Abstract
C14H12F2O2, orthorhombic, P212121 (no. 19), a = 10.7266(4) Å, b = 17.4494(8) Å, c = 24.9738(11) Å, V = 4674.4(3) Å3, Z = 16, Rgt(F) = 0.0457, wRref(F2) = 0.1170, T = 170.0 K.
Table 1 contains the crystallographic data. The list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Plate, colourless |
| Size: | 0.39 × 0.19 × 0.09 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.12 mm−1 |
| Diffractometer, scan mode: | Bruker D8 Venture, φ and ω-scans |
| θmax, completeness: | 27,1°, > 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 66158, 10327, 0.085 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ (Iobs), 7186 |
| N(param)refined: | 657 |
| Programs: | Bruker programs, 1 OLEX2, 2 SHELX, 3 , 4 PLATON 5 , 6 |
1 Source of materials
The crude title compound (2.5 g, 10 mmol) was dissolved in H2O (10 mL) and dioxane (30 mL), and the mixture was filtered to remove insoluble precipitates. The filtrate was concentrated in vacuo, and the resulting residue was partitioned between ethyl acetate and saturated aqueous NaHCO3. The organic phase was isolated, dried, and concentrated. Purification of the residue by flash column chromatography, eluting with a 2:3 mixture of ethyl acetate/hexane, yielded the title compound as a yellow solid. Single crystals were grown by slow evaporation. The purified solid (250 mg, 1.0 mmol) was dissolved in ethyl acetate (2 mL), and the solution was filtered into a small vial. The vial was covered with perforated film and left undisturbed at room temperature for a week, affording colorless crystals.
2 Experimental details
All hydrogen atoms were placed in geometrically calculated positions and refined using a riding model. Methyl groups were treated as idealized rotating rigid bodies, while aromatic C–H hydrogen atoms were constrained to ride on their parent carbon atoms. The isotropic displacement parameters of the hydrogen atoms were set to Uiso(H) = 1.5Ueq(C) for the methyl groups and Uiso(H) = 1.2Ueq(C) for all other hydrogen atoms.
3 Comment
Elagolix, marketed under the brand name Orilissa, represents a significant therapeutic advancement in women’s health as a first-in-class, orally active, non-peptide gonadotropin-releasing hormone (GnRH) receptor antagonist. 7 It is approved by the U. S. Food and Drug Administration for the management of moderate to severe pain associated with endometriosis and, in combination with estradiol/norethindrone acetate, for the management of heavy menstrual bleeding associated with uterine fibroids. 8
The title compound, 2,2′-difluoro-3,3′-dimethoxy-1,1′-biphenyl, is a crucial building block in the industrial synthesis of elagolix. 9 In this biphenyl unit, rotation about the C–C single bond connecting the two phenyl rings is severely restricted by the bulky ortho-substituents. This hindered rotation results in atropisomerism, locking the molecule into a specific, stable three-dimensional conformation that is essential for its high-affinity binding to the GnRH receptor. In continuation of our research on the structure-activity relationships and hydrogen-bonding schemes of pharmacologically active compounds, 10 , 11 we herein report the single-crystal X-ray structure determination of the title compound.
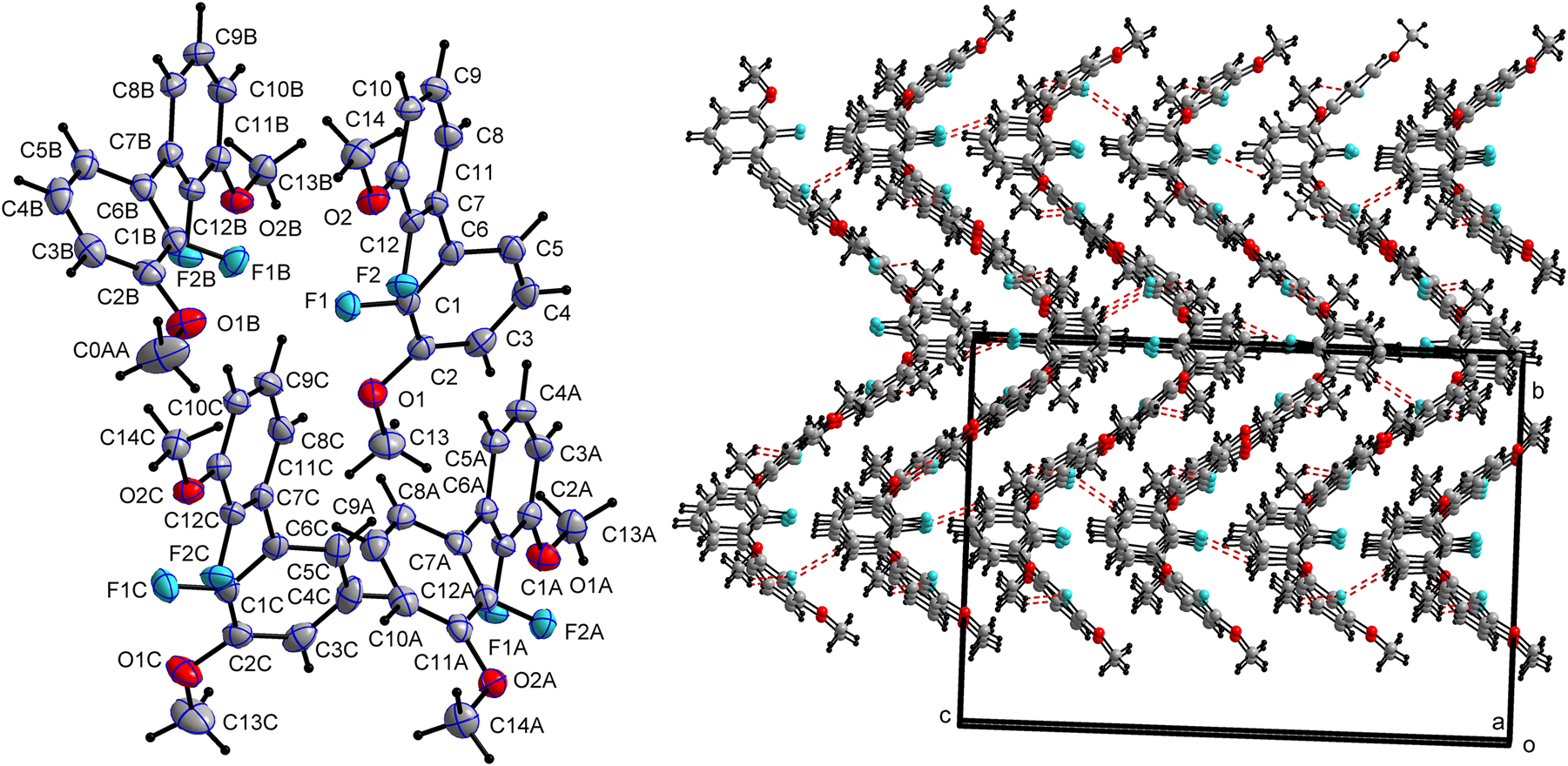
The asymmetric unit contains four crystallographically independent molecules of 2,2′-difluoro-3,3′-dimethoxy-1,1′-biphenyl (cf. left part of the figure). The atoms of these independent molecules are distinguished by the suffixes A, B, and C, while those of the first molecule bear no suffix.
The defining structural characteristic of each molecule is the significant twist around the C–C single bond connecting the two phenyl rings. The presence of four independent molecules (Z′ = 4) is a notable feature, suggesting that packing forces create slightly different local environments for each molecule.
First, the four molecules adopt slightly different conformations, primarily in the main biphenyl twist and the torsion angles of the methoxy groups, to optimize packing interactions. The dihedral angles between the planes of the two aromatic rings are 48.3° for molecule A, 46.5° for molecule B, 48.9° for molecule C, and 46.3° for the no suffix molecule.
In all four independent molecules, the methoxy groups are oriented nearly coplanar with their respective aromatic rings, though their precise conformations vary, highlighting the influence of different local packing environments. This conformational flexibility is evident when comparing the geometric parameters across the asymmetric unit. For instance, the molecule designated without a suffix is almost perfectly planar, with a C14–O2–C11–C10 torsion angle of 1.090(6) and a C11–O2–C14 bond angle of 116.945(3). In contrast, molecule A exhibits a greater deviation from planarity, with a corresponding C14A–O2A–C11A–C10A torsion angle of 8.193(6) and a C11A–O2A–C14A bond angle of 116.618(4). The remaining two molecules show similar, distinct conformational parameters.
Furthermore, the crystallographic independence of the four molecules in the asymmetric unit was confirmed by PLATON 5 , 6 analysis. The ADDSYM routine, which searches for additional symmetry elements, found no missed or pseudo symmetry that could relate these molecules. The analysis confirmed that the P212121 space group is correct and complete, with no higher symmetry possible.
The crystal packing is dominated by a network of weak intermolecular interactions (cf. right part of the figure), and no classical hydrogen bonds are present.
Several weak hydrogen bonds, C–H⋯O and C–H⋯F interactions, link the molecules. For example: A methyl C–H group acts as a donor to a methoxy oxygen atom (C0AA–H0AB⋯O2) with a H⋯A distance of 2.56 Å, connecting molecules along the crystallographic a-axis. A methyl C–H group interacts with a fluorine atom (C13B–H13I⋯F1) with a H⋯A distance of 2.52 Å. (Symmetry code: x, y, z, within the ASU interaction). The C–H⋯π interactions play a significant role in the packing. For instance: The hydrogen atom H13G of a methyl group on molecule B interacts with the center of a phenyl ring of molecule A, with a H⋯Cg distance of 2.62 Å. The hydrogen H14H of a methyl group on molecule C interacts with the phenyl ring of molecule A, with a H⋯Cg distance of 2.65 Å. These interactions link molecules into chains propagating along the a-axis.
The combination of these weak interactions creates a complex 3D network. The C–H⋯π interactions link molecules head-to-tail into chains that run along the a-axis.
These chains are further connected by C–H⋯O and C–H⋯F interactions, which are generated by the 21 screw axis running along b. This assembles the chains into corrugated layers that lie in the ab-plane. The layers stack along the c-axis, with offset π-π interactions between phenyl rings of adjacent layers providing cohesion and completing the three-dimensional structure.
The overall packing is dense and intricate, driven by the need to satisfy multiple weak directional interactions for the four independent molecules.
Acknowledgments
We gratefully acknowledge support by Ningbo Polytechnic University Zhejiang Collaborative Innovation Center, and Ningbo Polytechnic Academician Workstation.
-
All the authors have accepted: responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
References
1. SMART and SAINT; Bruker AXS Inc.: Madison, Wisconsin, USA, 2019.Suche in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42 (2), 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. C 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Sheldrick, G. M. Shelxt–Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar PubMed PubMed Central
5. Spek, A. L. Single-Crystal Structure Validation with the Program Platon. J. Appl. Cryst. 2003, 36 (1), 7–13; https://doi.org/10.1107/s0021889802022112.Suche in Google Scholar
6. Spek, A. L. Structure Validation in Chemical Crystallography. Acta Crystallog. D 2009, D65, 148–155; https://doi.org/10.1107/s090744490804362x.Suche in Google Scholar
7. Schlaff, W. D.; Ackerman, R. T.; Al–Hendy, A.; Archer, D. F.; Barnhart, K. T.; Bradley, L. D.; Carr, B. R.; Feinberg, E. C.; Hurtado, S. M.; Kim, J.; Liu, R.; Mabey, R. G.; Owens, C. D.; Poindexter, A.; Puscheck, E. E.; Rodiguez-Ginorio, H.; Simon, J. A.; Soliman, A. M.; Stewart, E. A.; Watts, N. B.; Muneyyirci-Delale, O. Elagolix for Heavy Menstrual Bleeding in Women with Uterine Fibroids. N. Engl. J. Me. 2020, 382 (4), 328–340; https://doi.org/10.1056/nejmoa1904351.Suche in Google Scholar PubMed
8. Taylor, H. S.; Giudice, L. C.; Lessey, B. A.; Abrao, M. S.; Kotarski, J.; Archer, D. F.; Diamond, M. P.; Surrey, E.; Johnson, N. P.; Watts, N. B.; Gallagher, J. C.; Simon, J. A.; Carr, B. R.; Dmowski, W. P.; Leyland, N.; Rowan, J. P.; Duan, W. R.; Ng, J.; Schwefel, B.; Thomas, J. W.; Jain, R. I.; Chwalisz, K. Treatment of Endometriosis–Associated Pain with Elagolix, an Oral GnRH Antagonist. N. Engl. J. Me. 2017, 377 (1), 28–40; https://doi.org/10.1056/nejmoa1700089.Suche in Google Scholar
9. Guo, Z.; Chen, Y.; Wu, D.; Chen, C.; Wade, W.; Dwight, W. J.; Huang, C. Q.; Tucci, F. C. Pyrimidine-2, 4-dione Derivatives as Gonadotropin-Releasing Hormone Receptor Antagonists. World Intellect. Property Organ. (WIPO) WO2005007165A1, 2005.Suche in Google Scholar
10. Li, Y.; Wang, J. The Crystal Structure of 4-(Methoxycarbonyl)Benzoic Acid. C9H8O4. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 349–350; https://doi.org/10.1515/ncrs-2018-0408.Suche in Google Scholar
11. Li, Y.; Wu, Y.; Wang, J. The Crystal Structure of 3-((4-Chloro-N-(2-Methoxyethyl)Benzamido)Methyl)Phenyl Methanesulfonate. C18H20ClNO5S. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 1033–1036; https://doi.org/10.1515/ncrs-2022-0376.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

