Abstract
C13H15N3O4, monoclinic, P21/c (No. 4), a = 8.641(4) Å, b = 7.372(3) Å, c = 11.195(6) Å, β = 107.24(2)°, V = 681.2(6) Å3, Z = 2, T = 296 K, Rgt(F) = 0.0338, wRref(F2) = 0.0859.
Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Colourless plate |
| Size: | 0.40 × 0.23 × 0.04 mm |
| Wavelength: μ: |
Cu Kα radiation (1.54178 Å) 0.86 mm−1 |
| Diffractometer, scan mode: θmax, completeness: |
Bruker D8 Venture, φ and ω scans 74.0°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 10989, 2614, 0.042 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2506 |
| N(param)refined: | 183 |
| Programs: | Bruker, 1 Olex2, 2 SHELX 3 , 4 |
1 Source of materials
The title compound, 5-amino-4-(4-amino-1-oxoisoindolin-2-yl)-5-oxopentanoic acid, was synthesized from methyl 2-(bromomethyl)-3-nitrobenzoate. The crude product was purified by column chromatography on silica gel using an ethyl acetate/n-hexane (4:1, v/v) eluent and dried under vacuum at 40 °C for 16 h. For crystallization, the purified compound (277 mg, 1 mmol) was dissolved in a mixture of ethyl acetate and n-hexane (4:1, v/v). This solution was placed in a vial covered with perforated film to allow for slow evaporation of the solvent. Colorless, plate crystals were obtained at room temperature.
2 Experimental details
Hydrogen atom thermal parameters were fixed at 1.2 times the parent atom values for C(H), C(H,H), and N(H,H) groups, and 1.5 times for O(H) groups. The primary amine N1 was treated as a rotating group with freely rotating H1A and H1B atoms. Riding coordinate models were applied to tertiary carbon C9(H9), methylene groups C8, C11, and C12, and aromatic carbons C1, C2, and C3. The hydroxyl O4 was modeled as an idealized tetrahedral rotating group, allowing optimal H4 orientation while maintaining standard bond parameters.
3 Comment
Lenalidomide is an immunomodulatory agent with antiangiogenic and antineoplastic properties. 5 It is commercially available for the treatment of myelodysplastic syndromes and multiple myeloma. The title compound, 5-amino-4-(4-amino-1-oxoisoindolin-2-yl)-5-oxopentanoic acid, represents one of the most significant impurities of Lenalidomide synthesis and has attracted our attention. We have successfully obtained crystals of this compound and report its crystal structure for the first time and its structure have already been deposited in the Cambridge Crystallographic Data Centre after applied by us, that is, CCDC 2480898.
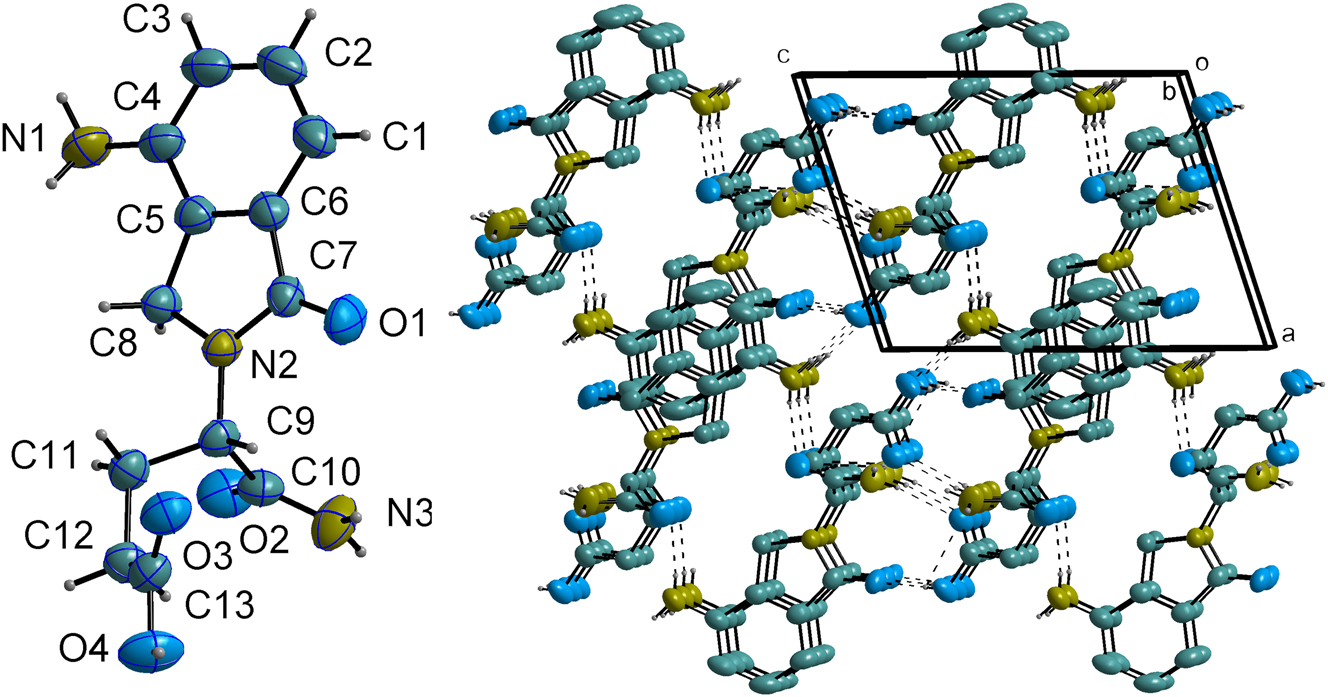
The asymmetric unit of the title compound, contains one discrete molecule (cf. left part of the figure). The molecular conformation is characterized by a relatively rigid and pseudo-planar core, which is comprised of a phenyl ring fused to a five-membered dihydro-isoindolone ring system (N2–C7–C6–C5–C8). The planarity of this fused system is a notable feature, with the average absolute torsion angle within the five-membered ring being only 1.743(6)°.
The overall molecular structure is stabilized by a network of interactions, including a strong intramolecular hydrogen bond between the hydroxyl group and a carbonyl oxygen.
In the crystal, the molecules are assembled into a complex three-dimensional architecture through an extensive network of intermolecular hydrogen bonds and π-π stacking interactions (cf. right part of the figure, some hydrogen atoms are omitted for clarity purpose.). The primary structural motif is generated by a strong O4–H4⋯O1′ hydrogen bond (D⋯A = 2.608(3) Å; ′=1 − x, −1/2 + y, 2 − z), which links molecules head-to-tail into infinite chains propagating along the crystallographic b-axis.
These chains are further interconnected into a robust 3D framework by multiple N–H⋯O interactions. The primary amino group (N1) serves as a bifurcated donor, forming hydrogen bonds to a carbonyl oxygen (N1–H1A⋯O2) and a carboxylic acid oxygen (N1–H1B⋯O4) of two different adjacent molecules. Concurrently, the terminal amide group (N3) donates two hydrogen bonds (N3–H3A⋯O3 and N3–H3B⋯O3) to the carbonyl oxygen atoms of neighboring molecules, further reinforcing the network.
In addition to these strong interactions, the crystal packing is stabilized by weak parallel-displaced π-π stacking between the phenyl rings of adjacent molecules, characterized by a centroid-centroid distance of 3.922(3) Å. Bond lengths and all other geometric parameters are in the expected range. 6 , 7
Acknowledgments
We gratefully acknowledge support by Jiangsu Vcare Pharmatech CO., LTD.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
References
1. Bruker Instrument Service. vV6.2.14; SAINT, V8.40B; APEX5, v2023.9-4; Bruker Nano, Inc.: Madison, WI, 2019–2023.Suche in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42 (2), 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. C 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Sheldrick, G. M. Shelxt – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar PubMed PubMed Central
5. Seton–Rogers, S. Destruction of Ikaros. Nat. Rev. Cancer 2014, 14 (1), 9; https://doi.org/10.1038/nrc3663.Suche in Google Scholar
6. Wang, Q.; Gao, Y.; Tong, H.; Tang, W. The Crystal Structure of 5-amino-5-oxo-4-(1-oxo-4-(2-oxopyrrolidin-1-yl)isoindolin-2-yl) Pentanoic Acid, C17H19N3O5. Z. Kristallogr. - N. Cryst. Struct. 2024, 239, 43; https://doi.org/10.1515/ncrs-2023-0431.Suche in Google Scholar
7. Otogawa, K.; Ishikawa, K.; Shiro, M.; Asahi, T. Crystal Structure of (S)-4-Carbamoyl-4-(1,3-dioxo-isoindolin-2-yl)butanoic acid. Acta Crystallogr., Sect. E:: Crystallogr. Commun. 2015, 71, 107; https://doi.org/10.1107/S2056989014027121.Suche in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

