Solvothermal synthesis and crystal structure of poly((μ 3-2-amino-1,3-benzenedicarboxylato-κ 4 O:O′,O″,O‴)-bis(μ 4-2-amino-1,3-benzenedicarboxylato-κ 5 O:O:O′,O″,O‴)-(N,N-dimethylformamide)-aqua-di-europium(III)-N,N-dimethylformamide solvate, C30H31N5O7Eu2
Abstract
C30H31N5O7Eu2, orthorhombic, Pna21 (no. 34), a = 18.1766(8) Å, b = 11.0796(5) Å, c = 16.5109(6) Å, V = 3325.1(2) Å3, Z = 4, R gt (F) = 0.0230, wR ref (F 2) = 0.0611, T = 150(2) K.
Table 1 contains the crystallographic data. The list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Block, colourless |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.5 mm−1 |
| Diffractometer, scan mode: | Bruker SMART, ω-scans |
| θ max, completeness: | 26,4°, > 99 % |
| N(hkl)measured , N(hkl)unique, R int: | 40066, 6704, 0.049 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 6,370 |
| N(param)refined: | 476 |
| Programs: | Bruker programs, 1 Olex2, 2 Shelx 3 , 4 |
1 Source of materials
2–Amino-1,3-benzenedicarboxylic acid (0.2 mmol) and europium nitrate hexahydrate (0.1 mmol) were placed in a reactor lined with polytetrafluoroethylene, then 10 mL DMF (N,N-dimethylformamide) were added. The reaction was carried out in an oven at 393 K for 72 h before natural cooling. After cooling, the crystals were filtered, washed with anhydrous ethanol and dried at room temperature to obtain the colorless target europium organic framework material with a yield of 89.2 % (based on 2-amino-1,3-benzenedicarboxylic acid).
2 Experimental details
The structure was solved by Direct Methods with the SHELXS-2018 program. All H-atoms from C and N atoms were positioned with idealized geometry and refined isotropically (U iso(H) = 1.2U eq(C), 1.2U eq(N) or 1.5U eq(O)) using a riding model with C–H = 0.950 and N–H = 0.880 Å for 2-amino-1,3-benzenedicarboxylate, C–H = 0.950, 0.979, 0.980, and 0.981 Å for DMF molecules, O–H = 0.870 Å, respectively.
3 Comment
To date, some crystal structures based on 2-amino-1,3-benzenedicarboxylic acid, 5 its salts, 6 and transition metal complexes such as UO2 2+, 7 , 8 Mn(II), 9 Cu(II), 10 , 11 and Cd(II) 12 have been reported. However, no corresponding lanthanide complexes or lanthanide-organic frameworks (LnOFs) have been reported anywhere. A number of 5-amino-1,3-benzenedicarboxylate sometimes act as co-ligands with acetate, oxalate and other carboxylate-containing ligands, or neutral nitrogen-containing ligands, such as 1,10-phenanthroline based LnOFs have been reported. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 Almost all of them were synthesised under hydrothermal conditions, except the 5-amino-2,4,6-triiodoisophthalate-based LnOFs by reacting starting materials in DMF solution. 21 Therefore, we apply for a new europium-organic framework with the formula C30H31N5O15Eu2 under solvothermal condition by choosing 2-amino-1,3-phthalate as the linker.
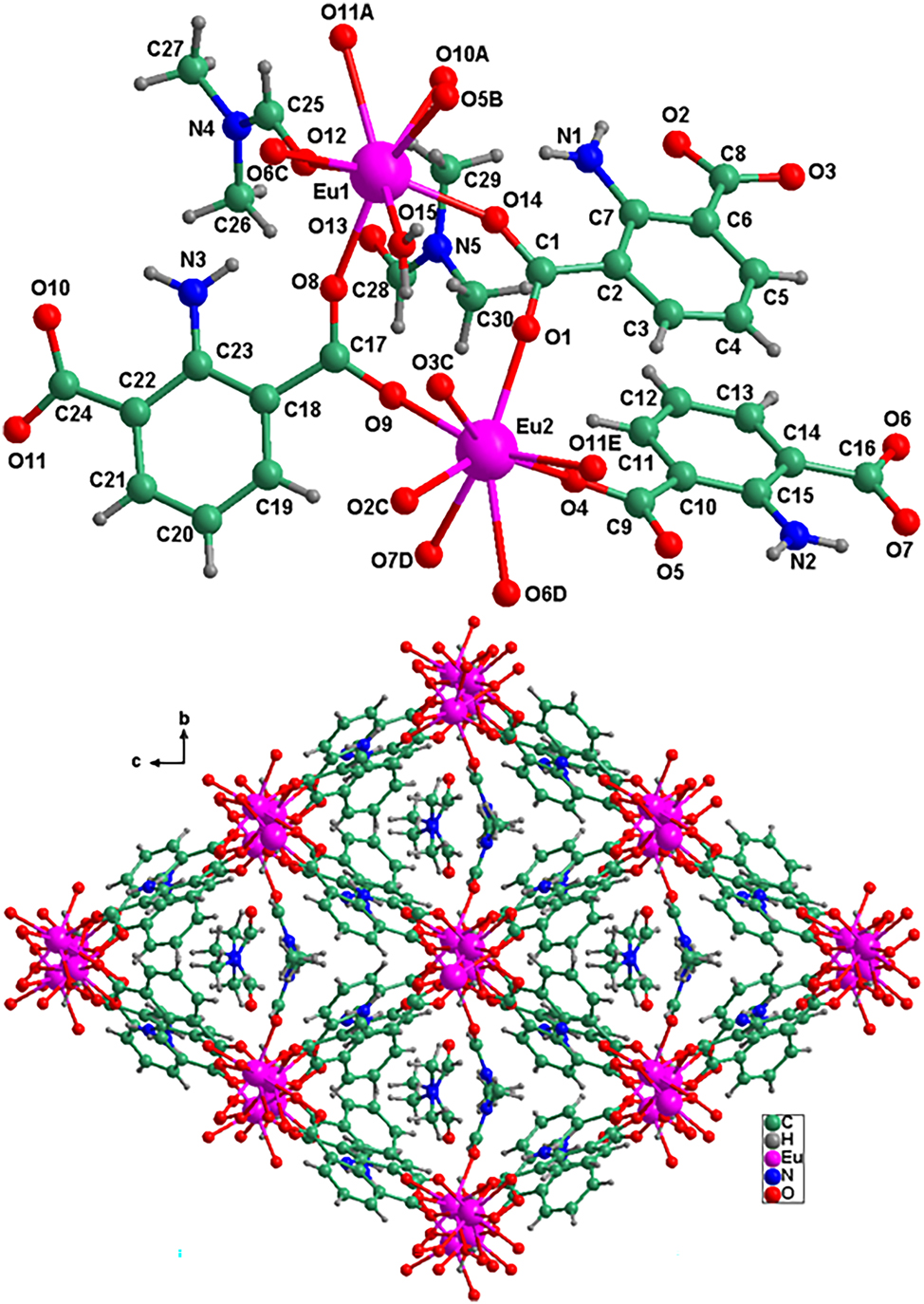
As shown in the Figure, the asymmetric unit of the title compound consists of three fully deprotonated 2-amino-1,3-benzenedicarboxylate ligands, one coordinated DMF molecule, one coordinated water molecule, and one crystalline DMF molecule and two Eu2+. The 2-amino-1,3-benzenedicarboxylate ligand adopts three types of coordinate modes, by which the 2-amino-1,3-benzenedicarboxylate ligands connect three Eu(III) atoms (two Eu1 and one Eu2), four Eu(III) atoms (two Eu1 and two Eu2), and three Eu(III) atoms (two Eu1 and one Eu2), respectively. From the Figure, we can see that it contains two crystallographically independent Eu(III), named Eu1 and Eu2, respectively. Eu1 is coordinated with eight O atoms from six 2-amino-1,3-benzenedicarboxylate ligands, while Eu2 is coordinated with six O atoms from five 2-amino-1,3-benzenedicarboxylate ligands, one O atom from one DMF molecule and one O atom from one water molecule to generate an eight-coordination environment. The bond lengths of Eu1–O12, Eu1–O13, Eu1–O14, Eu1–O10A, Eu1–O11A (code A: 1−x, −y, 0.5+z), Eu1–O15, Eu1–O5B (code B: −0.5+x, 0.5−y, z, Eu1–O6C (code C: 1.5−x, −0.5+y, −0.5+z) are 2.4747(49), 2.3912(53), 2.3226(53), 2.4594(52), 2.5801(38), 2.3587(48), 2.4272(47), and 2.3451(50) Å; while the bond lengths of Eu2–O1, Eu2–O4, Eu2–O9, Eu2–O2C, Eu2–O3C, Eu2–O6D, Eu2–O7D, Eu2– O11 (code E: 1.5−x, −0.5+y, 0.5+z) are 2.3345(52), 2.2829(53), 2.2834(54), 2.4613(52), 2.4507(55), 2.6475(54), 2.4566(53) and 2.4176(44) Å respectively, which are comparable with the reported values for its analogues. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 As demonstrated in the figure, Eu1 and Eu2 are bridged by three 2-amino-1,3-benzenedicarboxylate ligands to form a di–Eu(III)cluster, which are further connected by 2-amino-1,3-benzenedicarboxylate ligands to generate a 3D europium-organic framework, showing one-dimensional channels along the a direction (see the right part of the figure). In addition, the coordinated DMF and the crystalline DMF molecules are filled in the channels of the structure.
Acknowledgments
We are grateful for financial support from Postgraduate Education Reform and Quality Improvement Project of Henan Province (YJS2023JD65).
References
1. Bruker, SAINT v8.37A, Bruker AXS Inc, Madison, Wisconsin, USA, 2015.Suche in Google Scholar
2. Bourhis, L. J.; Dolomanov, O. V.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. The Anatomy of a Comprehensive Constrained, Restrained Refinement Program for the Modern Computing Environment–Olex2 Dissected. Acta Crystallogr. 2015, A71, 59–75; https://doi.org/10.1107/s2053273314022207.Suche in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Sheldrick, G. Using Phases to Determine the Space Group. Acta Crystallogr. 2018, A74, A353; https://doi.org/10.1107/s0108767318096472.Suche in Google Scholar
5. Cai, B.; Zhu, M. E.; Meng, Y. N.; Li, K. The Crystal Structure of 2-Aminoisophthalic Acid, C8H7NO4. Z. Kristallogr.–N. Cryst. Struct. 2020, 235, 543–544; https://doi.org/10.1515/ncrs-2019-0783.Suche in Google Scholar
6. Huang, X. F.; Zhang, Z. H.; Zhang, Q. Q.; Wang, L. Z.; He, M. Y.; Chen, Q.; Song, G. Q.; Wei, L.; Wang, F.; Du, M. Norfloxacin Salts with Benzenedicarboxylic Acids: Charge-Assisted Hydrogen-Bonding Recognition and Solubility Regulation. CrystEngComm 2013, 15, 6090–6100; https://doi.org/10.1039/c3ce40567b.Suche in Google Scholar
7. Meng, Y.; Liu, R. X.; Bao, H.; Jin, R. Y.; Song, S. H.; Feng, G. J. The Crystal Structure of poly[(μ2-2-Aminoisophthalato-Κ4 O,O′:O″:O‴)-(N-methylpyrrolidone κ1O)-dioxido-Uranium(VI)], C13H14N2O7. U. Z. Kristallogr.–N. Cryst. Struct. 2024, 239, 1085–1087; https://doi.org/10.1515/ncrs-2024-0309.Suche in Google Scholar
8. Meng, Y.; Liu, D.; Lan, Q.; Xie, Z.; Niu, F.; Zhang, X.; Yang, Y. Synthesis, Structure, and Photocatalytic Properties of a Two-Dimensional Uranyl Organic Framework. Z. Kristallogr. Cryst. Mater. 2023, 238 (1–2), 65–71; https://doi.org/10.1515/zkri-2022-0012.Suche in Google Scholar
9. Huang, G.; Chen, X. Crystal Structure of Catena-Poly[qua- (μ-2-2-Aminoisophthalat-κ3O,O’:O”) (1,10-phenanthroline-κ2N,N’)Manganese(II)] C20H15MnN3O5. Z. Kristallogr.–N. Cryst. Struct. 2022, 237, 797–799; https://doi.org/10.1515/ncrs-2022-0182.Suche in Google Scholar
10. Peikert, K.; Hoffmann, F.; Froba, M. Amino Substituted Cu3(btc)2: A New Metal-Organic Framework with a Versatile Functionality. Chem. Commun. 2012, 48, 11196–11198; https://doi.org/10.1039/c2cc36220a.Suche in Google Scholar PubMed
11. Tang, J.; Cai, M.; Xie, G.; Bao, S.; Ding, S.; Wang, X.; Tao, J.; Li, G. Amino-induced 2D Cu-Based Metal-Organic Framework as an Efficient Heterogeneous Catalyst for Aerobic Oxidation of Olefins. Chem.–Eur. J. 2020, 26, 4333–4340; https://doi.org/10.1002/chem.201905249.Suche in Google Scholar PubMed
12. Yang, Y.; Feng, Y.; Li, Y.; Shen, W.; Li, Z.; Mao, Z.; Lu, T.; Zhao, S.; Zhao, Z. Visible Light-Assisted Photocatalytic Degradation of Methylene Blue by 1D/2D CdxOy Clusters Based on Metal-Organic Frameworks without H2O2. J. Solid State Chem. 2023, 325, 124134; https://doi.org/10.1016/j.jssc.2023.124134.Suche in Google Scholar
13. Qiu, Y.; Deng, H.; Yang, S.; Mou, J.; Daiguebonne, C.; Kerbellec, N.; Guillou, O.; Batten, S. R. S. Crystal Structures, and Gas Storage Studies in New Three-Dimensional 5-aminoisophthalate Praseodymium Polymeric Complexes. Anorg. Chem. 2009, 48, 3976–3981; https://doi.org/10.1021/ic8020518.Suche in Google Scholar PubMed
14. Chandran, S. P. R.; Soumya, U. S.; Drisya, M. R.; Sudarsanakumar, M. R.; Kurup, M. R. P. Structural Studies of poly[(μ2-Acetato)(μ3-5-aminoisophthalato) diaquacerium(III) Monohydrate]: A New Three Dimensional Fluorescent Metal-Organic Framework Constructed from Dimers of CeO9 Polyhedra with Hydrophilic ’S′ Shaped Channels. J. Mol. Struct. 2017, 1137, 396–402.10.1016/j.molstruc.2017.02.024Suche in Google Scholar
15. Wang, J. J.; Si, P. P.; Yang, J.; Zhao, S. S.; Li, P. P.; Li, B.; Wang, S. Y.; Lu, M.; Yu, S. Y. La(III)-Based MOFs with 5-Aminoisophthalic Acid for Optical Detection and Degradation of Organic Molecules in Water. Polyhedron 2019, 162, 255–262; https://doi.org/10.1016/j.poly.2019.01.045.Suche in Google Scholar
16. Wang, H. M.; Liu, H. P.; Chu, T. S.; Yang, Y. Y.; Hu, Y. S.; Liu, W. T.; Ng, S. W. A Luminescent Terbium Coordination Polymer for Sensing Methanol. RSC Adv. 2015, 4, 14035–14041; https://doi.org/10.1039/c4ra00745j.Suche in Google Scholar
17. Xu, H.; Zheng, N.; Jin, X.; Yang, R.; Li, Z. Hydrothermal Synthesis of Highly Robust 2–D Layered Coordination Polymer of La. Chem. Lett. 2002, 31, 1144–1145; https://doi.org/10.1246/cl.2002.1144.Suche in Google Scholar
18. Kariem, M.; Kumar, M.; Yawer, M.; Sheikh, H. N. Solvothermal Synthesis and Structure of Coordination Polymers of Nd(III) and Dy(III) with Rigid Isophthalic Acid Derivatives and Flexible Adipic Acid. J. Mol. Struct. 2017, 1150, 438–446; https://doi.org/10.1016/j.molstruc.2017.08.111.Suche in Google Scholar
19. Sarma, D.; Prabu, M.; Biju, S.; Reddy, M. L. P.; Natarajan, S. Synthesis, Structure and Optical Studies of a Family of Three-Dimensional Rare-Earth Aminoisophthalates [M(μ2–OH) (C8H5NO4)] (M = Y3+, La3+, Pr3+, Nd3+, Sm3+, Eu3+, Gd3+, Dy3+, and Er3+). Eur. J. Inorg. Chem. 2010, 24, 3813–3822; https://doi.org/10.1002/ejic.201000225.Suche in Google Scholar
20. Huang, Y.; Yan, B.; Shao, M. Hydrothermal Synthesis, Structures and Photoluminescent Properties of Two Lanthanide 5-aminoisophthalate Coordination Polymers with Layer Structures. Solid State Sci. 2008, 10, 90–98; https://doi.org/10.1016/j.solidstatesciences.2007.07.035.Suche in Google Scholar
21. Dai, F.; Cui, P.; Ye, F.; Sun, D. An Open Neodymium-Organic Framework with the NbO Structure Type Based on Binuclear SBU Involved In Situ Generated Formate. Cryst. Growth Des. 2010, 10, 1474–1477; https://doi.org/10.1021/cg1000914.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

