Abstract
C19H14NO4, monoclinic, P21/c (no. 14), a = 16.8973(12) Å, b = 5.6188(3) Å, c = 18.1088(15) Å, β = 117.145(10)°, V = 1529.9(2) Å3, Z = 4, R gt (F) = 0.0420 wR ref (F2) = 0.1050, T = 200(10) K.
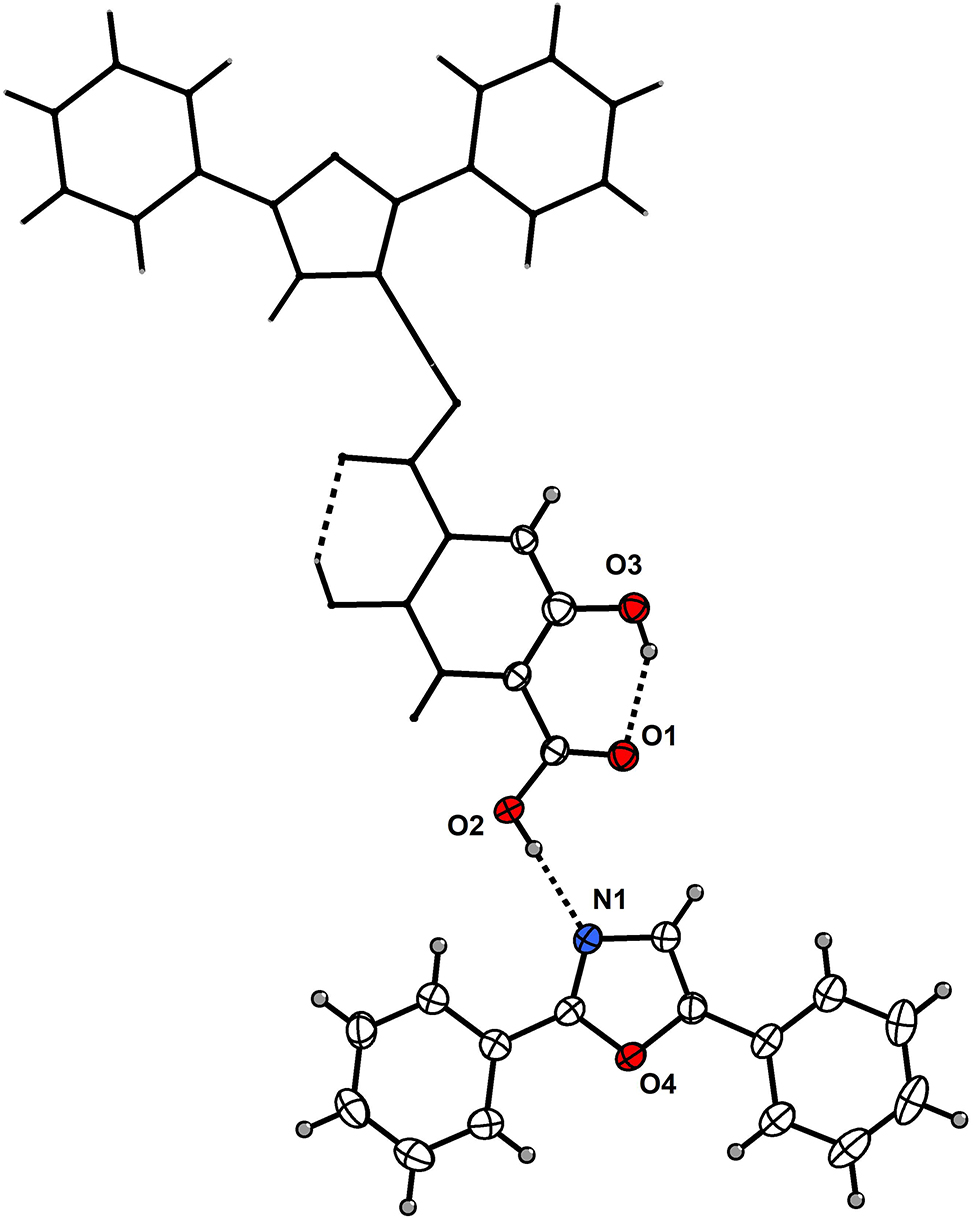
Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Clear light yellow block |
| Size: | 0.27 × 0.25 × 0.23 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Bruker Apex2, φ and ω scans |
| θmax, completeness: | 26.4°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 10013, 3106, 0.026 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2579 |
| N(param)refined: | 219 |
| Programs: | Bruker, 1 SHELX, 2 , 3 Olex2 4 |
1 Source of materials
Equimolar amounts of 2,5-diphenyloxazole (DPO, 0.05 mmol) and 2,5-dihydroxyterephthalic acid (0.05 mmol) were dissolved in a mixed solvent system of methanol and acetonitrile (v/v = 1:1, total volume 10 mL). The solution was stirred at room temperature for 2 h to ensure complete dissolution and facilitate molecular assembly, followed by filtration to remove any insoluble particulates. The filtrate was subjected to slow evaporation under ambient conditions over a period of 7–10 days, yielding colorless block crystals. The crystals were manually isolated, rinsed with a minimal volume of cold methanol to eliminate residual mother liquor, and subsequently characterized by structural analysis.
2 Experimental details
The initial structural model was solved by intrinsic phasing methods employing ShelXT 2 and was subsequently refined by full-matrix least-squares minimization on F2 using ShelXL. 3 All structural solution and refinement procedures were conducted within the Olex2 software package. 4
3 Comment
Cocrystallization has emerged as a powerful strategy in crystal engineering for modulating the physicochemical properties of organic materials without covalent modification. By combining two or more complementary molecules into a single crystalline lattice, cocrystals can exhibit tailored functionalities such as enhanced solubility, optimized mechanical behavior, and improved stability, making them highly attractive for pharmaceutical, optoelectronic, and energetic material applications. 5 , 6 DPO is a well-known fluorophore with strong emission properties, widely used in scintillation counters and optoelectronic devices. 7 However, its tendency to form densely packed, π–π stacked architectures often limits exciton migration and emission efficiency. On the other hand, 2,5-dihydroxyterephthalic acid offers multiple hydrogen-bonding sites and conformational flexibility due to its carboxyl and hydroxyl groups, making it an ideal coformer for constructing extended supramolecular networks through synergistic non-covalent interactions. 8 Although both compounds have been individually studied, their cocrystal formation remains unexplored. We hypothesize that the integration of the rigid, π-conjugated DPO with the hydrogen-bond-rich 2,5-dihydroxyterephthalic acid will facilitate the formation of a novel cocrystal structure directed by O–H⋯N, C–H⋯O, and π–π interactions. Such a structure is anticipated to exhibit unique photophysical properties and packing motifs, potentially enabling new applications in organic luminescence and sensing.
The asymmetric unit of the title structure contains one DPO molecule and one half-molecule of 2,5-dihydroxyterephthalic acid (see the figure). The title molecule is located around an inversion center. The three rings (two benzene rings and one 4,5-dihydrooxazole ring) of the DPO molecule are nearly coplanar. The DPO molecules exhibit π-π stacking interactions among themselves, and similarly, the 2,5-dihydroxyterephthalic acid molecules also display π-π stacking. An intermolecular hydrogen bond is observed between the N atom of the oxazoline moiety (DPO) and the hydroxyl group of the carboxylic acid function (2,5-dihydroxyterephthalic acid), with an N⋯O distance of 2.6718(18) Å. Within the 2,5-dihydroxyterephthalic acid molecule, the carbonyl oxygen of the carboxyl group forms two intramolecular hydrogen bonds with the ortho-hydroxy group, both with a bond distance of 2.6071(18) Å. Nearly all atoms of this molecule are coplanar. The benzene ring of the DPO molecule forms dihedral angles of 70.00° and 26.35° with two adjacent 2, 5-dihydroxyterephthalic acid molecules, resulting in an ordered arrangement of the two molecular species. The coordination environment in the present complex closely resembles that observed in reported cocrystals incorporating DPO or 2,5-dihydroxyterephthalic acid. 9 , 10 , 11
Acknowledgments
This work was financially supported by the projects of Natural Science Foundation of Shaanxi Province (2024JC–YBMS-884); The 2024 Key Scientific Research Program Projects of the Shaanxi Provincial Department of Education (Key Laboratory Projects, 24JS004); The Xianyang key laboratory of molecular imaging and drug synthesis (2021QXNL–PT-0008); The 2023 key research and development project of the Xianyang Science and Technology Bureau (L2023–ZDYF–SF-030); Doctoral research fund project of Xianyang Vocational and Technical College (2021BK01).
References
1. Bruker Saint, Apex2 and Sadabs; Bruker AXS Inc.: Madison, WI, USA, 2012.Suche in Google Scholar
2. Sheldrick, G. M. Shelxt-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A: Found. Adv. 2015, 71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. Sect. C: Struct. Chem. 2015, 71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
5. Pantwalawalkar, J.; Kale, N.; Nangare, S.; Patil, S.; Pawar, S.; Jadhav, N. Pharmaceutical Cocrystals: Unlocking the Potential of Challenging Drug Candidates. J. Drug Delivery Sci. Technol. 2025, 104, 106572; https://doi.org/10.1016/j.jddst.2024.106572.Suche in Google Scholar
6. Liu, G.; Bu, R.; Huang, X.; Zhong, K.; Jiao, F.; Wei, S. –H.; Li, H.; Zhang, C. Energetic Cocrystallization as the Most Significant Crystal Engineering Way to Create New Energetic Materials. Cryst. Growth Des. 2022, 22, 954–970; https://doi.org/10.1021/acs.cgd.1c01090.Suche in Google Scholar
7. Asemi, N. N.; Aljaafreh, M. J.; Prasad, S.; Aldawood, S.; AlSalhi, M. S.; Aldaghri, O. Efficient Liquid Scintillator Loaded with a Light-Emitting Conjugated Oligomer for Beta- and Gamma-Ray Spectroscopic Measurements. Radiat. Meas. 2022, 156, 106826; https://doi.org/10.1016/j.radmeas.2022.106826.Suche in Google Scholar
8. Kavali, R. P.; Tonannavar, J.; Bhovi, J.; Tonannavar, J. Study of OH…O bonded-Cyclic Dimer for 2,5-Dihydroxyterephthalic Acid as Aided by MD, DFT Calculations and IR, Raman, NMR Spectroscopy. J. Mol. Struct. 2022, 1264, 133174; https://doi.org/10.1016/j.molstruc.2022.133174.Suche in Google Scholar
9. Fan, G.; Yang, X.; Liang, R.; Zhao, J.; Li, S.; Yan, D. Molecular Cocrystals of Diphenyloxazole with Tunable Fluorescence, Up-Conversion Emission and Dielectric Properties. CrystEngComm 2016, 18, 240–249; https://doi.org/10.1039/c5ce02019k.Suche in Google Scholar
10. Yan, D.; Yang, H.; Meng, Q.; Lin, H.; Wei, M. Two-Component Molecular Materials of 2,5-Diphenyloxazole Exhibiting Tunable Ultraviolet/Blue Polarized Emission, Pump-Enhanced Luminescence, and Mechanochromic Response. Adv. Funct. Mater. 2014, 24 (5), 587–594; https://doi.org/10.1002/adfm.201302072.Suche in Google Scholar
11. Yin, P.-P.; Chen, Y.-E.; Diao, J.-W.; Cheng, Y.-Y.; Yang, X.-G.; Liu, B.-Z.; Ma, L.-F. Facile Synthesis of a 2,5-Dihydroxyterephthalic Acid-based Charge Transfer Cocrystal for Photoelectric Applications. CrystEngComm 2024, 26, 2746–2750; https://doi.org/10.1039/d4ce00338a.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

