In a recent issue of Matter, Wang et al. present macrophage cell membrane (CM)-cloaked nanoparticles (PN-AIE MØs) specifically designed for treating monkeypox (Mpox) [1]. Incorporating photosensitizers with aggregation-induced emission (AIE) properties for imaging and phototherapy, this approach efficiently targets and eradicates Mpox.
The global resurgence of Mpox as a health emergency highlights the urgent need for novel therapeutic strategies [2]. Traditional treatments, though somewhat effective, need more specificity and targeted delivery capabilities, especially against rapidly mutating viruses. Innovations in nanotechnology [3], particularly CM-cloaked engineered precision nanoparticles (NPs) [4], represent significant advancements in infectious disease management. Conventional nano-delivery systems often face rapid immune clearance, typically mitigated with hydrophilic polymer coatings like polyethylene glycol (PEG) [5]. In contrast, biomimetic CM coatings provide a better alternative by enhancing biocompatibility and avoiding immune detection [6]. These coatings encapsulate NPs with natural CMs, enhancing their integration into biological systems and targeting infection sites, exemplified by Wang et al.’s application of macrophage CM (MCM) coatings in Mpox treatment [1] (Figure 1).
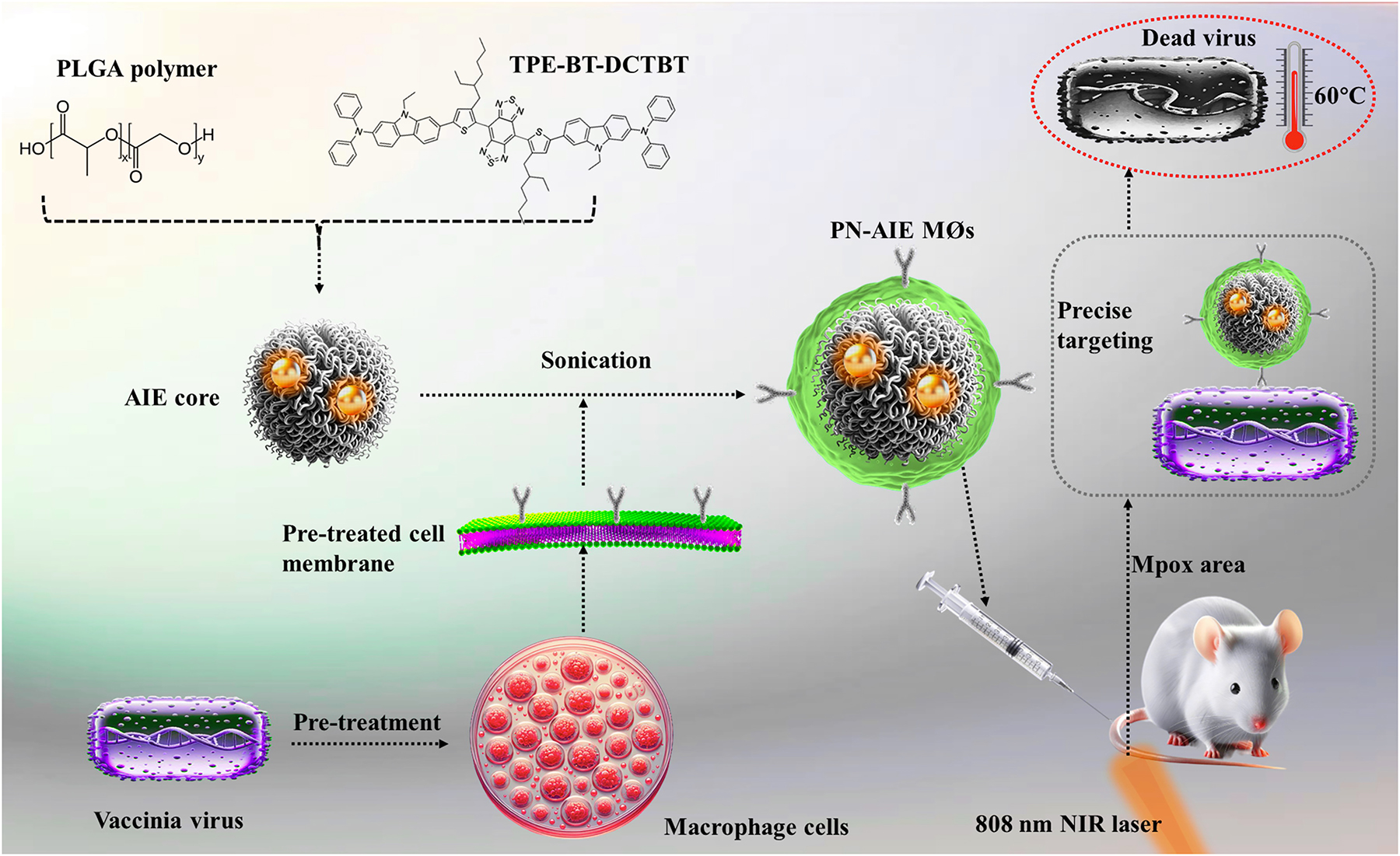
Schematic representation of the development and function of pre-treated macrophage-derived biomimetic nanoparticles (NPs) targeting Mpox. This figure depicts the preparation of PLGA NPs encapsulating NIR-II emissive photosensitizer TPE-BT-DCTBT with aggregation-induced emission (AIE) properties. These NPs are coated with vaccinia virus pre-treated macrophage cell membrane to form PN-AIE MØs. The pre-treatment enhances macrophage receptor expression, enabling precise targeting of Mpox virus by PN-AIE MØs. These PN-AIE MØs facilitate targeted phototherapy upon NIR-II laser irradiation and imaging, effectively localizing and eradicating the Mpox virus while minimizing impact on surrounding healthy tissues.
NP technologies enhance infectious disease management through targeted drug delivery and improved diagnostics. Notable types include liposomes, polymeric, and metal-based NPs, each offering distinct benefits in biocompatibility and delivery precision as shown in Table 1. Safety concerns vary, focusing on cytotoxicity, immunogenicity, and biodistribution. For example, metallic NPs, although effective antimicrobials, can pose toxicity risks and accumulate in organs. In contrast, biodegradable polymeric NPs, such as poly (lactic-co-glycolic acid) (PLGA) are favored for their lower toxicity and safer long-term use, providing precise targeting that reduces systemic side effects and adapts to pathogen mutations.
Comparison of nanoparticle technologies in infectious disease management.
| NP type | Characteristics | Safety considerations |
|---|---|---|
| Liposomes | Results in biocompatible carriers; enhances drug solubility | Low immunogenicity; risk of fusion with endogenous vesicles |
| Polymeric NPs | Controlled release capabilities; customizable for targeted delivery | Biodegradable with low toxicity; varies with polymer composition |
| Metallic NPs | Some of them offer antimicrobial properties; can offer imaging/diagnosis; magnetic NPs can offer magnetic targeting | Potential for heavy metal toxicity; accumulation in organs might cause health issues |
| Dendrimers | Uniform size and shape; high drug loading efficiency | High surface charge can cause cytotoxicity |
| Quantum dots | High photostability; suitable for long-term imaging studies | Contain heavy metals, raising concerns about body safety |
-
NP, nanoparticle.
Central to Wang et al.’s innovative approach is the synthesis of a photosensitizer endowed with AIE properties, enhancing both phototherapy and imaging capabilities significantly. AIE distinguishes itself from traditional fluorophores by increasing luminescence upon aggregation rather than diminishing. This unique characteristic has recently been harnessed in AIE-enabled PLGA NPs employed within advanced fluorescence lifetime imaging applications [7]. Such techniques provide detailed visualization and mapping of cellular dynamics at the subcellular level, revealing complex biological processes beyond the reach of conventional imaging methods.
The strategic selection of MCM coatings is pivotal for their inherent ability to recognize and neutralize pathogens, making them highly effective for therapeutic agent delivery directly to infection sites. This choice is not arbitrary; macrophages play a critical role in the body’s immune response by identifying, engulfing, and destroying pathogens. The use of MCM exploits these natural properties, enhancing the NP’s ability to target and treat infections effectively [8]. Furthermore, MCM-coated NPs have shown success in combating even bacterial (M. tuberculosis) infection, as reported in a recent study, underscoring their versatility and broad potential in treating various infectious diseases [9]. Other CMs offer unique therapeutic benefits: erythrocyte membranes enhance systemic circulation, leukocytes target inflammatory sites, platelets home to damaged vasculature, and cancer CMs ensure tumor-specific targeting. Each type is chosen based on the desired therapeutic outcome.
Wang et al.’s research presents the innovative photosensitizer TPE-BT-DCTBT, characterized by its near-infrared II (NIR-II) emission and AIE properties. The synthesized compound exhibited peak photoluminescence at 1008 nm and UV-visible absorption at 735 nm in THF, making it suitable for deep tissue imaging and photothermal and photodynamic therapies (PTT and PDT). It was designed to enhance fluorescence in aggregated states, overcoming the limitation of traditional fluorophores’ aggregation-caused quenching. To manage its hydrophobic nature, TPE-BT-DCTBT was encapsulated in FDA-approved PLGA via nanoprecipitation [7], enhancing its solubility and stability for delivery. The resulting NPs, named PN-AIE MØs, incorporated MCM coatings pre-treated with the vaccinia virus. This pre-treatment significantly amplified the expression of viral recognition receptors such as CCR2, CD206, and CD14 on the macrophages, enhancing the NPs’ specificity and efficiency in targeting and neutralizing Mpox infections. Dynamic light scattering confirmed the successful MCM coating, with PN-AIE MØs measuring 156.6 nm in size and -32.6 mV in zeta potential. Upon NIR laser exposure at 808 nm for 7 minutes, these NPs demonstrated remarkable photothermal conversion, reaching temperatures up to ∼60°C and achieving a photothermal conversion efficiency of 34.1%, substantially surpassing that of some typical photothermal agents.
In their study, Wang et al. developed a mouse model mimicking Mpox by injecting the vaccinia virus into scratched mouse tails to induce characteristic pustular lesions. The engineered NPs, PN-AIE MØs, showed remarkable targeting and retention at infection sites for over 72 h due to enhanced receptor expression on pre-treated MCM. Following 808-nm laser irradiation, these sites displayed significantly higher infrared intensity, indicating the NPs’ effective PTT. This led to nearly complete scab resolution and a substantial reduction in viral loads, demonstrating the therapeutic potential of PN-AIE MØs. Post-treatment histological analysis showed diminished viral antigen density and tissue necrosis alongside improved epidermal healing. Additionally, the study highlighted PN-AIE MØs’ role in preventing viral transmission, significantly reducing viral spread from treated lesions. Biocompatibility tests confirmed the safety of PN-AIE MØs for both in vitro and in vivo applications, with no significant cytotoxic effects or adverse impacts on major organs, underscoring their viability for therapeutic applications.
Wang et al.’s research significantly advance Mpox therapeutics, offering insights into using nanotechnology for infectious diseases. Their methodology holds promise for treating various viral infections, especially those resistant to standard therapies. Integrating PTT with existing Mpox treatments could enhance efficacy; co-loading NPs with traditional antivirals might reduce drug dosages and improve outcomes. Future enhancements could include boosting targeting precision by adding peptides or aptamers to MCM coatings, increasing specificity over conventional coatings [10]. Additionally, exploring other types of NPs [11] or the NPs’ effectiveness against other pathogens could expand their therapeutic scope [12]. Notably, the high photothermal conversion efficiency of TPE-BT-DCTBT suggests potential applications in cancer treatment. However, regulatory challenges must be addressed as these NPs, potentially classified as biologics, require rigorous safety and efficacy evaluations to transition from lab to clinical use.
In conclusion, Wang et al.’s research lays the groundwork for next-generation infectious disease therapeutics, emphasizing precision, efficacy, and safety.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: Q.T.H.S. initiated the conception and design, Q.T.H.S., L.F., and A.K.M.M.A were responsible for topic selection and writing the manuscript. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no conflict of interest.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Wang, W, Li, B, Wu, Y, Li, M, Ma, S, Yan, D, et al.. Macrophage-derived biomimetic nanoparticles for light-driven theranostics toward Mpox. Matter 2024;7:1187–206. https://doi.org/10.1016/j.matt.2024.01.004.Suche in Google Scholar
2. Shomuyiwa, DO, Manirambona, E. Monkeypox virus declared as a global health emergency: what next for Africa’s preparedness? Trav Med Infect Dis 2023;53:102577. https://doi.org/10.1016/j.tmaid.2023.102577.Suche in Google Scholar PubMed PubMed Central
3. Xiang, J, Shao, S, Zhou, Z, Shen, Y. “One-for-All” approach: a black technology for nanomedicine development? Mediev Rev 2023;3:184–7. https://doi.org/10.1515/mr-2023-0003.Suche in Google Scholar PubMed PubMed Central
4. Shubhra, QTH. Iron oxide nanoparticles in magnetic drug targeting and ferroptosis-based cancer therapy. Mediev Rev 2023;3:444–7. https://doi.org/10.1515/mr-2023-0029.Suche in Google Scholar PubMed PubMed Central
5. Liu, Y, Ding, M, Guo, K, Wang, Z, Zhang, C, Shubhra, QTH. Systemic co-delivery of drugs by a pH- and photosensitive smart nanocarrier to treat cancer by chemo-photothermal-starvation combination therapy. Smart Mater Med 2022;3:390–403. https://doi.org/10.1016/j.smaim.2022.05.003.Suche in Google Scholar
6. Guo, K, Xiao, N, Liu, Y, Wang, Z, Tóth, J, Gyenis, J, et al.. Engineering polymer nanoparticles using cell membrane coating technology and their application in cancer treatments: opportunities and challenges. Nano Mater Sci 2022;4:295–321. https://doi.org/10.1016/j.nanoms.2021.12.001.Suche in Google Scholar
7. Cai, Q, Musiol, R, Shubhra, QTH. Advancing fluorescence imaging with dual-mode AIE nanoparticles. Chem 2024;10:429–32. https://doi.org/10.1016/j.chempr.2024.01.010.Suche in Google Scholar
8. Chen, S, Saeed, AFUH, Liu, Q, Jiang, Q, Xu, H, Xiao, GG, et al.. Macrophages in immunoregulation and therapeutics. Signal Transduct Targeted Ther 2023;8:207. https://doi.org/10.1038/s41392-023-01452-1.Suche in Google Scholar PubMed PubMed Central
9. Cai, Q, Tian, Y, Shubhra, QTH. Pre-activated macrophage membrane-encased aggregation-induced emission featuring nanoparticles: a novel possibility for tuberculosis treatment. Signal Transduct Targeted Ther 2023;8:207.10.1038/s41392-024-01855-8Suche in Google Scholar PubMed PubMed Central
10. Pan, H, Yang, S, Gao, L, Zhou, J, Cheng, W, Chen, G, et al.. At the crossroad of nanotechnology and cancer cell membrane coating: expanding horizons with engineered nanoplatforms for advanced cancer therapy harnessing homologous tumor targeting. Coord Chem Rev 2024;506:215712. https://doi.org/10.1016/j.ccr.2024.215712.Suche in Google Scholar
11. Shubhra, QTH, Oyane, A, Nakamura, M, Puentes, S, Marushima, A, Tsurushima, H. Rapid one-pot fabrication of magnetic calcium phosphate nanoparticles immobilizing DNA and iron oxide nanocrystals using injection solutions for magnetofection and magnetic targeting. Mater Today Chem 2017;6:51–61. https://doi.org/10.1016/j.mtchem.2017.10.001.Suche in Google Scholar
12. Li, B, Wang, W, Zhao, L, Wu, Y, Li, X, Yan, D, et al.. Photothermal therapy of tuberculosis using targeting pre-activated macrophage membrane-coated nanoparticles. Nat Nanotechnol 2024;19:834–45. https://doi.org/10.1038/s41565-024-01618-0.Suche in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Reviews
- Biophysical stimuli for promoting bone repair and regeneration
- A stepwise approach to deriving functional β-cells from human embryonic or induced pluripotent stem cells
- Eco-fertility: examining the climate change-total fertility rate nexus in the context of sustainable developmental goals in a systematic review approach
- Redefining chronic mountain sickness: insights from high-altitude research and clinical experience
- Review of organ damage from COVID and Long COVID: a disease with a spectrum of pathology
- Perspective
- Combining transcriptomic and metabolomic insights to guide the clinical application of adipose- and bone marrow-derived mesenchymal stem cells
- Research Highlight
- Precision phototherapy and imaging with aggregation-induced emission-based nanoparticles cloaked in macrophage membrane
Artikel in diesem Heft
- Frontmatter
- Reviews
- Biophysical stimuli for promoting bone repair and regeneration
- A stepwise approach to deriving functional β-cells from human embryonic or induced pluripotent stem cells
- Eco-fertility: examining the climate change-total fertility rate nexus in the context of sustainable developmental goals in a systematic review approach
- Redefining chronic mountain sickness: insights from high-altitude research and clinical experience
- Review of organ damage from COVID and Long COVID: a disease with a spectrum of pathology
- Perspective
- Combining transcriptomic and metabolomic insights to guide the clinical application of adipose- and bone marrow-derived mesenchymal stem cells
- Research Highlight
- Precision phototherapy and imaging with aggregation-induced emission-based nanoparticles cloaked in macrophage membrane


