Abstract
G protein-coupled receptors (GPCRs) modulate a variety of physiological functions and have been proven to be outstanding drug targets. However, approximately one-third of all non-olfactory GPCRs are still orphans in respect to their signal transduction and physiological functions. Receptors of the class of Adhesion GPCRs (aGPCRs) are among these orphan receptors. They are characterized by unique features in their structure and tissue-specific expression, which yields them interesting candidates for deorphanization and testing as potential therapeutic targets. Capable of G-protein coupling and non-G protein-mediated function, aGPCRs may extend our repertoire of influencing physiological function. Besides their described significance in the immune and central nervous systems, growing evidence indicates a high importance of these receptors in metabolic tissue. RNAseq analyses revealed high expression of several aGPCRs in pancreatic islets, adipose tissue, liver, and intestine but also in neurons governing food intake. In this review, we focus on aGPCRs and their function in regulating metabolic pathways. Based on current knowledge, this receptor class represents high potential for future pharmacological approaches addressing obesity and other metabolic diseases.
Introduction
G protein-coupled receptors (GPCRs) are important drug targets based on their well-known signaling pathways and good accessibility for ligands. Although two-thirds of non-olfactory GPCRs have been intensively characterized and assigned to endogenous agonists there is still a reasonable portion that remains orphan (Schöneberg and Liebscher 2021). The entire adhesion-type class of GPCRs (aGPCRs) belongs to these unstudied receptors, yet, their remarkable characteristics have the potential to establish new paradigms in treatment of metabolic diseases. These receptors share the common structure of all GPCRs containing an extracellular N-terminus, an intracellular C-terminus, and seven α-helical transmembrane helices connected by three extra- and intracellular loops (7TM domain) (Liebscher et al. 2013). Their large N-termini contain several functional domains not found in most other GPCRs, which are essential for several of the unusual functions that they display (Figure 1A) (Hamann et al. 2015; Langenhan 2020; Vizurraga et al. 2020). The structural hallmark of the aGPCR N-terminus is the GPCR autoproteolysis-inducing (GAIN) domain (Araç et al. 2012; Araç et al. 2016), which features the GPCR proteolytic site (GPS) at which the receptor undergoes autoproteolytic processing in the endoplasmic reticulum (Chang et al. 2003; Lin et al. 2004).
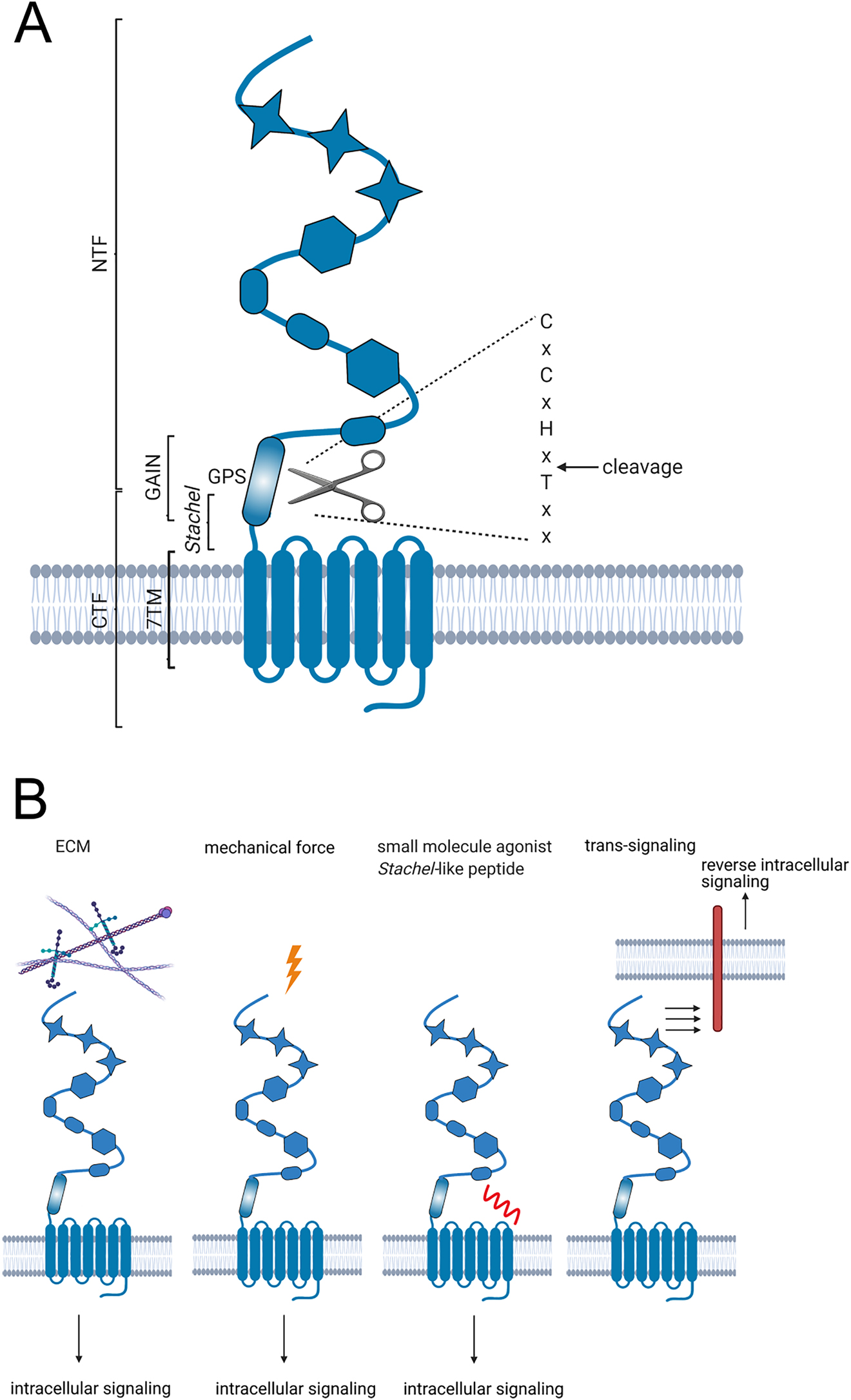
Adhesion GPCR structure and activation.
(A) Schematic presentation of the aGPCR structure. Adhesion GPCRs possess a 7-transmembrane (7TM) domain and a large N-terminus containing several adhesive domains, which are depicted here schematically as star, ellipse, and hexagon. The characteristic feature of aGPCRs is the GAIN (GPCR autoproteolysis-inducing) domain where autoproteolytic cleavage occurs within the GPS (GPCR proteolytic site). The resulting N-terminal fragment (NTF) and C-terminal fragment (CTF) remain non-covalently attached to each other. The extracellular portion of the CTF harbors the tethered agonist sequence, called the Stachel. (B) Activation of aGPCRs can occur by interaction with extracellular matrix (ECM), mechanical force, or small molecule agonists. Artificial activation is achieved by peptides derived from the tethered agonist sequence. Another feature of aGPCRs is the possibility of trans-signaling by interaction with and activation of receptors from other cells.
Besides splitting the receptor protein into two functional units (the N-terminal fragment and the C-terminal fragment), the GAIN domain like some of the other domains of the N-terminus, can bind extracellular matrix (ECM) components (Figure 1B) (Dunn et al. 2019; Luo et al. 2011; Petersen et al. 2015; Wandel et al. 2012). Also, interactions between the aGPCR N-terminus and surface antigens of neighboring cells were observed (Wang et al. 2005). Some of these ligands, such as collagen IV on GPR126 (Paavola et al. 2014), appear to activate the respective receptor directly, while others require additional mechanical forces to achieve a downstream signal. This has been demonstrated for the activation of GPR126 with laminin-211, which is dependent on mechano-stimulation (Petersen et al. 2015). Furthermore, it has been proposed for GPR56 that mechanical force together with the interaction of collagen III is required for platelet activation (Yeung et al. 2020). However, mechanical stimuli can also activate aGPCRs without the addition of a ligand (Figure 1B). Mechano-stimulation of GPR114 results in adenylyl cyclase activation and cAMP formation (Wilde et al. 2016) and the drosophila Latrophilin homolog CIRL decreases intracellular cAMP in response to mechano-stimulation (Scholz et al. 2015; Scholz et al. 2017). Thus, the large extracellular portion of these receptors integrates several context-specific signals via an encrypted tethered agonist sequence embedded within the GPS, referred to as the Stachel sequence (Balenga et al. 2017; Brown et al. 2017; Liebscher and Schöneberg 2016; Liebscher et al. 2014; Müller et al. 2015; Stoveken et al. 2015; Wilde et al. 2016). Peptides derived from this sequence can be used to activate aGPCRs (Figure 1B), thus giving rise to potential therapeutics, while mechano-sensing could provide a more localized approach to modulate receptor activity levels. Another highly interesting aspect of aGPCR function is the so-called trans-signaling, which is mediated solely by the interaction of the receptor’s N-terminus with membrane receptors of neighboring cells (Figure 1B) (Prömel et al. 2012; Tu et al. 2018). The targets of this external signaling are largely unknown but future studies will further elucidate this fascinating signaling mechanism.
Given the growing interest in aGPCRs their nomenclature has recently been homogenized (Hamann et al. 2015). However, the new nomenclature features various inconsistencies (Scholz et al. 2019), thus, the historical denomination is still commonly used, including in this review. So far, functional studies on aGPCRs address mainly their roles in the neuronal (Ackerman et al. 2018; Bolliger et al. 2011; Giera et al. 2015; Krasnoperov et al. 1996; Mogha et al. 2013; Piao et al. 2004; Sugita et al. 1998) and immune systems (Lin et al. 2017) as well as in developmental processes (Langenhan et al. 2009; Tissir and Goffinet 2013). Six human pathologies have been shown to be directly caused by mutations in aGPCRs (Schöneberg and Liebscher 2021). For example, the Usher Syndrome is a combined blind-deafness caused by malfunctioning of VLGR1 (Weston et al. 2004) and the bilateral frontal polymicrogyria results from mutations within GPR56 (Piao et al. 2004). In contrast, much less is known about aGPCR involvement in metabolic functions although many of these receptors are highly expressed in adipose tissue, the liver, the endocrine pancreas, and the gut. This review aims to summarize what is known about the contribution of aGPCRs to healthy and disease-state tissue functions that are recognized for their major role in metabolism.
Adipose tissue
Adipose tissue was long considered as an inert organ with the sole purpose of storing excess energy. However, in the last decades it has been identified as a key endocrine organ with an active role in maintaining the body’s energy homeostasis (Coelho et al. 2013). It is categorized in brown (BAT) and white (WAT) subtypes with the latter being subdivided into subcutaneous (SAT) and visceral (VAT) adipose tissue with distinct functions, morphology, and development (Belaj and Eller 2012). While primary functions of WAT are storing energy-rich triacylglycerols and hormonal signaling, BAT is mainly active in non-shivering thermogenesis. WAT and BAT are located in distinct depots all over the body, yet some WAT depots are susceptible to browning in response to stimuli, such as cold exposure. The browning results in beige (also referred to as “brite”) adipocytes with shared characteristics of both brown and white adipocytes (Jong et al. 2015; Young et al. 1984).
In adipose tissue, expression of numerous GPCRs has been demonstrated and the activation of G protein-signaling pathways regulates adipocyte function (Amisten et al. 2015). Increased cAMP levels due to activation of the Gs protein are indispensable for inducing adipogenesis (Petersen et al. 2008) and have also been shown to reduce hormone secretion and glucose uptake while increasing lipolysis (Frühbeck et al. 2014). Accordingly, the inhibition of adenylyl cyclases by Gi/o proteins has the opposite effects on lipolysis and hormone secretion (Caron et al. 2018; Stich et al. 1999). Interestingly, activation of Gi as well as Gs signaling in adipose tissue has been reported to positively impact glucose homeostasis under obese conditions (Caron et al. 2019; Wang et al. 2020). Furthermore, cAMP levels are connected to non-shivering thermogenesis in BAT as well as WAT browning (Barbatelli et al. 2010; Cannon and Nedergaard 2004; Jiang et al. 2017). Activation of Gq/11-protein signaling increases glucose uptake and interferes with WAT browning (Balasubramanian et al. 2014; Klepac et al. 2016). In general, Gq/11 signaling inhibits adipogenesis and BAT functioning, and induces BAT whitening (Klepac et al. 2016).
White adipose tissue
Initially, the expression of 11 aGPCRs was demonstrated in human WAT using a qPCR approach (Amisten et al. 2015). Additional studies have provided deeper insight into aGPCR expression by proteomic approaches (Fagerberg et al. 2014; Uhlén et al. 2015) and RNAseq of human and mouse WAT including changes depending on dietary conditions (Dalgaard et al. 2016; Suchý et al. 2020). Ultimately, expression has been observed for 14 aGPCRs in mouse VAT (Suchý et al. 2020; Vernia et al. 2016) and 17 aGPCRs in human SAT (Dalgaard et al. 2016) (Table 1).
Expression of aGPCRs in human (Dalgaard et al. 2016) and mouse (Vernia et al. 2016) WAT under changed nutritional conditions determined by RNAseq as well as expression in mouse brown preadipocytes and mature adipocytes determined by qPCR analysis (Klepac et al. 2016). RNAseq expression is given in FPKM values (+++ > 50; ++ > 10; + > 1; − < 1) and expression determined by qPCR is given in ΔCt values (+++ < 19; ++ < 23; + < 27; − > 32).
| aGPCR | Human WAT | Mouse WAT | Mouse BAT | |||
|---|---|---|---|---|---|---|
| Lean | Obese | Chow | High-fat | Preadipocytes | Adipocytes | |
| Adgrl1/Lphn1 | ++ | ++ | + | + | +++ | +++ |
| Adgrl2/Lphn2 | ++ | ++ | + | ++ | +++ | +++ |
| Adgrl3/Lphn3 | + | + | − | − | ++ | + |
| Adgrl4/Eltd1 | +++ | +++ | ++ | ++ | + | − |
| Adgre1/Emr1 | + | + | ++ | +++ | + | ++ |
| Adgre2/Emr2 | + | + | ||||
| Adgre3/Emr3 | + | + | ||||
| Adgre4/Emr4 | + | + | − | − | − | − |
| Adgre5/Cd97 | ++ | ++ | +++ | +++ | +++ | +++ |
| Adgra1/Gpr123 | − | − | − | − | − | − |
| Adgra2/Gpr124 | ++ | ++ | + | + | +++ | +++ |
| Adgra3/Gpr125 | ++ | ++ | + | + | +++ | ++ |
| Adgrc1/Celsr1 | − | + | − | − | + | − |
| Adgrc2/Celsr2 | + | + | + | + | + | + |
| Adgrc3/Celsr3 | − | − | − | − | − | − |
| Adgrd1/Gpr133 | ++ | + | ++ | ++ | + | ++ |
| Adgrd2/Gpr144 | − | − | ||||
| Adgrf1/Gpr110 | − | − | − | − | − | − |
| Adgrf2/Gpr111 | − | − | − | − | − | − |
| Adgrf3/Gpr113 | − | − | − | − | − | − |
| Adgrf4/Gpr115 | − | − | − | − | + | + |
| Adgrf5/Gpr116 | +++ | +++ | +++ | ++ | + | + |
| Adgrb1/Bai1 | − | − | − | − | + | − |
| Adgrb2/Bai2 | + | + | + | − | + | + |
| Adgrb3/Bai3 | + | + | − | − | − | − |
| Adgrg1/Gpr56 | ++ | ++ | +++ | ++ | ++ | + |
| Adgrg2/Gpr64 | + | + | ++ | + | + | + |
| Adgrg3/Gpr97 | + | + | + | − | − | − |
| Adgrg4/Gpr112 | − | − | − | − | − | − |
| Adgrg5/Gpr114 | − | − | − | − | + | − |
| Adgrg6/Gpr126 | + | + | + | + | − | − |
| Adgrg7/Gpr128 | − | − | − | − | − | − |
| Adgrv1/Vlgr1 | − | − | − | − | − | − |
Only limited studies are available regarding the function of aGPCRs in adipose tissue (Figure 2). A comprehensive analysis identified 11 aGPCRs to be significantly expressed during differentiation of the adipocyte model cell line 3T3-L1 (Suchý et al. 2020). Reducing the expression of six aGPCRs (Lphn2, Gpr124, Gpr125, Gpr116, Gpr64, and Gpr126) via transient knock-down impeded adipogenesis (Suchý et al. 2020) (Figure 2A). Furthermore, the knock-down of Gpr126 and Gpr64 altered the pattern of free fatty acids accumulated in 3T3-L1 cells (Suchý et al. 2020). Analysis of preadipocytes in Haiyang Yellow chicken revealed dynamic expression of Gpr133 during adipogenesis combined with an interaction with gga-miR-135a-5p (Chen et al. 2019), a regulator of adipogenesis in 3T3-L1 cells (Chen et al. 2014). However, a regulatory function of Gpr133 in WAT has not been demonstrated, yet.
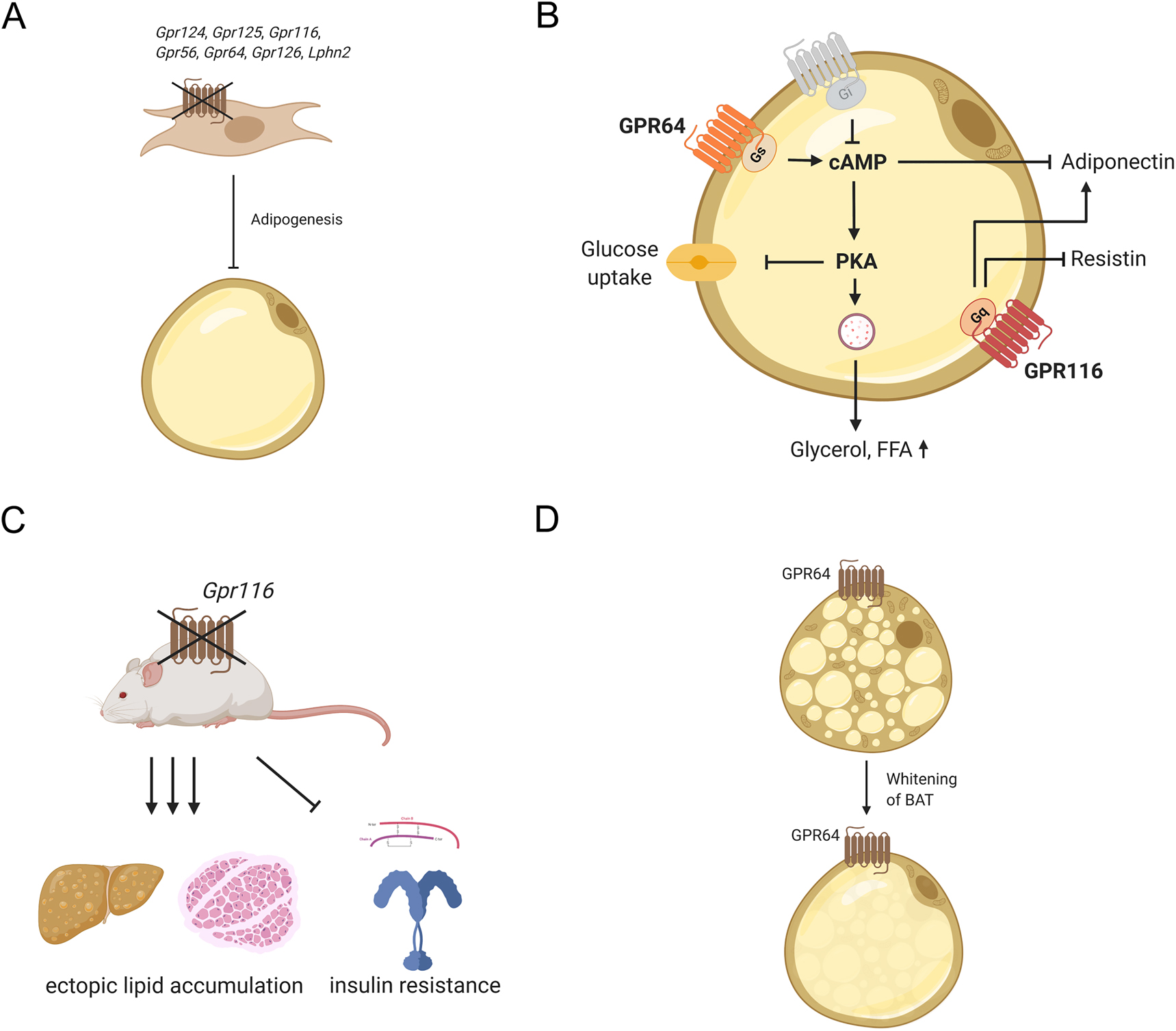
Adhesion GPCR functions in WAT and BAT.
(A) Gpr124, Gpr125, Gpr116, Gpr56, Gpr64, Gpr126, and Lphn2 are involved in adipogenesis and knock-down of these receptors results in impaired differentiation of preadipocytes. (B) GPR64 and GPR116 are expressed on mature adipocytes and influence their function. Activation of GPR64 elevates intracellular cAMP and, such, stimulates protein kinase A, which regulates glucose uptake and lipolysis. Furthermore, elevated cAMP levels reduce adiponectin secretion. GPR116 modulates secretion of adiponectin and resistin most likely by activation of the Gq/11 pathway. (C) Adipose tissue-specific deletion of Gpr116 results in insulin resistance and ectopic fat accumulation in liver and skeletal muscle underlining the importance of this receptor in maintaining body’s energy homeostasis. (D) GPR64 is discussed to induce whitening of BAT.
Gpr116 was found to be abundantly expressed in mouse primary adipocytes (Nie et al. 2012) as well as in differentiated 3T3-L1 cells, and its upregulation during adipogenesis was the highest among all aGPCRs (Suchý et al. 2020). This suggested a functional role for Gpr116 in adipogenesis and mature adipocytes. Interestingly, this receptor is coupled to Gq/11 proteins (Brown et al. 2017; Demberg et al. 2017) and inhibition of Gpr116 expression reduced lipid accumulation in primary mouse adipocytes (Nie et al. 2012) and 3T3-L1 cells (Suchý et al. 2020). Furthermore, adipose tissue-specific knock-out of Gpr116 resulted in insulin resistance and glucose intolerance while reducing adipocyte size and adiponectin secretion (Nie et al. 2012) (Figure 2B). Additionally, when challenged with high-fat diet, knock-out animals manifested ectopic lipid accumulation in liver and muscle as well as higher levels of circulating free fatty acids and triacylglycerol (Nie et al. 2012), highlighting the important role of Gpr116 in lipid metabolism (Figure 2C). Unfortunately, the endogenous signal that leads to activation of GPR116 is still unknown, even though interaction with α5 integrin has been suggested in vascular development (Lu et al. 2017). Because α5 integrin expression declines from preadipocytes to mature adipocytes (Liu et al. 2005a), it seems unlikely that the adipose tissue function of GPR116 is induced by interaction with this integrin.
The most abundant aGPCR in WAT is Gpr56, which is dynamically regulated during differentiation (Al Hasan et al. 2020; Suchý et al. 2020) and downregulated in VAT of obese rats (Al Hasan et al. 2020). Collagen III, a ligand of GPR56, is an integral part of the adipose tissue ECM (Khan et al. 2009). Remodeling of ECM including an up-regulation of Col3aI has been shown in obesity (Khan et al. 2009), which could be assumed to increase GPR56 signaling. Thus, the observed down-regulation of Gpr56 might constitute a mechanism counteracting this, considering that knock-down of this receptor in 3T3-L1 cells resulted in reduced differentiation and a complete knock-out decreased adhesion and proliferation of these cells (Al Hasan et al. 2020). Interestingly, Gpr56 knock-out also displayed altered expression of ECM components (Al Hasan et al. 2020) yielding GPR56 a potential target to interfere with ECM remodeling and induction of adipose tissue fibrosis.
Also, the functional role of Gpr64 in adipocytes has been demonstrated in 3T3-L1 cells (Suchý et al. 2020). The receptor couples to Gs-, Gi-, and Gq proteins (Demberg et al. 2015). Activation with a tethered agonist-derived peptide increased intracellular cAMP levels in 3T3-L1 cells, thereby accelerating lipolysis and reducing glucose uptake and adiponectin secretion (Suchý et al. 2020) (Figure 2B).
Conclusively, significant progress was achieved in deciphering the roles of aGPCR in WAT. However, many aspects like the identification of receptor ligands and activation mechanisms remain to be subject for further research to entirely elucidate the function of aGPCRs in WAT physiology.
Brown adipose tissue
Similar to WAT, a multitude of GPCRs, including aGPCRs, are expressed in BAT (Klepac et al. 2016; Li et al. 2020). A comparative analysis of isolated human pre- and mature adipocytes found 14 aGPCRs to be expressed in both and four aGPCRs only in preadipocytes (Klepac et al. 2016) (Table 1). Furthermore, proteome analysis of murine BAT has demonstrated LPHN2, LPHN3, CD97, and GPR116 expression (Li et al. 2020). As G protein-signaling pathways have been connected to BAT activation and, subsequently, regulate non-shivering thermogenesis, these receptors are promising targets to increase energy expenditure and reduce blood glucose levels.
However, the role of aGPCRs in BAT function was not intensively investigated, although, there is evidence that aGPCRs are involved therein. For example, GPR64 has been connected to whitening of BAT, which is generally associated with activation of Gq/11 protein-signaling pathways (Klepac et al. 2016) (Figure 2D). This connection is based on the observation that Gpr64 expression, which is usually lower in mature brown adipocytes compared to preadipocytes, increases upon knock-out of Prdm16, a transcription factor regulating thermogenic genes (Harms et al. 2014; Klepac et al. 2016). Upregulation of Gpr64 expression is accompanied by inducing a white fat-like phenotype of BAT (Harms et al. 2014). Considering the multiple signaling options of this receptor (see above), one can speculate that selectively activating its G-protein preference may be an interesting strategy to inhibit whitening of BAT or even induce WAT browning. In this line, the upregulation of the Gs protein-coupled aGPCR Gpr133 (Bohnekamp and Schöneberg 2011) in mature brown adipocytes compared to brown preadipocytes (Klepac et al. 2016) may indicate an involvement of this receptor in browning and thermogenesis.
In sum, information about aGPCRs expressed in BAT is still scarce and future investigations will need to shed further light on the specific role of these receptors in this adipose tissue.
Pancreatic islets
Blood glucose levels are mainly regulated by the hormones insulin and glucagon secreted from pancreatic islets, also known as islets of Langerhans, which were discovered by Paul Langerhans in 1869 (Quesada et al. 2006). They are organized in cell clusters composed of five different types of endocrine cells, which secrete hormones required for maintaining glucose homeostasis. The most abundant cells (80%) are insulin-producing β cells which are found in the core of mouse pancreatic islets. Glucagon-secreting α cells, which represent 15% of mouse pancreatic islet cells, and somatostatin-releasing δ cells (5%) are located in the islet periphery (Arrojo e Drigo et al. 2015; Cabrera et al. 2006). Pancreatic polypeptide (PP) cells and ε cells secreting pancreatic polypeptide and ghrelin, respectively, represent only small cell populations located in the islet mantle (Andralojc et al. 2009; Wang et al. 2013).
The blood glucose level is the main signal for the regulated secretion of insulin or glucagon. While insulin is secreted after a meal to lower blood glucose levels, glucagon’s function is to raise blood glucose levels during fasting periods. Both hormones have pivotal roles in glucose homeostasis, and their correct interplay is required for physiological blood glucose levels (Aronoff et al. 2004). It is well-established that GPCRs modulate pancreatic hormone secretion via activation of different G protein-signaling pathways (Ahrén 2009). Gq/11-coupled receptors activate phospholipase C resulting in elevated IP3 concentrations, which induce release of Ca2+ from intracellular storage. Increasing cytosolic Ca2+ concentrations enhance the secretory response of pancreatic hormones (Winzell and Ahrén 2007). In addition, the second messenger cAMP influences hormone release by activation of protein kinase A and Epac, which stimulate the exocytosis of vesicles. Thereby, Gs protein-coupled receptors stimulate and Gi/o protein-coupled receptors inhibit hormone secretion from endocrine pancreatic cells (Winzell and Ahrén 2007). The influence of G12/13-protein signaling is not well-understood, but it is discussed that this pathway contributes to vesicle discharge and activation increases glucose-stimulated insulin release (Rives et al. 2018). Besides their impact on pancreatic hormone secretion there is evidence that G protein-signaling pathways are involved in islet development. For example, the Gs-coupled adenosine receptor A2a promotes β-cell proliferation and regeneration (Schulz et al. 2016). Furthermore, while Gi-protein signaling reduces β-cell mass during development (Berger et al. 2015), activation of the Gq/11-protein pathway increases β-cell amount and insulin content in adult mice (Jain et al. 2013).
Pancreatic islet cells express numerous GPCRs, which have been widely discussed to be potential therapeutic targets for type 2 diabetes (T2D) (Amisten et al. 2017; Amisten et al. 2013; Madiraju and Poitout 2007). Recent transcriptome analyses also revealed considerable expression of 16 aGPCRs (Meister et al. 2014) (Table 2), of which Celsr3, Lphn1, Lphn3, Bai3, and Gpr56 have been functionally characterized.
Publicly available and own RNAseq data was analyzed to determine aGPCR expression in human pancreatic islets (Dunér et al. 2016) and sorted α- and β cells (Blodgett et al. 2015) as well as mouse pancreatic islets (Meister et al. 2014) and sorted islet cells (Adriaenssens et al. 2016; DiGruccio et al. 2016). RNAseq expression is given in FPKM values (+++ > 20; ++ > 2; + > 0.5; − < 0.5) or TPM values (+++ > 2; ++ > 0.2; + > 0.02; − < 0.02).
| aGPCR | Human | Mouse | ||
|---|---|---|---|---|
| Whole islet | Cell type | Whole islet | Cell type | |
| Adgrl1/Lphn1 | + | α = β | ++ | α = β = δ |
| Adgrl2/Lphn2 | ++ | α > β | + | δ > α = β |
| Adgrl3/Lphn3 | − | − | + | β > α > δ |
| Adgrl4/Eltd1 | ++ | − | ++ | − |
| Adgre1/Emr1 | − | − | + | − |
| Adgre2/Emr2 | − | − | ||
| Adgre3/Emr3 | − | − | ||
| Adgre4/Emr4 | − | − | − | − |
| Adgre5/Cd97 | + | α > β | ++ | δ > α > β |
| Adgra1/Gpr123 | − | − | − | − |
| Adgra2/Gpr124 | + | α > β | + | α > δ > β |
| Adgra3/Gpr125 | ++ | α = β | ++ | δ > β = α |
| Adgrc1/Celsr1 | − | − | + | β = δ > α |
| Adgrc2/Celsr2 | + | α > β | ++ | α = δ > β |
| Adgrc3/Celsr3 | + | α > β | ++ | α = δ > β |
| Adgrd1/Gpr133 | − | − | − | − |
| Adgrd2/Gpr144 | − | − | ||
| Adgrf1/Gpr110 | ++ | − | − | − |
| Adgrf2/Gpr111 | − | − | − | − |
| Adgrf3/Gpr113 | − | − | − | − |
| Adgrf4/Gpr115 | + | − | − | − |
| Adgrf5/Gpr116 | ++ | − | ++ | δ > α = β |
| Adgrb1/Bai1 | − | − | − | − |
| Adgrb2/Bai2 | − | − | − | − |
| Adgrb3/Bai3 | − | − | ++ | δ > β = α |
| Adgrg1/Gpr56 | +++ | β > α | +++ | δ > α = β |
| Adgrg2/Gpr64 | − | α > β | − | − |
| Adgrg3/Gpr97 | − | α > β | − | − |
| Adgrg4/Gpr112 | − | − | − | − |
| Adgrg5/Gpr114 | + | α > β | − | − |
| Adgrg6/Gpr126 | + | − | + | − |
| Adgrg7/Gpr128 | − | − | − | − |
| Adgrv1/Vlgr1 | + | β > α | + | β > α = δ |
The most abundant aGPCR in mouse and human pancreatic islets is Gpr56 (Amisten et al. 2013, 2017; Dunér et al. 2016; Meister et al. 2014). Its expression is down-regulated by long-term glucose stimulation and reduced in islets of T2D patients (Dunér et al. 2016). Activation with its ligand collagen III stimulates glucose-dependent insulin secretion of isolated pancreatic islets (Figure 3A) (Dunér et al. 2016; Olaniru et al. 2018). However, the exact mechanism is still not completely solved. The coupling preferences of GPR56 include Gq/11- and G12/13 signaling, however, elevation of cAMP and protein kinase A activation have been suggested as well (Dunér et al. 2016; Olaniru et al. 2018). Furthermore, Gpr56 expression and collagen interaction regulate apoptosis and viability of pancreatic β cells. With collagen III being part of the ECM surrounding pancreatic islets (Korpos et al. 2013), these results indicate that ECM molecules regulate β-cell function.
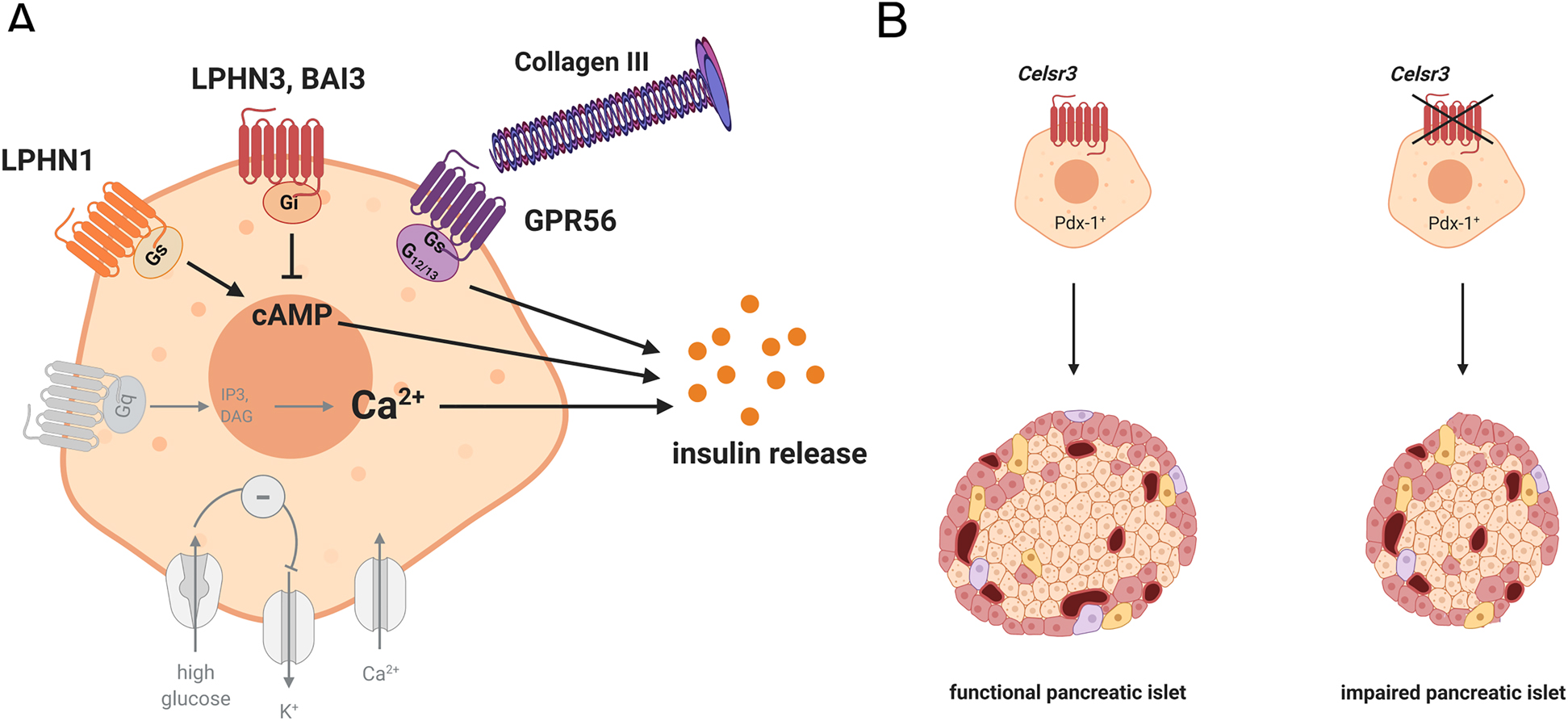
Impact of aGPCRs on pancreatic islet function and development.
(A) Insulin secretion is modulated by G protein-signaling pathways induced by aGPCRs expressed on pancreatic β cells. Activation of LPHN1 increases intracellular cAMP and, therefore, insulin secretion, while LPHN3 and BAI3 reduce intracellular cAMP and insulin release upon activation. Gpr56 regulates β-cell viability and increases insulin secretion when activated by its ligand collagen III. (B) Deletion of Celsr3 in Pdx-positive islet progenitor cells results in reduced β-cell amount, eventually leading to impaired insulin secretion.
High levels of expression have also been observed for receptors of the Latrophilin family. Interestingly, transcriptome analysis of sorted pancreatic islet cells has revealed equal expression of Lphn1 in α-, β-, and δ cells, whereas Lphn2 and Lphn3 are most abundant in δ- and β cells, respectively (Adriaenssens et al. 2016; DiGruccio et al. 2016) (Table 2). Already more than 20 years ago, it was demonstrated that α-Latrotoxin, a ligand of LPHN1, induces insulin secretion in primary islets and β-cell lines (Lang et al. 1998). However, there are controversial reports whether this effect is receptor-mediated (Lajus et al. 2006; Liu et al. 2005b; Silva et al. 2005). Recently, tethered peptide-based activation demonstrated coupling of LPHN1 and LPHN3 to Gs- and Gi proteins in pancreatic islets, respectively (Müller et al. 2015; Röthe et al. 2019). In vitro and ex vivo analyses have revealed increased insulin secretion following LPHN1 activation, whereas activation of LPHN3 resulted in reduced insulin release (Figure 3A). Furthermore, down-regulation of Lphn3 increased basal insulin secretion in the β-cell line MIN6, indicating that the receptor has a regulative function in β cells even without activation (Röthe et al. 2019).
BAI3 activation by its ligand complement 1q-like-3 protein (C1ql3) has been shown to reduce insulin secretion in primary islets and β-cell lines by decreasing intracellular cAMP levels and PKA activity (Gupta et al. 2018) (Figure 3A). Similar to Lphn3, Bai3 knock-down increases insulin secretion, suggesting a ligand-independent inhibitory effect of the receptor. Interestingly, C1ql3 expression is increased in plasma of obese patient indicating that BAI3 might be an interesting target in the regulation of insulin secretion in obesity-associated T2D (Shanaki et al. 2016). However, it needs to be noted that Bai3 expression in human islets is very low (Dunér et al. 2016) (Table 2).
Even though aGPCRs have been implicated in developmental processes in other tissues little is known about their impact on islet development. Thus far, only Celsr3 has been linked to β-cell development. Deletion of Celsr3 in Pdx-positive pancreatic progenitor cells diminishes β-cell amount leading to an impaired glucose tolerance (Cortijo et al. 2012) (Figure 3B). Transcriptome analysis of human fetal β cells revealed the highest aGPCR expression for Gpr97, although the receptor is not expressed in adult human and mouse islets (Blodgett et al. 2015) (Table 2). It has been demonstrated that glucocorticoid-activated GPR97 reduces intracellular cAMP levels via the Gi/o protein-signaling pathway (Gupte et al. 2012; Ping et al. 2021). Interestingly, glucocorticoids as well as activation of Gi/o proteins have been shown to interfere with proper islet development leading to reduced amount of mature β cells (Berger et al. 2015; Dumortier et al. 2011; Gesina et al. 2004). Thus, one can speculate that GPR97 might have a role during β-cell development.
Expression in other tissues connected to energy homeostasis
The liver is one of the main organs controlling and coordinating glucose homeostasis, lipid and amino acid metabolism, and several other processes which are modulated by GPCRs. Gs-protein signaling induced by, for example, glucagon, results in protein kinase A activation and subsequently in glycogen degradation and gluconeogenesis whereas activation of Gi/o proteins has opposite effects (Rossi et al. 2018). Gluconeogenesis and glycogenolysis are also induced by receptors coupled to Gq/11 proteins like the V1b vasopressin receptor (Li et al. 2013).
To date, there is no report of the functional relevance of any aGPCR in hepatic metabolic processes available. However, many aGPCRs are expressed in the liver (Fagerberg et al. 2014; Yang et al. 2016) and, specifically, in hepatocytes (MacParland et al. 2018; Schaum et al. 2018) (Table 3). Evidence for functional relevance has been found in different mouse models of hepatosteatosis, in which Gpr110 has been identified among the 50 strongest regulated genes referred to as common indicators of non-alcoholic fatty liver disease (Lede et al. 2017). Further support comes from several genome-wide association studies. An intergenic single nucleotide polymorphism (SNP) close to the 3′ UTR of the Celsr2 gene has been associated with altered levels of LDL cholesterol. This LDL-associated SNP was strongly correlated with Celsr2 transcript levels in human liver (Kathiresan et al. 2008). Similarly, several other SNPs in or in close proximity to aGPCR genes are associated with liver metabolism. For example, GWAS and QTL analyses linked Lphn3 to HDL cholesterol levels, Gpr125 to LDL particle size, and Vlgr1 to apolipoprotein A2 levels (summarized in Kovacs and Schöneberg 2016). However, causal or mechanistic links between liver metabolism and aGPCR gene variants remain to be determined.
Meta-analysis of aGPCR expression in human liver (Fagerberg et al. 2014) and hepatocytes (MacParland et al. 2018) as well as mouse liver (Yang et al. 2016) and hepatocytes (Schaum et al. 2018). RNAseq expression is given in FPKM values (+++ > 20; ++ > 2; + > 0.5; − < 0.5). Hepatocyte-specific expression was determined using single cell expression atlas for human and mouse liver.
| aGPCR | Human | Mouse | ||
|---|---|---|---|---|
| Liver | Hepatocyte | Liver | Hepatocyte | |
| Adgrl1/Lphn1 | + | − | + | − |
| Adgrl2/Lphn2 | +++ | +++ | ++ | + |
| Adgrl3/Lphn3 | + | − | − | − |
| Adgrl4/Eltd1 | ++ | ++ | ++ | + |
| Adgre1/Emr1 | + | − | +++ | − |
| Adgre2/Emr2 | ++ | − | ||
| Adgre3/Emr3 | − | − | ||
| Adgre4/Emr4 | − | − | ++ | − |
| Adgre5/Cd97 | ++ | − | +++ | − |
| Adgra1/Gpr123 | − | − | − | − |
| Adgra2/Gpr124 | ++ | − | ++ | − |
| Adgra3/Gpr125 | +++ | +++ | ++ | ++ |
| Adgrc1/Celsr1 | ++ | ++ | ++ | + |
| Adgrc2/Celsr2 | + | ++ | − | + |
| Adgrc3/Celsr3 | − | + | − | − |
| Adgrd1/Gpr133 | ++ | + | − | − |
| Adgrd2/Gpr144 | − | − | ||
| Adgrf1/Gpr110 | − | − | − | + |
| Adgrf2/Gpr111 | − | − | − | − |
| Adgrf3/Gpr113 | − | + | − | − |
| Adgrf4/Gpr115 | − | − | − | − |
| Adgrf5/Gpr116 | ++ | + | ++ | + |
| Adgrb1/Bai1 | − | − | − | − |
| Adgrb2/Bai2 | − | − | − | − |
| Adgrb3/Bai3 | − | − | − | − |
| Adgrg1/Gpr56 | ++ | + | + | + |
| Adgrg2/Gpr64 | − | − | + | + |
| Adgrg3/Gpr97 | ++ | − | ++ | − |
| Adgrg4/Gpr112 | − | − | − | − |
| Adgrg5/Gpr114 | − | − | − | − |
| Adgrg6/Gpr126 | +++ | +++ | + | − |
| Adgrg7/Gpr128 | ++ | ++ | − | − |
| Adgrv1/Vlgr1 | ++ | ++ | − | + |
Food intake and energy homeostasis are regulated by hypothalamic neurons of the arcuate nucleus, namely agouti-related peptide (AgRP) and pro-opiomelanocortin (POMC) neurons. POMC neurons release melanocyte-stimulating hormone activating melanocortin type 4 receptor (MC4R) expressing neurons central for inhibiting food intake and increasing energy expenditure. AgRP secreted from AgRP neurons is an inverse agonist of MC4R, thus, inducing food intake. Therefore, activated AgRP neurons promote food intake and activation of POMC neurons has anorexigenic effects (Adan et al. 2006). The function of these neurons is modulated by G protein-signaling pathways. While induction of Gs- and Gq/11-protein pathways increases neuron activity, Gi protein-coupled receptors inhibit it (Krashes et al. 2011; Zhan et al. 2013). Transcriptome analysis has revealed expression of several aGPCRs in these neurons (Table 4) (Henry et al. 2015). Interestingly, Bai3 is expressed in both types of neurons and during fasting periods its expression is down-regulated in AgRP neurons and up-regulated in POMC neurons (Henry et al. 2015). Such, it appears likely that BAI3 has a regulative role in food intake. As BAI3 signaling has been linked to Gi/o-protein activation (Gupta et al. 2018), it can be presumed that its activation silences POMC neurons under fasting conditions and inhibits AgRP neurons under feeding.
Expression of aGPCRs in hypothalamic AgRP and POMC neurons under fasted and fed conditions in the mice (Henry et al. 2015). RNAseq expression is given in TPM values (+++ > 50; ++ > 10; + > 1; − < 1).
| aGPCR | AgRP | POMC | ||
|---|---|---|---|---|
| Fasted | Fed | Fasted | Fed | |
| Adgrl1/Lphn1 | +++ | +++ | +++ | +++ |
| Adgrl2/Lphn2 | ++ | ++ | ++ | ++ |
| Adgrl3/Lphn3 | ++ | ++ | +++ | +++ |
| Adgrl4/Eltd1 | − | − | − | − |
| Adgre1/Emr1 | − | − | − | − |
| Adgre4/Emr4 | − | − | − | − |
| Adgre5/Cd97 | − | − | − | − |
| Adgra1/Gpr123 | +++ | +++ | +++ | +++ |
| Adgra2/Gpr124 | − | + | + | − |
| Adgra3/Gpr125 | ++ | ++ | + | ++ |
| Adgrc1/Celsr1 | − | − | − | − |
| Adgrc2/Celsr2 | − | − | − | − |
| Adgrc3/Celsr3 | − | − | − | − |
| Adgrd1/Gpr133 | − | + | + | + |
| Adgrf1/Gpr110 | − | − | − | − |
| Adgrf2/Gpr111 | + | − | + | − |
| Adgrf3/Gpr113 | − | − | − | + |
| Adgrf4/Gpr115 | − | + | + | + |
| Adgrf5/Gpr116 | − | − | − | − |
| Adgrb1/Bai1 | ++ | +++ | ++ | ++ |
| Adgrb2/Bai2 | ++ | + | ++ | ++ |
| Adgrb3/Bai3 | ++ | +++ | +++ | ++ |
| Adgrg1/Gpr56 | +++ | +++ | +++ | +++ |
| Adgrg2/Gpr64 | +++ | + | + | + |
| Adgrg3/Gpr97 | − | − | − | − |
| Adgrg4/Gpr112 | − | − | − | − |
| Adgrg5/Gpr114 | − | − | − | − |
| Adgrg6/Gpr126 | + | ++ | − | + |
| Adgrg7/Gpr128 | − | − | − | − |
| Adgrv1/Vlgr1 | − | + | + | + |
Expression of aGPCRs has also been demonstrated throughout the gastro-intestinal tract (Badiali et al. 2012; Fagerberg et al. 2014). High expression in intestine has been reported for Gpr128 and knock-out of the receptor resulted in reduced body weight (Ni et al. 2014). Of specific interest are enteroendocrine cells which secrete hormones, such as the gastric inhibitory polypeptide (GIP), and regulate whole-body metabolic homeostasis. GPCRs expressed in these cells are described to function as sensors for metabolites. Interestingly, RNAseq analysis has revealed a high expression of Gpr112 in enteroendocrine cells compared to enterocytes (Sommer and Mostoslavsky 2014) indicating a specific function of this aGPCR in these cells.
Future perspectives and concluding remarks
Whole body energy homeostasis depends on the rigorous regulation of tissue interplay and GPCRs have been demonstrated to be involved in several of these processes. Their specific expression in metabolically relevant tissues has made GPCRs promising targets to treat obesity and its associated pathologies, among them the free fatty acid receptors 1–4 (Christiansen et al. 2008; Da Oh et al. 2010; Li et al. 2019; McNelis et al. 2015; Milligan et al. 2015; Miyamoto et al. 2017; Mohammad 2016; Park et al. 2016; Shimpukade et al. 2012; Son et al. 2021; Ueno et al. 2019), the glucose-dependent insulinotropic peptide receptor (Killion et al. 2020; Sachs et al. 2021), the glucagon-like peptide 1 receptor (Campbell 2020; Chadda et al. 2020; Maselli and Camilleri 2021; McLean et al. 2020; Willard et al. 2020), and MC4R (Clément et al. 2020; Gonçalves et al. 2018; Kievit et al. 2013; Kühnen et al. 2019). Only limited information is available for the class of aGPCRs albeit their high expression in metabolically relevant tissues. Ongoing research on these receptors will further elucidate their signaling properties and physiological relevance and may expose their potential targetability to treat metabolic disorders such as obesity, T2D, fatty liver disease, and cardiovascular disease. As outlined above, some aGPCRs specifically modulate functions of adipocytes and pancreatic islet cells. Their dual structural characteristics of having a large N-terminus and a 7TM domain opens the possibility to modulate their functions, classically by 7TM-binding drugs or Stachel-derived peptides but also through targeting the adhesive extracellular domains. These binding partners can be identified using affinity purification approaches, as demonstrated for GPR56 and collagen III (Luo et al. 2011). Small molecules binding at the 7TM domain of aGPCRs and modulating receptor’s signal transduction have been identified for GPR56, GPR97, GPR114, and GPR126 using drug screening studies (Bradley et al. 2019; Gupte et al. 2012; Stoveken et al. 2016). Autoantibodies against the N-terminus of GPCRs modulating receptor activity are well-known from autoimmune diseases (Schöneberg and Liebscher 2021) but are more and more generated as specific therapeutics to treat diverse pathologies (Hutchings 2020). The large N-termini are ideal epitopes to develop specific antibodies blocking aGPCR interactions with other cells and/or ECM or even influencing aGPCR signaling. Examples for successful applications have been shown for CD97, whose interaction with its binding partner CD55 can be abolished using an antibody targeting the EGF domains of the receptor (Eichler et al. 2019) and EMR2, which can be activated by incubation with an antibody targeting its GAIN domain (Bhudia et al. 2020; Yona et al. 2008).
Funding source: Deutsche Forschungsgemeinschaft 10.13039/501100001659
Award Identifier / Grant number: CRC1052/B6
Award Identifier / Grant number: CRC1423
Award Identifier / Grant number: FOR2149
Funding source: European Social Fund 10.13039/501100004895
Acknowledgments
Figures were created using BioRender.com.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Our research related to this topic is funded by the German Research Foundation (CRC1052/B6 project number 209933838 (TS and IL), CRC1423 project number 421152132 (IL; TS, and SP), FOR2149 project number 246212759 (IL; SP, and TS)) and the European Union (European Social Fund) (DT, SP, and IL).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Ackerman, S.D., Luo, R., Poitelon, Y., Mogha, A., Harty, B.L., D’Rozario, M., Sanchez, N.E., Lakkaraju, A.K.K., Gamble, P., Li, J., et al. (2018). GPR56/ADGRG1 regulates development and maintenance of peripheral myelin. J. Exp. Med. 215: 941–961, https://doi.org/10.1084/jem.20161714.Suche in Google Scholar PubMed PubMed Central
Adan, R.A.H., Tiesjema, B., Hillebrand, J.J.G., La Fleur, S.E., Kas, M.J.H., and de Krom, M. (2006). The MC4 receptor and control of appetite. Br. J. Pharmacol. 149: 815–827, https://doi.org/10.1038/sj.bjp.0706929.Suche in Google Scholar PubMed PubMed Central
Adriaenssens, A.E., Svendsen, B., Lam, B.Y.H., Yeo, G.S.H., Holst, J.J., Reimann, F., and Gribble, F.M. (2016). Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia 59: 2156–2165, https://doi.org/10.1007/s00125-016-4033-1.Suche in Google Scholar PubMed PubMed Central
Ahrén, B. (2009). Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat. Rev. Drug Discov. 8: 369–385, https://doi.org/10.1038/nrd2782.Suche in Google Scholar PubMed
Al Hasan, M., Roy, P., Dolan, S., Martin, P.E., Patterson, S., and Bartholomew, C. (2020). Adhesion G-protein coupled receptor 56 is required for 3T3-L1 adipogenesis. J. Cell. Physiol. 235: 1601–1614, https://doi.org/10.1002/jcp.29079.Suche in Google Scholar PubMed
Amisten, S., Atanes, P., Hawkes, R., Ruz-Maldonado, I., Liu, B., Parandeh, F., Zhao, M., Huang, G.C., Salehi, A., and Persaud, S.J. (2017). A comparative analysis of human and mouse islet G-protein coupled receptor expression. Sci. Rep. 7: 46600, https://doi.org/10.1038/srep46600.Suche in Google Scholar PubMed PubMed Central
Amisten, S., Neville, M., Hawkes, R., Persaud, S.J., Karpe, F., and Salehi, A. (2015). An atlas of G-protein coupled receptor expression and function in human subcutaneous adipose tissue. Pharmacol. Ther. 146: 61–93, https://doi.org/10.1016/j.pharmthera.2014.09.007.Suche in Google Scholar PubMed
Amisten, S., Salehi, A., Rorsman, P., Jones, P.M., and Persaud, S.J. (2013). An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacol. Ther. 139: 359–391, https://doi.org/10.1016/j.pharmthera.2013.05.004.Suche in Google Scholar PubMed
Andralojc, K.M., Mercalli, A., Nowak, K.W., Albarello, L., Calcagno, R., Luzi, L., Bonifacio, E., Doglioni, C., and Piemonti, L. (2009). Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia 52: 486–493, https://doi.org/10.1007/s00125-008-1238-y.Suche in Google Scholar PubMed
Araç, D., Boucard, A.A., Bolliger, M.F., Nguyen, J., Soltis, S.M., Südhof, T.C., and Brunger, A.T. (2012). A novel evolutionarily conserved domain of cell-adhesion GPCRs mediates autoproteolysis. EMBO J. 1364–1378, https://doi.org/10.1038/emboj.2012.26.Suche in Google Scholar PubMed PubMed Central
Araç, D., Sträter, N., and Seiradake, E. (2016). Understanding the structural basis of adhesion GPCR functions. Handb. Exp. Pharmacol. 234: 67–82, https://doi.org/10.1007/978-3-319-41523-9_4.Suche in Google Scholar PubMed
Aronoff, S.L., Berkowitz, K., Shreiner, B., and Want, L. (2004). Glucose metabolism and regulation: beyond insulin and glucagon. Diabetes Spectr. 17: 183–190, https://doi.org/10.2337/diaspect.17.3.183.Suche in Google Scholar
Arrojo e Drigo, R., Ali, Y., Diez, J., Srinivasan, D.K., Berggren, P.-O., and Boehm, B.O. (2015). New insights into the architecture of the islet of Langerhans: a focused cross-species assessment. Diabetologia 58: 2218–2228, https://doi.org/10.1007/s00125-015-3699-0.Suche in Google Scholar PubMed
Badiali, L., Cedernaes, J., Olszewski, P.K., Nylander, O., Vergoni, A.V., and Schiöth, H.B. (2012). Adhesion GPCRs are widely expressed throughout the subsections of the gastrointestinal tract. BMC Gastroenterol. 12: 134, https://doi.org/10.1186/1471-230x-12-134.Suche in Google Scholar PubMed PubMed Central
Balasubramanian, R., Robaye, B., Boeynaems, J.-M., and Jacobson, K.A. (2014). Enhancement of glucose uptake in mouse skeletal muscle cells and adipocytes by P2Y6 receptor agonists. PLoS One 9: e116203, https://doi.org/10.1371/journal.pone.0116203.Suche in Google Scholar PubMed PubMed Central
Balenga, N., Azimzadeh, P., Hogue, J.A., Staats, P.N., Shi, Y., Koh, J., Dressman, H., and Olson, J.A. (2017). Orphan adhesion GPCR GPR64/ADGRG2 is overexpressed in parathyroid tumors and attenuates calcium-sensing receptor-mediated signaling. J. Bone Miner. Res. 32: 654–666, https://doi.org/10.1002/jbmr.3023.Suche in Google Scholar PubMed PubMed Central
Barbatelli, G., Murano, I., Madsen, L., Hao, Q., Jimenez, M., Kristiansen, K., Giacobino, J.P., de Matteis, R., and Cinti, S. (2010). The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 298: E1244–E1253, https://doi.org/10.1152/ajpendo.00600.2009.Suche in Google Scholar PubMed
Belaj, K.J. and Eller, P. (2012). The fate of fat. Gerontology 58: 120–122, https://doi.org/10.1159/000331798.Suche in Google Scholar PubMed
Berger, M., Scheel, D.W., Macias, H., Miyatsuka, T., Kim, H., Hoang, P., Ku, G.M., Honig, G., Liou, A., Tang, Y., et al. (2015). Gαi/o-coupled receptor signaling restricts pancreatic β-cell expansion. Proc. Natl. Acad. Sci. U.S.A. 112: 2888–2893, https://doi.org/10.1073/pnas.1319378112.Suche in Google Scholar PubMed PubMed Central
Bhudia, N., Desai, S., King, N., Ancellin, N., Grillot, D., Barnes, A.A., and Dowell, S.J. (2020). G protein-coupling of adhesion GPCRs ADGRE2/EMR2 and ADGRE5/CD97, and activation of G protein signalling by an anti-EMR2 antibody. Sci. Rep. 10: 1004, https://doi.org/10.1038/s41598-020-57989-6.Suche in Google Scholar PubMed PubMed Central
Blodgett, D.M., Nowosielska, A., Afik, S., Pechhold, S., Cura, A.J., Kennedy, N.J., Kim, S., Kucukural, A., Davis, R.J., Kent, S.C., et al. (2015). Novel observations from next-generation RNA sequencing of highly purified human adult and fetal islet cell subsets. Diabetes 64: 3172–3181, https://doi.org/10.2337/db15-0039.Suche in Google Scholar PubMed PubMed Central
Bohnekamp, J. and Schöneberg, T. (2011). Cell adhesion receptor GPR133 couples to Gs protein. J. Biol. Chem. 286: 41912–41916, https://doi.org/10.1074/jbc.c111.265934.Suche in Google Scholar
Bolliger, M.F., Martinelli, D.C., and Südhof, T.C. (2011). The cell-adhesion G protein-coupled receptor BAI3 is a high-affinity receptor for C1q-like proteins. Proc. Natl. Acad. Sci. Unit. States Am. 108: 2534–2539, https://doi.org/10.1073/pnas.1019577108.Suche in Google Scholar PubMed PubMed Central
Bradley, E.C., Cunningham, R.L., Wilde, C., Morgan, R.K., Klug, E.A., Letcher, S.M., Schöneberg, T., Monk, K.R., Liebscher, I., and Petersen, S.C. (2019). In vivo identification of small molecules mediating Gpr126/Adgrg6 signaling during Schwann cell development. Ann. N. Y. Acad. Sci. 1456: 44–63, https://doi.org/10.1111/nyas.14233.Suche in Google Scholar PubMed PubMed Central
Brown, K., Filuta, A., Ludwig, M.-G., Seuwen, K., Jaros, J., Vidal, S., Arora, K., Naren, A.P., Kandasamy, K., Parthasarathi, K., et al. (2017). Epithelial Gpr116 regulates pulmonary alveolar homeostasis via Gq/11 signaling. JCI Insight 2, https://doi.org/10.1172/jci.insight.93700.Suche in Google Scholar PubMed PubMed Central
Cabrera, O., Berman, D.M., Kenyon, N.S., Ricordi, C., Berggren, P.-O., and Caicedo, A. (2006). The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl. Acad. Sci. U.S.A. 103: 2334–2339, https://doi.org/10.1073/pnas.0510790103.Suche in Google Scholar PubMed PubMed Central
Campbell, J.E. (2020). Targeting the GIPR for obesity: to agonize or antagonize? Potential mechanisms. Mol. Metab. 101139, https://doi.org/10.1016/j.molmet.2020.101139.Suche in Google Scholar PubMed PubMed Central
Cannon, B. and Nedergaard, J. (2004). Brown adipose tissue: function and physiological significance. Physiol. Rev. 84: 277–359, https://doi.org/10.1152/physrev.00015.2003.Suche in Google Scholar PubMed
Caron, A., Dungan Lemko, H.M., Castorena, C.M., Fujikawa, T., Lee, S., Lord, C.C., Ahmed, N., Lee, C.E., Holland, W.L., Liu, C., et al. (2018). POMC neurons expressing leptin receptors coordinate metabolic responses to fasting via suppression of leptin levels. eLife 7: e33710, https://doi.org/10.7554/eLife.33710.Suche in Google Scholar PubMed PubMed Central
Caron, A., Reynolds, R.P., Castorena, C.M., Michael, N.J., Lee, C.E., Lee, S., Berdeaux, R., Scherer, P.E., and Elmquist, J.K. (2019). Adipocyte Gs but not Gi signaling regulates whole-body glucose homeostasis. Mol. Metab. 27: 11–21, https://doi.org/10.1016/j.molmet.2019.06.019.Suche in Google Scholar PubMed PubMed Central
Chadda, K.R., Cheng, T.S., and Ong, K.K. (2020). GLP-1 agonists for obesity and type 2 diabetes in children: systematic review and meta-analysis. Obes. Rev. 22: e13177.10.1111/obr.13177Suche in Google Scholar
Chang, G.-W., Stacey, M., Kwakkenbos, M.J., Hamann, J., Gordon, S., and Lin, H.-H. (2003). Proteolytic cleavage of the EMR2 receptor requires both the extracellular stalk and the GPS motif. FEBS Lett. 547: 145–150, https://doi.org/10.1016/s0014-5793(03)00695-1.Suche in Google Scholar
Chen, C., Peng, Y., Peng, Y., Peng, J., and Jiang, S. (2014). miR-135a-5p inhibits 3T3-L1 adipogenesis through activation of canonical Wnt/β-catenin signaling. J. Mol. Endocrinol. 52: 311–320, https://doi.org/10.1530/jme-14-0013.Suche in Google Scholar
Chen, L., Zhang, T., Zhang, S., Huang, J., Zhang, G., Xie, K., Wang, J., Wu, H., and Dai, G. (2019). Identification of long non-coding RNA-associated competing endogenous RNA network in the differentiation of chicken preadipocytes. Genes (Basel) 10: 795.10.3390/genes10100795Suche in Google Scholar
Christiansen, E., Urban, C., Merten, N., Liebscher, K., Karlsen, K.K., Hamacher, A., Spinrath, A., Bond, A.D., Drewke, C., Ullrich, S., et al. (2008). Discovery of potent and selective agonists for the free fatty acid receptor 1 (FFA1)GPR40, a potential target for the treatment of type II diabetes. J. Med. Chem. 51: 7061–7064, https://doi.org/10.1021/jm8010178.Suche in Google Scholar
Clément, K., van den Akker, E., Argente, J., Bahm, A., Chung, W.K., Connors, H., de Waele, K., Farooqi, I.S., Gonneau-Lejeune, J., Gordon, G., et al. (2020). Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 8: 960–970, https://doi.org/10.1016/s2213-8587(20)30364-8.Suche in Google Scholar
Coelho, M., Oliveira, T., and Fernandes, R. (2013). State of the art paper biochemistry of adipose tissue: an endocrine organ. Arch. Med. Sci. 2: 191–200, https://doi.org/10.5114/aoms.2013.33181.Suche in Google Scholar PubMed PubMed Central
Cortijo, C., Gouzi, M., Tissir, F., and Grapin-Botton, A. (2012). Planar cell polarity controls pancreatic beta cell differentiation and glucose homeostasis. Cell Rep. 2: 1593–1606, https://doi.org/10.1016/j.celrep.2012.10.016.Suche in Google Scholar PubMed PubMed Central
Dalgaard, K., Landgraf, K., Heyne, S., Lempradl, A., Longinotto, J., Gossens, K., Ruf, M., Orthofer, M., Strogantsev, R., Selvaraj, M., et al. (2016). Trim28 haploinsufficiency triggers bi-stable epigenetic obesity. Cell 164: 353–364, https://doi.org/10.1016/j.cell.2015.12.025.Suche in Google Scholar PubMed PubMed Central
Da Oh, Y., Talukdar, S., Bae, E.J., Imamura, T., Morinaga, H., Fan, W., Li, P., Lu, W.J., Watkins, S.M., and Olefsky, J.M. (2010). GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142: 687–698, https://doi.org/10.1016/j.cell.2010.07.041.Suche in Google Scholar PubMed PubMed Central
Demberg, L.M., Rothemund, S., Schöneberg, T., and Liebscher, I. (2015). Identification of the tethered peptide agonist of the adhesion G protein-coupled receptor GPR64/ADGRG2. Biochem. Biophys. Res. Commun. 464: 743–747, https://doi.org/10.1016/j.bbrc.2015.07.020.Suche in Google Scholar PubMed
Demberg, L.M., Winkler, J., Wilde, C., Simon, K.-U., Schön, J., Rothemund, S., Schöneberg, T., Prömel, S., and Liebscher, I. (2017). Activation of Adhesion G Protein-coupled Receptors: agonist specificity of stachel sequence-derived peptides. J. Biol. Chem. 292: 4383–4394, https://doi.org/10.1074/jbc.m116.763656.Suche in Google Scholar
de Jong, J.M.A., Larsson, O., Cannon, B., and Nedergaard, J. (2015). A stringent validation of mouse adipose tissue identity markers. Am. J. Physiol. Endocrinol. Metab. 308: E1085–E1105, https://doi.org/10.1152/ajpendo.00023.2015.Suche in Google Scholar PubMed
DiGruccio, M.R., Mawla, A.M., Donaldson, C.J., Noguchi, G.M., Vaughan, J., Cowing-Zitron, C., van der Meulen, T., and Huising, M.O. (2016). Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Mol. Metab. 5: 449–458, https://doi.org/10.1016/j.molmet.2016.04.007.Suche in Google Scholar PubMed PubMed Central
Dumortier, O., Theys, N., Ahn, M.-T., Remacle, C., and Reusens, B. (2011). Impairment of rat fetal beta-cell development by maternal exposure to dexamethasone during different time-windows. PLoS One 6: e25576, https://doi.org/10.1371/journal.pone.0025576.Suche in Google Scholar PubMed PubMed Central
Dunér, P., Al-Amily, I.M., Soni, A., Asplund, O., Safi, F., Storm, P., Groop, L., Amisten, S., and Salehi, A. (2016). Adhesion G protein-coupled receptor G1 (ADGRG1/GPR56) and pancreatic β-cell function. J. Clin. Endocrinol. Metab. 101: 4637–4645, https://doi.org/10.1210/jc.2016-1884.Suche in Google Scholar PubMed
Dunn, H.A., Orlandi, C., and Martemyanov, K.A. (2019). Beyond the ligand: extracellular and transcellular G protein-coupled receptor complexes in physiology and pharmacology. Pharmacol. Rev. 71: 503–519, https://doi.org/10.1124/pr.119.018044.Suche in Google Scholar PubMed PubMed Central
Eichler, W., Lohrenz, A., Simon, K.-U., Krohn, S., Lange, J., Bürger, S., and Liebscher, I. (2019). The role of ADGRE5/CD97 in human retinal pigment epithelial cell growth and survival. Ann. N. Y. Acad. Sci. 1456: 64–79, https://doi.org/10.1111/nyas.14210.Suche in Google Scholar PubMed
Fagerberg, L., Hallström, B.M., Oksvold, P., Kampf, C., Djureinovic, D., Odeberg, J., Habuka, M., Tahmasebpoor, S., Danielsson, A., Edlund, K., et al. (2014). Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteomics 13: 397–406, https://doi.org/10.1074/mcp.m113.035600.Suche in Google Scholar
Frühbeck, G., Méndez-Giménez, L., Fernández-Formoso, J.-A., Fernández, S., and Rodríguez, A. (2014). Regulation of adipocyte lipolysis. Nutr. Res. Rev. 27: 63–93, https://doi.org/10.1017/s095442241400002x.Suche in Google Scholar
Gesina, E., Tronche, F., Herrera, P., Duchene, B., Tales, W., Czernichow, P., and Breant, B. (2004). Dissecting the role of glucocorticoids on pancreas development. Diabetes 53: 2322–2329, https://doi.org/10.2337/diabetes.53.9.2322.Suche in Google Scholar PubMed
Giera, S., Deng, Y., Luo, R., Ackerman, S.D., Mogha, A., Monk, K.R., Ying, Y., Jeong, S.-J., Makinodan, M., Bialas, A.R., et al. (2015). The adhesion G protein-coupled receptor GPR56 is a cell-autonomous regulator of oligodendrocyte development. Nat. Commun. 6: 6121, https://doi.org/10.1038/ncomms7121.Suche in Google Scholar PubMed PubMed Central
Gonçalves, J., Pereira, L., Palmer, D., and Meldal, M. (2018). MC4R agonists: structural overview on antiobesity therapeutics. Trends Pharmacol. Sci. 39: 402–423, https://doi.org/10.1016/j.tips.2018.01.004.Suche in Google Scholar PubMed
Gupta, R., Nguyen, D.C., Schaid, M.D., Lei, X., Balamurugan, A.N., Wong, G.W., Kim, J.-A., Koltes, J.E., Kimple, M.E., and Bhatnagar, S. (2018). Complement 1q-like-3 protein inhibits insulin secretion from pancreatic β-cells via the cell adhesion G protein-coupled receptor BAI3. J. Biol. Chem. 293: 18086–18098, https://doi.org/10.1074/jbc.ra118.005403.Suche in Google Scholar PubMed PubMed Central
Gupte, J., Swaminath, G., Danao, J., Tian, H., Li, Y., and Wu, X. (2012). Signaling property study of adhesion G-protein-coupled receptors. FEBS Lett. 586: 1214–1219, https://doi.org/10.1016/j.febslet.2012.03.014.Suche in Google Scholar PubMed
Hamann, J., Aust, G., Araç, D., Engel, F.B., Formstone, C., Fredriksson, R., Hall, R.A., Harty, B.L., Kirchhoff, C., Knapp, B., et al. (2015). International Union of Basic and Clinical Pharmacology. XCIV. Adhesion G protein-coupled receptors. Pharmacol. Rev. 67: 338–367, https://doi.org/10.1124/pr.114.009647.Suche in Google Scholar PubMed PubMed Central
Harms, M.J., Ishibashi, J., Wang, W., Lim, H.-W., Goyama, S., Sato, T., Kurokawa, M., Won, K.-J., and Seale, P. (2014). Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab. 19: 593–604, https://doi.org/10.1016/j.cmet.2014.03.007.Suche in Google Scholar PubMed PubMed Central
Henry, F.E., Sugino, K., Tozer, A., Branco, T., and Sternson, S.M. (2015). Cell type-specific transcriptomics of hypothalamic energy-sensing neuron responses to weight-loss. eLife 4, https://doi.org/10.7554/eLife.09800.Suche in Google Scholar PubMed PubMed Central
Hutchings, C.J. (2020). A review of antibody-based therapeutics targeting G protein-coupled receptors: an update. Expet Opin. Biol. Ther. 20: 925–935, https://doi.org/10.1080/14712598.2020.1745770.Suche in Google Scholar PubMed
Jain, S., Ruiz de Azua, I., Lu, H., White, M.F., Guettier, J.-M., and Wess, J. (2013). Chronic activation of a designer Gq-coupled receptor improves β cell function. J. Clin. Invest. 123: 1750–1762, https://doi.org/10.1172/jci66432.Suche in Google Scholar PubMed PubMed Central
Jiang, Y., Berry, D.C., and Graff, J.M. (2017). Distinct cellular and molecular mechanisms for β3 adrenergic receptor-induced beige adipocyte formation. eLife 6: e30329.10.7554/eLife.30329.031Suche in Google Scholar
Kathiresan, S., Melander, O., Guiducci, C., Surti, A., Burtt, N.P., Rieder, M.J., Cooper, G.M., Roos, C., Voight, B.F., Havulinna, A.S., et al. (2008). Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat. Genet. 40: 189–197, https://doi.org/10.1038/ng.75.Suche in Google Scholar PubMed PubMed Central
Khan, T., Muise, E.S., Iyengar, P., Wang, Z.V., Chandalia, M., Abate, N., Zhang, B.B., Bonaldo, P., Chua, S., and Scherer, P.E. (2009). Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell Biol. 29: 1575–1591, https://doi.org/10.1128/mcb.01300-08.Suche in Google Scholar
Kievit, P., Halem, H., Marks, D.L., Dong, J.Z., Glavas, M.M., Sinnayah, P., Pranger, L., Cowley, M.A., Grove, K.L., and Culler, M.D. (2013). Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes 62: 490–497, https://doi.org/10.2337/db12-0598.Suche in Google Scholar PubMed PubMed Central
Killion, E.A., Chen, M., Falsey, J.R., Sivits, G., Hager, T., Atangan, L., Helmering, J., Lee, J., Li, H., Wu, B., et al. (2020). Chronic glucose-dependent insulinotropic polypeptide receptor (GIPR) agonism desensitizes adipocyte GIPR activity mimicking functional GIPR antagonism. Nat. Commun. 11: 4981, https://doi.org/10.1038/s41467-020-18751-8.Suche in Google Scholar PubMed PubMed Central
Klepac, K., Kilić, A., Gnad, T., Brown, L.M., Herrmann, B., Wilderman, A., Balkow, A., Glöde, A., Simon, K., Lidell, M.E., et al. (2016). The Gq signalling pathway inhibits brown and beige adipose tissue. Nat. Commun. 7: 10895.10.1038/ncomms10895Suche in Google Scholar PubMed PubMed Central
Korpos, É., Kadri, N., Kappelhoff, R., Wegner, J., Overall, C.M., Weber, E., Holmberg, D., Cardell, S., and Sorokin, L. (2013). The peri-islet basement membrane, a barrier to infiltrating leukocytes in type 1 diabetes in mouse and human. Diabetes 62: 531–542, https://doi.org/10.2337/db12-0432.Suche in Google Scholar PubMed PubMed Central
Kovacs, P. and Schöneberg, T. (2016). The relevance of genomic signatures at adhesion GPCR loci in humans. Handb. Exp. Pharmacol. 234: 179–217, https://doi.org/10.1007/978-3-319-41523-9_9.Suche in Google Scholar PubMed
Krashes, M.J., Koda, S., Ye, C., Rogan, S.C., Adams, A.C., Cusher, D.S., Maratos-Flier, E., Roth, B.L., and Lowell, B.B. (2011). Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121: 1424–1428, https://doi.org/10.1172/jci46229.Suche in Google Scholar
Krasnoperov, V.G., Beavis, R., Chepurny, O.G., Little, A.R., Plotnikov, A.N., and Petrenko, A.G. (1996). The calcium-independent receptor of alpha-latrotoxin is not a neurexin. Biochem. Biophys. Res. Commun. 227: 868–875, https://doi.org/10.1006/bbrc.1996.1598.Suche in Google Scholar PubMed
Kühnen, P., Krude, H., and Biebermann, H. (2019). Melanocortin-4 receptor signalling: importance for weight regulation and obesity treatment. Trends Mol. Med. 25: 136–148, https://doi.org/10.1016/j.molmed.2018.12.002.Suche in Google Scholar PubMed
Lajus, S., Vacher, P., Huber, D., Dubois, M., Benassy, M.-N., Ushkaryov, Y., and Lang, J. (2006). Alpha-latrotoxin induces exocytosis by inhibition of voltage-dependent K+ channels and by stimulation of L-type Ca2+ channels via latrophilin in beta-cells. J. Biol. Chem. 281: 5522–5531, https://doi.org/10.1074/jbc.m510528200.Suche in Google Scholar PubMed
Lang, J., Ushkaryov, Y., Grasso, A., and Wollheim, C.B. (1998). Ca2+-independent insulin exocytosis induced by alpha-latrotoxin requires latrophilin, a G protein-coupled receptor. EMBO J. 17: 648–657, https://doi.org/10.1093/emboj/17.3.648.Suche in Google Scholar PubMed PubMed Central
Langenhan, T. (2020). Adhesion G protein-coupled receptors-candidate metabotropic mechanosensors and novel drug targets. Basic Clin. Pharmacol. Toxicol. 126: 5–16, https://doi.org/10.1111/bcpt.13223.Suche in Google Scholar PubMed
Langenhan, T., Prömel, S., Mestek, L., Esmaeili, B., Waller-Evans, H., Hennig, C., Kohara, Y., Avery, L., Vakonakis, I., Schnabel, R., et al. (2009). Latrophilin signaling links anterior-posterior tissue polarity and oriented cell divisions in the C. elegans embryo. Dev. Cell 17: 494–504, https://doi.org/10.1016/j.devcel.2009.08.008.Suche in Google Scholar PubMed PubMed Central
Li, J.Hua, Jain, S., McMillin, S.M., Cui, Y., Gautam, D., Sakamoto, W., Lu, H., Jou, W., McGuinness, O.P., Gavrilova, O., et al. (2013). A novel experimental strategy to assess the metabolic effects of selective activation of a Gq-coupled receptor in hepatocytes in vivo. Endocrinology 154: 3539–3551, https://doi.org/10.1210/en.2012-2127.Suche in Google Scholar PubMed PubMed Central
Lede, V., Meusel, A., Garten, A., Popkova, Y., Penke, M., Franke, C., Ricken, A., Schulz, A., Kiess, W., Huster, D., et al. (2017). Altered hepatic lipid metabolism in mice lacking both the melanocortin type 4 receptor and low density lipoprotein receptor. PLoS One 12: e0172000, https://doi.org/10.1371/journal.pone.0172000.Suche in Google Scholar PubMed PubMed Central
Li, Z., Hu, L., Wang, X., Zhou, Z., Deng, L., Xu, Y., and Zhang, L. (2019). Design, synthesis, and biological evaluation of novel dual FFA1 (GPR40)/PPARδ agonists as potential anti-diabetic agents. Bioorg. Chem. 92: 103254, https://doi.org/10.1016/j.bioorg.2019.103254.Suche in Google Scholar PubMed
Liebscher, I. and Schöneberg, T. (2016). Tethered agonism: a common activation mechanism of adhesion GPCRs. Handb. Exp. Pharmacol. 234: 111–125, https://doi.org/10.1007/978-3-319-41523-9_6.Suche in Google Scholar PubMed
Li, J., Li, J., Zhao, W.-G., Sun, H.-D., Guo, Z.-G., Liu, X.-Y., Tang, X.-Y., She, Z.-F., Yuan, T., Liu, S.-N., et al. (2020). Comprehensive proteomics and functional annotation of mouse brown adipose tissue. PLoS One 15: e0232084, https://doi.org/10.1371/journal.pone.0232084.Suche in Google Scholar PubMed PubMed Central
Liebscher, I., Schön, J., Petersen, S.C., Fischer, L., Auerbach, N., Demberg, L.Marie, Mogha, A., Cöster, M., Simon, K.-U., Rothemund, S., et al. (2014). A tethered agonist within the ectodomain activates the adhesion G protein-coupled receptors GPR126 and GPR133. Cell Rep. 9: 2018–2026.10.1016/j.celrep.2014.11.036Suche in Google Scholar PubMed PubMed Central
Liebscher, I., Schöneberg, T., and Prömel, S. (2013). Progress in demystification of adhesion G protein-coupled receptors. Biol. Chem. 394: 937–950, https://doi.org/10.1515/hsz-2013-0109.Suche in Google Scholar PubMed
Lin, H.-H., Chang, G.-W., Davies, J.Q., Stacey, M., Harris, J., and Gordon, S. (2004). Autocatalytic cleavage of the EMR2 receptor occurs at a conserved G protein-coupled receptor proteolytic site motif. J. Biol. Chem. 279: 31823–31832, https://doi.org/10.1074/jbc.m402974200.Suche in Google Scholar PubMed
Lin, H.-H., Hsiao, C.-C., Pabst, C., Hébert, J., Schöneberg, T., and Hamann, J. (2017). Adhesion GPCRs in regulating immune responses and inflammation. Adv. Immunol. 136: 163–201, https://doi.org/10.1016/bs.ai.2017.05.005.Suche in Google Scholar PubMed
Liu, J., DeYoung, S.M., Zhang, M., Zhang, M., Cheng, A., and Saltiel, A.R. (2005a). Changes in integrin expression during adipocyte differentiation. Cell Metab. 2: 165–177, https://doi.org/10.1016/j.cmet.2005.08.006.Suche in Google Scholar PubMed
Liu, J., Wan, Q., Lin, X., Zhu, H., Volynski, K., Ushkaryov, Y., and Xu, T. (2005b). Alpha-latrotoxin modulates the secretory machinery via receptor-mediated activation of protein kinase C. Traffic 6: 756–765, https://doi.org/10.1111/j.1600-0854.2005.00313.x.Suche in Google Scholar PubMed
Lu, S., Liu, S., Wietelmann, A., Kojonazarov, B., Atzberger, A., Tang, C., Schermuly, R.T., Gröne, H.-J., and Offermanns, S. (2017). Developmental vascular remodeling defects and postnatal kidney failure in mice lacking Gpr116 (Adgrf5) and Eltd1 (Adgrl4). PLoS One 12: e0183166, https://doi.org/10.1371/journal.pone.0183166.Suche in Google Scholar PubMed PubMed Central
Luo, R., Jeong, S.-J., Jin, Z., Strokes, N., Li, S., and Piao, X. (2011). G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination. Proc. Natl. Acad. Sci. U.S.A. 108: 12925–12930, https://doi.org/10.1073/pnas.1104821108.Suche in Google Scholar PubMed PubMed Central
MacParland, S.A., Liu, J.C., Ma, X.-Z., Innes, B.T., Bartczak, A.M., Gage, B.K., Manuel, J., Khuu, N., Echeverri, J., Linares, I., et al. (2018). Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat. Commun. 9: 4383, https://doi.org/10.1038/s41467-018-06318-7.Suche in Google Scholar PubMed PubMed Central
Madiraju, S.R.M. and Poitout, V. (2007). G protein-coupled receptors and insulin secretion: 119 and counting. Endocrinology 148: 2598–2600, https://doi.org/10.1210/en.2007-0336.Suche in Google Scholar PubMed PubMed Central
Maselli, D.B. and Camilleri, M. (2021). Effects of GLP-1 and its analogs on gastric physiology in diabetes mellitus and obesity. Adv. Exp. Med. Biol. 1307: 171–192, https://doi.org/10.1097/psy.0000000000000891.Suche in Google Scholar PubMed
McLean, B.A., Wong, C.K., Campbell, J.E., Hodson, D.J., Trapp, S., and Drucker, D.J. (2020). Revisiting the complexity of GLP-1 action-from sites of synthesis to receptor activation. Endocr. Rev. 42: 101–132.10.1210/endrev/bnaa032Suche in Google Scholar PubMed PubMed Central
McNelis, J.C., Lee, Y.S., Mayoral, R., van der Kant, R., Johnson, A.M.F., Wollam, J., and Olefsky, J.M. (2015). GPR43 potentiates β-cell function in obesity. Diabetes 64: 3203–3217, https://doi.org/10.2337/db14-1938.Suche in Google Scholar PubMed PubMed Central
Meister, J., Le Duc, D., Ricken, A., Burkhardt, R., Thiery, J., Pfannkuche, H., Polte, T., Grosse, J., Schöneberg, T., and Schulz, A. (2014). The G protein-coupled receptor P2Y14 influences insulin release and smooth muscle function in mice. J. Biol. Chem. 289: 23353–23366, https://doi.org/10.1074/jbc.m114.580803.Suche in Google Scholar
Milligan, G., Alvarez-Curto, E., Watterson, K.R., Ulven, T., and Hudson, B.D. (2015). Characterizing pharmacological ligands to study the long-chain fatty acid receptors GPR40/FFA1 and GPR120/FFA4. Br. J. Pharmacol. 172: 3254–3265, https://doi.org/10.1111/bph.12879.Suche in Google Scholar PubMed PubMed Central
Miyamoto, J., Kasubuchi, M., Nakajima, A., and Kimura, I. (2017). Anti-inflammatory and insulin-sensitizing effects of free fatty acid receptors. Handb. Exp. Pharmacol. 236: 221–231, https://doi.org/10.1007/164_2016_47.Suche in Google Scholar PubMed
Mogha, A., Benesh, A.E., Patra, C., Engel, F.B., Schöneberg, T., Liebscher, I., and Monk, K.R. (2013). Gpr126 functions in Schwann cells to control differentiation and myelination via G-protein activation. J. Neurosci. 33: 17976–17985, https://doi.org/10.1523/jneurosci.1809-13.2013.Suche in Google Scholar
Mohammad, S. (2016). GPR40 agonists for the treatment of type 2 diabetes mellitus: benefits and challenges. Curr. Drug Targets 17: 1292–1300, https://doi.org/10.2174/1389450117666151209122702.Suche in Google Scholar PubMed
Müller, A., Winkler, J., Fiedler, F., Sastradihardja, T., Binder, C., Schnabel, R., Kungel, J., Rothemund, S., Hennig, C., Schöneberg, T., et al. (2015). Oriented cell division in the C. elegans Embryo is coordinated by G-protein signaling dependent on the adhesion GPCR LAT-1. PLoS Genet. 11: e1005624, https://doi.org/10.1371/journal.pgen.1005624.Suche in Google Scholar PubMed PubMed Central
Ni, Y.-Y., Chen, Y., Lu, S.-Y., Sun, B.-Y., Wang, F., Wu, X.-L., Dang, S.-Y., Zhang, G.-H., Zhang, H.-X., Kuang, Y., et al. (2014). Deletion of Gpr128 results in weight loss and increased intestinal contraction frequency. World J. Gastroenterol. 20: 498–508, https://doi.org/10.3748/wjg.v20.i2.498.Suche in Google Scholar PubMed PubMed Central
Nie, T., Hui, X., Gao, X., Li, K., Lin, W., Xiang, X., Ding, M., Kuang, Y., Xu, A., Fei, J., et al. (2012). Adipose tissue deletion of Gpr116 impairs insulin sensitivity through modulation of adipose function. FEBS Lett. 586: 3618–3625, https://doi.org/10.1016/j.febslet.2012.08.006.Suche in Google Scholar PubMed
Olaniru, O.E., Pingitore, A., Giera, S., Piao, X., Castañera González, R., Jones, P.M., and Persaud, S.J. (2018). The adhesion receptor GPR56 is activated by extracellular matrix collagen III to improve β-cell function. Cell. Mol. Life Sci. 75: 4007–4019, https://doi.org/10.1007/s00018-018-2846-4.Suche in Google Scholar PubMed PubMed Central
Paavola, K.J., Sidik, H., Zuchero, J.B., Eckart, M., and Talbot, W.S. (2014). Type IV collagen is an activating ligand for the adhesion G protein-coupled receptor GPR126. Sci. Signal. 7: ra76, https://doi.org/10.1126/scisignal.2005347.Suche in Google Scholar PubMed PubMed Central
Park, B.-O., KimHeon, S., Kong, G.Y., Da Kim, H., Kwon, M.S., Lee, S.U., Kim, M.-O., Cho, S., Lee, S., Lee, H.-J., et al. (2016). Selective novel inverse agonists for human GPR43 augment GLP-1 secretion. Eur. J. Pharmacol. 771: 1–9, https://doi.org/10.1016/j.ejphar.2015.12.010.Suche in Google Scholar PubMed
Petersen, S.C., Luo, R., Liebscher, I., Giera, S., Jeong, S.-J., Mogha, A., Ghidinelli, M., Feltri, M.L., Schöneberg, T., Piao, X., et al. (2015). The adhesion GPCR GPR126 has distinct, domain-dependent functions in Schwann cell development mediated by interaction with laminin-211. Neuron 85: 755–769, https://doi.org/10.1016/j.neuron.2014.12.057.Suche in Google Scholar PubMed PubMed Central
Petersen, R.K., Madsen, L., Pedersen, L.M., Hallenborg, P., Hagland, H., Viste, K., Døskeland, S.O., and Kristiansen, K. (2008). Cyclic AMP (cAMP)-mediated stimulation of adipocyte differentiation requires the synergistic action of Epac- and cAMP-dependent protein kinase-dependent processes. Mol. Cell Biol. 28: 3804–3816, https://doi.org/10.1128/mcb.00709-07.Suche in Google Scholar
Piao, X., Hill, R.S., Bodell, A., Chang, B.S., Basel-Vanagaite, L., Straussberg, R., Dobyns, W.B., Qasrawi, B., Winter, R.M., Innes, A.M., et al. (2004). G protein-coupled receptor-dependent development of human frontal cortex. Science 303: 2033–2036, https://doi.org/10.1126/science.1092780.Suche in Google Scholar PubMed
Ping, Y.-Q., Mao, C., Xiao, P., Zhao, R.-J., Jiang, Y., Yang, Z., An, W.-T., Shen, D.-D., Yang, F., Zhang, H., et al. (2021). Structures of the glucocorticoid-bound adhesion receptor GPR97-Go complex. Nature 589: 620–626, https://doi.org/10.1038/s41586-020-03083-w.Suche in Google Scholar PubMed
Prömel, S., Frickenhaus, M., Hughes, S., Mestek, L., Staunton, D., Woollard, A., Vakonakis, I., Schöneberg, T., Schnabel, R., Russ, A.P., et al. (2012). The GPS motif is a molecular switch for bimodal activities of adhesion class G protein-coupled receptors. Cell Rep. 321–331, https://doi.org/10.1016/j.celrep.2012.06.015.Suche in Google Scholar PubMed PubMed Central
Quesada, I., Todorova, M.G., and Soria, B. (2006). Different metabolic responses in alpha-, beta-, and delta-cells of the islet of Langerhans monitored by redox confocal microscopy. Biophys. J. 90: 2641–2650, https://doi.org/10.1529/biophysj.105.069906.Suche in Google Scholar PubMed PubMed Central
Rives, M.-L., Rady, B., Swanson, N., Zhao, S., Qi, J., Arnoult, E., Bakaj, I., Mancini, A., Breton, B., Lee, S.P., et al. (2018). GPR40-mediated Gα12 activation by allosteric full agonists highly efficacious at potentiating glucose-stimulated insulin secretion in human islets. Mol. Pharmacol. 93: 581–591, https://doi.org/10.1124/mol.117.111369.Suche in Google Scholar PubMed
Rossi, M., Zhu, L., McMillin, S.M., Pydi, S.P., Jain, S., Wang, L., Cui, Y., Lee, R.J., Cohen, A.H., Kaneto, H., et al. (2018). Hepatic Gi signaling regulates whole-body glucose homeostasis. J. Clin. Invest. 128: 746–759, https://doi.org/10.1172/jci94505.Suche in Google Scholar PubMed PubMed Central
Röthe, J., Thor, D., Winkler, J., Knierim, A.B., Binder, C., Huth, S., Kraft, R., Rothemund, S., Schöneberg, T., and Prömel, S. (2019). Involvement of the adhesion GPCRs latrophilins in the regulation of insulin release. Cell Rep. 26: 1573–1584.e5, https://doi.org/10.1016/j.celrep.2019.01.040.Suche in Google Scholar PubMed
Sachs, S., Niu, L., Geyer, P., Jall, S., Kleinert, M., Feuchtinger, A., Stemmer, K., Brielmeier, M., Finan, B., DiMarchi, R.D., et al. (2021). Plasma proteome profiles treatment efficacy of incretin dual agonism in diet-induced obese female and male mice. Diabetes Obes. Metab. 23: 195–207, https://doi.org/10.1111/dom.14215.Suche in Google Scholar PubMed
Schaum, N., Karkanias, J., Neff, N.F., May, A.P., Quake, S.R., Wyss-Coray, T., Darmanis, S., Batson, J., Botvinnik, O., Chen, M.B., et al. (2018). Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562: 367–372, https://doi.org/10.1038/s41586-018-0590-4.Suche in Google Scholar PubMed PubMed Central
Scholz, N., Gehring, J., Guan, C., Ljaschenko, D., Fischer, R., Lakshmanan, V., Kittel, R.J., and Langenhan, T. (2015). The adhesion GPCR latrophilin/CIRL shapes mechanosensation. Cell Rep. 11: 866–874, https://doi.org/10.1016/j.celrep.2015.04.008.Suche in Google Scholar PubMed
Scholz, N., Guan, C., Nieberler, M., Grotemeyer, A., Maiellaro, I., Gao, S., Beck, S., Pawlak, M., Sauer, M., Asan, E., et al. (2017). Mechano-dependent signaling by Latrophilin/CIRL quenches cAMP in proprioceptive neurons. Elife 6:e28360.10.7554/eLife.28360Suche in Google Scholar PubMed PubMed Central
Scholz, N., Langenhan, T., and Schöneberg, T. (2019). Revisiting the classification of adhesion GPCRs. Ann. N. Y. Acad. Sci. 1456: 80–95, https://doi.org/10.1111/nyas.14192.Suche in Google Scholar PubMed PubMed Central
Schöneberg, T. and Liebscher, I. (2021). Mutations in G protein-coupled receptors: mechanisms, pathophysiology and potential therapeutic approaches. Pharmacol. Rev. 73: 89–119, https://doi.org/10.1124/pharmrev.120.000011.Suche in Google Scholar PubMed
Schulz, N., Liu, K.-C., Charbord, J., Mattsson, C.L., Tao, L., Tworus, D., and Andersson, O. (2016). Critical role for adenosine receptor A2a in β-cell proliferation. Mol. Metab. 5: 1138–1146, https://doi.org/10.1016/j.molmet.2016.09.006.Suche in Google Scholar PubMed PubMed Central
Shanaki, M., Fadaei, R., Moradi, N., Emamgholipour, S., and Poustchi, H. (2016). The circulating CTRP13 in type 2 diabetes and non-alcoholic fatty liver patients. PLoS One 11: e0168082, https://doi.org/10.1371/journal.pone.0168082.Suche in Google Scholar PubMed PubMed Central
Shimpukade, B., Hudson, B.D., Hovgaard, C.K., Milligan, G., and Ulven, T. (2012). Discovery of a potent and selective GPR120 agonist. J. Med. Chem. 55: 4511–4515, https://doi.org/10.1021/jm300215x.Suche in Google Scholar PubMed
Silva, A.M., Liu-Gentry, J., Dickey, A.S., Barnett, D.W., and Misler, S. (2005). alpha-Latrotoxin increases spontaneous and depolarization-evoked exocytosis from pancreatic islet beta-cells. J. Physiol. 565: 783–799, https://doi.org/10.1113/jphysiol.2005.082586.Suche in Google Scholar PubMed PubMed Central
Sommer, C.A. and Mostoslavsky, G. (2014). RNA-Seq analysis of enteroendocrine cells reveals a role for FABP5 in the control of GIP secretion. Mol. Endocrinol. 28: 1855–1865, https://doi.org/10.1210/me.2014-1194.Suche in Google Scholar PubMed PubMed Central
Son, S.-E., Kim, N.-J., and Im, D.-S. (2021). Development of free fatty acid receptor 4 (FFA4/GPR120) agonists in health science. Biomol. Ther. (Seoul) 29: 22–30, https://doi.org/10.4062/biomolther.2020.213.Suche in Google Scholar PubMed PubMed Central
Stich, V., de Glisezinski, I., Crampes, F., Suljkovicova, H., Galitzky, J., Riviere, D., Hejnova, J., Lafontan, M., and Berlan, M. (1999). Activation of antilipolytic alpha(2)-adrenergic receptors by epinephrine during exercise in human adipose tissue. Am. J. Physiol. 277: R1076–R1083, https://doi.org/10.1152/ajpregu.1999.277.4.r1076.Suche in Google Scholar
Stoveken, H.M., Bahr, L.L., Anders, M.W., Wojtovich, A.P., Smrcka, A.V., and Tall, G.G. (2016). Dihydromunduletone is a small-molecule selective adhesion G protein-coupled receptor antagonist. Mol. Pharmacol. 90: 214–224, https://doi.org/10.1124/mol.116.104828.Suche in Google Scholar PubMed PubMed Central
Stoveken, H.M., Hajduczok, A.G., Xu, L., and Tall, G.G. (2015). Adhesion G protein-coupled receptors are activated by exposure of a cryptic tethered agonist. Proc. Natl. Acad. Sci. U.S.A. 288: 6194–6199, https://doi.org/10.1073/pnas.1421785112.Suche in Google Scholar PubMed PubMed Central
Suchý, T., Zieschang, C., Popkova, Y., Kaczmarek, I., Weiner, J., Liebing, A.-D., Çakir, M.Volkan, Landgraf, K., Gericke, M., Pospisilik, J.A., et al. (2020). The repertoire of Adhesion G protein-coupled receptors in adipocytes and their functional relevance. Int. J. Obes. (Lond) 44: 2124–2136, https://doi.org/10.1038/s41366-020-0570-2.Suche in Google Scholar PubMed PubMed Central
Sugita, S., Ichtchenko, K., Khvotchev, M., and Südhof, T.C. (1998). Alpha-latrotoxin receptor CIRL/latrophilin 1 (CL1) defines an unusual family of ubiquitous G-protein-linked receptors. G-protein coupling not required for triggering exocytosis. J. Biol. Chem. 273: 32715–32724, https://doi.org/10.1074/jbc.273.49.32715.Suche in Google Scholar PubMed
Tissir, F. and Goffinet, A.M. (2013). Atypical cadherins Celsr1-3 and planar cell polarity in vertebrates. Prog. Mol. Biol. Transl. Sci. 116: 193–214, https://doi.org/10.1016/b978-0-12-394311-8.00009-1.Suche in Google Scholar
Tu, Y.-K., Duman, J.G., and Tolias, K.F. (2018). The adhesion-GPCR BAI1 promotes excitatory synaptogenesis by coordinating bidirectional trans-synaptic signaling. J. Neurosci. 38: 8388–8406, https://doi.org/10.1523/jneurosci.3461-17.2018.Suche in Google Scholar PubMed PubMed Central
Ueno, H., Ito, R., Abe, S.-I., Ookawara, M., Miyashita, H., Ogino, H., Miyamoto, Y., Yoshihara, T., Kobayashi, A., Tsujihata, Y., et al. (2019). SCO-267, a GPR40 full agonist, improves glycemic and body weight control in rat models of diabetes and obesity. J. Pharmacol. Exp. Therapeut. 370: 172–181, https://doi.org/10.1124/jpet.118.255885.Suche in Google Scholar PubMed
Uhlén, M., Fagerberg, L., Hallström, B.M., Lindskog, C., Oksvold, P., Mardinoglu, A., Sivertsson, Å., Kampf, C., Sjöstedt, E., Asplund, A., et al. (2015). Proteomics. Tissue-based map of the human proteome. Science 347: 1260419, https://doi.org/10.1126/science.1260419.Suche in Google Scholar PubMed
Vernia, S., Edwards, Y.J., HanSook, M., Cavanagh-Kyros, J., Barrett, T., Kim, J.K., and Davis, R.J. (2016). An alternative splicing program promotes adipose tissue thermogenesis. Elife 5, https://doi.org/10.7554/eLife.17672.Suche in Google Scholar PubMed PubMed Central
Vizurraga, A., Adhikari, R., Yeung, J., Yu, M., and Tall, G.G. (2020). Mechanisms of adhesion G protein-coupled receptor activation. J. Biol. Chem. 295: 14065–14083, https://doi.org/10.1074/jbc.rev120.007423.Suche in Google Scholar
Wandel, E., Saalbach, A., Sittig, D., Gebhardt, C., and Aust, G. (2012). Thy-1 (CD90) is an interacting partner for CD97 on activated endothelial cells. J. Immunol. 188: 1442–1450, https://doi.org/10.4049/jimmunol.1003944.Suche in Google Scholar PubMed
Wang, L., Pydi, S.P., Zhu, L., Barella, L.F., Cui, Y., Gavrilova, O., Bence, K.K., Vernochet, C., and Wess, J. (2020). Adipocyte Gi signaling is essential for maintaining whole-body glucose homeostasis and insulin sensitivity. Nat. Commun. 11: 2995, https://doi.org/10.1038/s41467-020-16756-x.Suche in Google Scholar PubMed PubMed Central
Wang, T., Ward, Y., Tian, L., Lake, R., Guedez, L., Stetler-Stevenson, W.G., and Kelly, K. (2005). CD97, an adhesion receptor on inflammatory cells, stimulates angiogenesis through binding integrin counterreceptors on endothelial cells. Blood 105: 2836–2844, https://doi.org/10.1182/blood-2004-07-2878.Suche in Google Scholar PubMed
Wang, X., Zielinski, M.C., Misawa, R., Wen, P., Wang, T.-Y., Wang, C.-Z., Witkowski, P., and Hara, M. (2013). Quantitative analysis of pancreatic polypeptide cell distribution in the human pancreas. PLoS One 8: e55501, https://doi.org/10.1371/journal.pone.0055501.Suche in Google Scholar PubMed PubMed Central
Weston, M.D., Luijendijk, M.W.J., Humphrey, K.D., Möller, C., and Kimberling, W.J. (2004). Mutations in the VLGR1 gene implicate G-protein signaling in the pathogenesis of Usher syndrome type II. Am. J. Hum. Genet. 74: 357–366, https://doi.org/10.1086/381685.Suche in Google Scholar
Wilde, C., Fischer, L., Lede, V., Kirchberger, J., Rothemund, S., Schöneberg, T., and Liebscher, I. (2016). The constitutive activity of the adhesion GPCR GPR114/ADGRG5 is mediated by its tethered agonist. FASEB J. 30: 666–673, https://doi.org/10.1096/fj.15-276220.Suche in Google Scholar
Willard, F.S., Douros, J.D., Gabe, M.Bn, Showalter, A.D., Wainscott, D.B., Suter, T.M., Capozzi, M.E., van der Velden, W.Jc., Stutsman, C., Cardona, G.R., et al. (2020). Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight 5.10.1172/jci.insight.140532Suche in Google Scholar
Winzell, M.S. and Ahrén, B. (2007). G-protein-coupled receptors and islet function-implications for treatment of type 2 diabetes. Pharmacol. Ther. 116: 437–448, https://doi.org/10.1016/j.pharmthera.2007.08.002.Suche in Google Scholar
Yang, G., Chen, L., Grant, G.R., Paschos, G., Song, W.-L., Musiek, E.S., Lee, V., McLoughlin, S.C., Grosser, T., Cotsarelis, G., et al. (2016). Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci. Transl. Med. 8: 324ra16, https://doi.org/10.1126/scitranslmed.aad3305.Suche in Google Scholar
Yeung, J., Adili, R., Stringham, E.N., Luo, R., Vizurraga, A., Rosselli-Murai, L.K., Stoveken, H.M., Yu, M., Piao, X., Holinstat, M., et al. (2020). GPR56/ADGRG1 is a platelet collagen-responsive GPCR and hemostatic sensor of shear force. Proc. Natl. Acad. Sci. U.S.A. 117: 28275–28286, https://doi.org/10.1073/pnas.2008921117.Suche in Google Scholar
Yona, S., Lin, H.-H., Dri, P., Davies, J.Q., Hayhoe, R.P.G., Lewis, S.M., Heinsbroek, S.E.M., Brown, K.A., Perretti, M., Hamann, J., et al. (2008). Ligation of the adhesion-GPCR EMR2 regulates human neutrophil function. FASEB J. 22: 741–751, https://doi.org/10.1096/fj.07-9435com.Suche in Google Scholar
Young, P., Arch, J.R., and Ashwell, M. (1984). Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 167: 10–14, https://doi.org/10.1016/0014-5793(84)80822-4.Suche in Google Scholar
Zhan, C., Zhou, J., Feng, Q., Zhang, J.-E., Lin, S., Bao, J., Wu, P., and Luo, M. (2013). Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J. Neurosci. 33: 3624–3632, https://doi.org/10.1523/jneurosci.2742-12.2013.Suche in Google Scholar
© 2021 Isabell Kaczmarek et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Highlight: Signal Transduction in Health and Disease
- Signal transduction in health and disease
- Arrestin-dependent internalization of rhodopsin-like G protein-coupled receptors
- Post-translational lysine ac(et)ylation in health, ageing and disease
- The relevance of adhesion G protein-coupled receptors in metabolic functions
- The phytochemical plumbagin reciprocally modulates osteoblasts and osteoclasts
- Infection, inflammation and thrombosis: a review of potential mechanisms mediating arterial thrombosis associated with influenza and severe acute respiratory syndrome coronavirus 2
- Functional characterisation of two receptor interaction determinants in human thymic stromal lymphopoietin
- Corrigendum
- Corrigendum to: Emerging mechanisms of drug-induced phospholipidosis
Artikel in diesem Heft
- Frontmatter
- Highlight: Signal Transduction in Health and Disease
- Signal transduction in health and disease
- Arrestin-dependent internalization of rhodopsin-like G protein-coupled receptors
- Post-translational lysine ac(et)ylation in health, ageing and disease
- The relevance of adhesion G protein-coupled receptors in metabolic functions
- The phytochemical plumbagin reciprocally modulates osteoblasts and osteoclasts
- Infection, inflammation and thrombosis: a review of potential mechanisms mediating arterial thrombosis associated with influenza and severe acute respiratory syndrome coronavirus 2
- Functional characterisation of two receptor interaction determinants in human thymic stromal lymphopoietin
- Corrigendum
- Corrigendum to: Emerging mechanisms of drug-induced phospholipidosis


