once diagnosed, malnutrition is stratified into moderate or severe depending on phenotypic criteria.
This study aimed to evaluate the value of pretreatment immunonutritional status and CT-derived body composition parameters on feasibility, safety, efficacy, and survival of patients undergoing PIPAC for gastrointestinal peritoneal metastases (PM).
Materials and methods
Study design
We prospectively recorded the clinical data of patients undergoing PIPAC for PM of gastrointestinal origin between September 2018 to May 2020 at the Foundation Policlinico A. Gemelli IRCCS, Rome, Italy. For each patient age, gender, American Society of Anesthesiology (ASA) score, Eastern Cooperative Oncology Group Performance Status (ECOG PS), previous oncological and surgical history, were prospectively recorded. Laboratory test results included absolute counts of white blood cells, absolute neutrophil count (NEU), absolute lymphocyte count (LYM), platelet count (PLT), and albumin (ALB) levels. Moreover, starting from laboratory data taken, the following parameters were calculated: PNI [20]: ALB [g/L] + 0.005 × LYM, NLR: NEU/LYM, and PLR: PLT/LYM. At baseline, a complete nutritional evaluation, including weight, height, body mass index (BMI), was carried out. All patients who had already received any type of nutritional intervention (i.e. oral nutritional supplements, enteral nutrition, parenteral nutrition, etc.) were excluded from the analysis. CT scan images, taken within one month before the PIPAC procedure, were collected for body composition assessment. The following perioperative data were recorded: the number of PIPAC cycles administered per patient and the incidence of no-entry at laparoscopy, peritoneal cancer index (PCI), ascites volume, intraoperative complications, length of hospital stay, readmission rate, 30-days postoperative adverse events according to the National Cancer Institute Common Terminology Criteria for Adverse Events classification version 5.0 (CTCAE). Pathological response was assessed according to the peritoneal regression grading score (PRGS) and any reduction of the PRGS was considered a response to treatment. Each patient was recalled and followed up to death whenever possible. The Ethical Committee of the Foundation Policlinico A. Gemelli approved the study (ID: 2541; Prot. 16328/19), according to the principles of the Declaration of Helsinki. All patients provided written informed consent.
PIPAC procedure
A PM interdisciplinary tumor board gave the indication for PIPAC on an individual basis considering several factors: ECOG PS, past chemotherapy lines and responses, previous surgery, clinical evaluation of abdominal accessibility, and disease extension on CT scan. Exclusion criteria were ECOG PS higher than 2, bowel obstruction, limited accessibility to the abdominal cavity on clinical evaluation, presence of other distant metastases, severe renal, hepatic or bone marrow impairment. The PIPAC procedure was performed according to the standard technique [21, 22]. An exploratory laparoscopy with peritoneal disease assessment with PCI was carried out. If present, ascitic fluid was drained and at least four peritoneal biopsies were taken for pathological response assessment. A nebulizer (Capnopen-MIP, Reger Medizintechnik, Rottweil, Germany) connected to a high-pressure injector (Injektron 82M, MedTron, Saarbrücken) creates a pressurized aerosol containing doxorubicin 1.5 mg/m2 body surface area in 50 mL of NaCl 0.9%, with cisplatin 7.5 mg/m2 in 150 mL NaCl 0.9% or oxaliplatin 92 mg/m2 body surface in 200 mL NaCl 0.9%. Since the beginning of 2020, cisplatin-doxorubicin dosages were updated to 10.5–2.1 mg/m2 on the basis of the dedicated dose-escalation study [23]. The injector flow is set to 6 mL/s with a maximum upstream pressure of 200 psi and a 12 mmHg capnoperitoneum. The injector flow is set to 30 mL/min with a maximum upstream pressure of 200 psi and an intraabdominal pressure of 12 mmHg. The injection is remote-controlled, and it is monitored by a laparoscopic camera held in place by a self-retaining retractor. The capnoperitoneum is then maintained for 30 min at 37 °C. After aerosol evacuation via a closed aerosol waste system, the trocars are removed. The fascia and skin were closed with absorbable sutures. Patients were discharged on the first or second postoperative day. A PIPAC course globally consists of three cycles, scheduled every 6–8 weeks.
CT-derived body composition parameters
A specific image analysis software (SliceOmatic v5.0, Tomovision, Montreal, Canada) was used to examine CT images, by an operator trained in musculoskeletal anatomy, to define different tissues, according to the following Hounsfield Unit (HU) thresholds: −29 to +150 for muscle, −190 to −30 for intermuscular adipose tissue (IMAT), −150 to −50 for visceral adipose tissue (VAT), and −190 to −30 for subcutaneous adipose tissue (SAT). Skeletal muscle area (SMA) was analyzed on a single axial slice at the third vertebral level aiming to include following muscular groups: psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and rectus abdominis. Muscle attenuation (MA) was obtained by the mean HU of SMA. Tissue boundaries were manually corrected as needed. Normalizing the previously measured parameters by height squared, skeletal muscle index (SMI), visceral adipose tissue index (VATI), and subcutaneous adipose tissue (SATI) were obtained, while the total fat area (TFA) was calculated adding all the fat tissues. According to previously published studies on PM patients [24, 25], 52.4 cm2/m2 for men and 38.5 cm2/m2 for women were used as cut-off values to define low-SMI patients.
Statistical analysis
The objective of the study was to describe the immunonutritional status of patients undergoing PIPAC and to assess its relation with procedure-related, oncological and survival outcomes. In particular, we explored the immunonutritional variables related to the following endpoints: receiving multiple PIPAC cycles, PIPAC-related adverse events, pathological response on PM biopsies, and overall survival (OS). Kolmogorov–Smirnov test was used to assess normal distribution. Continuous variables were expressed as median (25 and 75th percentiles), categorical ones as number (percentage). Wilcoxon rank-sum test was used to assess differences between two groups; Chi-square or Fisher Exact test were appropriately used for categorical variables. ROC curves were used to find the cut-off of the parameters statistically significant at univariate analysis, reporting area under the curve (AUC), and cut off, were necessary. OS was calculated using Kaplan–Meier curves and differences between them were assessed through the log-rank test. All significant parameters at univariate analysis (p<0.05) were used to construct a Cox proportional regression analysis. Statistical analysis was performed using STATA® Software (Version 14.0, Stata Corporation; College Station, TX, USA).
Results
The baseline characteristics of the 51 patients are summarized in Table 1. The cohort study was composed of 26 males (51%) and 25 females (49%), with a median age of 63 years (54–71), and a median BMI of 20.9 kg/m2 (18.6–24.6). Fourty one patients (80.4%) were malnourished according to GLIM criteria. Five patients (10%) were in the third class of the ASA score, and 6 (11%) were in the second ECOG PS class. Primary tumors were gastric (39%), colorectal (33%), and hepato–pancreatic–biliary (HPB) (24%), with a 43% rate of synchronous PM. Almost all patients had already undergone one line of systemic chemotherapy, and 31 (60%) underwent two or more lines of systemic chemotherapy. PIPAC-related data are presented in Table 2. The access to the abdominal cavity and the first PIPAC cycle was feasible in all cases. Thirty (58.8%) patients repeated PIPAC procedure, and the median hospital stay was two days (1–3) without any readmission.
Baseline characteristics of the study sample.
| n (%) or median (IQR) | |
|---|---|
| Female | 25 (49) |
| Age, years | 63 (54–71) |
| Weight, kg | 59 (52–71) |
| Height, cm | 168 (163–173) |
| BMI, kg/m2 | 20.9 (18.6–24.6) |
| Malnourished according to GLIM | 41 (80.4) |
| ASA score | |
|
7 (14) |
|
39 (76) |
|
5 (10) |
| ECOG PS | |
|
11 (22) |
|
34 (67) |
|
6 (11) |
| Primary neoplasm | |
|
19 (37) |
|
20 (39) |
|
12 (24) |
|
22 (43) |
|
29 (57) |
| Previous systemic chemotherapy | |
|
1 (2) |
|
50 (98) |
|
31 (60) |
-
ASA, American Society of Anesthesiology; BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group performance status scale; GLIM, global leadership initiative on malnutrition; HPB, hepato-pancreatic-biliary cancer; IQR, interquartile range; PIPAC, pressurized intraperitoneal aerosol chemotherapy.
Operative and postoperative PIPAC-related data.
| n (%) or median (IQR) | |
|---|---|
| Total number of PIPAC | 102 |
| Only I PIPAC cycle | 21 (41) |
| Multiple PIPAC cycles | 30 (58) |
| Laparoscopic entry failures | 0 (0) |
| PCI | 22 (12–30) |
| Ascites, mL | 500 (28–1.350) |
| Cisplatin–doxorubicin 7.5–1.5, mg/mq | 28 (55)a |
| Oxaliplatin 92, mg/mq | 20 (39) |
| Operative time, min | 98 (74–131) |
| Intraoperative complications | 1 |
| Hospital stay, days | 2 (1–3) |
| Readmission rate | 0 |
| Adverse events (CTCAE v. 5.0) | |
|
17 (17) |
|
1 (1)b |
|
0 (0) |
|
28 (55) |
-
IQR, interquartile range; PIPAC, pressurized intraperitoneal aerosol chemotherapy; CTCAE, common terminology criteria for adverse events. aOne patient underwent cisplatin 7.5 mg/mq only due to previous adverse reaction to doxorubicin; six patients received cisplatin-doxorubicin 10.5–2.1 mg/mq after dosage update in 2020. bSkin effusion and abdominal pain due to trocar-site chemotherapy infiltration.
Receiving multiple PIPAC cycles
Data regarding patients receiving multiple PIPAC cycles are shown in Table 3. In particular, 30 patients (58.8%) did not complete the third PIPAC cycle; the main reasons were disease progression (50.0%), no access to abdominal cavity (23.5%), patient’s refusal (14.5%), patients waiting for the next cycle (6.0%), complete pathological response (3.0%), others (3.0%). Median SMI was 42.3 cm2/m2 (37.6–49.7), with an incidence of low-SMI rate of 72.6%. No differences between patients who received one or more PIPAC cycles about body composition parameters were found. ALB, LYM, and PNI were lower in patients who received only one PIPAC, while NEU and NLR were higher. Cut offs were as follow: 27.5 for ALB, 3.55 for NEU, 0.90 for LYM, 36.5 for PNI, and 4.8 for NLR. AUC was higher for PNI and ALB with the value of 0.907 (p: 0.0001) and 0.911 (p: 0.0001), respectively.
Multiple PIPAC procedures data.
| Total (51 patients) | 1 PIPAC (21 patients) | ≥2 PIPAC (30 patients) | p-Value | AUC | Cutoff | Sens | Spec | |
|---|---|---|---|---|---|---|---|---|
| SMA, cm2 | 120.1 (102.5–136.3) | 121.4 (96.3–133.5) | 119.4 (106.1–141.2) | 0.76 | ||||
| SMI, cm2/m2 | 42.3 (37.6–49.7) | 42.8 (35.2–51.2) | 41.6 (38.1–49.4) | 0.93 | ||||
| Low-SMI rate | 37 (72.6%) | 14 (66.7%) | 23 (76.7%) | 0.52 | ||||
| MA, HU | 41.3 (37.5–47.8) | 42.6 (35.4–48.1) | 40.6 (37.5–47.1) | 0.84 | ||||
| VAT, cm2 | 36.6 (19.7–75.8) | 33.0 (16.4–75.8) | 39.5 (19.7–83.7) | 0.64 | ||||
| VATI, cm2/m2 | 13.1 (6.5–28.9) | 10.2 (6.2–32.3) | 13.2 (6.5–28.9) | 0.76 | ||||
| SAT, cm2 | 92.3 (60.3–148.4) | 90.9 (45.1–186.2) | 94.3 (62.3–119.6) | 0.83 | ||||
| SATI, cm2/m2 | 32.9 (19.8–49.6) | 34.5 (16.1–59.4) | 32.6 (22.2–43.4) | 0.90 | ||||
| IMAT, cm2 | 6.4 (2.7–8.4) | 6.7 (3.5–8.4) | 5.6 (2.5–8.7) | 0.79 | ||||
| TFA, cm2 | 149.8 (98.5–257.8) | 160.5 (70.1–298.9) | 140.1 (99.9–230.6) | 0.85 | ||||
| Malnutrition according to GLIM | 41 (80.4%) | 17 (80.9%) | 24 (80.0%) | 0.93 | ||||
| Creatinine, mg/dL | 0.85 (0.73–1.15) | 0.86 (0.78–1.53) | 0.83 (0.69–1.11) | 0.35 | ||||
| Albumin, g/L | 29 (22–36) | 22 (20–23) | 33.5 (29–37) | <0.0001 | 0.907 | 27.5 | 87 | 90 |
| Neutrophils, 103 cells/mm3 | 4.26 (2.60–5.32) | 4.51 (4.10–7.50) | 3.45 (2.43–5.00) | 0.03 | 0.679 | 3.55 | 53 | 86 |
| Lymphocytes, 103 cells/mm3 | 1.04 (0.76–1.48) | 0.83 (0.67–1.29) | 1.23 (0.86–1.57) | 0.02 | 0.687 | 0.90 | 73 | 62 |
| Platelets, 103 cells/mm3 | 199 (135–284) | 213 (123–311) | 197 (141–276) | 0.77 | ||||
| PNI | 34.9 (26.2–42.1) | 25.9 (24.3–28.5) | 40.7 (35.5–44.6) | <0.0001 | 0.911 | 36.5 | 97 | 86 |
| NLR | 4.6 (2.1–6.7) | 6.2 (5.1–7.4) | 2.4 (1.8–4.7) | 0.001 | 0.771 | 4.8 | 77 | 81 |
| PLR | 179.2 (128.8–276.5) | 252.1 (138.2–428.8) | 175.7 (108.7–275.8) | 0.12 |
-
Data in bold indicate a statistically significant association. AUC, area under the ROC curve; GLIM, global leadership initiative on malnutrition; IMAT, intermuscular adipose tissue; MA, muscle attenuation; NLR, neutrophil-to-lymphocyte ratio; PIPAC, pressurized intraperitoneal aerosol chemotherapy; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; SAT, subcutaneous adipose tissue; SATI, subcutaneous adipose tissue index; Sens, sensitivity; SMA, skeletal muscle area; SMI, skeletal muscle index; Spec, specificity; TFA, total fat area; VAT, visceral adipose tissue; VATI, visceral adipose tissue index.
PIPAC-related adverse events
Of 102 total PIPAC procedures performed, 18 (17.6%) adverse events were developed, of which only 1 (0.9%) was Grade 3 according to CTCAE. The only severe AE consisted of diffuse abdominal cutaneous and subcutaneous inflammation due to the infiltration of oxaliplatin from the trocar sites at the second PIPAC cycle. There were no grade four and five adverse events. Due to the very limited number of severe AE, no further analysis was performed on this issue.
Pathological response
A pathological response according to the PRGS was documented in 28 out of 30 patients receiving more than one PIPAC and available for evaluation, which accounts for 55% of the overall cohort. Table 4 reports data correlated to pathological response. No differences between responders and non-responders according to PRGS were found in terms of blood tests. MA was higher in responding half of patients, while VAT, VATI, SAT, SATI, and TFA were lower in the same population. Cut offs were as follow: 39.5 for MA, 35.4 for VAT, 13.1 for VATI, 89 for SAT, 32.1 for SATI, and 149.8 for TFA. The highest AUC value was for SAT (0.739; p: 0.005).
Laboratory and body composition data correlated to pathological response.
| No pathological response (23 patients) | Pathological response (28 patients) | p-Value | AUC | Cut off | Sens | Spec | |
|---|---|---|---|---|---|---|---|
| SMA, cm2 | 119.3 (104.2–144.8) | 121.3 (102.3–130.6) | 0.62 | ||||
| SMI, cm2/m2 | 42.4 (38.0–51.3) | 42.0 (37.6–44.6) | 0.73 | ||||
| Low-SMI rate | 15 (65.2%) | 22 (78.6%) | 0.35 | ||||
| MA, HU | 38.2 (33.7–43.7) | 43.6 (40.4–48.5) | 0.02 | 0.693 | 39.5 | 84 | 62 |
| VAT, cm2 | 62.1 (34.8–86.9) | 25.8 (15.5–57.7) | 0.02 | 0.691 | 35.4 | 76 | 61 |
| VATI, cm2/m2 | 22.4 (13.2–28.9) | 9.5 (5.7–21.0) | 0.03 | 0.688 | 13.1 | 75 | 77 |
| SAT, cm2 | 118.3 (90.9–168.6) | 74.6 (50.7–102.7) | 0.005 | 0.739 | 89.0 | 71 | 82 |
| SATI, cm2/m2 | 41.5 (32.6–59.4) | 27.3 (17.4–38.6) | 0.007 | 0.731 | 32.1 | 71 | 77 |
| IMAT, cm2 | 7.1 (2.7–10.8) | 5.4 (2.8–7.7) | 0.24 | ||||
| TFA, cm2 | 194.9 (124.2–290.7) | 103.9 (77.6–175.8) | 0.01 | 0.703 | 149.8 | 71 | 73 |
| Malnutrition according to GLIM | 19 (82.6%) | 22 (78.6%) | 0.72 | ||||
| Haemoglobin, g/dL | 11.8 (11.7–12.9) | 12.8 (11.8–14.2) | 0.39 | ||||
| Creatinine, mg/dL | 0.85 (0.76–1.07) | 0.87 (0.69–1.51) | 0.83 | ||||
| Albumin, g/L | 28 (20–34) | 29 (22–38) | 0.26 | ||||
| Neutrophils, 103 cells/mm3 | 4.36 (2.43–7.94) | 4.10 (3.08–5.02) | 0.62 | ||||
| Lymphocytes, 103 cells/mm3 | 1.11 (0.76–1.57) | 1.03 (0.73–1.44) | 0.51 | ||||
| Platelets, 103 cells/mm3 | 203 (129–282) | 200 (152–289) | 0.86 | ||||
| PNI | 34.9 (25.6–41.5) | 35.2 (27.3–43.0) | 0.55 | ||||
| NLR | 4.83 (1.92–8.37) | 3.95 (2.94–5.45) | 0.87 | ||||
| PLR | 238.3 (132.6–411.8) | 186.4 (110.4–285.3) | 0.64 |
-
Data in bold indicate a statistically significant association. AUC, area under the ROC curve; GLIM, global leadership initiative on malnutrition; IMAT, intermuscular adipose tissue; MA, muscle attenuation; NLR, neutrophil-to-lymphocyte ratio; PIPAC, pressurized intraperitoneal aerosol chemotherapy; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; SAT, subcutaneous adipose tissue; SATI, subcutaneous adipose tissue index; Sens, sensitivity; SMA, skeletal muscle area; SMI, skeletal muscle index; Spec, specificity; TFA, total fat area; VAT, visceral adipose tissue; VATI, visceral adipose tissue index.
Overall survival
Within the median follow-up period of 36.0 months (range: 27.6–44.4), 38 (74.5%) patients died, with a median OS of 8.33 months (95% CI 5.90–9.47) (Figure 1). Table 5 reported univariate Kaplan–Meier analysis for all the tested variables and the Cox regression multivariate analysis performed. For PNI analysis the same cut off found in Table 3 was used. In particular, ascites [HR 2.50 (95% CI 1.17–5.30); p: 0.01], dysphagia [HR 2.83 (95% CI 1.11–7.19); p: 0.02], and PNI less than 36.5 [HR 3.43 (95% CI 1.65–7.15); p: 0.0005] resulted associated with a poor OS (Figure 2). At the Cox regression model, a low PNI [HR 2.41 (95% CI 1.08–5.46); p: 0.034] remained the only independent factor for OS.
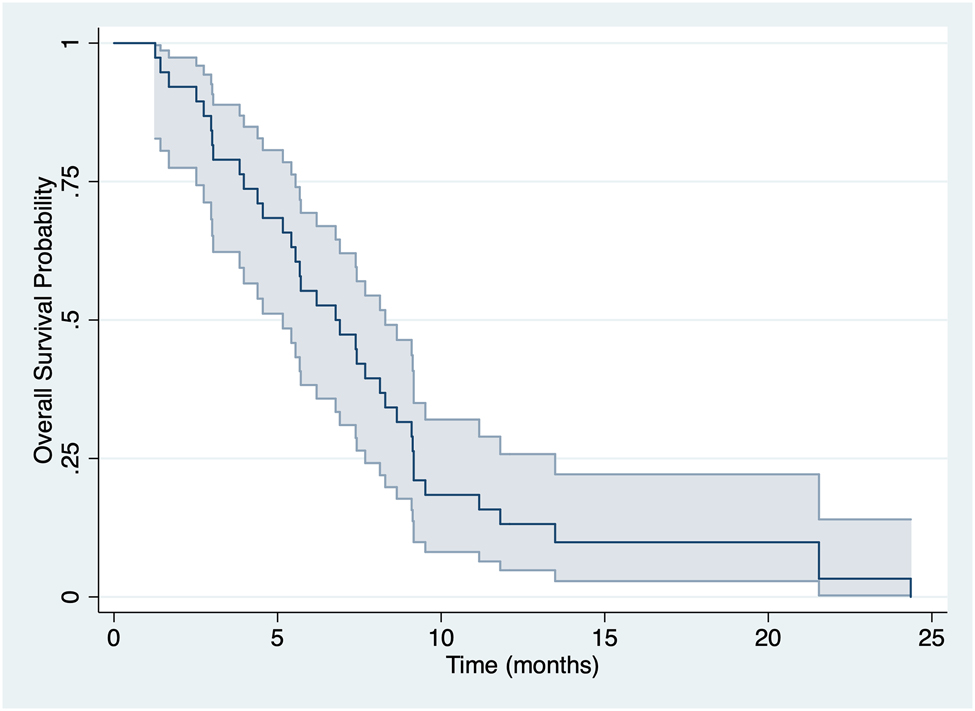
Overall survival analysis.
Univariate and multivariate analysis for overall survival.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age≥65 | 1.49 (0.73–3.04) | 0.26 | ||
| Sex | 1.68 (0.86–3.28) | 0.11 | ||
| BMI>18.5 | 0.83 (0.37–1.85) | 0.64 | ||
| Malnutrition according to GLIM | 1.08 (0.48–2.41) | 0.85 | ||
| ECOG≥2 | 0.81 (0.25–2.66) | 0.73 | ||
| ASA≥3 | 0.76 (0.23–2.49) | 0.64 | ||
| Ascites | 2.50 (1.17–5.30) | 0.01 | 2.18 (0.91–5.24) | 0.08 |
| Dysphagia | 2.83 (1.11–7.19) | 0.02 | 3.17 (0.94–8.98) | 0.07 |
| Nausea | 1.29 (0.60–2.78) | 0.49 | ||
| CHT cycles≥12 | 0.65 (0.33–1.25) | 0.18 | ||
| PNI<36.5 | 3.43 (1.65–7.15) | 0.0005 | 2.41 (1.08–5.46) | 0.034 |
| Low-SMI rate | 1.15 (0.56–2.39) | 0.69 | ||
| MA | 1.21 (0.61–2.43) | 0.57 | ||
| PRGS | 0.69 (0.36–1.35) | 0.29 | ||
-
Data in bold indicate a statistically significant association. ASA, American Society of Anesthesiology; BMI, body mass index; CHT, chemotherapy; ECOG PS, Eastern Cooperative Oncology Group performance status scale; GLIM, global leadership initiative on malnutrition; MA, muscle attenuation; PIPAC, pressurized intraperitoneal aerosol chemotherapy; PNI, prognostic nutritional index; SMI, skeletal muscle index.

Univariate Kaplan–Meier analysis for PNI.
Discussion
To our best knowledge, this is the first study evaluating the impact of immunonutritional status and CT-derived body composition analysis on patients undergoing PIPAC for gastrointestinal PM. In fact, the only one study recently published, evaluating body composition parameters in PM patients, used bioelectrial impedance analysis rather than CT scan derived analysis [26].
PIPAC is a novel method of intraperitoneal chemotherapy administration with promising results for peritoneal surface malignancies, but, despite its safety and efficacy data are available, a precise indication is still under debate [27]. One of the most appealing features of PIPAC is the possibility to undergo multiple cycles and thus progressively hit a higher quantity of tumor cells. Since only half of patients are able to complete the programmed treatment cycle [9, 28], there is a need to find new objective selection criteria to decrease the number of patients undergoing only one PIPAC.
In our study, 58% of patients received multiple PIPAC cycles, in line with literature data [5, 29]. Among the pre-treatment immunonutritional parameters assessed, ALB, NEU, and LYM were significantly associated with the capability for PM patients to tolerate more than one PIPAC. Moreover, for their derived indexes PNI and NLR, cut offs of 36.5 and 4.8 were respectively calculated, but, due to the small sample size, they need further validation on a larger and more selected population. PNI is an unexpensive and easy to calculate parameter, which reflects the nutrition and inflammatory status of patients [30], whose role as a prognostic index for OS in many oncological settings has been widely recognised [31], [32], [33]. In our study, a PNI under the identified cut off value (36.5) resulted the only independently correlated factor with a worse OS (HR 2.41), and similar results were reported in a recent large retrospective study conducted on PM patients [34]. Starting from these findings, an immunonutritional evaluation should be routinely performed before programming a PIPAC treatment, and patients with an impaired status referred to a clinical nutrition unit.
A compromised nutritional status in PM patients, mostly depending on both mechanical and metabolic factors, can determine a body composition impairment [35]. In the last decade there has been an increasing interest in body composition analysis in cancer patients, since a quantitative and qualitative impairment of skeletal muscle mass determines a limited patient capacity to cope with surgical and antiblastic treatments [36, 37]. In fact, in almost all oncological settings, a low skeletal muscle mass, derived from SMI, is considered an independent prognostic factor for increased postoperative morbidity, chemotherapy toxicity, and worse OS [38, 39]. As previously reported, patients’ selection for PIPAC depends on several factors, including immunonutritional status, and in this study was defined after an interdisciplinary tumor board. The absolute contraindications to PIPAC were known hypersensitivity reaction to antiblastic agents, advanced metastatic disease with clinical deterioration and clinical or radiological evidence of gastrointestinal occlusion. To date, in literature there are no well-defined nutritional criteria or symptoms that preclude PIPAC procedure, so this field should be further investigated in next studies. In the complexity of this scenario, in which PIPAC improves survivals of PM patients, enhances quality of life, and relieves symptoms related to PM [40], the ideal patient for PIPAC is everyone who completes a three-cycle course in order to maximize the effects of this therapy. Consequently, an adequate nutritional support initiated at an earlier stage could improve clinical outcomes and treatment compliance, particularly in malnourished patients with metastatic disease.
While in previously published studies in gastrointestinal PM patients, the incidence of low SMI was 40–55% [24, 25, 40–42], in our study there was a higher prevalence of low SMI patient, reaching 73%. However, no SMI differences were found among the analyzed outcomes, while MA, describing the quality of muscle tissue, was correlated to pathological response after PIPAC, predicting a reduction in PRGS score on subsequent peritoneal biopsies. Also other studies on advanced ovarian cancer correlated MA, but not SMI, with oncological and survival outcomes [43, 44], introducing the possibility that in PM patients muscle quality might be considered a better predictor than muscle quantity. The CT scan analysis of fat tissues in PM patients showed that lower VAT, SAT, and TFA values were correlated to a better pathological response. Our hypothesis is that a higher quantity of fat mass, above all VAT, can act as a physical barrier for chemotherapeutic agents nebulized with PIPAC, reducing in the short term the pathological response.
This study is affected by several limitations: i) the sample size needs to be increased to confirm our findings; ii) since changes in body composition during chemotherapy administration are frequently reported [45], [46], [47], further longitudinal analysis is planned to investigate the impact of repeated PIPAC cycles; iii) GI primary tumors may differently condition nutritional status of PM patients.
In conclusion, the European Society of Clinical Nutrition Guidelines on cancer patients [48, 49] suggest a nutritional evaluation at the beginning of any oncological pathway to early identify patients at risk of malnutrition and those already malnourished, in which a nutritional intervention could reduce the risk of therapies discontinuation [50]. Since PIPAC procedure is administrated in very advanced oncological patients, an accurate nutritional evaluation is even more advisable. Further studies on a larger population of gastrointestinal PM patients receiving PIPAC are needed both to define the optimal selection criteria and to identify the best nutritional support to sustain them during the procedures.
-
Research funding: None declared.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: This research, involving human subjects, complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as revised in 2013), and has been approved by the authors’ Institutional Review Board (Fondazione Policlinico Universitario Agostino Gemelli IRCCS) or equivalent committee. (protocol code 16328/19, ID 2541, 09.05.2019).
References
1. Alyami, M, Hübner, M, Grass, F, Bakrin, N, Villeneuve, L, Laplace, N, et al.. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol 2019;20:e368–77. https://doi.org/10.1016/s1470-2045(19)30318-3.Suche in Google Scholar
2. Di Giorgio, A, Abatini, C, Attalla El Halabieh, M, Vita, E, Vizzielli, G, Gallotta, V, et al.. From palliation to cure: PIPAC for peritoneal malignancies. Minerva Med 2019;110:385–98. https://doi.org/10.23736/S0026-4806.19.06081-6.Suche in Google Scholar PubMed
3. Struller, F, Horvath, P, Solass, W, Weinreich, FJ, Strumberg, D, Kokkalis, MK, et al.. Pressurized intraperitoneal aerosol chemotherapy with low-dose cisplatin and doxorubicin (PIPAC C/D) in patients with gastric cancer and peritoneal metastasis: a phase II study. Ther Adv Med Oncol 2019;11:1758835919846402. https://doi.org/10.1177/1758835919846402.Suche in Google Scholar PubMed PubMed Central
4. Ceelen, W. Pressurized intraperitoneal aerosol chemotherapy in peritoneal carcinomatosis: is it all up in the air? Br J Surg 2021;108:456–7. https://doi.org/10.1093/bjs/znab076.Suche in Google Scholar PubMed
5. Di Giorgio, A, Sgarbura, O, Rotolo, S, Schena, CA, Bagalà, C, Inzani, F, et al.. Pressurized intraperitoneal aerosol chemotherapy with cisplatin and doxorubicin or oxaliplatin for peritoneal metastasis from pancreatic adenocarcinoma and cholangiocarcinoma. Ther Adv Med Oncol 2020;12:1758835920940887. https://doi.org/10.1177/1758835920940887.Suche in Google Scholar PubMed PubMed Central
6. Nadiradze, G, Giger-Pabst, U, Zieren, J, Strumberg, D, Solass, W, Reymond, MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with low-dose cisplatin and doxorubicin in gastric peritoneal metastasis. J Gastrointest Surg 2016;20:367–73. https://doi.org/10.1007/s11605-015-2995-9.Suche in Google Scholar PubMed PubMed Central
7. Di Giorgio, A, Schena, CA, El Halabieh, MA, Abatini, C, Vita, E, Strippoli, A, et al.. Systemic chemotherapy and pressurized intraperitoneal aerosol chemotherapy (PIPAC): a bidirectional approach for gastric cancer peritoneal metastasis. Surg Oncol 2020;34:270–5. https://doi.org/10.1016/j.suronc.2020.05.006.Suche in Google Scholar PubMed
8. Rovers, KP, Wassenaar, ECE, Lurvink, RJ, Creemers, GM, Burger, JWA, Los, M, et al.. Pressurized intraperitoneal aerosol chemotherapy (oxaliplatin) for unresectable colorectal peritoneal metastases: a multicenter, single-arm, phase II trial (CRC-PIPAC). Ann Surg Oncol 2021;28:5311–26. https://doi.org/10.1245/s10434-020-09558-4.Suche in Google Scholar PubMed
9. Ploug, M, Graversen, M, Pfeiffer, P, Mortensen, MB. Bidirectional treatment of peritoneal metastasis with pressurized intraperitoneal aerosol chemotherapy (PIPAC) and systemic chemotherapy: a systematic review. BMC Cancer 2020;20:105. https://doi.org/10.1186/s12885-020-6572-6.Suche in Google Scholar PubMed PubMed Central
10. Odendahl, K, Solass, W, Demtröder, C, Giger-Pabst, U, Zieren, J, Tempfer, C, et al.. Quality of life of patients with end-stage peritoneal metastasis treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC). Eur J Surg Oncol 2015;41:1379–85. https://doi.org/10.1016/j.ejso.2015.06.001.Suche in Google Scholar PubMed
11. Rotolo, S, Ferracci, F, Santullo, F, Lodoli, C, Inzani, F, Abatini, C, et al.. Systemic chemotherapy and pressurized intraperitoneal aerosol chemotherapy (PIPAC): a case report of a multimodal treatment for peritoneal metastases of pancreatic origin. Int J Surg Case Rep 2020;77S:S75–8. https://doi.org/10.1016/j.ijscr.2020.10.054.Suche in Google Scholar PubMed PubMed Central
12. Rahimi-Gorji, M, Van de Sande, L, Debbaut, C, Ghorbaniasl, G, Braet, H, Cosyns, S, et al.. Intraperitoneal aerosolized drug delivery: technology, recent developments, and future outlook. Adv Drug Deliv Rev 2020;160:105–14. https://doi.org/10.1016/j.addr.2020.10.015.Suche in Google Scholar PubMed
13. Li, J, Xu, R, Hu, DM, Zhang, Y, Gong, TP, Wu, XL. Prognostic nutritional index predicts outcomes of patients after gastrectomy for cancer: a systematic review and meta-analysis of nonrandomized studies. Nutr Cancer 2019;71:557–68. https://doi.org/10.1080/01635581.2019.1577986.Suche in Google Scholar PubMed
14. Orditura, M, Galizia, G, Diana, A, Saccone, C, Cobellis, L, Ventriglia, J, et al.. Neutrophil to lymphocyte ratio (NLR) for prediction of distant metastasis-free survival (DMFS) in early breast cancer: a propensity score-matched analysis. ESMO Open 2016;1:e000038. https://doi.org/10.1136/esmoopen-2016-000038.Suche in Google Scholar PubMed PubMed Central
15. Templeton, AJ, McNamara, MG, Seruga, B, Vera-Badillo, FE, Aneja, P, Ocana, A, et al.. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. https://doi.org/10.1093/jnci/dju124.Suche in Google Scholar PubMed
16. Hong-Bo Yang, MX, Ma, L-N, Feng, L-X, Zhuang, Y. Prognostic significance of neutrophil-lymphocyteratio/platelet-lymphocyteratioin lung cancers: a meta-analysis. Oncotarget 2016;22:76769–78. https://doi.org/10.18632/oncotarget.12526.Suche in Google Scholar PubMed PubMed Central
17. Rinninella, E, Cintoni, M, Raoul, P, Pozzo, C, Strippoli, A, Bria, E, et al.. Effects of nutritional interventions on nutritional status in patients with gastric cancer: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr ESPEN 2020;38:28–42. https://doi.org/10.1016/j.clnesp.2020.05.007.Suche in Google Scholar PubMed
18. Rinninella, E, Fagotti, A, Cintoni, M, Raoul, P, Scaletta, G, Quagliozzi, L, et al.. Nutritional interventions to improve clinical outcomes in ovarian cancer: a systematic review of randomized controlled trials. Nutrients 2019;11:1404. https://doi.org/10.3390/nu11061404.Suche in Google Scholar PubMed PubMed Central
19. Sugiyama, K, Narita, Y, Mitani, S, Honda, K, Masuishi, T, Taniguchi, H, et al.. Baseline sarcopenia and skeletal muscle loss during chemotherapy affect survival outcomes in metastatic gastric cancer. Anticancer Res 2018;38:5859–66. https://doi.org/10.21873/anticanres.12928.Suche in Google Scholar PubMed
20. Onodera, T, Goseki, N, Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984;85:1001–5.Suche in Google Scholar
21. Solaß, W, Hetzel, A, Nadiradze, G, Sagynaliev, E, Reymond, MA. Description of a novel approach for intraperitoneal drug delivery and the related device. Surg Endosc 2012;26:1849–55. https://doi.org/10.1007/s00464-012-2148-0.Suche in Google Scholar PubMed
22. Sgarbura, O, Villeneuve, L, Alyami, M, Bakrin, N, Torrent, JJ, Eveno, C, et al.. Current practice of pressurized intraperitoneal aerosol chemotherapy (PIPAC): still standardized or on the verge of diversification? Eur J Surg Oncol 2021;47:149–56. https://doi.org/10.1016/j.ejso.2020.08.020.Suche in Google Scholar PubMed
23. Robella, M, De Simone, M, Berchialla, P, Argenziano, M, Borsano, A, Ansari, S, et al.. A phase I dose escalation study of oxaliplatin, cisplatin and doxorubicin applied as PIPAC in patients with peritoneal carcinomatosis. Cancers 2021;13:1060. https://doi.org/10.3390/cancers13051060.Suche in Google Scholar PubMed PubMed Central
24. Agalar, C, Sokmen, S, Arslan, C, Altay, C, Basara, I, Canda, AE, et al.. The impact of sarcopenia on morbidity and long-term survival among patients with peritoneal metastases of colorectal origin treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a 10-year longitudinal analysis of a single-center experience. Tech Coloproctol 2020;24:301–8. https://doi.org/10.1007/s10151-020-02159-z.Suche in Google Scholar PubMed
25. Banaste, N, Rousset, P, Mercier, F, Rieussec, C, Valette, PJ, Glehen, O, et al.. Preoperative nutritional risk assessment in patients undergoing cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for colorectal carcinomatosis. Int J Hyperther 2018;34:589–94. https://doi.org/10.1080/02656736.2017.1371342.Suche in Google Scholar PubMed
26. Hilal, Z, Rezniczek, GA, Klenke, R, Dogan, A, Tempfer, CB. Nutritional status, cachexia, and anorexia in women with peritoneal metastasis and intraperitoneal chemotherapy: a longitudinal analysis. J Gynecol Oncol 2017;28:e80. https://doi.org/10.3802/jgo.2017.28.e80.Suche in Google Scholar PubMed PubMed Central
27. Nowacki, M, Alyami, M, Villeneuve, L, Mercier, F, Hubner, M, Willaert, W, et al.. Multicenter comprehensive methodological and technical analysis of 832 pressurized intraperitoneal aerosol chemotherapy (PIPAC) interventions performed in 349 patients for peritoneal carcinomatosis treatment: an international survey study. Eur J Surg Oncol 2018;44:991–6. https://doi.org/10.1016/j.ejso.2018.02.014.Suche in Google Scholar PubMed
28. Tempfer, C, Giger-Pabst, U, Hilal, Z, Dogan, A, Rezniczek, GA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal carcinomatosis: systematic review of clinical and experimental evidence with special emphasis on ovarian cancer. Arch Gynecol Obstet 2018;298:243–57. https://doi.org/10.1007/s00404-018-4784-7.Suche in Google Scholar PubMed
29. Khosrawipour, T, Khosrawipour, V, Giger-Pabst, U. Pressurized intra peritoneal aerosol chemotherapy in patients suffering from peritoneal carcinomatosis of pancreatic adenocarcinoma. PLoS One 2017;12:e0186709. https://doi.org/10.1371/journal.pone.0186709.Suche in Google Scholar PubMed PubMed Central
30. Takamizawa, Y, Shida, D, Boku, N, Nakamura, Y, Ahiko, Y, Yoshida, T, et al.. Nutritional and inflammatory measures predict survival of patients with stage IV colorectal cancer. BMC Cancer 2020;20:1092. https://doi.org/10.1186/s12885-020-07560-3.Suche in Google Scholar PubMed PubMed Central
31. Liao, G, Zhao, Z, Yang, H, Chen, M, Li, X. Can prognostic nutritional index be a prediction factor in esophageal cancer?: a meta-analysis. Nutr Cancer 2020;72:187–93. https://doi.org/10.1080/01635581.2019.1631859.Suche in Google Scholar PubMed
32. Xue, Y, Zhou, X, Xue, L, Zhou, R, Luo, J. The role of pretreatment prognostic nutritional index in esophageal cancer: a meta-analysis. J Cell Physiol 2019;234:19655–62. https://doi.org/10.1002/jcp.28565.Suche in Google Scholar PubMed PubMed Central
33. Dai, Y, Liu, M, Lei, L, Lu, S. Prognostic significance of preoperative prognostic nutritional index in ovarian cancer: a systematic review and meta-analysis. Medicine (Baltim) 2020;99:e21840. https://doi.org/10.1097/md.0000000000021840.Suche in Google Scholar PubMed PubMed Central
34. Nie, R, Yuan, S, Chen, S, Chen, X, Chen, Y, Zhu, B, et al.. Prognostic nutritional index is an independent prognostic factor for gastric cancer patients with peritoneal dissemination. Chin J Cancer Res 2016;28:570–8. https://doi.org/10.21147/j.issn.1000-9604.2016.06.03.Suche in Google Scholar PubMed PubMed Central
35. Archid, R, Solass, W, Tempfer, C, Königsrainer, A, Adolph, M, Reymond, MA, et al.. Cachexia anorexia syndrome and associated metabolic dysfunction in peritoneal metastasis. Int J Mol Sci 2019;20:5444. https://doi.org/10.3390/ijms20215444.Suche in Google Scholar PubMed PubMed Central
36. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyère, O, Cederholm, T, et al.. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:601. https://doi.org/10.1093/ageing/afz046.Suche in Google Scholar PubMed PubMed Central
37. Fielding, RA, Vellas, B, Evans, WJ, Bhasin, S, Morley, JE, Newman, AB, et al.. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011;12:249–56. https://doi.org/10.1016/j.jamda.2011.01.003.Suche in Google Scholar PubMed PubMed Central
38. Mele, MC, Rinninella, E, Cintoni, M, Pulcini, G, Di Donato, A, Grassi, F, et al.. Nutritional support in lung cancer patients: the state of the art. Clin Lung Cancer 2020;22:e584–94. https://doi.org/10.1016/j.cllc.2020.10.008.Suche in Google Scholar PubMed
39. Rinninella, E, Cintoni, M, Raoul, P, Pozzo, C, Strippoli, A, Bria, E, et al.. Muscle mass, assessed at diagnosis by L3-CT scan as a prognostic marker of clinical outcomes in patients with gastric cancer: a systematic review and meta-analysis. Clin Nutr 2020;39:2045–54. https://doi.org/10.1016/j.clnu.2019.10.021.Suche in Google Scholar PubMed
40. Giger-Pabst, U, Demtröder, C, Falkenstein, TA, Ouaissi, M, Götze, TO, Rezniczek, GA, et al.. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for the treatment of malignant mesothelioma. BMC Cancer 2018;18:442. https://doi.org/10.1186/s12885-018-4363-0.Suche in Google Scholar PubMed PubMed Central
41. Chemama, S, Bayar, MA, Lanoy, E, Ammari, S, Stoclin, A, Goéré, D, et al.. Sarcopenia is associated with chemotherapy toxicity in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer. Ann Surg Oncol 2016;23:3891–8. https://doi.org/10.1245/s10434-016-5360-7.Suche in Google Scholar PubMed
42. Galan, A, Rousset, P, Mercier, F, Képénékian, V, Valette, PJ, Glehen, O, et al.. Overall survival of pseudomyxoma peritonei and peritoneal mesothelioma patients after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy can be predicted by computed tomography quantified sarcopenia. Eur J Surg Oncol 2018;44:1818–23. https://doi.org/10.1016/j.ejso.2018.07.060.Suche in Google Scholar PubMed
43. McSharry, V, Mullee, A, McCann, L, Rogers, AC, McKiernan, M, Brennan, DJ. The impact of sarcopenia and low muscle attenuation on overall survival in epithelial ovarian cancer: a systematic review and meta-analysis. Ann Surg Oncol 2020;27:3553–64. https://doi.org/10.1245/s10434-020-08382-0.Suche in Google Scholar PubMed
44. Rinninella, E, Cintoni, M, Raoul, P, Mele, MC, De Gaetano, AM, Marini, MG, et al.. Minimal impact of lenvatinib (Lenvima®) on muscle mass in advanced hepatocellular carcinoma and implications for treatment duration. Two cases from the REFLECT study. Eur Rev Med Pharmacol Sci 2019;23:10132–8. https://doi.org/10.26355/eurrev_201911_19583.Suche in Google Scholar PubMed
45. Sandini, M, Patino, M, Ferrone, CR, Alvarez-Pérez, CA, Honselmann, KC, Paiella, S, et al.. Association between changes in body composition and neoadjuvant treatment for pancreatic cancer. JAMA Surg 2018;153:809–15. https://doi.org/10.1001/jamasurg.2018.0979.Suche in Google Scholar PubMed PubMed Central
46. Rinninella, E, Strippoli, A, Cintoni, M, Raoul, P, Vivolo, R, Di Salvatore, M, et al.. Body composition changes in gastric cancer patients during preoperative FLOT therapy: preliminary results of an Italian cohort study. Nutrients 2021;13:960. https://doi.org/10.3390/nu13030960.Suche in Google Scholar PubMed PubMed Central
47. Arends, J, Baracos, V, Bertz, H, Bozzetti, F, Calder, PC, Deutz, NEP, et al.. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr 2017;36:1187–96. https://doi.org/10.1016/j.clnu.2017.06.017.Suche in Google Scholar PubMed
48. Arends, J, Bachmann, P, Baracos, V, Barthelemy, N, Bertz, H, Bozzetti, F, et al.. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11–48. https://doi.org/10.1016/j.clnu.2016.07.015.Suche in Google Scholar PubMed
49. Rinninella, E, Persiani, R, D’Ugo, D, Pennestrì, F, Cicchetti, A, Di Brino, E, et al.. NutriCatt protocol in the enhanced recovery after surgery (ERAS) program for colorectal surgery: the nutritional support improves clinical and cost-effectiveness outcomes. Nutrition 2018;50:74–81. https://doi.org/10.1016/j.nut.2018.01.013.Suche in Google Scholar PubMed
50. Blackwood, HA, Hall, CC, Balstad, TR, Solheim, TS, Fallon, M, Haraldsdottir, E, et al.. A systematic review examining nutrition support interventions in patients with incurable cancer. Support Care Cancer 2020;28:1877–89. https://doi.org/10.1007/s00520-019-04999-4.Suche in Google Scholar PubMed
© 2022 Stefano Rotolo et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Consensus statement for treatment protocols in pressurized intraperitoneal aerosol chemotherapy (PIPAC)
- Body composition and immunonutritional status in patients treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC) for gastrointestinal peritoneal metastases: a prospective single-center analysis
- Association of intraoperative gross hematuria with acute kidney injury after cytoreductive surgery
- Some pleural effusions labeled as idiopathic could be produced by the inhalation of silica
- Short Communication
- A severe oxaliplatin immune-induced syndrome after oxaliplatin-based pressurized intraperitoneal aerosol chemotherapy (PIPAC)
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Consensus statement for treatment protocols in pressurized intraperitoneal aerosol chemotherapy (PIPAC)
- Body composition and immunonutritional status in patients treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC) for gastrointestinal peritoneal metastases: a prospective single-center analysis
- Association of intraoperative gross hematuria with acute kidney injury after cytoreductive surgery
- Some pleural effusions labeled as idiopathic could be produced by the inhalation of silica
- Short Communication
- A severe oxaliplatin immune-induced syndrome after oxaliplatin-based pressurized intraperitoneal aerosol chemotherapy (PIPAC)

