Abstract
New symmetrically para-substituted tetraphenylporphyrin Tl(III) salicylate complexes, having the general formula SA/5-SSATl(III)t(4-Y)PP (Y=-H, -CH3, -OCH3); SA=salicylates and 5-SSA=5-sulfosalicylates, were synthesized and characterized by elemental analysis, optical absorption/emission studies, infrared spectrum, NMR studies (1H, 205Tl), thermal analysis and mass spectra; the complexes are in good consistency with experimental results. All the newly synthesized complexes exhibited good optical property, and thermogravimetric analysis (TGA) results showed their excellent thermal stability (>400°C). Macrocyclic complexes of Tl(III) ligate to axial ligand to form six-coordinate complex, the nature of which influences the photophysical properties of the former depending upon the spin-orbital coupling character of the axial ligand. A square-based pyramidal structure has been proposed on the basis of these studies. In addition, some of the synthesized complexes of thallium porphyrins were also screened for anti-bacterial activity. Cytotoxic activity of 5-SSATl(III)t (4-OCH3)PP evaluated against four human cancer cell lines exhibited strong (up to 90%) growth inhibition.
Introduction
Tetrapyrrolic macrocycles like porphyrin and phthalocyanines have attracted considerable interest and hold a special place in modern chemistry functioning as reaction catalysts (Karimipour et al., 2007, 2012, 2013), oxygen transporters, chemical sensors (Cosma et al., 2009; Musturappa et al., 2013), or in molecular electronic devices (Drain et al., 2002). Porphyrins were reported to exhibit a variety of biological activities. This is due to the fact that natural and synthetic porphyrins have relatively low toxicity in vitro and in vivo, and they possess antitumor (Antonova et al., 2010; Fadda et al., 2013) and antioxidant effects (Stojiljkovic et al., 2001; Yuasa et al., 2007) and have a good potential for ion complexation. The ability for numerous chemical modifications and the large number of different mechanisms by which porphyrins affect microbial and viral pathogens place porphyrins into a group of compounds with an outstanding potential for discovery of novel agents, procedures and materials active against pathogenic microorganisms. Porphyrins with only one, two, or more substituents present a compact architecture that suits a wide variety of applications or further synthetic elaboration. To synthesize porphyrins bearing a molecular recognition site, porphyrin synthons that have functional groups at the p-positions of the meso-phenyl groups are usually employed, since these functional groups might be further modified by chemical treatments to enhance selectivity in the porphyrin-mediated reactions. Although the coordination of most of the metallic elements to the porphyrin macrocycle has been demonstrated for many years, it was transition-metal porphyrin complexes which were the focus of the most intense attention. More recently, there has been a resurgence of interest in main-group chemistry, driven by the search for new conducting materials and new chemotherapeutic agents and the recent discovery of previously unsuspected bonding modes for main-group elements. Porphyrin complexes of the main-group elements have not been excluded from this renewed interest, and there has been recent activity in the study of elements from groups 13 and 14 coordinated to the porphyrin macrocycle. Gallium(III), indium(III) and thallium(III) porphyrins have been receiving more attention due to their good medical application and non-linear optical (NLO) properties (Rajesh et al., 2010; Fitzgerald et al., 2015). Sn(IV) compounds possessed diversified applications in industries and agriculture and acts as good photosensitizer (Manke et al., 2014). Thallium(III) porphyrins show a range of properties, among the most striking of which is the remarkable structural diversity which differs from typical coordination geometries observed for most metalloporphyrin complexes. Another favorable feature of these complexes is their capability of hosting tri- or tetravalent central atoms that require the further coordination of one and two axial substituents, respectively. In fact, axial substituents indirectly stabilize the electronic excited states which are involved in the multiphoton absorption processes through either preventing or reducing intermolecular aggregation in solution with the increase of molecule concentration. Following the preceding discussion, we have synthesized the series of axially substituted thallium(III) porphyrins.
In particular, we prepared meso-tetraphenylporphyrin (H2tPP) and its para-substituted derivative H2t(4-Y)PP Y=p-CH3, p-OCH3) aquo-meso-(5,10,15,20-tetraarylporphyrinato)thallium(III)hydroxide, [(OH)(H2O)Tlt(4-Y)PP], axially ligated Tl(III) porphyrins; meso-tetraphenylporphyrinato salicylato thallium(III), Tlt(4-Y)PPSA and meso-tetraphenylporphyrinato 5-sulphosalicylatothallium(III), Tlt(4-Y)PP 5-SSA. Finally, the biological activity of the newly synthesized porphyrin ligands and their complexes was tested against some Gram (+) and Gram (-) bacteria (Escherichia coli, Pseudomonas florescens, Staphylococcus aureus and Bacillus subtilis).
Results and discussion
Elemental analysis
All the resulting solids are soluble in CHCl3, CH2Cl2, CH3OH and DMSO. The analytical data of the complexes of thallium(III) metal complexes are given in Table 1.
Analytical data of some of the axially ligated complexes of Tl(III) porphyrins.
| Elemental analysis | Calculated percentage | Found percentage | ||||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | S | C | H | N | S | |
| SATltpp | 62.87 | 3.60 | 5.73 | 0.00 | 62.36 | 3.68 | 5.72 | 0.00 |
| 5-SSATltpp | 59.04 | 3.08 | 5.40 | 3.08 | 59.02 | 3.06 | 5.48 | 3.10 |
| SATlt(4-CH3)PP | 63.18 | 3.90 | 5.36 | 0.00 | 63.38 | 3.73 | 5.30 | 0.00 |
| 5-SSATlt(4-CH3)PP | 60.52 | 3.75 | 5.13 | 2.93 | 60.23 | 3.62 | 5.23 | 2.74 |
| SATlt(4-OCH3)PP | 60.13 | 3.73 | 5.10 | 0.00 | 60.25 | 3.69 | 5.33 | 0.00 |
| 5-SSATlt(4-OCH3)PP | 47.6 | 3.55 | 4.85 | 2.77 | 47.59 | 3.43 | 4.78 | 2.83 |
Absorption spectroscopy
The B and the Q bands in the spectrum of typical porphyrin both arise from π-π* transitions and can be explained by considering the four frontier orbitals (Milgrom, 1997) (HOMO and LUMO orbitals) (the Gouterman four-orbital model). From the UV-Vis absorption values given in the Experimental section, it can be clearly observed that variation of the peripheral substituents on the porphyrin ring causes minor changes in the intensity and wavelengths of these absorptions, and their spectral data in different solvents (Table 2) shows that many of the absorption bands of para-substituted derivatives exhibit bathochromic (red shift) as compared to the unsubstituted porphyrin. The electronic absorption spectra of aquo-meso-tetraaryl porphyrinato thallium(III) hydroxide (OH)(H2O)T1(III)tpp and (OH)(H2O)T1(III)t(4-Y)PP, Y=-CH3, -OCH3 are of normal two-banded type. It is apparent from the spectra that there is no major distortion of the macrocycle ring upon metalation and that the large metal atom is situated above rather than in the plane of the porphyrin ring. Both B bands (350–450 nm) and Q bands (500–700 nm) were found to be red-shifted in all thallium porphyrins (Figure 1). Also, the molar absorbance of both the main Soret and the Q bands of the metalloporphyrins are higher than the corresponding values for the free-base porphyrin (Tables 2 and 3). According to earlier observations (Horvath et al., 2004, 2006; Valicsek et al., 2004, Valicsek et al., 2011) this type of spectral properties is unambiguously characteristic for OOP (out of plane) or Sat (sitting atop) complexes, confirming the expectations based on the size (95 pm ionic radius) of Tl(III). Not only metalation but also axial coordination is accompanied by red shifts of the characteristic absorption bands. As the corresponding values for B(0,0) itself indicate, the larger OOP distance in case of axially ligated porphyrin results in the higher dome distortion of the porphyrin ligand. For the Q(0,0) band the effect of the axial ligand is much stronger, indicating that the energy of the S1 state is more influenced by this structural change. The f values (oscillator strength) of some of the metalated porphyrin and their axially ligated derivatives (Table 3) show that change in polarity of the solvent does not significantly alter the position of transitions but result in an enhanced full width at half maximum (fwhm) (1/2) value indicating weak interactions with the solvent.
Optical absorption data of free base porphyrins and their Tl(III) derivatives in different solvents.
| Compound | Solvent | B band λmax (nm) | Q bands λmax (nm) |
|---|---|---|---|
| H2tPP | Acetone | 422 | 514, 549, 590, 646, |
| Chloroform | 414 | 512, 546, 592, 644 | |
| H2t(4-CH3)TPP | Acetone | 422 | 517, 533, 592, 648 |
| Chloroform | 420 | 515, 533, 590, 646 | |
| H2t(4-OCH3)PP | Acetone | 422 | 516, 557, 594, 651 |
| Chloroform | 420 | 515, 556, 592, 649 | |
| (OH)(H2O)Tlt(4-H)PP | Acetone | 438 | 568, 606 |
| Chloroform | 438 | 566, 605 | |
| (OH)(H2O)Tlt(4-CH3)PP | Acetone | 449 | 453, 671 |
| Chloroform | 447 | 451, 672 | |
| (OH)(H2O)Tlt(4-OCH3)PP | Acetone | 431 | 518, 568 |
| Chloroform | 430 | 517, 566 |
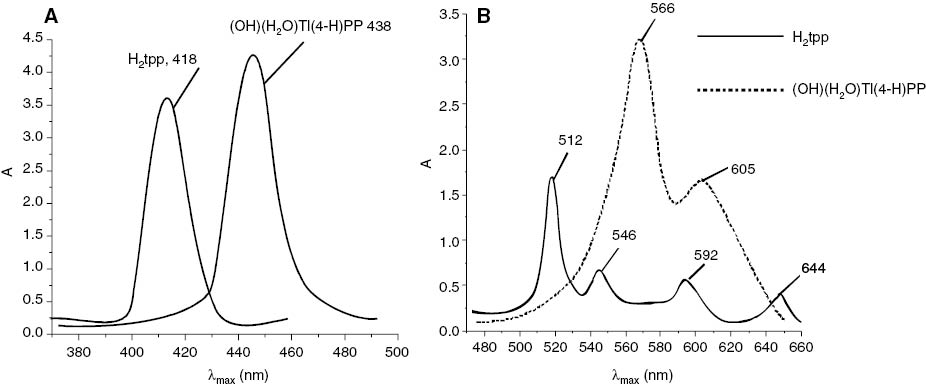
UV visible spectra of free base porphyrin and thallium(III) porphyrin.
(a) UV-visible overlapped B-bands of H2tpp and (OH)(H2O)Tltpp. (b) UV-visible overlapped Q-bands of H2tpp and (OH)(H2O)Tltpp.
Optical absorption data of axially ligated thallium porphyrins in different solvents together with logε and υ1/2.
| Compound | Solvent | λmax, nm (logε, m-1 cm-1) | υ1/2 (cm-1) | F=4.33×10-9 εΔν1/2 | ||||
|---|---|---|---|---|---|---|---|---|
| B(0,0) | Q(1,0) | Q(0,0) | B(0,0) | Q(1,0) | B bands | Q bands | ||
| SATltpp | Acetone | 444(4.553) | 588(4.452) | 603(4.218) | 1319 | 432 | 0.0561 | 0.0568 |
| Chloroform | 442(3.875) | 588(4.230) | 605(4.107) | 1224 | 463 | 0.0413 | 0.0341 | |
| 5-SSATltpp | Acetone | 445(4.553) | 651(4.861) | 649(4.434) | 1591 | 647 | 0.0546 | 0.0534 |
| Chloroform | 445(4.550) | 651(4.332) | 647(4.603) | 1530 | 238 | 0.0623 | 0.0221 | |
| SATlt(4-CH3)PP | Acetone | 449(4.571) | 572(4.243) | 606(4.421) | 1310 | 644 | 0.0431 | 0.0483 |
| Chloroform | 447(3.571) | 570(4.243) | 605(4.314) | 1227 | 425 | 0.2269 | 0.0322 | |
| 5-SSATlt(4-CH3)PP | Acetone | 451(4.043) | 596(4.341) | 673(4.210) | 1398 | 565 | 0.2269 | 0.0404 |
| Chloroform | 451(4.044) | 594(4.235) | 671(4.130) | 1322 | 326 | 0.1342 | 0.0243 | |
| SATlt(4-OCH3)PP | Acetone | 435(4.670) | 567(4.307) | 611(4.313) | 1354 | 683 | 0.2570 | 0.0600 |
| Chloroform | 432(4.672) | 566(4.302) | 609(4.221) | 1269 | 602 | 0.1725 | 0.0523 | |
| 5-SSATlt(4-OCH3)PP | Acetone | 449(4.747) | 568(4.241) | 671(4.04) | 1373 | 588 | 0.3233 | 0.0591 |
| Chloroform | 447(4.735) | 568(4.113) | 669(4.232) | 1302 | 758 | 0.0869 | 0.0425 | |
The optical absorption spectra of axially ligated Tl(III) porphyrins when recorded in different solvents show only marginal change in λmax values. The spectrum of the complex SATl(III)t(4-CH3)PP is shown in Figure 2.
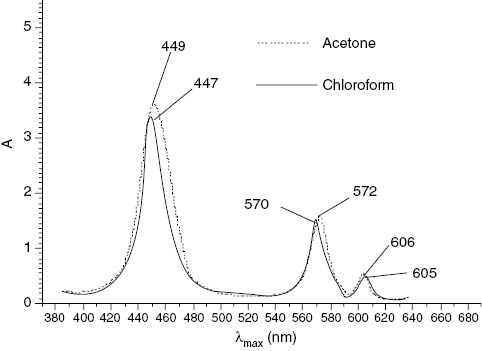
UV-visible overlapped B and Q bands of SATlt(4-CH3)PP.
Infrared spectroscopy
The infrared (IR) spectral data of various synthesized complexes of thallium porphyrins are listed in Table 4. It is found that the ν(N-H) stretching frequency of free base porphyrins are located at ~3400–3320 cm-1 (Sun et al., 2011) and disappeared when thallium metal ion was inserted into the porphyrin ring. There is an additional Tl-N stretch vibration at 400–500 cm-1 in metalated complexes indicating the formation of Tl(III) porphyrins. The band in the complexes at 1633 cm-1 [νasymmetric(OCO)] are assigned to the chelating bidentate salicylate ligand. The bidentate nature of salicylates in the IR spectra of the complexes was confirmed on the basis of Stoilovo et al. results that unidentate CO2 exhibits three bands (COO deformation) at 920–720 cm-1 and a strong band at 540 cm-1 (Suen et al., 1992). All these four bands were absent in axially ligated thallium porphyrin complexes. Further, the band observed near 1724 cm-1 is assigned to ν(C=O) stretching frequency. The frequency corresponding to free O-H group is observed at 3418 cm-1 confirming the coordination of axial ligand through carboxylate oxygen atoms. The decrease in vibrational frequency of OH group is due to the weakening of O-H bond as -CO2 in its vicinity from salicylate ring ligates to the metal atom.
Main infrared absorption frequencies corresponding to the simple porphyrin H2tpp and various groups in XTlt(4-Y)PP (X=SA, 5-SSA) and (Y=-H, -CH3, -OCH3).
| Porphyrin | ν(O-H) (cm-1) | ν(N-H) (cm-1) | ν(C-H)asym (cm-1) | ν(sp3-CH) | ν(=C-H)sym | ν(C-N) (cm-1) | ν(C=C) (cm-1) | ν(C=O) | ν(Tl-N)(cm-1) |
|---|---|---|---|---|---|---|---|---|---|
| H2tpp | 3425 | – | – | 2923 | 1353 | 1582 | – | – | |
| (OH)(H2O)Tltpp | 3523 | – | – | – | 2920 | 1351 | 1552 | – | 488 |
| SATlt(4-H)PP | 3419 | – | 2960 | 2854 | 2925 | 1328 | 1533 | 1725 | 472 |
| 5-SSATlt(4-H)PP | 3401 | – | 2962 | 2859 | 2929 | 1336 | 1535 | 1728 | 470 |
| SATlt(4-CH3)PP | 3418 | – | 2960 | 2855 | 2919 | 1329 | 1534 | 1724 | 473 |
| 5-SSATlt(4-CH3)PP | 3401 | – | 2962 | 2859 | 2929 | 1335 | 1533 | 1728 | 470 |
| SATlt(4-OCH3)PP | 3419 | – | 2960 | 2854 | 2925 | 1329 | 1532 | 1725 | 472 |
| 5-SSATlt(4-OCH3)PP | 3401 | – | 2961 | 2859 | 2929 | 1336 | 1534 | 1728 | 472 |
1H NMR spectroscopy
The thallium porphyrin complexes with salicylates(SA/5-SSA) as axial ligands all exhibit sharp 1H NMR spectra. The characteristic data for the NMR of the free base porphyrins, corresponding metalated complexes of thallium(III) and their axially ligated derivatives are summarized in Table 5.
1H NMR data showing chemical shift (in ppm) values of axially ligated thallium porphyrins in CDCl3 at 300 K.
| Porphyrins | Pyrrole protons | Meso-aryl protons | Other protons |
|---|---|---|---|
| SATlt(4-H)PP | 9.06 (s) | 8.89s and 8.75s (H2,6) | 6.78t, H4″ |
| 5.6 (d) | 7.72s and 7.71s (H3,5) | 6.08d, H3″ | |
| 7.68m (H4) | 6.31d, H6″ | ||
| 6.16 t, H5″, 5.0(s, 1H, O-H) | |||
| 5-SSATlt(4-H)PP | 8.78(s) | 8.15 and 8.06 (d, H2,6) | 5.1–6.0m H6″, H4″, H3″ |
| 7.19(s), 5.6(dd) | 7.99 and 7.78(d, H2′,6′) | 5.0(s, 1H, O-H) | |
| 7.7 (bs, H3.5) | 2.0(bs, 1H, SO3H) | ||
| SATlt(4-CH3)PP | 9.01(s) | 8.76s and 8.63s(H2,6) | 6.78t, H4″ |
| 5.5(d) | 7.63s and 7.61(H3,5) | 6.07d, H3″ | |
| 6.29d, H6″ | |||
| 6.13t, H5″, 5.0(s, 1H, O-H) 2.83(s, 12H, CH3) | |||
| 5-SSATlt(4-CH3)PP | 8.72(s) | 8.02 and 7.98(d, H2,6) | 4.5–5.6m H6″, H4″, H3″ |
| 7.14(s) | 7.63 and 7.34(d, H2′,6′) | 5.0(s, 1H, O-H), 2.0(bs, 1H, SO3H), 2.83(s, 12, CH3) | |
| 5.5(m) | |||
| SATlt(4-OCH3)PP | 8.83(s) | 8.77 and 8.73(s, H2,6) | 6.78t, H4″ |
| 5.5(m) | 7.62 and 7.59(s, H3,5) | 6.06d, H3″ | |
| 6.30d, H6″ | |||
| 6.11t, H5″, 5.0(s, 1H, O-H) 4.15(s, 12H, OCH3) | |||
| 5-SSATlt(4-OCH3)PP | 8.69 and 7.14(s) | 7.83 and 7.13(d, H2,6) | 4.6–6.0m, H6″, H4″, H3″ |
| 5.5(m) | 7.64–7.33(d, H2′,6′) 7.3(bs, H3,5) | 5.0(s, 1H, O-H), 2.0(1s, 1H, SO3H) |
δ in ppm, the nature of splitting patterns(s) (s, singlet; d, doublet; m, multiplet; bs, broad singlet; dd, doublet of doublet; o, ortho; p, para; m, meta).
1H NMR spectra are fruitful in clarifying the insertion of metal ion in the porphyrin core as the signal related to N-H protons was found to be absent, and also there was a shift in the signals of other protons in the NMR spectra of metalated porphyrins. In the axially ligated derivatives of thallium porphyrins, the singlet at 5.0 ppm of medium intensity was observed assigned to the -OH group which confirms that axial ligation was taking place through the oxygen atom of carboxylate group. All the δ values correspond to the proton signals of axial ligands in the axially ligated complexes that exhibited upfield shift when compared with the signals of porphyrin protons and the proton signal of free axial ligands. This upfield shift is attributed to the ring current effect (Lu et al., 1999) of the porphyrin macrocycle.
205Tl NMR spectroscopy
The range for 205Tl NMR chemical shift values is very large, i.e. about 7000 ppm. As far as oxidation states are concerned, for Tl(I) complexes chemical shift values are in the range -200 to +200 ppm, and for Tl(III) these lie in the range +2000 to +3000 ppm. 205Tl NMR spectra of SATl(III)t(4-CH3)PP when recorded in CDCl3 exhibits a sharp resonance at δ 2588 ppm which confirms the presence of Tl(III) ion in the complex. On comparing this value with the chemical shift value of the complexes already reported in literature (Ma et al., 2003), it was found that this value has shifted towards lower frequency which can be attributed to the presence of four electron-releasing -CH3 groups on the meso-phenyl ring. This parameter causes shielding of the thallium nucleus and hence shifts towards lower frequency (Figure 3).
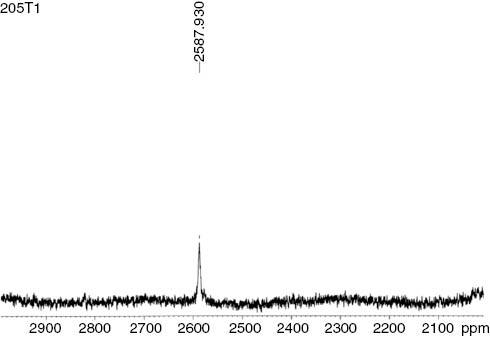
205Tl NMR spectrum of the complex SATlt(4-CH3)PP.
Thermal analysis
The thermogravimetric (TG) curve of one of the synthesized complex 5-SSATl(III)t(4-H)pp (Figure 4) shows a continuous weight loss starting from 150°C to 750°C. The curve shows an initial weight loss of a sulphosalicylate group and one phenyl ring at 158.2°C (obs. wt. loss=27.7%, calc. wt. loss=27.2%). This is followed by a loss of three phenyl rings and thallium metal ion at 408.5°C (obs. wt. loss=40.2%, calc. wt. loss=40.2%). There are some exothermal peaks observed in the range of 300–600°C showing major weight loss in this region. It is a continuous decomposition process; the small exothermic peaks correspond to the loss of chains of the porphyrin ring (Zhuang et al., 2009), and the large exothermic peaks at nearly 580°C corresponds to the collapse of the porphyrins skeleton. The residual mass was 0 at 751.2°C.
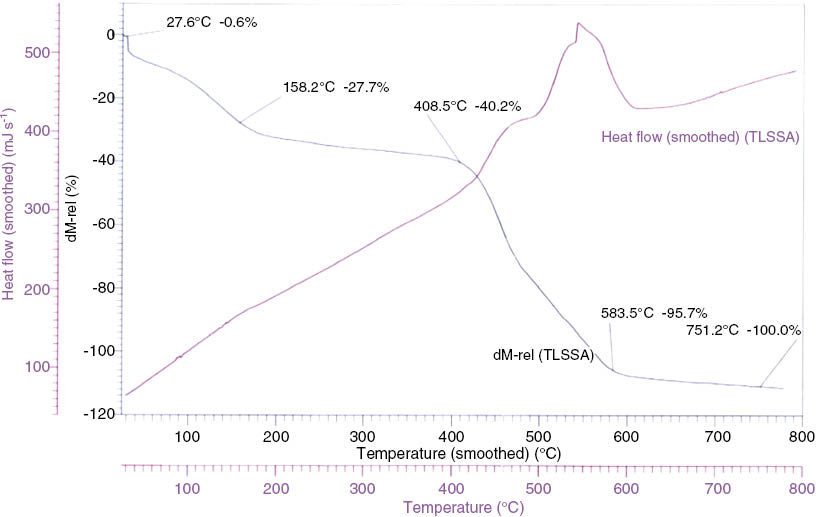
Thermogravimetric analysis (TGA)-differential thermal analysis (DTA) curve of 5-SSATltpp.
Fluorescence spectroscopy
The variation of emission properties in free base porphyrin H2t(4-Y)PP (Y: -CH3, -OCH3) and their axially ligated Tl(III) porphyrins have been investigated by means of fluorescence spectroscopy; data are given in Table 6. Porphyrins show two emission bands, a strong Q(0,0) band at higher energy accompanied by a weak Q(0,1) band at a lower energy. The free-base porphyrins H2t(4-CH3)PP, with excitation at 450nm, exhibits two emission bands at 620 and 672 nm corresponding to Q(0,0) and Q(0,1) transitions, respectively, the intensity of the Q(0,0) being higher than the Q(0,1) transition. Meso-subsitution of the porphyrin ring leads to red shift of all the emission bands relative to that of unsubstituted tetraphenylporphyrins. However, the emission bands of axially ligated Tl(III) porphyrins are blue shifted (Horvath et al., 2006) compared to free base porphyrins. This behavior is attributed to an enhanced spin-orbit coupling induced by the presence of the heavy central metal atom in thallium porphyrin complexes, which leads to a more efficient S1→T1 intersystem crossing and thus reduces the probability of fluorescent emission (Knöra and Strasser, 2002). Thus, the excitation spectrum of fluorescence is in agreement with the absorption spectrum (Figure 5).
Summary of the characteristic emission data of free base porphyrin and axially ligated Tl(III) porphyrin at 28K in DMSO.
| Complexes | λmax (nm) | |
|---|---|---|
| Q(0,0) | Q(0,1) | |
| H2t(4-CH3)PP | 620 | 672 |
| Tlt(4-CH3)PP | 606 | 653 |
| SATlt(4-CH3)PP | 580 | 630 |
| H2t(4-OCH3)PP | 620 | 671 |
| 5-SSATlt(4-OCH3)PP | 582 | 630 |
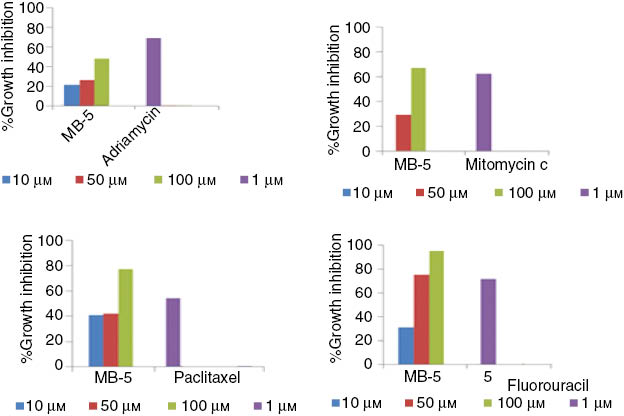
In vitro cytotoxicity of 5-SSATlt(4-OCH3)PP complex against human cancer cell lines.
Mass spectrometry
The mass spectra of some of the investigated axially ligated thallium porphyrin complexes are characterized by the presence of the molecular ion peak followed by different specific fragmentation pattern. The molecular ion peak of the investigated complexes was found to be 44 units less than the corresponding molecular weight of the complex, which is attributed to the loss of stable CO2 molecule from the complex. The major losses are 304, 615, 616 and 81. Besides the molecular ion, an identical peak m/z=304 fragment, corresponding to the tetrapyrrole moiety, appears in each spectrum (Cosma et al., 2006). Two groups of peaks were observed (main signals accompanied by isotopic patterns confirming the chemical composition of the ions) at m/z 615 and 616 that correspond to the fragments of H2tpp+1 and H2tpp+2 (H2tpp=meso-tetraphenyl porphyrin), and the peak at 81 corresponds to the SO3H group.
In addition, the intensities of the registered peaks are significantly higher. The base peak of thallium porphyrin complexes was observed 100% intensity giving evidence about the stability of complexes of thallium porphyrins.
Antibacterial studies
Antibacterial activity of the synthesized Tl(III) porphyrin complexes was tested by agar well diffusion method. The free base prophyrins, their corresponding metalated and axially ligated thallium(III) porphyrin complexes viz., H2t(4-OCH3)PP, H2t(4-CH3)PP, (OH)(H2O)Tlt(4-CH3)PP, (OH)(H2O)Tlt(4-OCH3)PP, SATlt(4-CH3)PP, 5-SSATlt(4-CH3)PP and 5-SSATlt(4-OCH3)PP were tested at three concentrations (10-3, 10-4 and 10-5m) against five bacterial strains viz., B. subtilis, Micrococcus luteus, S. aureus, Pseudomonas florescens and E. coli. The thallium(III) complex 5-SSATlt(4-OCH3)PP was found sensitive only to B. subtilis (Gram positive) and P. florescens (Gram negative) bacteria at the concentrations of 10-3 and 10-4m. Other complex SATlt(4-CH3)PP) showed sensitivity against B. subtilis and M. luteus. The remaining complexes, viz., H2t(4-CH3)PP, (OH)(H2O)Tlt(4-CH3)PP and 5-SSATlt(4-CH3)PP showed activity only against Gram negative bacteria E. coli with zone of inhibition ranging from 6 to 14 mm. The rest of the complexes did not show any activity against bacterial strains. The results were observed in dose-dependent manner as demonstrated in Table 7.
In vitro antibacterial evaluation of free base porphyrin and the corresponding thallium porphyrin complexes.
| Bacterial strains | Conc. | B. subtilis | M. luteus | S. aureus | P. flourescens | E. coli |
|---|---|---|---|---|---|---|
| Zones of inhibition (mm) | ||||||
| H2t(4-OCH3)PP | 10-3 | 8 | ||||
| 10-4 | 6 | |||||
| 10-5 | – | |||||
| SATlt(4-OCH3)PP | 10-3 | 9 | 6 | |||
| 10-4 | 7 | – | ||||
| 10-5 | – | – | ||||
| 5-SSATlt(4-OCH3)PP | 10-3 | 10 | 8 | |||
| 10-4 | 6 | 6 | ||||
| 10-5 | – | – | ||||
| H2t(4-CH3)PP | 10-3 | 6 | ||||
| 10-4 | 4 | |||||
| 10-5 | – | |||||
| (OH)(H2O)Tlt(4-CH3)PP | 10-3 | 9 | ||||
| 10-4 | 6 | |||||
| 10-5 | – | |||||
| SATlt(4-CH3)PP | 10-3 | 7 | 9 | 10 | 9 | |
| 10-4 | – | 6 | ||||
| 10-5 | – | – | ||||
| 5-SSATlt(4-CH3)PP | 10-3 | 9 | 9 | 10 | 10 | |
| 10-4 | – | – | – | – | ||
| 10-5 | – | – | – | – | ||
| Positive control | 19 | 20 | 21 | 18 | 24 | |
| Control | ||||||
| Cmp+ 10 μg | ||||||
Anticancer activity
In the present work, the cytotoxicities of 5-SSATl(III)t (4-OCH3)PP, SATl(III)t(4-OCH3)PP and SATl(III)t(4-CH3)PP were evaluated against four human cancer cell lines, viz., breast (MCF-7), leukemia (THP-1), prostate (PC-3) and lung (A549) at different concentrations. One of the complexes, namely, 5-Sulphosalicylato (meso-tetra-p-methoxyporphyrinato)thallium(III) (MB-5) shows significant activity (Figure 5) against lung and leukemia, classifying these complexes as chemotherapeutically significant. We also tested other synthesized compounds against the above mentioned panel of cancer cell lines; however, none of the compounds exhibited cytotoxicity even at 100 μm concentration (results not shown).
Conclusion
The present paper was concerned about the synthesis of symmetrically substituted meso-tetraphenylporphyrin, their complexation with thallium metal ion and then axial ligation with salicylates, SA/5-SSA. The complexes were characterized by elemental analysis, UV-Vis, IR, fluorescence and NMR studies. In the UV-Vis spectra, both the Soret and Q bands were significantly red shifted on metalation, and further large shift of these bands on axial ligation was related to the distortion of the porphyrin core. The IR spectrum of these compounds showed that salicylic acids (SA/5-SSA) ligate in a bidentate mode through the carboxylate group. 1H NMR spectroscopic study of these compounds showed that due to ring current effect of the porphyrin macrocycle, the proton signals of the axial ligands (SA/5-SSA) are shifted to higher field in comparison to the signals of porphyrin protons and proton signals of free axial ligands. The production of the monomeric form of thallium porphyrin complexes was well confirmed by means of electronspray ionization mass spectrometry (ESI-MS). The coordination of salicylates through COO to these complexes in methanol solution was clarified using ESI-MS. The ensuing complexes show admirable thermal stability (>400°C), and the decomposition of the complexes comes to an end at 750°C. 205Tl NMR studies confirm the presence of Tl(III) ion in the complexes. The biological activities were also investigated, and the synthesized compounds showed varying degrees of inhibitory effects: low (up to 6 mm) and moderate (up to 11 mm). The parent compounds possessed a low activity against the bacterial strains at low concentration but indicated a slight rise in the activity at 100 μg mL-1. Similarly, thallium porphyrins and their axially ligated derivatives show rise in the activity when compared with their parent compounds. Such increase in the activity of the complexes compared to that of ligands could be explained on the basis of Overton’s concept (Overton, 1901) and Tweedy’s chelation theory (Tweedy, 1964). One of the complexes screened for anticancer activity showed up to 90% growth inhibition. On the basis of the elemental analysis and spectral studies, confirmed by mass spectra showing characteristic molecular ion peak at their m/z value for their monomeric form, a square-pyramidal structure (Figure 6) for SATl(III)t(4-Y)PP and 5-SSATlt(4-Y)PP complexes has been proposed. All complexes are soluble in DMSO, CHCl3 and CH3OH.
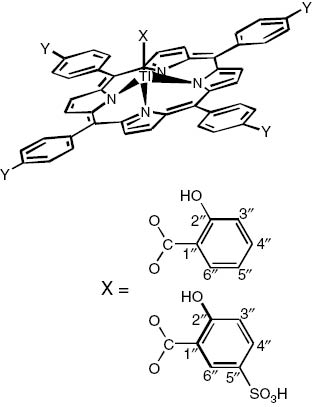
Suggested structure of XTlt(4-Y)PP(X=SA, 5-SSA).
Experimental
Materials and instrumentation
All the chemicals were of analytical grade and were used as received unless otherwise noted. All the reagents were purchased from Himedia (Mumbai, India). Pyrrole was distilled over potassium hydroxide pellets under vacuum prior to use. All the organic solvents that were used for the synthesis and for chromatographic separations were dried before use. Elemental analysis (C, H, N and S) was performed on a Vario EL III and CHNS-932 LECO Elemental Analyzer (IIIM, Jammu). UV-Vis spectra were recorded on a Systonic 119 UV Visible spectrophotometer (University of Jammu, Jammu) in the range 350–700 nm. The oscillator strength (f) of the transitions in absorption spectra were calculated from the expression
where ε is the molar absorption coefficient in dm3 mol-1 cm-1 and Δν½ is the fwhm in cm-1. IR spectra were recorded on a Perkin Elmer-spectrum 400 FTIR spectrophotometer using KBr pellets in the range of 4000–400 cm-1. The 1H NMR spectra were recorded on a Bruker Avance II 500 (500 MHz) using tetramethylsilane as internal standard and CDCl3 as solvent. 205Tl NMR spectra were recorded on a Bruker Avance 400 MHz spectrometer in CDCl3. TGA and DTA were recorded on Linseis STA PT-100 thermometer using dry samples at the heating rate of 10°C min-1 in an air atmosphere. Fluorescence measurements were performed on Synergy MX BIOTEK Multimode Reader. The porphyrin solution prepared in CH2Cl2 was 10-6m.
Biological studies
Antibacterial studies:
Qualitative analysis for screening of antimicrobial activity of the complexes was carried out by agar well diffusion method with modifications. The complexes were tested against two Gram positive bacteria (B. subtilis MTCC2389 and S. aureus MTCC7443) and three Gram negative bacteria (M. luteus MTCC4821, E. coli MTCC2127 and P. florescens MTCC4828). Twenty milliliters of sterilized nutrient agar were inoculated with 100 mL of bacterial suspension (108 CFU mL-1) and then poured on to sterilized petri plate. The agar plate was left to solidify at room temperature. A well of 6 mm was aseptically bored into the agar plate. Then 20 mL of the complexes (diluted with DMSO, 1:1) was added in each well. Chloremphenicol (10 μg) was used as a positive reference to determine the sensitivity of bacteria. The plates were kept at 4°C for 2 h to allow the dispersal and then incubated at 37°C for 24 h.
In vitro cytotoxicity against human cancer cell lines
Cell lines and cell cultures:
The human prostrate (PC-3), lung (A-549) and acute lymphoblastic leukemia (THP-1) cell lines were grown and maintained in RPMI-1640 medium, pH 7.4, whereas DMEM was used for breast (MCF-7). The media were supplemented with FCS (10%), penicillin (100 units mL-1), streptomycin (100 μg mL-1) and glutamine (2 mm), and cells were grown in CO2 incubator (Heraeus, GmbH, Germany) at 37°C with 90% humidity and 5% CO2. Cells were treated with samples dissolved in DMSO, while the untreated control cultures received only the vehicle (DMSO, <0.2%).
Cytotoxicity assay
In vitro cytotoxicity against human cancer cell lines was determined using sulphorhodamine B dye assay (Thiantanawat et al., 2003; Tong et al., 2004). Both test sample stock solutions were prepared in DMSO and serially diluted with growth medium to obtain desired concentrations.
Synthesis of axially ligated thallium(III) porphyrin complexes
Synthesis of macrocycles
H2tPP and H2t(4-Y)TPP (Y=-CH3 and -OCH3) were synthesized. The synthesis of the porphyrins has been adapted from the synthesis reported by Adler et al. (1967). The appropriate benzaldehyde is refluxed for 30 min with 0.08 mL of pyrrole in 12 mL of propionic acid in a 50-mL round bottom flask fitted with a water condenser. The pyrrole must be from a freshly opened bottle or have recently been purified by vacuum distillation. After refluxing, the reaction mixture is cooled to room temperature, and 10 mL of cold methanol is added. The deep-purple solid is filtered by vacuum filtration with a Buchner funnel. The flask and crude solid is washed with three portions of cold methanol followed by boiling distilled water. The crystals are air-dried on the Buchner funnel for 15 min, dried in a vacuum desiccator and purified by column chromatography (Scheme 1). The syntheses of the substituted porphyrins, i.e. H2t(4-CH3)TPP and H2t(4-OCH3)TPP, are performed in a similar manner. Average yields are about 40 mg (23%). All the benzaldehydes are commercially available. The resulting porphyrins can be characterized by visible absorption or 1H NMR spectroscopy.
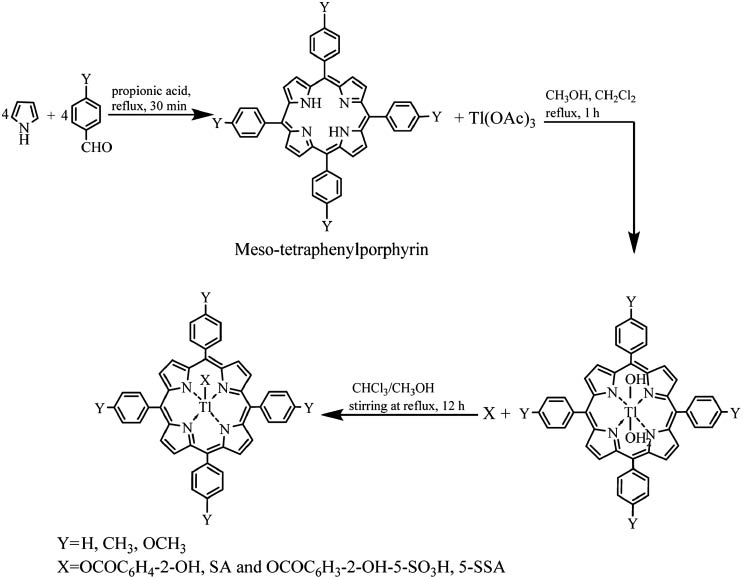
Synthesis of meso-tetraphenylporphyrins and their corresponding metalated and axially ligated derivatives.
Synthesis of aquo-meso-(5,10,15, 20-tetraarylporphyrinato)thallium(III)hydroxide, [(OH)(H2O)Tltpp and (OH)(H2O)Tlt(4-Y)PP] (Y: -CH3, -OCH3)
A mixture of meso-tetraphenylporphyrins 0.0068 mmol in CH2Cl2 (10 mL) and Tl(OAC)3 0.12 mmol in MeOH (2.5 mL) was refluxed for 1 h. After concentration, the residue was dissolved in CHCl3, dried with anhydrous Na2SO4 and filtered. The filtrate was concentrated and recrystallized from CH2Cl2-MeOH (1:5 v/v) yielding purple solid of complex, (0.037 mmol, 55%), which was again dissolved in CH2Cl2-ether [1:1 (v/v)] and layered with MeOH to get purple crystals of thallium(III) porphyrins (Scheme 1).
Synthesis of axially ligated Tl(III) Porphyrins: Tlt(4-Y)PP, SA and Tlt(4-Y)PP, 5-SSA
(OH)(H2O)Tl(III)t(4-Y)PP 0.15 mmol in 30 mL CHCl3 was treated with respective salicylates 0.56 mmol in 25 mL CH3OH and stirred under reflux for 12 h. After concentration, the mixture was dissolved in minimum quantity of CH2Cl2 and extracted four times with distilled water to remove excess unreacted salicylates (SA, 5-SSA). The resulting solution was then filtered through anhydrous Na2SO4 in order to remove water molecule. The CH2Cl2 layer was then concentrated to dryness, producing purple prism. The same procedure was applied for the synthesis of all axially ligated complexes of thallium porphyrins. The overall route for the synthesis of axially ligated thallium (III) porphyrin complexes is given in Scheme 1.
Acknowledgments:
We thank Department of Chemistry and Department of Biotechnology, University of Jammu, IIIM Jammu, IISC Banglore, India for providing support.
References
Adler, A. D.; Longo, F. R.; Finarelli, J. D.; Goldmacher, J.; Assour, J.; Korsakoff, L. A simplified synthesis for meso-tetraphenylporphine. J. Org. Chem.1967, 32, 476–476.10.1021/jo01288a053Suche in Google Scholar
Antonova, N. A.; Osipova, V. P.; Kolyada, M. N.; Movchan, N. O.; Milaeva, E. R.; Pimenov, Y. T. Study of the antioxidant properties of porphyrins and their complexes with metals. Macroheterocycles2010, 3, 139–144.10.6060/mhc2010.2-3.139Suche in Google Scholar
Cosma, F. G.; Badea, V.; Vlascici, D.; Cosma, F.-E.; Simon, M. The 13th Symposium on Analytical and Environmental Problems, Szeged. 2006, 84–87.Suche in Google Scholar
Cosma, E. F.; Mirica, M. C.; Balcu, I.; Bucovicean, C.; Cretu, C.; Armeanu, I.; Cosma, G. F. Syntheses, spectroscopic and AFM characterization of some manganese porphyrins and their hybrid silica nanomaterials. Molecules2009, 14, 1370–1388.10.3390/molecules14041370Suche in Google Scholar
Drain, C. M.; Hupp, J. T.; Suslick, K. S.; Wasielewski, M. R.; Chen, X. A perspective on four new porphyrin-based functional materials and devices. J. Porphy. Phthal.2002, 6, 243–258.10.1142/S1088424602000282Suche in Google Scholar
Fadda, A. A.; El-Mekawy, R. E.; El-Shafei, A.; Freeman, H. S.; Hinks, D.; El-Fedawy, M. Design, synthesis, and pharmacological screening of novel porphyrin derivatives. J. Chem. 2013, 2013, 11.10.1155/2013/340230Suche in Google Scholar
Fitzgerald, J. P.; Huffman, P. D.; Brenner, I. A.; Wathen, J. J.; Beadie, G.; Pong, R. G. S.; Shirk, J. S.; Flom, S. R. Synthesis, chemical characterization and nonlinear optical properties of thallium(III) phthalocyanine halide complexes. Opt. Mater. Express.2015, 5, 19.10.1364/OME.5.001560Suche in Google Scholar
Horvath, O.; Valicsek, Z.; Vogler, A. Unique photoreactivity of mercury (II) 5,10,15,20-tetrakis (4-sulfonatophenyl)porphyrins. Inorg. Chem. Commun.2004, 7, 854–857.10.1016/j.inoche.2004.05.004Suche in Google Scholar
Horvath, O.; Huszank, R.; Valicsek, Z.; Lendvay, G. Photophysics and photochemistry of kinetically labile, water-soluble porphyrin complexes. Coord. Chem. Rev. 2006, 250, 1792–1803.10.1016/j.ccr.2006.02.014Suche in Google Scholar
Karimipour, G.; Karami B.; Montazerozohori M.; Zakavi S. Oxidative decarboxylation of carboxylic acids with tetrabutylammonium periodate catalyzed by manganese (III) meso-tetraarylporphyrins: effect of metals, meso-substituents, and anionic axial ligands. Chinese J. Catal. 2007, 28, 940–946.10.1016/S1872-2067(07)60080-1Suche in Google Scholar
Karimipour, G.; Ghaedi, M.; Behfar, M.; Andikaey, Z.; Kowkabi, S.; Orojloo, A. H. Synthesis and application of new porphyrin derivatives for preparation of copper selective electrodes: influence of carbon nanotube on their responses. IEEE Sens. J. 2012, 12, 2638–2647.10.1109/JSEN.2012.2187333Suche in Google Scholar
Karimipour, G.; Rezaei M.; Ashouri D. Zeolite encapsulated Fe-porphyrin for catalytic oxidation with iodobenzene diacetate (PhI(OAc)2). J. Mex. Chem. Soc. 2013, 57, 276–282.Suche in Google Scholar
Knöra, G.; Strasser, A. Coexisting intraligand fluorescence and phosphorescence of hafnium(IV) and thorium(IV) porphyrin complexes in solution. Inorg. Chem. Commun. 2002, 5, 993–995.10.1016/S1387-7003(02)00612-3Suche in Google Scholar
Lu, Y. Y.; Tung, J. Y.; Chen, J. H.; Liao, F. L.; Wang, S. L.; Wang, S. S.; Hwang, L. P. Salicylate exchange in meso-tetraphenylporphyrinato salicylato thallium (III), Tl (tpp) (2-OH-C6H4CO2) and 13C NMR investigation of its homolog thiocyanato (meso-tetra-p-tolyl-porphyrinato)thallium(III), Tl (tptp)(SCN). Polyhedron.1999, 1, 145–150.Suche in Google Scholar
Ma, G.; Fischer, A.; Ilyukhin, A.; Glaser, J. Formation and structure of novel ternary complexes of thallium(III)-cyanide–amine (ethylenediamine and triethylenetetramine) in solution and in solid. Inorg. Chim. Acta. 2003, 344, 117–122.10.1016/S0020-1693(02)01315-4Suche in Google Scholar
Manke, A. M.; Geisel, K.; Fetzer, A.; Kurz, P. A water-soluble tin(IV) porphyrin as a bioinspired photosensitiser for light-driven proton-reduction. Phys. Chem. Chem. Phys.2014, 16, 12029–12042.10.1039/C3CP55023KSuche in Google Scholar
Milgrom, L. R. The Colors of Life, an Introduction to the Chemistry of Porphyrins and Related Compounds. Oxford University Press: Oxford, 1997.Suche in Google Scholar
Musturappa, P. K.; Reddy, V. K. R.; Kotresh, H. M. N.; Basappa, C.; Jayanna, M. B.; Devendrachari, M. C.; Fasiulla. New metallophthalocyanines posture pyridine pendants via 1,3,4-oxadiazole bridge: synthesis, optical and electrical studies. Chem. Sci. J.2013, 2013, 11.Suche in Google Scholar
Overton CE. Studien uber die Narkose zugleich ein Beitrag zur allgemeinen Pharmakology. Gustav Fisher, Jena: Switzerland, 1901.Suche in Google Scholar
Rajesh, K.; Rahiman, A. K.; Bharathi, K. S.; Sreedaran, S.; Gangadevi, V.; Narayanan, V. Spectroscopic, redox and biological studies of push-pull porphyrins and their metal complexes. B. Kor. Chem. Soc. 2010, 31, 2656–2664.10.5012/bkcs.2010.31.9.2656Suche in Google Scholar
Stojiljkovic, I.; Evavold, B. D.; Kumar, V. Antimicrobial properties of porphyrins. Expert Opin. Invest. Drugs.2001, 10, 309–320.10.1517/13543784.10.2.309Suche in Google Scholar
Suen, S. C.; Lee, W. B.; Hong, F. E.; Jong, T. T.; Chen, J. H. Molecular structure of thallium (III) meso-tetraphenylporphyrin acetate Tltpp(OAc). Polyhedron.1992, 11, 3025–3030.10.1016/S0277-5387(00)80171-0Suche in Google Scholar
Sun, Z.-C.; She, Y.-B.; Zhou, Y.; Song, X.-F.; Li, K. Synthesis, characterization and spectral properties of substituted tetraphenylporphyrin iron chloride complexes. Molecules. 2011, 16, 2960–2970.10.3390/molecules16042960Suche in Google Scholar PubMed PubMed Central
Thiantanawat, A.; Long, B. J.; Bordie, A. M. Signaling pathways of apoptosis activated by aromatase inhibitors and antiestrogens. Cancer Res.2003, 63, 8037–8050.Suche in Google Scholar
Tong, X.; Lin, S.; Fujii, M.; Hou, D. X. Erratum to Echinocystic acid induces apoptosis in HL-60 cells through mitochondria-mediated death pathway. Cancer Lett.2004, 212, 21–32.10.1016/j.canlet.2004.03.035Suche in Google Scholar PubMed
Tweedy, B. G. Plant extracts with metal ions as potential antimicrobial agents. Phytopathology. 1964, 55, 910–917.Suche in Google Scholar
Valicsek, Z.; Horvath O.; Stevenson, K. L. Photophysics and photochemistry of water-soluble, sitting-atop bis-thallium(I) 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin. Photochem. Photobiol. Sci. 2004, 3, 669–673.10.1039/B405105JSuche in Google Scholar
Valicsek, Z.; Horvath, O.; Lendvay, G.; Kikas, I.; Skoric, I. Formation, photophysics, and photochemistry of cadmium(II) complexes with 5,10,15,20-tetrakis(4-sulfonatophenyl) porphyrin and its octabromo derivative: The effects of bromination and the axial hydroxo ligand. J. Photoch. Photobio. A.2011, 218, 143–155.10.1016/j.jphotochem.2010.12.014Suche in Google Scholar
Yuasa, M.; Oyaizu, K.; Murata, H.; Sahara, Y.; Hatsugai, T.; Ogata, A. Antioxidant and anticancer properties of metalloporphyrins embedded in liposomes. J. Oleo Sci. 2007, 56, 87–93.10.5650/jos.56.87Suche in Google Scholar PubMed
Zhuang, C.; Tang, X.; Wang, D.; Xia, A.; Lian, W.; Shi, Y.; Shi, T. An unsymmetrical porphyrin and its metal complexes: synthesis, spectroscopy, thermal analysis and liquid crystal properties. J. Serb. Chem. Soc.2009, 74, 10.10.2298/JSC0910097ZSuche in Google Scholar
©2016 by De Gruyter
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Synthesis, characterization and antimicrobial activity of diorganotin(IV) derivatives of some bioactive bifunctional tridentate Schiff base ligands
- Some heteroleptic boron derivatives and their isomers derived from Schiff bases and glycol: synthesis, characterization and antimicrobial activities
- Synthesis and characterization of some new thallium(III) macrocyclic complexes and their biological studies
- Synthesis, characterization, antimicrobial, and DNA cleavage evaluation of some organotin(IV) complexes derived from ligands containing the 1H-indole-2,3-dione moiety
- Is calomel truly a poison and what happens when it enters the human stomach? A study from the thermodynamic viewpoint
- Sequential extraction procedure for fractionation of Pb and Cr in artificial and contaminated soil
- Characterization and physical properties of hydrated zinc borates synthesized from sodium borates
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Synthesis, characterization and antimicrobial activity of diorganotin(IV) derivatives of some bioactive bifunctional tridentate Schiff base ligands
- Some heteroleptic boron derivatives and their isomers derived from Schiff bases and glycol: synthesis, characterization and antimicrobial activities
- Synthesis and characterization of some new thallium(III) macrocyclic complexes and their biological studies
- Synthesis, characterization, antimicrobial, and DNA cleavage evaluation of some organotin(IV) complexes derived from ligands containing the 1H-indole-2,3-dione moiety
- Is calomel truly a poison and what happens when it enters the human stomach? A study from the thermodynamic viewpoint
- Sequential extraction procedure for fractionation of Pb and Cr in artificial and contaminated soil
- Characterization and physical properties of hydrated zinc borates synthesized from sodium borates

