Abstract
C49H72O18, triclinic,
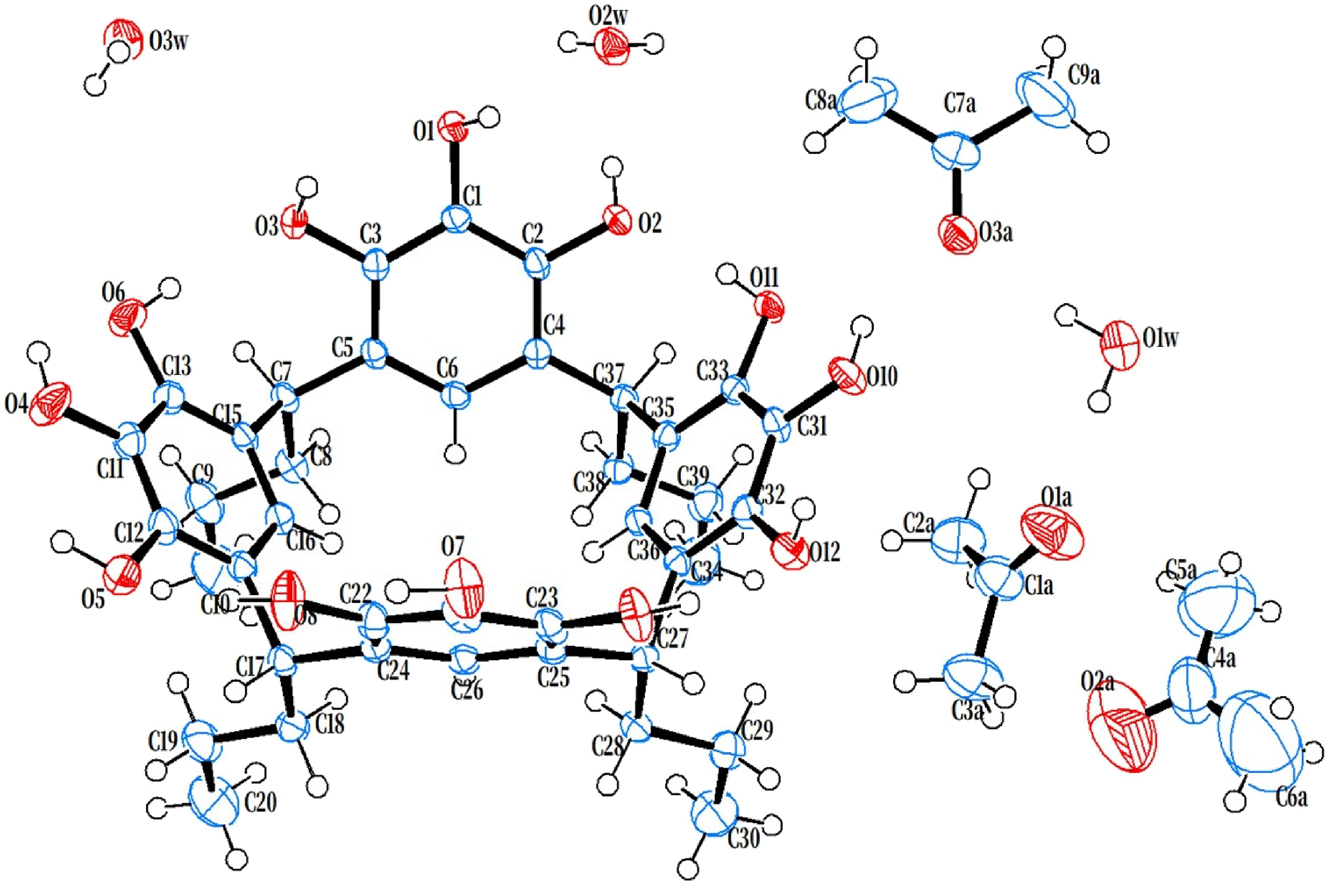
The molecular structure is shown in the figure (50 % ellipsoids). Table 1 contains the crystallographic data. The list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Rhombohedral, yellow |
| Size: | 0.50 × 0.40 × 0.20 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Siemens, ω-scans |
| θmax, completeness: | 28,3°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 15182, 11255, 0.036 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 7566 |
| N(param)refined: | 676 |
| Programs: | Bruker programs, 1 SHELX, 2 WinGX and ORTEP 3 |
1 Source of materials
The synthesis of the title compound, 2,8,14,20-tetrapropylpyrogallol[4]arene was reported previously. 4 For crystallization, 50 mg (1.39 mmol) of the compound was dissolved in 1 mL of acetone, overlaid onto 1 mL of water, followed by careful addition of 0.5 mL of toluene on top of the acetone layer, yielding yellow rhombohedral crystals.
2 Experimental details
The data were corrected for Lorentz and polarization effects (SAINT), and semiempirical absorption corrections based on equivalent reflections were applied (SADABS). 1 The structure was solved by Direct Methods and refined on F2 (SHELXTL). 2 Hydrogen atoms of 2,8,14,20-tetrapropylpyrogallol[4]arene were added to their geometrically ideal positions, and other hydrogen atoms of solvents were found from the difference map. Hydrogen atoms were found from the difference map. C-bound H atoms were refined using a riding model, with Uiso(H) = 1.5 Ueq(C) for methyl H atoms and 1.2 Ueq(C) for all others. C–H distances were constrained to 0.95 Å for aryl H atoms, 0.98 Å for methyl H, and 0.99 Å for methylene H atoms.
3 Comment
Pyrogallol[4]arenes, a subclass of resorcin[4]arenes, have attracted considerable attention due to their ability to form molecular capsules in both solution and the solid state, as well as their guest-inclusion properties. Research in this area was stimulated by Rebek’s pioneering studies on hydrogen-bonded capsules, which established these assemblies as model systems for investigating weak noncovalent interactions, particularly hydrogen bonding. 5 Since the initial report by Gerkensmeier and co-workers on hexameric capsule formation of C-alkyl-substituted pyrogallol[4]arenes in acetonitrile, 4 subsequent studies by Atwood, Cohen, Rebek, and others have demonstrated a wide range of capsule architectures. These include hydrogen-bonded hexameric and dimeric capsules influenced by substituent variation and solvent effects, 6 , 7 , 8 , 9 metal-coordinated dimeric capsules, 10 , 11 hexameric capsules, 12 and inclusion complexes with diverse organic guests. 10 , 13 , 14 , 15 , 16 , 17
The crystal structure of the title compound reveals one pyrogallol[4]arene molecule in the asymmetric unit, accompanied by three acetone and three water molecules. Notably, one acetone molecule occupies the pocket region of the pyrogallol[4]arene. This acetone appears to interact not only with a water and hydroxyl group through hydrogen bonding but also with a pocket through van der Waals forces. Its presence induces a distortion of the macrocyclic framework. Torsional angle analysis indicates that the cis-oriented set of four pyrogallol units adopts a distorted geometry: two opposite pyrogallol moieties (C1–C6 and C21–C26) exhibit narrower orientations, tilted by 29.88° and 30.32° relative to C7–C8 and C27–C28, respectively, whereas the other two (C11–C16 and C31–C36) are more widely splayed, with tilt angles of 50.56° and 46.63°. Comparable crystal structures of C-substituted pyrogallol[4]arenes incorporating methanol, 16 or 4,4′-bipyridine and acetone 17 as guests have been reported.
-
Research funding: This work was supported by Konkuk University.
References
1. Bruker. Apex2, Saint and Sadabs; Bruker AXS Inc.: Madison, Wisconsin, USA, 2004.Suche in Google Scholar
2. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
3. Farrugia, L. J. WinGX and Ortep for Windows: An Update. J. Appl. Cryst. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Suche in Google Scholar
4. Gerkensmeier, T.; Iwanek, W.; Agena, C.; Fröhlich, R.; Kotila, S.; Näther, C.; Mattay, J. Self-Assembly of 2,8,14,20-Tetraisobutyl-5,11,17,23-Tetrahydroxyresorc[4]Arene. Eur. J. Org. Chem. 1999, 1999 (9), 2257–2262; https://doi.org/10.1002/(sici)1099-0690(199909)1999:9<2257::aid-ejoc2257>3.0.co;2-h.10.1002/(SICI)1099-0690(199909)1999:9<2257::AID-EJOC2257>3.0.CO;2-HSuche in Google Scholar
5. Branda, N.; Wyler, R.; Rebek, J.Jr Encapsulation of Methane and Other Small Molecules in a Self-Assembling Superstructure. Science 1994, 263 (5151), 1267–1268; https://doi.org/10.1126/science.8122107.Suche in Google Scholar
6. Atwood, J. L.; Barbour, L. J.; Jerga, A. Hydrogen-Bonded Molecular Capsules are Stable in Polar Media. Chem. Commun. 2001, 2376–2377; https://doi.org/10.1039/b106250f.Suche in Google Scholar
7. Barrett, E. S.; Dale, T. J.; Rebek, J.Jr Stability, Dynamics, and Selectivity in the Assembly of Hydrogen-Bonded Hexameric Capsules. J. Am. Chem. Soc. 2008, 130 (7), 2344–2350; https://doi.org/10.1021/ja078009p.Suche in Google Scholar
8. Avram, L.; Cohen, Y. Molecules at Close Range: Encapsulated Solvent Molecules in Pyrogallol[4]Arene Hexameric Capsules. Org. Lett. 2006, 8 (2), 219–222; https://doi.org/10.1021/ol052459+.10.1021/ol052459+Suche in Google Scholar
9. Avram, L.; Cohen, Y. Self-Recognition, Structure, Stability, and Guest Affinity of Pyrogallol[4]Arene and Resorcin[4]Arene Capsules in Solution. J. Am. Chem. Soc. 2004, 126 (37), 11556–11563; https://doi.org/10.1021/ja047698r.Suche in Google Scholar
10. Power, N. P.; Dalgarno, S. J.; Atwood, J. L. Robust and Stable Pyrogallol[4]Arene Molecular Capsules Facilitated via an Octanuclear Zinc Coordination Belt. New J. Chem. 2007, 31, 17–20; https://doi.org/10.1039/b615947h.Suche in Google Scholar
11. Power, N. P.; Dalgarno, S. J.; Atwood, J. L. Guest and Ligand Behavior in Zinc-Seamed Pyrogallol[4]Arene Molecular Capsules. Angew. Chem. Int. Ed. 2007, 46 (45), 8601–8604; https://doi.org/10.1002/anie.200702941.Suche in Google Scholar
12. McKinlay, R. M.; Cave, G. W.; Atwood, J. L. Supramolecular Blueprint Approach to Metal-Coordinated Capsules. Proc. Natl. Acad. Sci. U. S. A. 2005, 102 (17), 5944–5948; https://doi.org/10.1073/pnas.0408113102.Suche in Google Scholar PubMed PubMed Central
13. Palmer, L. C.; Rebek, J.Jr Hydrocarbon Binding Inside a Hexameric Pyrogallol[4]Arene Capsule. Org. Lett. 2005, 7 (5), 787–789; https://doi.org/10.1021/ol047673x.Suche in Google Scholar PubMed
14. Bassil, D. B.; Dalgarno, S. J.; Cave, G. W.; Atwood, J. L.; Tucker, S. A. Spectroscopic Investigations of ADMA Encapsulated in Pyrogallol[4]Arene Nanocapsules. J. Phys. Chem. B 2007, 111 (30), 9088–9092; https://doi.org/10.1021/jp0707642.Suche in Google Scholar PubMed
15. Ahman, A.; Luostarinen, M.; Rissanen, K.; Nissinen, M. Complexation of C-Methyl Pyrogallarene with Small Quaternary and Tertiary Alkyl Ammonium Cations. New J. Chem. 2007, 31, 169–177; https://doi.org/10.1039/b609117b.Suche in Google Scholar
16. Shivanyuk, A.; Friese, J. C.; Döring, S.; Rebek, J. Solvent-Stabilized Molecular Capsules. J. Org. Chem. 2003, 68 (17), 6489–6496; https://doi.org/10.1021/jo034791+.10.1021/jo034791+Suche in Google Scholar PubMed
17. Cave, G. W. V.; Ferrarelli, M. C.; Atwood, J. L. Nano-Dimensions for the Pyrogallol[4]Arene Cavity. Chem. Commun. 2005, 2787–2789; https://doi.org/10.1039/b413829e.Suche in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

