Abstract
Our understanding of β-cell differentiation from pluripotent stem cells (PSCs) is rapidly evolving. Although progress has been made, challenges remain, particularly in achieving glucose-stimulated insulin secretion (GSIS). Human embryonic stem cells (hESCs) are valuable due to their pluripotent ability. A fixed protocol targeting master regulatory genes initiates stem cells into pancreatic lineage commitment. Due to the observations that a single stem cell can differentiate into multiple cell types depending on various factors and conditions, non-linear differentiation pathways exist. Co-expression of key factors remains essential for successful β-cell differentiation. The mature β-cell marker MAFA plays a critical role in maintaining the differentiation state and preventing dedifferentiation. Recapitulating pancreatic islet clustering enhances physiological responses, offering potential avenues for diabetes treatment. On the other hand, several enhanced differentiation protocols from induced pluripotent stem cells (iPSCs) have improved the functional insulin producing β-cells generated. These findings, with their potential to revolutionize diabetes treatment, highlight the complexity of β-cell differentiation and guide further advancements in regenerative medicine.
Introduction
The pancreas is a vital organ comprising both endocrine and exocrine roles. Among its various cellular constituents, the endocrine clusters known as islets of Langerhans have a pivotal role in controlling blood glucose concentrations. Beta (β) cells are essential pancreatic cells responsible for glucose homeostasis by matching fluctuating insulin demands and adjusting rates of insulin secretion [1]. Insulin is the most potent anabolic hormone in the human body, counteracting the hyperglycemic effects of glucagon and adrenaline. Following food intake, glucose levels increase in the bloodstream and induce a rise in the circulating insulin levels. The latter stimulates insulin receptors that are mostly abundant in the liver, muscle, and adipose tissue. This activation enhances anabolic glucose-consuming pathways like glycolysis, glycogen production, and lipid synthesis. Therefore, insulin release is essential for proper glucose uptake in various tissues.
Consequently, insulin release is acutely regulated as β cells adapt the rate of insulin secretion to metabolic needs. Glucose-stimulated insulin secretion (GSIS) occurs in a biphasic manner: it is composed of an immediate primary phase and a slower secondary phase. First, a sharp increase of circulating insulin is observed as the hormone is discharged from readily releasable granules. Glucose diffusion into the β-cell facilitated by GLUT2 induces a rapid rise in intracellular ATP levels (through glycolysis and the Krebs cycle). Following the increased cytoplasmic ATP/ADP ratio, the K-ATP channels close, causing membrane depolarization and intracellular calcium release. Ca2+ triggers decreased electrostatic repulsion between the secretory granules and the plasma membrane, activating exocytosis of the pre-existing insulin granules [2]. In addition to the triggering role of Ca2+, elevated cAMP levels are also required for a normal insulin secretory response to glucose [3]. The second phase of insulin release is slower: it requires continuous glucose stimulation, which induces transcriptional modifications leading to proinsulin peptide synthesis, granule build-up, and vesicle priming (Figure 1). In the Golgi complex of β cells, proinsulin is cleaved to form mature/active insulin. The cleavage product known as connecting peptide (C-peptide) is a short peptide of 31 amino acid residues that gets stored in secretory vesicles alongside insulin. Its short, 30-min half-life makes C-peptide a reliable marker for β cell function [4].
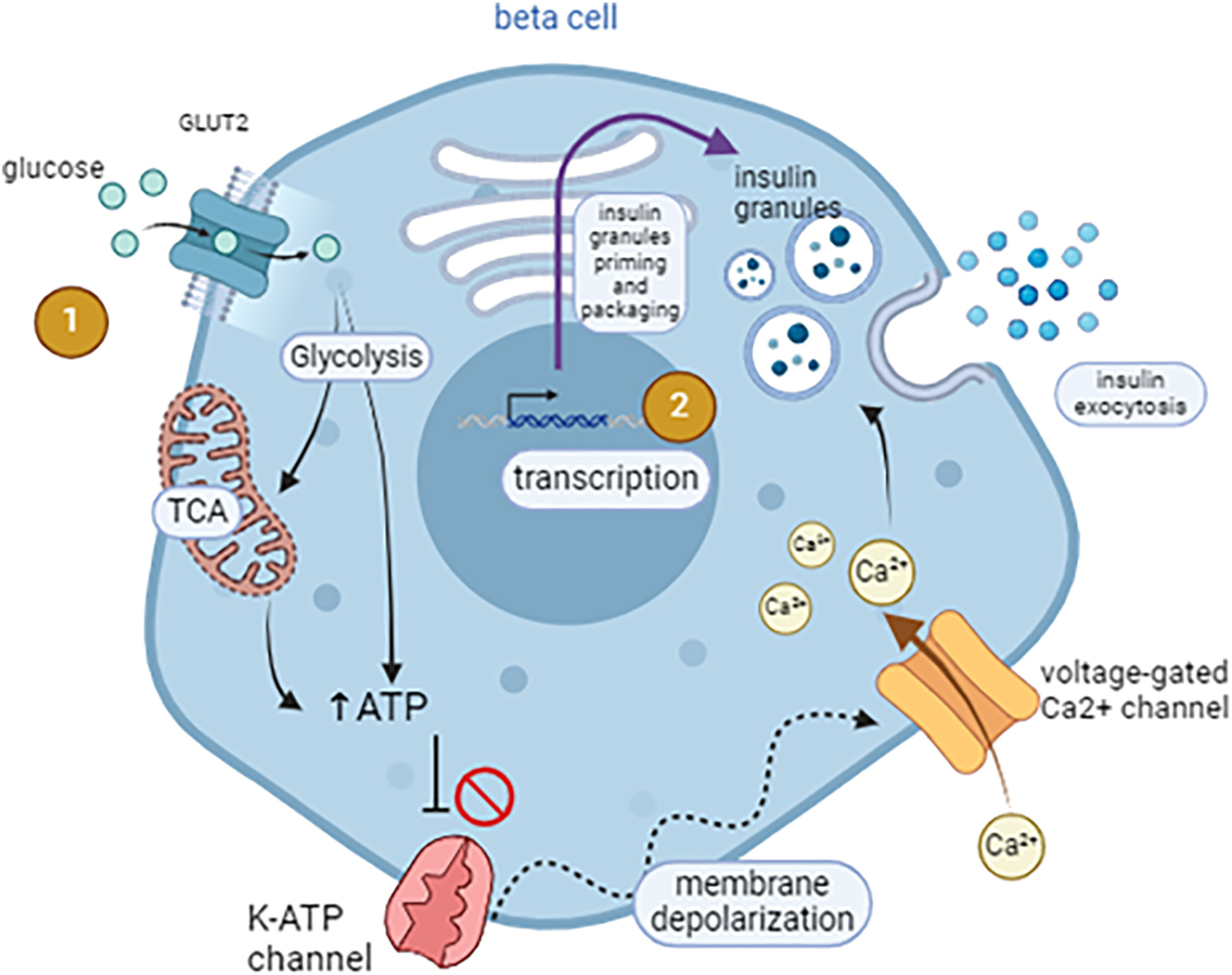
Islet cell architecture and signaling pathways mediating glucose-stimulated insulin secretion (GSIS). (1) Glucose uptake into β cells occurs via diffusion through the GLUT2 transporter. It undergoes glycolysis and the tricarboxylic acid (TCA) cycle, resulting in ATP production. Increased ATP to ADP ratio leads to the closure of the K-ATP channels, membrane depolarization, and influx of Ca2+ through the voltage-gated calcium channels. Ca2+ triggers exocytosis of the insulin granules. (2) Prolonged stimulation by glucose induces transcription of insulin gene in the β cell nucleus. Insulin is processed and packaged into vesicles that become primed for immediate release – figure generated on BioRender by C.F.
Thus, functional β-cells should acutely respond to an elevation of circulating glucose and stop insulin release once blood glucose levels return to baseline. These responsive β cells constitute the functional β cell mass. Healthy β cells shall adapt their number, size, and differentiation state to meet metabolic demands [5].
Functional β cell mass is depleted in cases of chronic metabolic distress like diabetes. With a rapidly rising prevalence, diabetes has been extensively studied and classified. Type 1 diabetes (T1D) is caused by autoimmune destruction of β-cells, leading to absolute insulin deficiency [6]. Type 2 diabetes (T2D) is characterized by dysfunctional β cells and insulin signaling, which is often associated with obesity and lifestyle factors [7], [8], [9]. We also note rare monogenic cases of diabetes (MODY) and neonatal diabetes, etc. [10]. While oral medications and lifestyle changes are the most prescribed remedy for T2D, poor diabetes control remains as most patients require a combination of multiple drugs [11]. Likewise, T1D management is not optimal as it requires exogenous insulin delivery. Such a procedure is coupled with challenging management of insulin dosing and leads to poor overall glycemic control [12], 13]. Traditional treatments for diabetes, including insulin therapy and oral hypoglycemic agents, primarily manage symptoms but do not address the underlying β-cell dysfunction or loss [14]. Therefore, developing regenerative therapies that can restore β-cell function or replace lost β-cells represents a promising approach to achieving long-term diabetes management and potential cure. This enhanced glucose homeostasis can be achieved by islet cell transplantation [15]. Deriving pancreatic β cells in vitro is essential for successful islet transplantation, for it addresses the following challenges and avenues for growth:
Limited Donor Availability. Cadaveric donors are the primary source of islets for transplantation. However, donor pancreases are limited, and the demand far exceeds the supply. In vitro-derived β cells can address this shortage.
Immunosuppression. Transplanted islets face immune rejection unless patients receive immunosuppressive drugs. In vitro-derived β cells can be genetically modified to reduce immunogenicity, thus improving transplantation outcomes.
Drug Testing and Research. In vitro-derived β cells serve as valuable models for studying diabetes pathogenesis, drug testing, and understanding β cell biology. Researchers can explore new therapies and optimize transplantation protocols.
Personalized Medicine. Patient-specific β cells can be generated from induced pluripotent stem cells (iPSCs). These β cells can then be used for personalized transplantation, minimizing immune rejection [12].
Quality Control. In vitro-derived β cells allow rigorous quality control, ensuring that transplanted islets are functional, viable, and safe.
Functional Restoration. Successful islet transplantation restores insulin secretion and glucose regulation, as well as reduces the need for exogenous insulin injections.
In short, deriving pancreatic β cells in vitro is essential for advancing islet transplantation as a viable treatment option for diabetes. It addresses donor shortages, improves transplantation outcomes, and accelerates research and drug development.
The following review focuses on the potential of embryonic stem cells (ESCs) and iPSCs to differentiate into functional β-cells. ESCs are pluripotent cells derived from the inner cell mass of blastocysts. They can differentiate into various cell types of the three germ layers (endoderm, mesoderm, and ectoderm) [16]. Their pluripotency makes them a valuable tool in regenerative medicine and holds significant promise for generating β cells. However, several problems remain with the use of ESCs, such as moral sensitivity regarding the use of human embryos and the immune rejection of the cells by recipients after transplantation. Human induced pluripotent stem cells (hiPSCs), on the other hand, are generated by reprogramming somatic cells to a pluripotent state. This reprogramming is achieved through the introduction of specific transcription factors such as OCT4, SOX2, KLF4, and c-MYC. hiPSCs offer several advantages over hESCs, including the ability to generate patient-specific cells, which reduces the risk of immune rejection and bypasses the ethical issues associated with hESCs. Recent advancements in hiPSC technology have significantly improved the efficiency and safety of the reprogramming process, making hiPSCs a promising alternative for β-cell generation [17], [18], [19].
This review synthesizes the latest advancements and persistent challenges in deriving functional β-cells from hESCs and hiPSCs. It explores differentiation pathways, key transcription factors, and novel experimental techniques, while also discussing the functional maturation of these cells and their translational and clinical applications. By integrating recent research findings, this review aims to highlight both the potential and limitations of stem cell-derived β-cell therapies for diabetes. The insights provided will contribute to a deeper understanding of β-cell differentiation and identify future research directions necessary for optimizing these regenerative therapies.
Differentiation stages (from ESC to insulin-producing cell)
In vitro engineered β-like cells possess limited functionality compared to primary human β-cells, from which we note deficient GSIS. However, Rezania et al. revolutionized the field with their seven-stage protocol that efficiently converts human embryonic stem cells (hESCs) into insulin-producing cells (IPCs) [17]. In the following section, we investigate the master genes controlling the differentiation process, and we expand upon them throughout the review. From stage 1 (definitive endoderm) to stage 7 (mature β cell), the researchers used several vitamins, growth factors, and special media to allow for cellular differentiation (Figure 2). While the endoderm-derived marker FOXA2 is expressed as early as in stage 1, the differentiating cells engage in the pancreatic lineage around stage 3 following the expression of PDX1. This pancreatic homeodomain transcription factor (PDX1) is a key marker of pancreatic progenitor. It is also known to precede the expression of NKX6.1 (homeobox transcription factor). However, their co-expression is restricted to β cell lineage [20]. The endocrine marker (NGN3) gives rise to hormone-expressing cells. While transiently expressed in the developing pancreas, its downstream target NEUROD1 persists in β cells as of stage 4 [17]. Mature β cells are finally characterized by high MAFA expression (a basic leucine zipper transcription factor). MAFA is absent in developing β cells and has an essential role in maintaining the homeostasis of mature β cells [21]. Such mature cells are expected to be insulin+/glucagon−/somatostatin−.
![Figure 2:
A 7-stage differentiation protocol from ES to β cell. Row 1: Differentiation stages, number and title. Row 2: Growth media used in the protocol. Row 3: Color-coded genes referencing their stages of expression. The most important β cell markers are PDX1 (pancreatic), NKX6.1 (pancreatic), NEUROD1 (endocrine), and MAFA (mature β cell). Adopted with permission from [17].](/document/doi/10.1515/mr-2024-0039/asset/graphic/j_mr-2024-0039_fig_002.jpg)
A 7-stage differentiation protocol from ES to β cell. Row 1: Differentiation stages, number and title. Row 2: Growth media used in the protocol. Row 3: Color-coded genes referencing their stages of expression. The most important β cell markers are PDX1 (pancreatic), NKX6.1 (pancreatic), NEUROD1 (endocrine), and MAFA (mature β cell). Adopted with permission from [17].
Characteristics of IPCs (from ESCs) [17]
Successful differentiation of IPCs
The previously mentioned protocol by Rezania et al. resulted in S7 cells that are β-like. In the following, we examine their characteristics and compare their functionality to human β cells. Given that MAFA is a key regulator of mature β cells, the researchers induced higher MAFA transcript levels through the complete S7 medium formulation (including AXL inhibitor, N-Cys, ALK5iII, and T3). Gene expression profiles of S7 cells were compared to human islets, revealing similarities in INS and MAFA transcript levels but also significantly decreased expression of other genes (CHGB, KCNK1, etc.) in S7 cells. The protocol successfully resulted in NKX6.1+/insulin+ S7 cells for every two hESCs, achieving a yield of 50 %, i.e., half of the S7 cells are β-like.
Insulin secretory mechanism in S7 cells
Having INS expressions similar to those in human islets, S7 cells successfully secrete amounts of insulin comparable to those of human β cells (Figure 3A). Thus, S7 cells exhibit functional insulin secretory mechanisms. However, they still have higher proinsulin content and lower C-peptide amounts compared with human islets. This data suggests inefficient proinsulin processing in S7 cells.
![Figure 3:
Stage 7 (S7) cells possess functional similarities to human β cells but are considered immature. (a) Insulin secretory mechanism in S7 and human islet cells. Hormone content relative to DNA content was measured; decreased C-peptide and increased proinsulin levels in S7 cells were noted. (b) Calcium signaling traces performed after 20 mmol/L glucose stimulation followed by 30 mmol/L KCl. Baseline glucose level was always 3 mmol/L. Percentages of responsive cells are indicated in grey. (c) S7 cells reverse diabetes in vivo. S7 cells were transplanted under the kidney capsule of SCID-beige mice with streptozotocin (STZ)-induced diabetes. Fasting blood glucose levels showed a reversal of diabetes 40 days post-transplantation and a fast return to hyperglycemia after the removal of the engrafted kidney (red arrow). Adopted with permission from [17].](/document/doi/10.1515/mr-2024-0039/asset/graphic/j_mr-2024-0039_fig_003.jpg)
Stage 7 (S7) cells possess functional similarities to human β cells but are considered immature. (a) Insulin secretory mechanism in S7 and human islet cells. Hormone content relative to DNA content was measured; decreased C-peptide and increased proinsulin levels in S7 cells were noted. (b) Calcium signaling traces performed after 20 mmol/L glucose stimulation followed by 30 mmol/L KCl. Baseline glucose level was always 3 mmol/L. Percentages of responsive cells are indicated in grey. (c) S7 cells reverse diabetes in vivo. S7 cells were transplanted under the kidney capsule of SCID-beige mice with streptozotocin (STZ)-induced diabetes. Fasting blood glucose levels showed a reversal of diabetes 40 days post-transplantation and a fast return to hyperglycemia after the removal of the engrafted kidney (red arrow). Adopted with permission from [17].
Glucose-stimulated insulin secretion in S7 cells
Next, researchers examined S7 cells’ ability to increase cytosolic Ca2+ concentrations in response to glucose stimulation rapidly. Only about 5–10 % of S7 cells significantly increased their intracellular Ca2+ level in response to 20 mmol/L of glucose. Also, these active cells reveal decreased Ca2+ discharge amplitude, slower release rate, and delayed signal termination (Figure 3B). However, S7 cells exhibited rapid and transient depolarization to 30 mmol/L KCl, proving functional voltage-gated Ca2+ channels and normal Ca2+ release. We understand that S7 cells exhibit delayed kinetics compared to human islets, resulting from quantitatively and qualitatively altered glucose sensitivity. Consequently, S7 cells release lower insulin levels in response to glucose and at a slower rate than human islets. In conclusion, S7 cells exhibit a successful insulin release mechanism. However, only a subpopulation responded to glucose and had delayed kinetics, proving that S7 cells are still considered functionally immature compared to normal human β cells.
In-vivo reversal of diabetes by S7 cells
Finally, though immature, S7 cells are capable of reversing diabetes in mice within 40 days post-transplantation. Injected into the recipient’s kidney, S7 cells lowered fasting blood glucose levels similarly to the baseline in healthy control. Return to hyperglycemia was observed following the removal of the engrafted kidney, supporting the essential role of S7 cells in reversing diabetes (Figure 3C). In conclusion, Rezania et al. fixed the protocol for ES differentiation into β cells. It resulted in functional IPCs capable of reversing diabetes in vivo, at the same time they exhibit decreased GSIS. These cells remain immature compared to human islets, and further research is needed to improve the outcome.
Temporal progression of gene expression in β cell differentiation [22]
The aforementioned protocol has been thoroughly implemented across different laboratories. Therefore, the need to understand the specifics of each differentiation stage increased. Researchers investigated the temporal progression of gene expression throughout the protocol. Do all cells enter the different stages at the same time? Do they all follow the same orderly path? A single-cell qPCR study conducted by Peterson et al. aims to understand the linearity of differentiation by researching the following: do cells always gain the pancreatic β cell-specific marker NKX6.1 before the endocrine progenitor marker NEUROD1 [22] ? More broadly, they are investigating possible lineage bifurcations and the impact of heterogeneity in hESC differentiation.
What came first, pancreatic or endocrine progenitor?
To temporally track the endocrine marker NEUROG3, researchers used an hESC line expressing EGFP under the control of NEUROG3 promoter (NEUROG3-EGFP). Due to GFP’s long half-life, cells that previously expressed NEUROG3 and since downregulated retain the GFP expression (NEUROG3−EGFP+). Conversely, cells that just upregulated NEUROG3 have not yet accumulated enough EGFP protein and are referred to as NEUROG3+EGFP−. Thus, early-born endocrine progenitors (EP) are NEUROG3−EGFP+ cells that expressed NEUROG3 a while ago, and late-born EP are NEURPG3+EGFP− cells that just upregulated the NEUROG3 marker [22]. Knowing that NKX6.1+ cells are born after stage 4, researchers were surprised to find two subpopulations of early stage 5 NKX6.1+ cells: NKX6.1+/early-born endocrine precursor and NKX6.1+/late-born endocrine precursor. Such finding reveals that NKX6.1 expression can be acquired either upstream or downstream of NEUROG3 expression.
Developmental potential of early and late EP
Furthermore, researchers questioned whether acquiring NKX6.1 downstream of NEUROG3 disrupts the generation of β-like cells. They cultured early and late EP cells and stained them for EGFP, NKX6.1, C-peptide, and glucagon. Both cell types revealed similar abilities in generating C-peptide+/NKX6.1+ β-like cells. They both additionally gave rise to NKX6.1+C-peptide- progeny. Finally, EGFP expression was detected in C-peptide+/glucagon-cells (representing β-like cells), C-peptide+/glucagon+ cells (representing polyhormonal cells) and few C-peptide-/glucagon+ cells (representing α cells). In conclusion, both early and late endocrine precursor cells have the ability to differentiate into the same cell types, from which we note INS+/GCG- β-like cells. This finding proves the presence of multiple bifurcations in the β cell differentiation lineage: the path is not linear as previously thought. The assumption that mature β cells derive from EP born out of NKX6.1+ pancreatic progenitors was challenged. NKX6.1 expression can be triggered in the endocrine progenitor cells to induce the β-like lineage. Further investigations are needed to discern additional triggers of this induction [22].
Genetic regulations by mafa in mature β cells
After establishing that the path towards β cell differentiation is non-linear, researchers aimed to answer the questions: what causes the final lineage commitment to β cells and what inhibits the cells’ dedifferentiation back to early stages? As GSIS is key in mature pancreatic β cells, and as this function remains flawed in S7 cells from Rezania et al. researchers investigated the voltage-gated Ca2+ channels crucial for insulin release [23]. Expectedly, these channels’ expression is associated with the transcription of MAFA, the master regulator of gene expression of mature β cells [24].
Voltage-gated Ca2+ channels
It has been known that voltage-gated Ca2+ channels (Cav) trigger intracellular Ca2+ influx, leading to exocytosis of docked insulin granules in β cells. Ca2+ channels are heteromeric, and several subunits control the differentiation of β cells. Impairment of specific Cav proteins was noted to reduce β cell mass, not involving β-cell death [25]. The gamma subunits (Cavγ) are the least studied [26]; Cavγ4 is the only gamma protein known to be involved in cell differentiation of myoblasts and fetal brain [27]. Luang et al. confirmed the essential role of Cavγ4 in assuring GSIS in differentiating β cells [23]. Indeed, they demonstrated that Cavγ4 regulates L-type Ca2+ channel gene expression. They proved Cavγ4’s important role not only in regulating Ca2+ influx but also in the trafficking of the L-type Ca2+ channel.
MAFA’s role in the regulation of Cavγ4
Pearson correlation coefficients were calculated by mRNA expression between Cavγ4 and the transcription factors known for pancreas development. Strong correlations were found between mRNA levels of Cavγ4 and MAFA, MAFB (possibly compensating for MAFA when needed) [28], NEUROD1 (endocrine progenitor), PX1 (pancreatic progenitor), and NKX6.1 (pancreatic β cell progenitor). Most importantly, MAFA’s impact on Cavγ4 was studied. β-cell-specific MAFA knockout mice exhibited largely reduced Cavγ4 protein levels. Also, chromatin immunoprecipitation analysis revealed two binding sites for MAFA on the promoter region of Cavγ4, confirming MAFA’s direct regulation of Cavγ4 expression.
Effects of Cavγ4 downregulation
Downregulation of Cavγ4 leads to β-cell dedifferentiation. New β-cell dedifferentiation marker aldehyde dehydrogenase 1a3 (Aldh1a3) [29] was found to be highly enriched in Cavγ4-silenced INS-1 cells. Aldh1a3 was initially recognized as a cancer precursor marker and later suggested as a β cell dedifferentiation marker in T2D [29]. Indeed, Cavγ4 deletion causes β cell dedifferentiation without affecting cell viability or proliferation. Thus, upon exposure of β cells to chronic oxidative stress, such as in glucose toxicity, MAFA levels become markedly reduced, inducing lower Cavγ4 levels and causing β cell dedifferentiation. The connection between glucotoxicity, differentiation, gene expression, and the functional consequences (Ca2+ signaling and insulin secretion) was elucidated. The evidence strongly suggests that Cavγ4 is a direct target of MAFA, which could be implicated in long-term regulation of β cell differentiation and functional status (Figure 4).
![Figure 4:
Schematic of MAFA’s regulation of Cavγ4 and the downstream cascade on insulin secretion in healthy and dedifferentiated β cells. Glucotoxicity in dedifferentiated β cell leads to reduced MAFA signaling, downregulation of its direct target Cavγ4, diminished L-type Ca2+ channels and intracellular Ca2+ signaling and finally impairment of glucose-stimulated insulin secretion (GSIS). Adopted with permission from [23].](/document/doi/10.1515/mr-2024-0039/asset/graphic/j_mr-2024-0039_fig_004.jpg)
Schematic of MAFA’s regulation of Cavγ4 and the downstream cascade on insulin secretion in healthy and dedifferentiated β cells. Glucotoxicity in dedifferentiated β cell leads to reduced MAFA signaling, downregulation of its direct target Cavγ4, diminished L-type Ca2+ channels and intracellular Ca2+ signaling and finally impairment of glucose-stimulated insulin secretion (GSIS). Adopted with permission from [23].
Endocrine cell clustering in β cell differentiation [30]
While in vitro cell culture conditions have been optimized to generate mature, functional β cells, the outcomes achieved thus far fall short of attaining perfection. Nair et al. investigated the importance of endocrine cell clustering during pancreatic islet organogenesis [30]. The researchers isolated and reaggregated immature β-like cells to form islets of enriched β-clusters (eBCs). In the following, we scrutinize the role of cellular reorganization involving endocrine clustering.
First, endocrine clustering was proven to be attained during human and rodent postnatal development, especially concerning β cell maturation. Indeed, in vivo studies have shown that EP first appear scattered throughout the pancreatic epithelium and only aggregate into islets once becoming mature [31]. Opposingly, in vitro hESC differentiation protocols result in mixed populations of PDX1+/NKX6.1+ combined with mature and immature endocrine cells subject to several lineage bifurcations (as mentioned in Section 4). Thus, the study conducted by Nair et al. proves the imminent role of endocrine cell clustering for hESC-derived β cell maturation.
To obtain C-peptide eBCs, researchers isolated INS−GFP+ immature β-like cells using fluorescence-activated cell sorting (FACS) and aggregated them into clusters. Islet-like cluster formation was further enhanced by extending the culture time for the reaggregated cells. To control for the extended culture, eBCs were compared to stage 7 cells from Rezania et al.’s protocol with prolonged cell cultivation. This negative control for endocrine clustering was referred to as non-enriched clusters (NECs).
eBCs resemble human islet cells
Functional characteristics of the β cells were analyzed. eBCs revealed dynamic secretion of C-peptide in response to 20 mmol/L glucose. The sharp rise of the response is similar yet slightly lower than that of human islets. Similarly, calcium influx was significant in eBCs following high glucose and KCl stimulations. It also returned rapidly to baseline when the stimulus was removed. Altogether, the data reveals that eBCs physiologically resemble human islet cells, with better glucose-induced response than the previously mentioned stage 7 cells by Rezania et al. (or NEC) (Figure 3B).
Transcriptome-wide analysis of eBCs
Gene expression profiles were assessed in eBCs using transcriptome-wide analysis. No significant differences were noted when comparing eBC transcripts to human islets. Between eBCs and NECs, no difference was noted in the canonical β cell markers such as PDX1 and NKX6.1. However, eBCs exhibited significantly higher levels of transcripts of mature β cell markers like MAFB, NEUROD1 and PAX6. To elucidate the cause behind the enhanced functional properties of eBCs compared to NECs, gene set enrichment analysis (GSEA) revealed that eBCs upregulated genes involved in oxidative phosphorylation, protein secretion and metabolic pathways like the TCA cycle (already proven to be essential in murine β cell maturation) [32].
β cell clustering improves mitochondrial function
As genes involved in mitochondrial function were upregulated in eBCs, researchers assessed mitochondrial respiratory function using a Seahorse XFe24 analyzer. Upon high glucose stimulation, eBCs and human islets increased their oxygen consumption rate (OCR), wile NECs did not. Transmission electron micrographs of mitochondria revealed increased folding in eBCs compared to NECs, indicating greater average inner-membrane length per mitochondrion. Metabolic maturation is therefore enhanced by β cell clustering, suggesting effective mitochondrial oxidative phosphorylation for glucose oxidation in mature β cells.
β cell clustering improves the maturity of insulin granules
Furthermore, researchers analyzed the role of upregulated protein secretion and ribosome genes in eBCs. By investigating the structure and constituents of the insulin granules, Nair et al. confirmed that eBCs contain decreased ratios of immature granules. The processed insulin to pro-insulin ratio was significantly elevated in eBCs compared to NECs, further endorsing the maturity of eBCs.
In conclusion, β cell clustering and reaggregation are critical for in vitro β cell differentiation and maturation. These steps allow for enhanced β cell functionality, such as increased sensibility to glucose, improved pro-insulin processing, robust C-peptide release, acute calcium signaling and signal ablation upon stimulus removal, and advanced mitochondrial energization.
Application of pluripotent stem cell-derived secretomes in β-cell differentiation
The use of secretomes derived from pluripotent stem cells (PSCs), which include a variety of bioactive molecules such as growth factors, cytokines, and extracellular vesicles, has emerged as a promising method to boost the efficiency and functionality of IPC differentiation.
Role of secretomes in differentiation
hPSC-derived secretomes can replicate the natural microenvironment, delivering crucial signals that aid in the effective differentiation of PSCs into IPCs. These bioactive molecules impact several vital aspects of cellular development and maturation:
Promotion of definitive endoderm formation. Secretomes aid in transitioning PSCs into definitive endoderm by providing signaling molecules like activin A and Wnt3a, which are essential for the initial steps toward pancreatic lineage differentiation [33].
Induction of pancreatic progenitor cells. During the intermediate stages, secretomes rich in retinoic acid and fibroblast growth factors (FGFs) promote the formation of pancreatic progenitors expressing markers such as PDX1 and NKX6.1, which are crucial for subsequent differentiation into IPCs [35].
Maturation and functional differentiation. Secretomes at later stages contain insulinotropic factors that enhance IPC maturation, upregulating the expression of insulin, glucokinase, and GLUT2, indicative of functional IPCs [36].
Recent advances and applications
Extracellular vesicles and microRNAs. Guo et al. explored the role of exosomes derived from pancreatic β-cells in promoting the differentiation of hiPSCs into IPCs. The study identified specific exosomal microRNAs crucial for this process, indicating that exosomes can significantly enhance IPC differentiation and functionality [37].
Bioactive peptides and canonical Wnt signaling. Heaton et al. investigated the use of a bioactive peptide from type V collagen, known as the WWASKS peptide, for differentiating iPSCs into pancreatic EP and islet organoids. Their research demonstrated that this peptide enhances the formation of essential pancreatic endocrine cells, including insulin-producing β-cells, and improves their glucose sensitivity and insulin secretion. The mechanism involves activating the canonical Wnt signaling pathway, which results in the translocation of β-catenin to the nucleus, a vital step for pancreatic progenitor development. This discovery offers promising prospects for new diabetes treatment strategies [38].
Novel protocols using IPSCs
Recent advancements in stem cell research have significantly enhanced protocols for differentiating iPSCs into functional insulin-producing β-cells. These advancements are pivotal as they strive to emulate the complex signaling environment necessary for β-cell development.
Supplements for enhanced differentiation
Enderami et al. presented an in-depth method for producing IPCs from iPSCs, utilizing a step-by-step differentiation protocol enhanced with platelet-rich plasma (PRP). PRP is a portion of the whole blood rich in platelets and fibrin. It has been shown to have a potent effect in stimulating the differentiation of stem cells into blood vessels, bone, and cartilage cells. Platelets release a variety of growth factors and adhesion molecules like platelet-derived growth factor (PDGF), insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), vitronectin, fibronectin, and others. This method includes several stages designed to emulate the natural developmental cues of pancreatic cells in vivo. The incorporation of PRP significantly improved both the differentiation efficiency and the functionality of thee resulting β-cells. First, the number of IPCs in the PRP group was significantly higher than the non-PRP group (46 % vs. 13 % insulin positive cells). Second, mRNA and protein levels of pancreatic-specific markers (Pdx1 and insulin) were also enhanced in the PRP group. As a conclusion, the use of patient-specific iPSCs and PRP led to enhanced IPC insulin secretion in response to glucose [39].
Horikawa et al. proposed a simplified approach for iPSC differentiation into IPCs by integrating vitamin C and the ALK5 inhibitor RepSox. This method aimed to improve differentiation efficiency and reduce costs. Their results showed that 3D culture conditions substantially enhanced glucose-stimulated insulin secretion compared to traditional 2D cultures [40].
Arroyave et al. further refined these methods by adding a combination of vitamin C and retinoic acid during the final stages of differentiation. Their study emphasized the epigenetic effects of these compounds, particularly their capacity to enhance gene expression by activating DNA demethylation proteins like TET enzymes. This technique led to the overexpression of specific β-cell genes, thereby improving the functionality of the derived β-cells [41].
Lee et al. showed that continuous inhibition of Sonic hedgehog signaling effectively leads to the differentiation of hiPSCs into functional insulin-producing β cells. This approach, using the small molecule SANT-1, yielded IPCs that demonstrated sustained insulin secretion in response to glucose stimulation [36].
Pellegrini et al. performed a detailed single-cell RNA sequencing (scRNA-seq) analysis to map transcriptional changes throughout the differentiation process. Their findings indicated that iPSCs fully commit to the endoderm lineage, resulting in a high degree of similarity to mature human islets. This comprehensive profiling helps identify critical transcriptional regulators and minimize potential off-target effects [42].
3D scaffold in differentiation
Ahmadi et al. investigated the differentiation of hiPSCs into pancreatic islet IPCs on a Silk/PES nanofiber 3D scaffold. Their study found that the 3D scaffold significantly enhanced the expression of pancreatic-specific markers and insulin secretion compared to traditional 2D cultures [43].
Encapsulation using elastin-like recombinamers (ELRs)
Montanucci et al. explored the application of ELRs to encapsulate iPSC-derived β-cell spheroids, aiming to enhance their stability and viability post-transplantation. This novel approach not only provides immunoprotection but also maintains the functional properties of the β-cells, showing potential for clinical use in diabetes treatment [44].
As a conclusion, recent studies have highlighted the importance of optimizing culture conditions and differentiation protocols to improve the yield and functionality of β-cells derived from iPSCs. For instance, the incorporation of specific molecules and growth factors, PSC-derived secretomes, as well as advancements in 3D culture and encapsulation techniques, have shown promise in enhancing the efficiency and maturity of derived β-cells.
Future avenues in β cell differentiation
As the field rapidly advances, the differentiation of β cells from PSCs remains complex. One of the long-term challenges in transplanting SC-derived β cells for therapeutic applications remains the selective differentiation into a β-cell only population. To this day, most protocols result in intermingled β-like, α-like, and δ-like cells [21]. Such unwanted mixtures have tumorigenic potentials, affecting the safety of cell therapies. Therefore, Nair et al.’s proposed eBCs [30] lead the way to a more secure alternative for islet cell therapy.
While researchers implement new protocols and study the genetic variations during β cell differentiation, it is important to note that the optimized route of cell transplantation is also extensively studied. Some argue that fully functional β cells will be sensible to the stresses associated with transplantation and recurrent autoimmunity in T1D [45]. However, immune isolation strategies against autoimmune destruction include macro- and micro-encapsulation devices. Yet, all these approaches also increase the distance between the grafted cells and the blood circulation, creating possibly damaging hypoxic environments for the graft [45]. As a cure for T1D, cell replacement therapy may still require conjunction with autoimmunity-suppressing therapies. As for T2D, a combination of antidiabetic drugs and stem cell-based therapy was revealed to be a promising treatment, at least in mice [46].
In recent decades, deriving pancreatic β cells from human stem cells has significantly progressed. Here are the major advancements not limited to our current discussion:
PSCs, including ESCs and iPSCs, serve as an ideal alternative source for generating pancreatic β cells. These cells can be differentiated into functional β cells in vitro.
Recent advances enable the differentiation of stem cell-derived islets (SC-islets) with high endocrine purity. These SC-islets include hormone-secreting β, α, and δ cells that undergo GSIS. In animal studies, these SC-islets have successfully treated or prevented diabetes [47].
Researchers can now study patient-derived SC-islets with diabetes. This approach allows the exploration of gene-editing strategies to reverse diabetes-causing gene variants.
Techniques like CRISPR-Cas9 have been employed to modify stem cells and enhance their functionality as β cells. These gene-edited cells hold promise for transplantation and diabetes treatment [48].
Efforts are ongoing to improve the maturation and functionality of stem cell-derived β cells. Researchers aim to achieve glucose responsiveness and insulin secretion comparable to primary human islets.
Our current knowledge of β cell differentiation from PSCs is rapidly evolving, hESCs and iPSCs have proven to be immensely valuable. A fixed protocol was established selecting for the main master regulatory genes of β cell differentiation. The protocol initiates the embryonic stem cell into pancreatic lineage commitment, fosters the acquisition of endocrine precursors and ultimately allows the expression of mature β cell markers. Yet, this protocol requires refinement for better yield and functional qualities of the generated β cells.
Furthermore, the β cell differentiation path was unveiled as non-linear and exhibited several bifurcations. The pancreatic β cell progenitor NKX6.1 and the endocrine precursor can be gained independently from one or the other, without any sequestered order required as previously believed. Yet, their co-expression persists to be essential for β cell differentiation. However, even at its last stage, β cell differentiation is susceptible to variations. The key mature β cell marker MAFA was proven imperative to maintain the differentiation state of the β cells. MAFA has been identified to act on Cavγ4 gene expression, preventing the cell’s dedifferentiation.
Though studying individual genetic markers of β cells is extremely helpful to understanding β cell morphogenesis, it was confirmed that recapitulating pancreatic islet clustering led to improved physiological responses of differentiating β cells (Figure 5). This approach mimics in vivo clustering of mature β cells and allows for enhanced glucose-stimulated insulin secretion.
![Figure 5:
Conclusive summary of β cell differentiation pathway. Middle row: Major steps of β cell differentiation from human embryonic stem cells (hESCs). Main transcription factors included based on Rezania et al.’s study [17]. Upper path: Possible bifurcation in the acquisition of NKX6.1 and NEUROG3 found by Peterson et al. [22]. Brown dash arrows: Possible dedifferentiation of end-stage β cells in case of MAFA downregulation [23]. Lower path: enriched β cell clusters improved β cell function [30]. Images generated on BioRender by C.F.](/document/doi/10.1515/mr-2024-0039/asset/graphic/j_mr-2024-0039_fig_005.jpg)
Conclusive summary of β cell differentiation pathway. Middle row: Major steps of β cell differentiation from human embryonic stem cells (hESCs). Main transcription factors included based on Rezania et al.’s study [17]. Upper path: Possible bifurcation in the acquisition of NKX6.1 and NEUROG3 found by Peterson et al. [22]. Brown dash arrows: Possible dedifferentiation of end-stage β cells in case of MAFA downregulation [23]. Lower path: enriched β cell clusters improved β cell function [30]. Images generated on BioRender by C.F.
Finally, several protocols were designed to improve the yield and functionality of β-cells derived from iPSCs. However, achieving GSIS comparable to primary human β-cells remains a critical challenge. These findings collectively underscore the intricacies of β cell differentiation, pointing toward avenues for further advancement in regenerative medicine for diabetes treatment.
-
Research ethics: The local Institutional Review Board deemed the study exempt from review.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: Authors state no conflict of interest.
-
Research funding: Government of Canada Project Grant PJT-175208 to JLL.
-
Data availability: Not applicable.
References
1. Gasa, R. Transcriptional control of pancreatic endocrine cell development. Drug News Perspect 2005;18:567–76. https://doi.org/10.1358/dnp.2005.18.9.953669.Suche in Google Scholar PubMed
2. Campbell, JE, Newgard, CB. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat Rev Mol Cell Biol 2021;22:142–58. https://doi.org/10.1038/s41580-020-00317-7.Suche in Google Scholar PubMed PubMed Central
3. Tian, G, Sol, E-RM, Xu, Y, Shuai, H, Tengholm, A. Impaired cAMP generation contributes to defective glucose-stimulated insulin secretion after long-term exposure to palmitate. Diabetes 2014;64:904–15. https://doi.org/10.2337/db14-1036.Suche in Google Scholar PubMed
4. Vejrazkova, D, Vankova, M, Lukasova, P, Vcelak, J, Bendlova, B. Insights into the physiology of C-peptide. Physiol Res 2020;69:S23743. https://doi.org/10.33549/physiolres.934519.Suche in Google Scholar PubMed PubMed Central
5. Guay, C, Regazzi, R. MicroRNAs and the functional β cell mass: for better or worse. Diabetes Metab 2015;41:369–77. https://doi.org/10.1016/j.diabet.2015.03.006.Suche in Google Scholar PubMed
6. Daneman, D. Type 1 diabetes. Lancet 2006;367:847–58. https://doi.org/10.1016/s0140-6736(06)68341-4.Suche in Google Scholar
7. Ahrén, B. Type 2 diabetes, insulin secretion and β-cell mass. Curr Mol Med 2005;5:275–86. https://doi.org/10.2174/1566524053766004.Suche in Google Scholar PubMed
8. DeFronzo, RA, Ferrannini, E, Groop, L, Henry, RR, Herman, WH, Holst, JJ, et al.. Type 2 diabetes mellitus. Nat Rev Dis Prim 2015;1:15019. https://doi.org/10.1038/nrdp.2015.19.Suche in Google Scholar PubMed
9. Chatterjee, S, Khunti, K, Davies, MJ. Type 2 diabetes. Lancet 2017;389:2239–51. https://doi.org/10.1016/s0140-6736(17)30058-2.Suche in Google Scholar
10. ADA. 2. Diagnosis and classification of diabetes: standards of care in diabetes – 2024. Diabetes Care 2024;47:S20–42. https://doi.org/10.2337/dc24-s002.Suche in Google Scholar PubMed PubMed Central
11. Tan, SY, Mei Wong, JL, Sim, YJ, Wong, SS, Mohamed Elhassan, SA, Tan, SH, et al.. Type 1 and 2 diabetes mellitus: a review on current treatment approach and gene therapy as potential intervention. Diabetes Metabol Syndr 2019;13:364–72. https://doi.org/10.1016/j.dsx.2018.10.008.Suche in Google Scholar PubMed
12. Akil, AA, Yassin, E, Al-Maraghi, A, Aliyev, E, Al-Malki, K, Fakhro, KA. Diagnosis and treatment of type 1 diabetes at the dawn of the personalized medicine era. J Transl Med 2021;19:137. https://doi.org/10.1186/s12967-021-02778-6.Suche in Google Scholar PubMed PubMed Central
13. Cito, M, Pellegrini, S, Piemonti, L, Sordi, V. The potential and challenges of alternative sources of β cells for the cure of type 1 diabetes. Endocr Connect 2018;7:R114–25. https://doi.org/10.1530/ec-18-0012.Suche in Google Scholar PubMed PubMed Central
14. Nathan, DM, Buse, JB, Davidson, MB, Ferrannini, E, Holman, RR, Sherwin, R, et al.. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009;52:17–30.10.1007/s00125-008-1157-ySuche in Google Scholar PubMed
15. Lemaire, K, Thorrez, L, Schuit, F. Disallowed and allowed gene expression: two faces of mature islet beta cells. Annu Rev Nutr 2016;36:45–71. https://doi.org/10.1146/annurev-nutr-071715-050808.Suche in Google Scholar PubMed
16. Dupont, G, Yilmaz, E, Loukas, M, Macchi, V, De Caro, R, Tubbs, RS. Human embryonic stem cells: distinct molecular personalities and applications in regenerative medicine. Clin Anat 2019;32:354–60. https://doi.org/10.1002/ca.23318.Suche in Google Scholar PubMed PubMed Central
17. Rezania, A, Bruin, JE, Arora, P, Rubin, A, Batushansky, I, Asadi, A, et al.. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014;32:1121–33. https://doi.org/10.1038/nbt.3033.Suche in Google Scholar PubMed
18. Feng, X, Zhang, H, Yang, S, Cui, D, Wu, Y, Qi, X, et al.. From stem cells to pancreatic β-cells: strategies, applications, and potential treatments for diabetes. Mol Cell Biochem 2024. https://doi.org/10.1007/s11010-024-04999-x.Suche in Google Scholar PubMed
19. Chernysheva, М, Ruchko, Е, Karimova, М, Vorotelyak, Е, Vasiliev, А. Development, regeneration, and physiological expansion of functional β-cells: cellular sources and regulators. Front Cell Dev Biol 2024;12:1424278. https://doi.org/10.3389/fcell.2024.1424278.Suche in Google Scholar PubMed PubMed Central
20. Aigha, II, Abdelalim, EM. NKX6.1 transcription factor: a crucial regulator of pancreatic β cell development, identity, and proliferation. Stem Cell Res Ther 2020;11:459. https://doi.org/10.1186/s13287-020-01977-0.Suche in Google Scholar PubMed PubMed Central
21. Nishimura, W, Iwasa, H, Tumurkhuu, M. Role of the transcription factor MAFA in the maintenance of pancreatic β-cells. Int J Mol Sci 2022;23:4478. https://doi.org/10.3390/ijms23094478.Suche in Google Scholar PubMed PubMed Central
22. Petersen, MBK, Azad, A, Ingvorsen, C, Hess, K, Hansson, M, Grapin-Botton, A, et al.. Single-cell gene expression analysis of a human ESC model of pancreatic endocrine development reveals different paths to β-cell differentiation. Stem Cell Rep 2017;9:1246–61. https://doi.org/10.1016/j.stemcr.2017.08.009.Suche in Google Scholar PubMed PubMed Central
23. Luan, C, Ye, Y, Singh, T, Barghouth, M, Eliasson, L, Artner, I, et al.. The calcium channel subunit gamma-4 is regulated by MafA and necessary for pancreatic beta-cell specification. Commun Biol 2019;2:106. https://doi.org/10.1038/s42003-019-0351-4.Suche in Google Scholar PubMed PubMed Central
24. Wu, R, Karagiannopoulos, A, Eliasson, L, Renström, E, Luan, C, Zhang, E. The calcium channel subunit gamma-4 as a novel regulator of MafA in pancreatic beta-cell controls glucose homeostasis. Biomedicines 2022;10:770. https://doi.org/10.3390/biomedicines10040770.Suche in Google Scholar PubMed PubMed Central
25. Namkung, Y, Skrypnyk, N, Jeong, MJ, Lee, T, Lee, MS, Kim, HL, et al.. Requirement for the L-type Ca(2+) channel alpha(1D) subunit in postnatal pancreatic beta cell generation. J Clin Invest 2001;108:1015–22. https://doi.org/10.1172/jci200113310.Suche in Google Scholar
26. Du, X, Wang, J, Zhu, H, Rinaldo, L, Lamar, KM, Palmenberg, AC, et al.. Second cistron in CACNA1A gene encodes a transcription factor mediating cerebellar development and SCA6. Cell 2013;154:118–33. https://doi.org/10.1016/j.cell.2013.05.059.Suche in Google Scholar PubMed PubMed Central
27. Kious, BM, Baker, CV, Bronner-Fraser, M, Knecht, AK. Identification and characterization of a calcium channel gamma subunit expressed in differentiating neurons and myoblasts. Dev Biol 2002;243:249–59. https://doi.org/10.1006/dbio.2001.0570.Suche in Google Scholar PubMed
28. Hang, Y, Stein, R. MafA and MafB activity in pancreatic β cells. Trends Endocrinol Metabol 2011;22:364–73. https://doi.org/10.1016/j.tem.2011.05.003.Suche in Google Scholar PubMed PubMed Central
29. Cinti, F, Bouchi, R, Kim-Muller, JY, Ohmura, Y, Sandoval, PR, Masini, M, et al.. Evidence of β-cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab 2016;101:1044–54. https://doi.org/10.1210/jc.2015-2860.Suche in Google Scholar PubMed PubMed Central
30. Nair, GG, Liu, JS, Russ, HA, Tran, S, Saxton, MS, Chen, R, et al.. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat Cell Biol 2019;21:263–74. https://doi.org/10.1038/s41556-018-0271-4.Suche in Google Scholar PubMed PubMed Central
31. Gregg, BE, Moore, PC, Demozay, D, Hall, BA, Li, M, Husain, A, et al.. Formation of a human β-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab 2012;97:3197–206. https://doi.org/10.1210/jc.2012-1206.Suche in Google Scholar PubMed PubMed Central
32. Yoshihara, E, Wei, Z, Lin, CS, Fang, S, Ahmadian, M, Kida, Y, et al.. ERRγ is required for the metabolic maturation of therapeutically functional glucose-responsive β cells. Cell Metabol 2016;23:622–34. https://doi.org/10.1016/j.cmet.2016.03.005.Suche in Google Scholar PubMed PubMed Central
33. Kim, HJ, Kim, G, Lee, J, Lee, Y, Kim, JH. Secretome of stem cells: roles of extracellular vesicles in diseases, stemness, differentiation, and reprogramming. Tissue Eng Regen Med 2022;19:19–33. https://doi.org/10.1007/s13770-021-00406-4.Suche in Google Scholar PubMed PubMed Central
34. Sionov, RV, Ahdut-HaCohen, R. A supportive role of mesenchymal stem cells on insulin-producing Langerhans islets with a specific emphasis on the secretome. Biomedicines 2023;11:2558. https://doi.org/10.3390/biomedicines11092558.Suche in Google Scholar PubMed PubMed Central
35. Kuo, YC, Lin, SY, De, S, Rajesh, R. Regeneration of pancreatic cells using optimized nanoparticles and l-glutamic acid-gelatin scaffolds with controlled topography and grafted activin A/BMP4. ACS Biomater Sci Eng 2023;9:6208–24. https://doi.org/10.1021/acsbiomaterials.3c00791.Suche in Google Scholar PubMed
36. Lee, S, Joo, JH, Oh, JY, Seo, EH, Kim, YH, Jun, E, et al.. Continuous inhibition of Sonic hedgehog signaling effectively leads to differentiation of human-induced pluripotent stem cells into functional insulin-producing β cells. Stem Cell Int 2021;2021:6681257.10.1155/2021/6681257Suche in Google Scholar
37. Guo, Q, Lu, Y, Huang, Y, Guo, Y, Zhu, S, Zhang, Q, et al.. Exosomes from β-cells promote differentiation of induced pluripotent stem cells into insulin-producing cells through microRNA-dependent mechanisms. Diabetes Metab Syndr Obes 2021;14:4767–82. https://doi.org/10.2147/dmso.s342647.Suche in Google Scholar PubMed PubMed Central
38. Heaton, ES, Hu, M, Liu, T, Hui, H, Tan, Y, Ye, K, et al.. Extracellular matrix-derived peptide stimulates the generation of endocrine progenitors and islet organoids from iPSCs. J Tissue Eng 2023;14. https://doi.org/10.1177/20417314231185858.Suche in Google Scholar PubMed PubMed Central
39. Enderami, SE, Mortazavi, Y, Soleimani, M, Nadri, S, Biglari, A, Mansour, RN. Generation of insulin-producing cells from human-induced pluripotent stem cells using a stepwise differentiation protocol optimized with platelet-rich plasma. J Cell Physiol 2017;232:2878–86. https://doi.org/10.1002/jcp.25721.Suche in Google Scholar PubMed
40. Horikawa, A, Mizuno, K, Tsuda, K, Yamamoto, T, Michiue, T. A simple method of hiPSCs differentiation into insulin-producing cells is improved with vitamin C and RepSox. PLoS One 2021;16:e0254373. https://doi.org/10.1371/journal.pone.0254373.Suche in Google Scholar PubMed PubMed Central
41. Arroyave, F, Uscategui, Y, Lizcano, F. From iPSCs to pancreatic β cells: unveiling molecular pathways and enhancements with vitamin C and retinoic acid in diabetes research. Int J Mol Sci 2024;25(17):9654.10.3390/ijms25179654Suche in Google Scholar PubMed PubMed Central
42. Pellegrini, S, Chimienti, R, Scotti, GM, Giannese, F, Lazarevic, D, Manenti, F, et al.. Transcriptional dynamics of induced pluripotent stem cell differentiation into β cells reveals full endodermal commitment and homology with human islets. Cytotherapy 2021;23:311–9. https://doi.org/10.1016/j.jcyt.2020.10.004.Suche in Google Scholar PubMed
43. Ahmadi, SF, Mansour, RN, Hassannia, H, Enderami, SE, Abediankenari, S, Hosseini-Khah, Z. Generation of glucose sensitive insulin-secreting cells from human induced pluripotent stem cells on optimized polyethersulfone hybrid nanofibrous scaffold. Artif Organs 2023;47:502–11. https://doi.org/10.1111/aor.14431.Suche in Google Scholar PubMed
44. Montanucci, P, Pescara, T, Greco, A, Basta, G, Calafiore, R. Human induced pluripotent stem cells (hiPSC), enveloped in elastin-like recombinamers for cell therapy of type 1 diabetes mellitus (T1D): preliminary data. Front Bioeng Biotechnol 2023;11:1046206. https://doi.org/10.3389/fbioe.2023.1046206.Suche in Google Scholar PubMed PubMed Central
45. Johnson, JD. The quest to make fully functional human pancreatic beta cells from embryonic stem cells: climbing a mountain in the clouds. Diabetologia 2016;59:2047–57. https://doi.org/10.1007/s00125-016-4059-4.Suche in Google Scholar PubMed
46. Bruin, JE, Saber, N, Braun, N, Fox, JK, Mojibian, M, Asadi, A, et al.. Treating diet-induced diabetes and obesity with human embryonic stem cell-derived pancreatic progenitor cells and antidiabetic drugs. Stem Cell Rep 2015;4:605–20. https://doi.org/10.1016/j.stemcr.2015.02.011.Suche in Google Scholar PubMed PubMed Central
47. Maxwell, KG, Kim, MH, Gale, SE, Millman, JR. Differential function and maturation of human stem cell-derived islets after transplantation. Stem Cells Transl Med 2022;11:322–31. https://doi.org/10.1093/stcltm/szab013.Suche in Google Scholar PubMed PubMed Central
48. Balboa, D, Prasad, RB, Groop, L, Otonkoski, T. Genome editing of human pancreatic beta cell models: problems, possibilities and outlook. Diabetologia 2019;62:1329–36. https://doi.org/10.1007/s00125-019-4908-z.Suche in Google Scholar PubMed PubMed Central
49. Luo, Z, Dong, Y, Yu, M, Fu, X, Qiu, Y, Sun, X, et al.. A novel insulin delivery system by β cells encapsulated in microcapsules. Front Chem 2022;10:1104979. https://doi.org/10.3389/fchem.2022.1104979.Suche in Google Scholar PubMed PubMed Central
50. Keymeulen, B, De Groot, K, Jacobs-Tulleneers-Thevissen, D, Thompson, DM, Bellin, MD, Kroon, EJ, et al.. Encapsulated stem cell–derived β cells exert glucose control in patients with type 1 diabetes. Nat Biotechnol 2023;1–8. https://doi.org/10.1038/s41587-023-02055-5.Suche in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Reviews
- Biophysical stimuli for promoting bone repair and regeneration
- A stepwise approach to deriving functional β-cells from human embryonic or induced pluripotent stem cells
- Eco-fertility: examining the climate change-total fertility rate nexus in the context of sustainable developmental goals in a systematic review approach
- Redefining chronic mountain sickness: insights from high-altitude research and clinical experience
- Review of organ damage from COVID and Long COVID: a disease with a spectrum of pathology
- Perspective
- Combining transcriptomic and metabolomic insights to guide the clinical application of adipose- and bone marrow-derived mesenchymal stem cells
- Research Highlight
- Precision phototherapy and imaging with aggregation-induced emission-based nanoparticles cloaked in macrophage membrane
Artikel in diesem Heft
- Frontmatter
- Reviews
- Biophysical stimuli for promoting bone repair and regeneration
- A stepwise approach to deriving functional β-cells from human embryonic or induced pluripotent stem cells
- Eco-fertility: examining the climate change-total fertility rate nexus in the context of sustainable developmental goals in a systematic review approach
- Redefining chronic mountain sickness: insights from high-altitude research and clinical experience
- Review of organ damage from COVID and Long COVID: a disease with a spectrum of pathology
- Perspective
- Combining transcriptomic and metabolomic insights to guide the clinical application of adipose- and bone marrow-derived mesenchymal stem cells
- Research Highlight
- Precision phototherapy and imaging with aggregation-induced emission-based nanoparticles cloaked in macrophage membrane


