Abstract
C22H30Cl2FeN10O8S4, orthorhombic, Pccn (no. 56), a = 17.905(6) Å, b = 13.141(4) Å, c = 14.918(5) Å, V = 3510.2(19) Å3, Z = 4, Rgt(F) = 0.0763, wRref(F2) = 0.2417, T = 296(2) K.
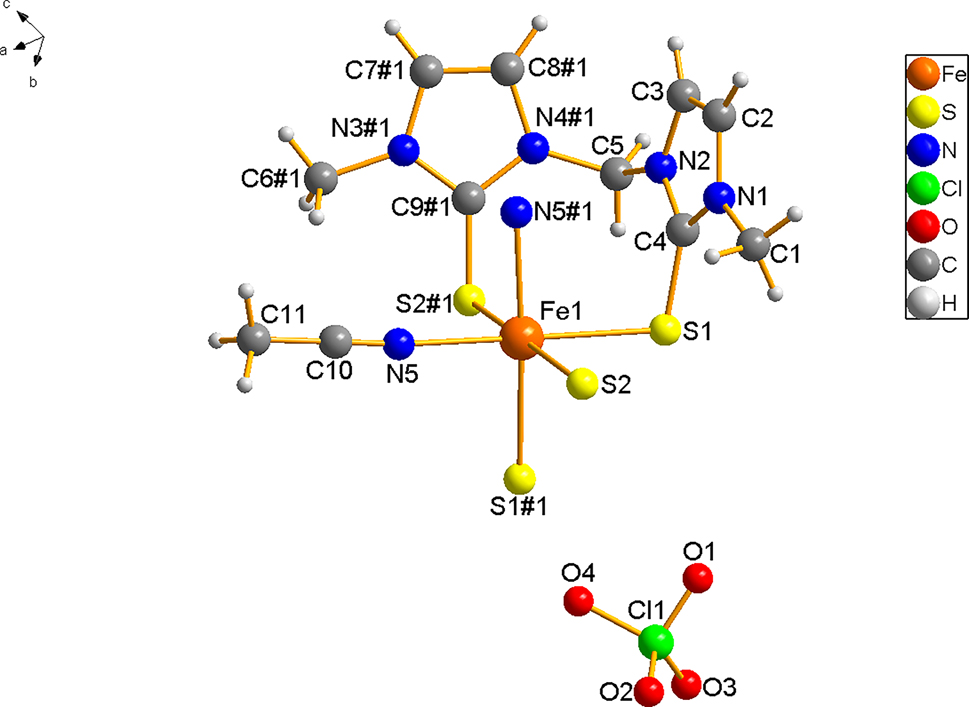
A part of the molecular structure of the title complex is shown in the figure. The symmetic code #1 is: 1/2−x, 1/2−y, z. Table 1 contains crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Red rodlike |
| Size: | 0.25 × 0.13 × 0.12 mm |
| Wavelength: | MoKα radiation (0.71073 Å) |
| μ: | 0.88 mm−1 |
| Diffractometer, scan mode: | APEX2, φ and ω scans |
| θmax, completeness: | 28.4°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 28309, 4110, 0.069 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2972 |
| N(param)refined: | 216 |
| Programs: | Bruker, 1 Olex2 , 2 SHELX 3 , 4 |
1 Source of material
The title complex [Fe(C9H12N4S2)2(CH3CN)2](ClO4)2 was synthesized by a direct synthesis. All reagents used were purchased from commercial sources and used without further purification. The 3,3′-methyl-enebis(1-methyl-1,3-dihydro-2H-imidazole-2-thione)methane (C9H12N4S2, abbreviated as mbit) was synthesized by the previously reported route. 4 , 5 , 6 A mixture of FeCl2 (63 mg, 0.5 mmol) and Ag(ClO4)2 (210 mg, 1 mmol) was dissolved in 10 mL CH3CN in a N2 atmosphere, stirred, then filtered to remove solid substances after the reaction was complete. Also under N2 atmosphere, mbit (240 mg, 1 mmol) was added to the filtrate, and stirring was performed for 8 h. Red rod shaped crystals were obtained by slowly evaporating the filtrate at room temperature.
2 Experimental details
The structure was solved by Direct Methods and refined with the Olex 1 , 2 and SHELXL 3 software package. The figure of the title crystal structure was drawn by the Diamond software. 7 Hydrogen atoms attached to C were positioned geometrically and refined as riding atoms. The U iso values were set to be 1.5U eq (C) of the carrier atom for methyl H atoms and 1.2U eq (C) for the remaining H atoms.
3 Comment
The structures of organic ligands usually play a decisive role in the configuration and properties of metal complexes. Therefore, in the synthesis process of functional metal complexes, it is very necessary to choose appropriate ligands. As a bidentate sulfur-containing ligand, mbit is often chosen to construct zero dimensional, one-dimensional, or three-dimensional complexes. 4 , 8 , 9 , 10 , 11 , 12 , 13 The S atoms in mbit can sometimes act as bridging atoms to form multinuclear complexes. 10 , 14 In the research process of transition metal complexes, iron has attracted much attention due to its abundant natural reservers, diverse coordination forms, excellent biocompatibility, and outstanding light absorption properties. Therefore, we designed and synthesized a new six coordinated mononuclear iron (II) complex with mbit as the ligand, and the crystal structure of this complex was tested.
X-ray crystallography analyses reveal that the asymmetric unit of the title complex contains one half of a Fe(II) cation, one mbit ligand, one CH3CN ligand located on a two-fold axis. One perchlorate counter anion is located in a general position of the space group Pccn. The Fe(II) ion is coordinated by two nitrogen atoms and four sulfur atoms. The two N atoms are derived from acetonitrile molecules, and the four sulfur atoms are derived from two mbit molecules. Both Fe–N bond distances are 2.207(3) Å, this value agrees well with previously reported Fe(II) complexes. 15 , 16 , 17 The Fe–S bond distances are 2.5157(12) and 2.5359(11) Å, respectively. These bond distances are slightly longer than those of the reported Fe(II) polymers with mbit as ligands. 9 In those polymers [L1FeCl2] n (L1 = bis(3-methyl-2-thione-imidazolyl) methane) and [L2FeCl2] n (L2 = bis(3-tert-butyl-2-thione- imidazolyl)methane), the Fe–S bond distances are in the range of 2.3725(5)–2.3906(5) Å. 9 The angles in the acetonitrile molecule and the metal-to-ligand bond are nearly linear (Bond angles: N5–C10–C11 is 178.8(5)°, Fe–N5–C10 is 174.8(3)°). In this complex, four N atoms from the same mbit ligand surround one N atom from the acetonitrile ligand, and these five N atoms are almost in the same plane. The angle of two such planes (plane1: N1, N2, N3#1, N4#1, N5#1; plane2: N1#1, N2#1, N3, N4, N5) is 83.7°. The shortest distance of the adjacent Fe(II) ions is 7.4590(27) Å. The stable packing of the title complex is mainly due to the presence of weak hydrogen bonding interactions of S···H bond (C(5)–H(5A)···S(1), 2.77 Å; C(5)–H(5A)···S(2)#2, 2.80 Å, #2: -x, -y, -z) and O⋯H bond (C(7)–H(7)···O(2)#3, 2.51 Å, #3: −1/2−x, 1+y, −1/2+z; C(8)–H(8)···S(3)#4, 2.55 Å, #4: 1/2+x, −y, 1/2−z; and C(11)–H(11B)⋯O(1)#5, 2.54 Å, #5: 1/2−x, 3/2−y, 1+z), forming a three dimensional network. It is worth noting that the structure of this iron complex is different from the Sn(IV) complex reported in 1998. 18 Although both complexes are six coordinated, and in each complex the two mbit molecules acting as bidentate chelating ligands through the sulfur atoms, in the Sn(IV) cation, the metal atom lying on a symmetry centre exhihits a slightly distorted octahedral coordination with the iodides at the apices in trans position. In the title complex, two acetonitrile molecules are in adjacent positions, the bond angle of N5–Fe–N5#1 is 83.82(17)°.
In order to evaluate the deviation degrees of the FeS4N2 moiety in the title complex from the ideal symmetry, continuous shape measurement analyses was performed using the Shape 2.1 program. 19 , 20 The calculated deviation parameters provide an estimation of the deviation from the ideal structure with 0 corresponding to the ideal polyhedron. The calculation result are as follows: Hexagon, 30.272; Pentagonal pyramid, 27.291; Octahedron, 0.537; Trigonal prism, 15.609; Johnson pentagonal pyramid J2, 31.195). According to the calculation results, the configuration of the FeS4N2 moiety in the title complex shows a slightly distorted octahedral configuration. In general the geometrical parameters are in the expected ranges. 21
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
-
Research funding: We gratefully acknowledge the financial support by the Natural Science Foundation of Henan Province (no. 252300421341), the Special Foundation of the Nanyang Normal University (no. 2023ZX002), the National Natural Science Foundation Project Cultivation Fund of Nanyang Normal University (no. 2025PY018, 2025PY032).
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
References
1. SAINT Apex2 and Sadabs; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Suche in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Cryst. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Chen, S.-Y.; Shi, Z.-Z.; Guo, J.; Li, Y.-X.; Yan, Y.-C.; Yu, B.-B. Crystal Structure of catena–poly[Bis(isothiocyanate κ1N)- (μ2-2-thione-3-methylimidazoly methane-κ2S:S′)cobalt(II)], C11H12CoN6S4. Z. Kristallogr. –N. Cryst. Struct. 2025, 240, 609–611; https://doi.org/10.1515/ncrs-2025-0129.Suche in Google Scholar
5. Williams, D. J.; Vanderveer, D.; Jones, R. L.; Menaldino, D. S. Main Group Metal Halide Complexes with Sterically Hindered Thioureas XI. Complexes of Antimony(III) and Bismuth(III) Chlorides with a New Bidentate Thiourea-1,1′–methylenebis(3-methyl-2H-imidazole-2-thione). Inorg. Chim. Acta 1989, 165, 173–178; https://doi.org/10.1016/s0020-1693-00-83235-1.Suche in Google Scholar
6. Jia, W.-G.; Huang, Y.-B.; Lin, Y.-J.; Wang, G.-L.; Jin, G.-X. Nickel Complexes and Cobalt Coordination Polymers with Organochalcogen (S, Se) Ligands Bearing an N-Methylimidazole Moiety: Syntheses, Structures, and Properties. Eur. J. Inorg. Chem. 2008, 4063–4073; https://doi.org/10.1002/ejic.200800559.Suche in Google Scholar
7. Brandenburg, K. Diamond. Visual Crystal Structure Information System. 3.0c; Crystal Impact: Bonn, Germany, 2005.Suche in Google Scholar
8. Pinder, T. A.; Derveer, D. V.; Rabinovich, D. Lead(II) in a Sulfur-Rich Environment: Synthesis and Molecular Structure of the First Dinuclear Bis(mercaptoimidazolyl)methane Complex. Inorg. Chem. Commun. 2007, 10, 1381–1384.10.1016/j.inoche.2007.08.015Suche in Google Scholar
9. Meyer, S.; Demeshko, S.; Dechert, S.; Meyer, F. Synthesis Structure and Mössbauer Characterization of Polymeric Iron(II) Complexes with Bidentate Thiourea Ligands. Inorg. Chim. Acta 2010, 363, 3088–3092; https://doi.org/10.1016/j.ica.2010.04.015.Suche in Google Scholar
10. Kimani, M. M.; Watts, D.; Graham, L. A.; Rabinovich, D.; Yap, G. P. A.; Brumaghim, J. L. Dinuclear Copper(I) Complexes with N-heterocyclic Thione and Selone Ligands: Synthesis, Characterization, and Electrochemical Studies. Dalton Trans. 2015, 44, 16313–16324; https://doi.org/10.1039/c5dt02232k.Suche in Google Scholar PubMed
11. Karri, R.; Chalana, A.; Das, R.; Rai, R. K.; Roy, G. Cytoprotective Effects of Imidazole-based [S1] and [S2]-Donor Ligands Against Mercury Toxicity: A Bioinorganic Approach. Metallomics 2019, 11, 213–225; https://doi.org/10.1039/c8mt00237a.Suche in Google Scholar PubMed
12. Beheshti, A.; Mousavifard, E. S.; Kubicki, M.; Grzeskiewicz, A.; Rezatofighi, S. E. Tuning the Structure of Mercury(II) Complexes as Antibacterial Agents by Varying the Halogen Atoms (Cl and Br) and Extending the Spacer Length of the Imidazole-2-thione Ligands. Inorg. Chim. Acta 2021, 514, 120010.10.1016/j.ica.2020.120010Suche in Google Scholar
13. Bahrani-Pour, M.; Beheshti, A.; Sedaghat, T.; Hoveizi, E.; Naseri, N.; Mayer, P.; Centore, R. New Hg(II) Coordination Polymers Based on a Thioimidazole Ligand with Good Performance to Detoxify Hg(II) and Reversibly Capture Iodine. Dalton Trans. 2023, 52, 683–695; https://doi.org/10.1039/d2dt03057h.Suche in Google Scholar PubMed
14. Slivarichova, M.; Costa, R. C. d.; Nunn, J.; Ahmad, R.; Haddow, M. F.; Sparkes, H. A.; Gray, T.; Owen, G. R. Two Synthetic Routes to Bis(1-methyl-Imidazole-2-thione)methane and Bis(1-benzyl-imidazole-2- thione)methane Complexes Including Sulfur Atom Insertion into Copper–NHC Bonds. J. Organoment. Chem. 2017, 847, 224–233.10.1016/j.jorganchem.2017.05.015Suche in Google Scholar
15. Bar, A. K.; Pichon, C.; Gogoi, N.; Duhayon, C.; Ramasesha, S.; Sutter, J. –P. Single-Ion Magnet Behaviour of Heptacoordinated Fe(II) Complexes: On the Importance of Supramolecular Organization. Chem. Commun. 2015, 51, 3616–3619; https://doi.org/10.1039/c4cc10182k.Suche in Google Scholar PubMed
16. Werncke, C. G.; Bouammali, M.-A.; Baumard, J.; Suaud, N.; Martins, C.; Guihery, N.; Vendier, L.; Zheng, J.; Sortais, J.-B.; Darcel, C.; Sabo-Etienne, S.; Sutter, J.-P.; Bontemps, S.; Pichon, C. Ising-Type Magnetic Anisotropy and Slow Relaxation of the Magnetization in Four–Coordinate Amido-pyridine FeII Complexes. Inorg. Chem. 2016, 51, 10968–10977; https://doi.org/10.1021/acs.inorgchem.6b01512.Suche in Google Scholar PubMed
17. Urtizberea, A.; Roubeau, O. Switchable Slow Relaxation of Magnetization in the Native Low Temperature Phase of a Cooperative Spin–Crossover Compound. Chem. Sci. 2017, 8, 2290–2295; https://doi.org/10.1039/c6sc04737h.Suche in Google Scholar PubMed PubMed Central
18. Bigoli, F.; Deplano, P.; Devillanova, F. A.; Lippolis, V.; Mercuri, M. L.; Pellinghelli, M. A.; Trogu, E. F. Synthesis, X-Ray and Spectroscopic Characterization of [SnI2(mbit)2](I3). 2/3I2 Obtained Through the One-Step Reaction of mbit·2I2with Tin Metal Powder (mbit = 1,1′-Bis (3-methyl-4-imidazoline-2-thione)methane). Inorg. Chim. Acta 1998, 267, 115–121; https://doi.org/10.1016/s0020-1693-97-05575-8.Suche in Google Scholar
19. Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. Shape Program, Version 2.1, 2013.Suche in Google Scholar
20. Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape Maps and Polyhedral Interconversion Paths in Transition Metal Chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708; https://doi.org/10.1016/j.ccr.2005.03.031.Suche in Google Scholar
21. Plaza–Lozano, D.; Ramírez–Palma, D.; Vela, A.; Olguín, J. High Spin iron(II) Complexes Based on Imidazolyl- and 1,2,3-Triazolyl-thione Ligands and NCE (E = S, Se or BH3) Co-Ligands: Effect of the S-Functional Group on the Structural and Magnetic Properties. New J. Chem. 2022, 46, 14910; https://doi.org/10.1039/D2NJ01989B.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

