Abstract
Background
To analyse the duration of parenteral nutrition (PN) in patients treated for peritoneal malignancy with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) over a 2 year period at a single UK National referral centre.
Methods
A retrospective analysis of prospective data for all patients (n=321) who underwent CRS and HIPEC for peritoneal malignancy at the Peritoneal Malignancy Institute Basingstoke between April 1, 2013 and March 31, 2015.
Duration of PN was compared between primary tumour site (appendix, colorectal, mesothelioma and other); completeness of CRS (complete CRS vs. major tumour debulking) and pre-operative nutritional assessment measures (including Mid Upper Arm Circumference).
Results
The median duration of PN was 9 days (range 2–87 days). A total of 13 % of patients had PN for less than 7 days and 6 % for 5 days or less. There was no significant difference in duration of PN between the different tumour sites. Two factors that may increase the duration of PN include having major tumour debulking (MTD) and a baseline MUAC<23.5 cm.
Conclusions
Most patients who underwent CRS and HIPEC for peritoneal malignancy required PN for more than 7 days with poor pre-operative nutritional status and inability to achieve complete cytoreduction predictors of prolonged PN requirements.
Introduction
Cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) was initially developed and popularized for Pseudomyxoma peritonei (PMP) of appendiceal origin [1, 2, 3] or primary peritoneal tumours with favourable biological behaviour, such as multicystic peritoneal mesothelioma [4, 5]. The key elements involve extensive peritonectomy and resection of involved organs, aiming for complete macroscopic tumour removal (complete CRS) combined with intraoperative HIPEC aiming to destroy any remaining microscopic disease. Favourable outcomes in many patients with PMP led to the extension of the treatment to other peritoneal malignancies, including those of ovarian or colorectal origin [6, 7]. The Peritoneal Malignancy Institute Basingstoke, part of Hampshire Hospitals Foundation Trust was commissioned as a national centre for the assessment and surgical management of PMP in 2000.
PMP is an uncommon malignancy which generally originates from a perforated tumour of the appendix. It is characterized by the presence of diffuse intra-abdominal gelatinous fluid collections and mucinous implants on peritoneal surfaces and the omentum, presenting the clinical picture of ‘jelly belly’. PMP may present unexpectedly at surgery, or as an incidental finding on cross-sectional imaging [3]. The optimal treatment for PMP is now considered complete CRS involving a series of peritonectomies and resection of involved organs [8]. If the tumour cannot be completely removed (generally because of extensive small bowel involvement or suboptimal patient performance status) MTD is performed [9]. This procedure involves removal of as much tumour as possible which generally includes the right colon with an ileocolic anastomosis or a total colectomy with an end ileostomy, an omentectomy and in females bilateral salpingo-oophorectomy. Progression of residual disease is almost inevitable. The traditional concept of repeat “debulking surgery” is rarely possible with a strategy of maximal tumour debulking at the initial operation. Traditional debulking involved minimal organ resection with evacuation of mucus but each attempt becomes more difficult and dangerous. The small bowel becomes increasingly involved due to adhesions following surgery and eventually surgery is impossible and is fraught with severe complications such as small bowel fistulae.
Nutritional support is a key component in the optimal treatment of patients with peritoneal malignancy. In 2003, a dedicated Nutrition Team was established at the Peritoneal Malignancy Institute consisting of specialist dieticians, a pharmacist and a consultant gastroenterologist. The nutrition strategy adopted for all patients undergoing CRS for peritoneal malignancy is that parenteral nutrition (PN) is commenced the day after surgery. This is usually at half of the patients’ nutritional requirements as per the National Institute for Health and Care Excellence guidelines [10] gradually increasing to full requirements and PN is continued until oral intake is adequate. The rationale is based on the extensive nature of the surgery and the effects on gastro-intestinal tract function of organ resection, peritonectomy and HIPEC with a resultant prolonged ileus.
All patients are seen by the nutrition team prior to surgery and undergo a thorough nutritional assessment. They are measured, weighed and scored as per the nationally recognised, Malnutrition Universal Screening Tool (MUST) [11]. MUST takes into account a patient’s Body Mass Index (BMI), unplanned weight loss and also an Acute Disease Effect (ADE). Patients score for the ADE if they are acutely ill and if there has been, or is likely to be, no nutritional intake for 5 days. Due to many patients having abdominal distension a Mid Upper Arm Circumference (MUAC) is also taken in order to estimate a dry body weight.
The weight (or dry weight for distended patients) is used for estimating nutritional requirements which are based on calculating Basal Metabolic Rate as per Henry’s Method [12] and then adding stress and activity factors as necessary. Due to the extensive nature of surgery a stress factor of 20 % and activity factor of 10 % is usually applied. The rate of the feed is increased gradually so patients are meeting their full nutritional requirements (goal rate) by day 4 after surgery. Nitrogen requirements of 0.17–0.2 g/kg/day are used. The PN used in this study was BBraun triple chamber bags, namely Nutriflex Lipid Peri and Nutriflex Omega Plus. The bags contain amino acids, lipid and glucose and the closest bag to the nutritional requirements of a patient is used. In some occasions, only a specific percentage of the bag is used to best fit a patient’s calorie and nitrogen requirement. Cernevit and additrace are added to each of the bags in order to provide patients with vitamins and micronutrients. PN is delivered via a dedicated lumen of a central venous catheter.
The decision to allow patients to commence oral food is made either by the nutrition team or the surgical team. Patients’ oral intake is assessed on a daily basis. Factors such as nasogastric output volumes, bowel function and patient’s appetite are all taken into consideration when deciding when oral intake can be increased from fluids to solid food. Oral intake is initiated with sips of water once a patient is extubated and awake. The amount of fluids allowed is gradually increased under the guidance of the surgical team until the patient is tolerating soup, ice cream and jelly, commonly referred to as “free fluids” in UK hospitals. Usually at this time the patient is gradually weaned off PN over a period of 1–2 days as they build up intake of solid food. Early enteral nutrition is poorly tolerated due to most patients having a greater omentectomy resecting the gastroepiploic arcade which can result in transient gastroparesis. In addition, handling the small bowel along with administration of HIPEC can lead to ileus.
The purpose of this study was to examine the duration of PN after CRS and HIPEC for peritoneal malignancy at the Peritoneal Malignancy Institute, in order to assess adherence to NICE guidelines for nutritional support and to inform future pathways for nutritional support in this patient group.
Materials and methods
We retrospectively reviewed prospectively collected nutrition and surgical data for all patients who had CRS and HIPEC for the treatment of peritoneal malignancy at the Peritoneal Malignancy Institute over a 2 year period (April 1, 2013 to March 31, 2015).
Data was extracted from the Peritoneal Malignancy Institute database and included the following parameters: Number of postoperative days on PN; MUAC measured by the dietitian during the nutritional assessment prior to surgery; main procedure (complete CRS vs. MTD); primary tumour site; duration (minutes) of open surgery; duration (days) of hospital stay; and Grade 3 and 4 Clavien Dindo complications.
Inferential statistics were computed in order to examine whether there was a statistically significant difference between duration of postoperative PN as a function of the main procedure, primary tumour site or MUAC.
Ethical approval
This article is a Quality Assurance report and it was deemed unnecessary to obtain ethical approval by the institute’s research and development department.
Results
Patient characteristics
A total of 321 patients underwent CRS and HIPEC for peritoneal malignancy at the Peritoneal Malignancy Institute between April 1, 2013 and March 31, 2015. Seven of these patients were excluded from the analysis due to either: 30 day mortality (n=3), discharged home on PN (n=1), the surgery being an ‘open and close’ case (n=2), the patient unable to have PN due to poor venous access (n=1). This left a study population of 314 patients.
Overall 6 % of patients had an MUAC of<23.5 cm (BMI is likely to be<20 kg/m2), 68 % of patients had a MUAC between 23.5–32 cm (BMI is likely to be between 20 and 30 kg/m2) and 26 % of patients had an MUAC of>32 cm (BMI is likely to be>30 kg/m2).
Surgical treatment categories
In total, 262 patients (83 %) underwent Complete CRS whilst the remaining 52 (17 %) underwent MTD. In 77 % of patients, the primary tumour site was the appendix, in 13 % colorectal, 5 % peritoneal mesothelioma and the remaining 5 % included ovarian, sarcoma and unknown/other (Table 1).
Surgical characteristics of 314 patients treated with cytoreductive surgery and HIPEC for peritoneal malignancy.
| Main Procedure | No. of patients | % |
|---|---|---|
| Complete CRS | 262 | 83 % |
| Major Debulk | 52 | 17 % |
| Primary Tumour Site | ||
| Appendix | 242 | 77 % |
| Colorectal | 40 | 13 % |
| Peritoneal mesothelioma | 16 | 5 % |
| Other | 16 | 5 % |
| Clavien Dindo Grade | ||
| ≤2 | 275 | 87.6 % |
| 3a/3b | 29 | 9 % |
| 4a/4b | 10 | 3.2 % |
The median duration of the 314 operative procedures was 481.5 min (range 119–870 min). The median length of hospital stay was 21 days (range 9–109 days).
Analysis of Clavien- Dindo complication data revealed that 275 (87.6 %) of patients had a Clavien- Dindo grade≤2, 29 (9 %) had a grade 3 complication and 10 (3.18 %) had a grade 4 complication (Table 2).
Postoperative clavien dindo complications grades.
| No. of patients (percentage) | |||
|---|---|---|---|
| Clavien Dindo grade | Complete CRS | Major Debulk | Total |
| ≤2 | 228 (87 %) | 47 (90.4 %) | 275 (87.58 %) |
| 3a/3b | 24 (9.2 %) | 5 (9.6 %) | 29 (9.24 %) |
| 4a/4b | 10 (3.8 %) | 0 | 10 (3.18 %) |
| Grand Total | 262 | 52 | 314 |
Nutrition support duration
The median duration of postoperative PN was 9 days (range 1–87 days). Nineteen patients (6 %) required PN for 5 days or less (Figure 1), and 42 patients (13 %) required PN for less than 7 days.
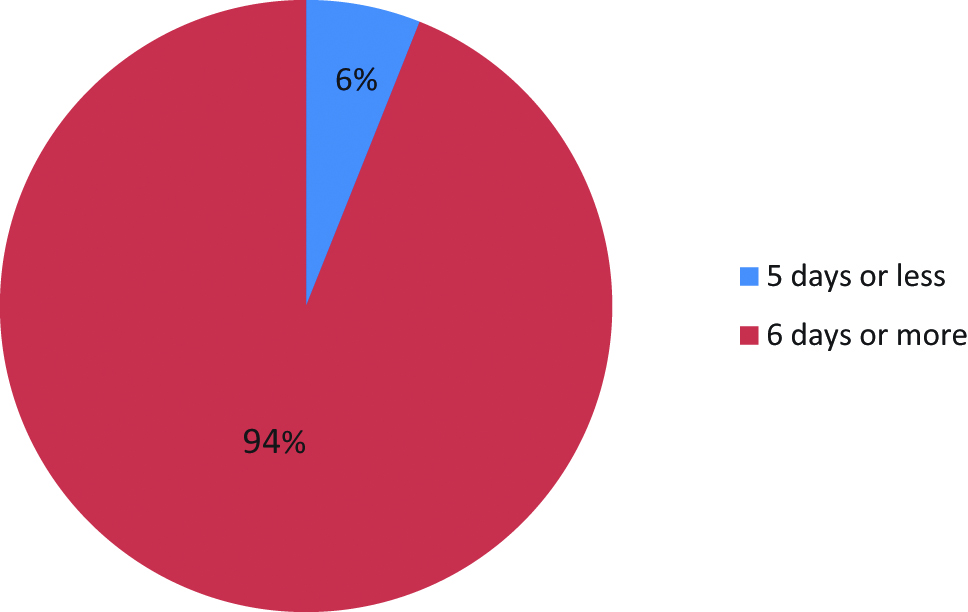
Percentage of patients on postoperative PN for 5 days or less.
Patients who had completed CRS received PN for a median duration of 9 days (range 1–87 days), compared with a median of 11 days (range 5–51) for patients who underwent MTD. This difference was not statistically significantly (U=5761, p=0.078).
There was no difference in the duration of postoperative PN between patients who had different primary tumour sites (H(5)=2.55, p>0.05) (Figure 2).
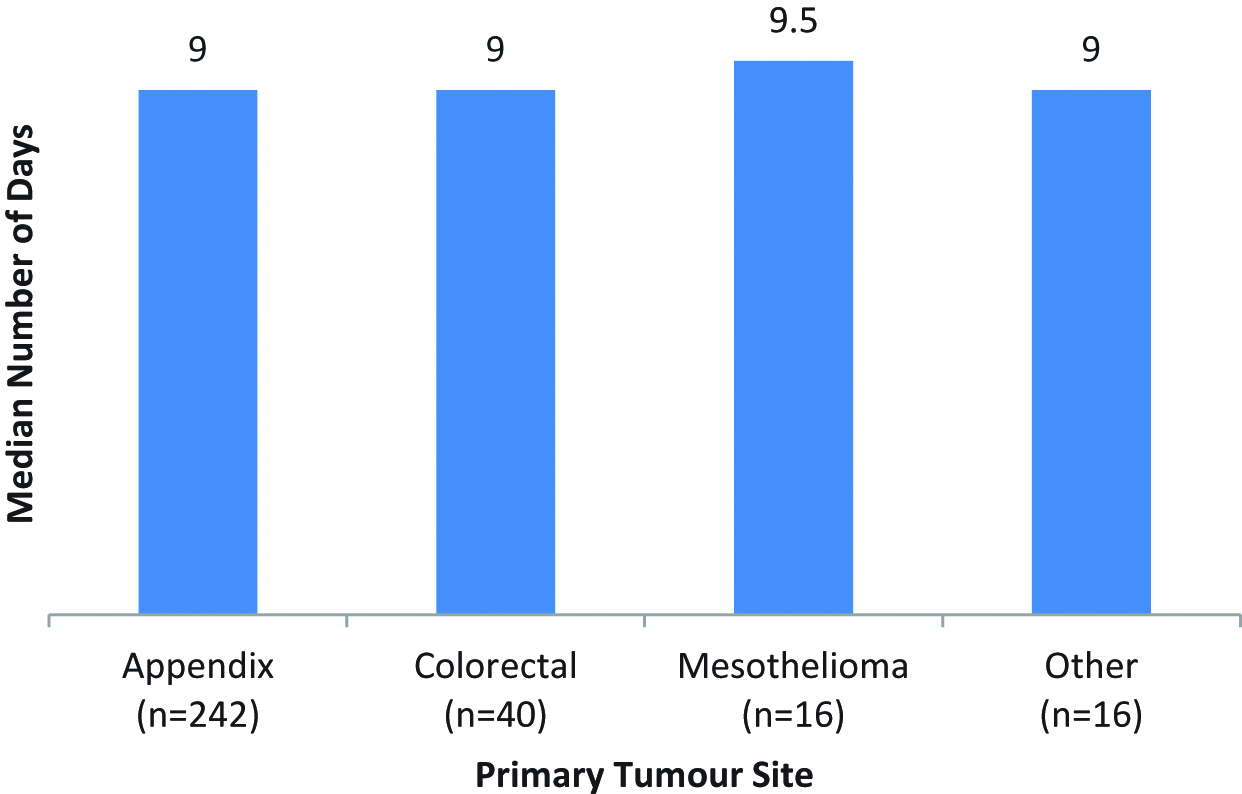
Comparison of number of days on PN by primary tumour site.
The duration of postoperative PN was not significantly longer (U=2003, p=0.076) for patients with an MUAC of<23.5 (Median=11.5) than for patients with an MUAC≥23.5 (Median=9).
Discussion
There is currently no agreed consensus as to the best route for nutrition support after CRS. In 2008 Mcquellon et al. [13] discussed ‘Recommendations for Nutritional Management’ and advised that prospective randomized studies were needed to explore and define the best timing and route of delivery for perioperative nutrition in this unique patient group.
The current study, despite being retrospective, outlines findings that may help to reach a consensus.
Firstly, very few patients (6 %) were able to tolerate oral intake before the fifth postoperative day. National UK guidelines suggest PN is indicated where the consequent intestinal failure is likely to last for 5 days or longer (NICE, 2006) [11].
It was somewhat surprising that there was no difference between duration of PN and primary site of tumour. It was assumed that colorectal peritoneal metastases (CPM) patients might need PN for a shorter period than those with classic PMP. This was assumed as these patients tend to have fewer peritonectomies, shorter duration of surgery and thus one would guess that PN requirements would be less. This may have been due to the relatively small amount of patients with CPM in this cohort.
Two factors that may well increase the duration of PN include having MTD rather than a complete CRS and having a MUAC<23.5 cm. Although both of these were not significantly different, this may also be due to the small number of patients in these groups. Only 17 % of the cohort had MTD, and only 6 % patients had a MUAC<23.5 cm.
We acknowledge that PN is not used routinely in all units worldwide performing CRS. Vashi et al. (2013) [14] used PN in to 31/60 (52 %) of patients. Their decision to use PN depended on the patients preoperative Subjective Global Assessment (SGA) score and presence of bowel obstruction. The conclusion was that preoperative SGA predicts length of stay and survival in cancer patients undergoing HIPEC. They did acknowledge that no definitive conclusions could be made regarding the role of PN in improving clinical outcomes in this group. It would also appear that placing feeding tubes also does not improve outcomes in this patient group. Dineen et al. 2016 [15] found that placement of enteral tubes did not improve postoperative nutrition and was associated with a longer length of stay and higher readmission rates. Our early experiences of inserting nasojejunal tubes resulted in tubes being displaced and feed not being absorbed.
It is our belief that routine PN best aids the patients’ recovery. By giving all patients PN after surgery it ensures that nutritional treatment is not delayed. It can also be beneficial to have standard protocols, particularly in an expanding service. A standard protocol ensures that nursing staff and all members of the multidisciplinary team understand the patient’s treatment plan and are more likely to ensure procedures are undertaken at specific times and in an organized manner. For instance by giving all patients postoperative PN, a dedicated lumen from a central venous catheter can be reserved immediately on line insertion. Furthermore, in light of the recent CALORIES trial [16], showing no difference in 30 day mortality between critically ill patients receiving enteral vs. PN, the data supports the use of PN in critically ill patients without any recognised major adverse effects.
To our knowledge there is no publication in the literature on the role, or methodology, of nutritional support in a large group of patients undergoing CRS and HIPEC. It is difficult to directly compare the experiences of Vashi et al. as patients in Vashi et al. report were all selected patients with peritoneal metastases, whereas the majority (77 %) of patients at Basingstoke underwent treatment for PMP [17]. It is apparent that more units are undertaking or contemplating CRS in the future, particularly for the treatment of colorectal peritoneal metastases.
In conclusion, our experience suggests that patients undergoing CRS and HIPEC should have routine post-operative PN as interventions are long and complex with a high risk of complications and post-operative ileus. The advantages of having a standard protocol aiming for all patients to meet their nutritional requirements outweighs the small risk of overuse of PN as illustrated by the small number of patients in our experience tolerating oral intake before the fifth postoperative day. To date there is no clear group of patients that seem to require PN for a shorter time, or not at all, although the number and location of bowel resections may well affect the duration of a paralytic ileus. Nutritional support is a key part in optimal management of patients with peritoneal malignancy and future clinical trials should investigate selective use of enteral vs. PN and with appropriate selection criteria randomization might be feasible.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: One of the authors, Faheez Mohamed, is on the Editorial Board of this Journal.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6:727–31.10.1007/s10434-999-0727-7Suche in Google Scholar PubMed
2. Youssef H, Newman C, Chandrakumaran K, Mohamed F, Cecil TD, Moran BJ. Operative findings, early complications and long-term survival in 456 patients with pseudomyxoma peritonei of appendiceal origin. Dis Colon Rectum. 2011;54:293–9.10.1007/DCR.0b013e318202f026Suche in Google Scholar PubMed
3. Moran BJ, Cecil TD. The aetiology, clinical presentation, and management of pseudomyxoma peritonei. Sug Oncol Clin North Am. 2003;12:385–603.Suche in Google Scholar
4. Yan TD, Deraco M, Baratti D, Kusamuna S, Elias D, Glehen O, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27:6237–42.10.1200/JCO.2009.23.9640Suche in Google Scholar PubMed
5. Chua TC, Yan TD, Deraco M, Glehen O, Moran BJ, Sugarbaker PH. Peritoneal Surface Oncology Group. Multi-institutional experience of diffuse intra-abdominal multicystic peritoneal mesothelioma. Br J Surg. 2011;98:60–4.10.1002/bjs.7263Suche in Google Scholar PubMed
6. Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–8.10.1200/JCO.2009.23.9285Suche in Google Scholar PubMed
7. Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L, et al. French Surgical Association. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116:5608–18.10.1002/cncr.25356Suche in Google Scholar PubMed
8. Moran BJ, Mukherjee A, Sexton R. Operability and early outcome in 100 consecutive laparotomies for peritoneal malignancy. Br J Surg. 2006;93:100–04.10.1002/bjs.5210Suche in Google Scholar PubMed
9. Dayal S, Taflampas P, Riss S, Chandrakumaran K, Cecil TD, Mohamed F, et al. Complete cytoreduction for pseudomyxoma peritonei is optimal but maximal tumor debulking may be beneficial in patients in whom complete tumor removal cannot be achieved. Dis Colon Rectum. 2013;56:1366–72.10.1097/DCR.0b013e3182a62b0dSuche in Google Scholar PubMed
10. NICE (National Institute for Health and Clinical Excellence). Nutrition support for adults. Clin Guidel. 2006;32:28–30.Suche in Google Scholar
11. BAPEN. Malnutrition Universal Screening Tool’ (The ‘MUST’). November 2003. Available at: www.bapen.org.uk/the-must.htmSuche in Google Scholar
12. Henry CJK. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr. 2005;8:1133–52.10.1079/PHN2005801Suche in Google Scholar PubMed
13. Mcquellon R, Gavazzi C, Piso P, Swain D, Levine E. Quality of life and nutritional assessment in Peritoneal Surface Malignancy (PSM): recommendations for care. J Surg Oncol. 2008;98:300–05.10.1002/jso.21050Suche in Google Scholar PubMed
14. Vashi P, Gupta D, Lammersfield C, Braun D, Popiel B, Misra S, et al. The relationship between baseline nutritional status with subsequent parenteral nutrition and clinical outcomes in cancer patients undergoing hyperthermic intraperitoneal chemotherapy. Nutrition. 2013;12:118.10.1186/1475-2891-12-118Suche in Google Scholar PubMed PubMed Central
15. Dineen SP, Robinson KA, Roland CL, Beaty KA, Rafeeq S, Mansfield PF, et al. Feeding tube placement during cytoreductive surgery and heated intraperitoneal chemotherapy does not improve postoperative nutrition and is associated with longer length of stay and higher admission rates. J Surg Res. 2016;200:158–63.10.1016/j.jss.2015.08.003Suche in Google Scholar PubMed
16. Harvey S, Parrott F, Harrison D, Bear DE, Seagaran E, Beale R, et al. Trial of the route of early nutritional support in critically Ill adults. N Engl J Med. 2014;371:1673–84.10.1056/NEJMoa1409860Suche in Google Scholar PubMed
17. Moran B, Cecil T, Chandrakumaran K, Arnold S, Mohamed F, Venkatasubramanium A. The results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1200 patients with peritoneal malignancy. INT J Colorectal Dis. 2015;17:772–8.10.1111/codi.12975Suche in Google Scholar PubMed
© 2018 Swain et al, published by De Gruyter
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Artikel in diesem Heft
- Editorial
- Cytoreductive Surgery (CRS) and Hyperthermic IntraPeritoneal Chemotherapy (HIPEC): don’t throw the baby out with the bathwater
- Review
- Photodynamic therapy and photothermal therapy for the treatment of peritoneal metastasis: a systematic review
- Original Articles
- Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) as an outpatient procedure
- Do patients undergoing cytoreductive surgery and HIPEC for peritoneal malignancy need parenteral nutrition?
- Biphasic learning curve of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy:technical competence and refinement of patient selection
Artikel in diesem Heft
- Editorial
- Cytoreductive Surgery (CRS) and Hyperthermic IntraPeritoneal Chemotherapy (HIPEC): don’t throw the baby out with the bathwater
- Review
- Photodynamic therapy and photothermal therapy for the treatment of peritoneal metastasis: a systematic review
- Original Articles
- Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) as an outpatient procedure
- Do patients undergoing cytoreductive surgery and HIPEC for peritoneal malignancy need parenteral nutrition?
- Biphasic learning curve of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy:technical competence and refinement of patient selection


